Abstract
A recent literature review of the field shows that tissue-engineered skin has been in clinical use for the last several decades and that, over this time the technology has advanced rapidly. Despite this progress no synthetic skin yet produced has completely replicated normal, healthy skin. Therefore, researchers must continue to develop materials that successfully overcome the problems with current skin tissue substitutes. This paper is a comprehensive review of the prospects for nanotechnology and nanomaterials to close this gap by mimicking surface properties for reconstruction of a variety of skin tissues. In addition, a number of commercially available products that regenerate different layers of the burn-damaged or chronically wounded skin are reviewed.
Keywords: Skin, regeneration, scaffold, tissue engineering
Introduction
Tissue engineering is an interdisciplinary area of nanomedicine in which biomaterial and medical science understands of pathological tissue and the principles used to achieve this understanding are applied to the improving or sustaining of tissue function through the development of biological substitutes. To achieve these goals, an artificial extra cellular matrix (ECM) and a source of cells are required. Although skin tissue constitutes a small amount of the body weight, skin damage such as burns and diabetic diseases can cause serious health complications. Tissue engineered replacements play on extremely important role in the treatment of chronic skin wounds. Skin tissue replacement techniques should be easy to perform for applying to the wound location. Skin tissue replacements must be readily adherent, have good physical and mechanical properties, and be non-antigenic [1],. The ultimate goal is to design and develop skin tissue engineering techniques that lead to novel and biomaterials-based skin replacement therapies. Skin tissue engineering generally requires a biomimetic ECM that can be assimilated into the body when the new tissue is regenerated. Biomaterials for such a matrix could be naturally occurring substances such as collagen or could be prepared from biodegradable synthetic polymers. Resorption, along with adequate cell adhesion onto the matrix, gives the biological materials an attractive potential in tissue regeneration.
A major part of any strategy for using an engineered skin tissue in which repair and regeneration takes place in an interaction with cells is to identify mechanisms and appropriate sources of cells by which such interaction can be most effective and productive. And, of course, the cells must be sufficiently abundant for complete regeneration to occur. Recently, some of the major advances in molecular biology have been applied to the understanding of wound healing, development and regenerative processes [1,2]. Tissue engineering as a discipline is becoming more aware of this knowledge base and there are now efforts towards designing artificial tissues using both cells and specifically designed biomaterials. This review article focuses on understanding the biomaterials, nanotechnology, regeneration biology and current advances in human skin substitutes. The review is divided into three parts: (1) biomaterials with some examples, (2) nanotechnology and its applications, and (3) commercially available skin substitutes.
Biomaterials
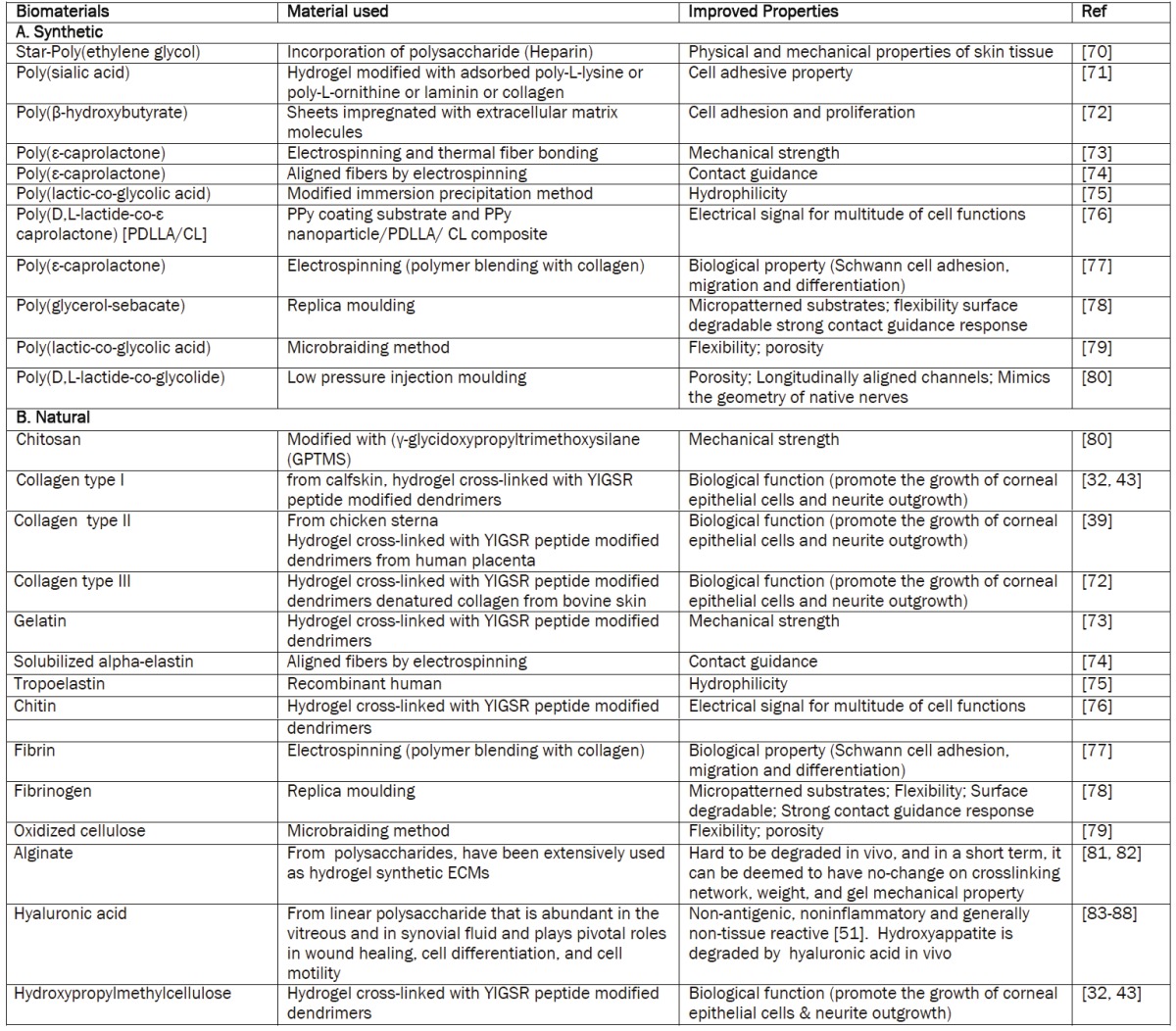
Tissue engineered skin replacements require a specific process to meet the main objectives [3,4]. The specification of this process in skin has depended on using both epidermal and dermal layers together to produce a replacement skin, which can then be grafted in place [3,4]. ECM is used as biomimetic update materials and is obtained from natural materials and also those manufactured synthetically. Examples of natural materials include fibronectin, collagen, chitosan, hyaluronan, polypeptides, hydroxyapatites, glycosaminoglycan, and alginates. These biomaterials have the excellent advantage of having low toxicity and a low chronic inflammatory response. Examples of synthetic materials include polylactide-co-glycolide, polylactide, and poly-glycolide which are used for sutures and meshes [5]. Other examples include polytetrafluoroethylene and polyethylene terephthalate (Table 1). Matrices used, often routinely, in healing applications are made from polymers that are often degraded in the body.
Table 1.
Biomaterials used or potentially suitable for skin replacement and improved properties)[35].
|
There are two primary fundamental challenges to obtaining degradable polymers. First, the first generation degradable polymers are widely used in tissue engineering but have poor mechanical and degradation properties [6]. To overcome these deficiencies, new classes of degradable materials are being developed.
The second fundamental challenge is how to process these polymers into scaffolds that have defined pore sizes and shapes that can direct tissue growth [7]. New nanotechnologies such as three-dimensional printing [8,9] and electrospinng [10,11] are emerging for accurate manufacturing of materials of defined pore size. Both the polymer implant and the subsequent generation of any degradation products in situ, must be non-toxic and non-immunogenic. It is also widely understood that with synthetic materials there are no cell-recognition signals. Progress may come in the form of the ongoing development of manufacturing processes that will incorporate cell-adhesion peptides into biomaterials. It is recognized that such peptides are involved in cellular interactions.
Natural materials
A number of natural materials have excellent properties that have led to their use in skin tissue engineering because of similar cellular properties to human skin tissues, including those pertaining to adhesion and infiltration Natural materials such as collagen [4], gelatin [3], laminin [5] and chitosan [6,7,9] et al. have been used as electrospun scaffolds for tissue engineering. Chitosan nanofiber meshes were analysed for their mechanical strength and permeability in the case of a rat tissue injury [7,9]. The poor mechanical properties of these constructs may restrict their application as nerve guide conduits. Collagen, a triple-helix protein, is one of the major components of the ECM and is found in all connective tissues. For this reason it is one of the most widely studied natural biomaterials employed in the field of tissue engineering. Natural biomaterials such as collagen are characterized by low mechanical strength, good biocompatibility, and low antigenicity. Collagen can be used in crude form or processed into porous sponges, gels, and sheets, and has the potential of being cross-linked with chemicals to make it stronger or to alter its degradation rate [12]. The use of a collagen-based matrix has been reported in several studies, not only in peripheral nerve regeneration [10,11], but also in spinal cord healing, scar formation [13], and tendon regeneration [14]. Despite their positive aspects, natural materials have several disadvantages. First, they may be immunological and inflammatory responses resulting from, as yet, undefined factors and pathogens and for which complete removal by purification before implantation is not possible. Second, natural materials are not homogeneous as a result of growth factors and the presence of residual constituents in the product. [15]. Third, synthetic materials are less costly and more readily characterized than natural materials.
Synthetic materials
Polymers have several advantages for the production of tissue engineering scaffolds, including (1) known compositions, (2) can be tailored vide a range of nerve prostheses by combining different copolymers in various proportions. Each combination produces tubular scaffolds with specific mechanical properties clearly; nerve guide conduits fabricated from biodegradable polymers are preferable to non-biodegradable polymers because of the advantage of avoiding a second surgery to remove the conduit. An important class of biomaterials used to develop tubular nerve guides is poly (α-hydroxy esters). These synthetic polymers are readily made into 3-D scaffolds that are bioresorped via hydrolysis in carbon dioxide and water. Researchers have tested several polyester nanofibrous fibers and scaffolds as nerve guides that have shown negligible inflammatory response, made of poly (glycolic acid) (PGA) [16], poly (lactide acid) (PLA) [17], poly (L-lactic acid) (PLLA) [18,19], or of a blend of poly (L-lactic acid)-caprolactone (PLLA PCL) [20,21] and poly (D,L-lactide-co-glycolide) and poly (ɛ caprolactone) (PLGA/PCL). Other synthetic materials for tissue engineering are hydrogels, insoluble hydrophilic polymers having high water content and tissue like mechanical properties that make them highly attractive scaffolds for implantation in empty tubular nerve prosthesis or for direct injection into the lesion site to enhance cell attachment and growth. Self-assembling peptides belong to the hydrogel class of biomaterials and comprise a well-defined amino acid sequence that, under physiological conditions, self-assembles to form a nanonscale fibrous scaffold.. Studies of these materials over several decades for use as biomaterials have demonstrated their usefulness not only for specific 3-D tissue cell cultures but also for tissue repair and regenerative therapies. These peptide scaffolds can be custom synthesized commercially and are readily modifiable inexpensively and quickly at the single-amino acid level. Furthermore, these designer self-assembling peptide scaffolds have recently become powerful tools for regenerative medicine to repair infarcted myocardia [22], to stop bleeding in seconds [23], and to repair nerve tissue [23]. Ellis-Behnke and colleagues [23] showed that the self-assembling peptide RADA16-I is a promising scaffold for neural regeneration in optic nerve lesions in hamster pups. Animals were treated by injection of RADA16-I in to their surgical wounds. Only in animals treated with multiple injections of the self-assembling scaffold did the brain tissue appear to have healed, whereas in untreated animals functional damage was still evident after weeks [23]. It was noted earlier in this paper that these synthetic materials have a major failing in that they lack cell recognition signals and, consequently, there are few cell interactions. Efforts to overcome this weakness are focussed on efforts to incorporate cell adhesion peptide motifs into synthetic biomaterials.
Nanotechnology
Nanotechnology is an emerging interdisciplinary technology whose applications have expanded into many areas over the last decade, including materials science, medicine and biomedical engineering. The essence of this new technology is the creation and utilization of surfaces, materials and devices at the molecular level [23]. Nanotechnology has had a great and significant impact in the field of medicine where it is used for diagnosis, tissue engineering and drug delivery. One of the biggest advantages of nanotechnology is the fast surgical recovery and its applications in tissue re-engineering. The properties of substances change dramatically when their size is reduced to the nanometric level. When a small amount of nanosize materials is mixed with a polymer matrix the performance of the resultant system is improved to an unprecedented level. These advances in nanotechnology based methods to medical technologies are a result of increased investments and directed research in nanotechnology.
Tissue Engineering
Tissue engineering is a development of biomaterials research and involves the production of various tissue substitutes from a range of biodegradable polymers and cells that are able to produce new tissues or restore existing ones to their original structural characteristics and functions. It is an interdisciplinary field that combines the knowledge of many sciences ranging from biology to materials science and medicine [24] and has been a subject of intensive research for human health care systems. The human body is a complex and well organized system of tissues and organs. Nutrients, oxygen and the suitable environment for cell growth are available in tissues. The extracellular matrix (ECM) component of tissues is a complex structure surrounding and supporting the cells within mammalian tissues. It is composed of three major classes of biomolecules: (1) structural proteins, mainly collagen and elastin, (2) specialized proteins such as fibrillin, fibronectin and laminin, and (3) proteoglycans which are proteins to which long chains of repeating disaccharide units called glycosaminoglycans (GAGs), are attached. Cell-extracellular matrix (ECM) and cell-cell interactions determine the ability of cells to build tissues and maintain tissue-specific functions. One of tissue engineering's primary objectives is to recreate an appropriate cellular environment that supports the control and regulation of cell functions [25]. During the last decade intensive research has been conducted in this field. There are two main components of a tissue engineered product cells and the carrier. The success rate of tissue engineering depends in part on carriers which are designed as scaffolds. The best approach is to design the scaffold, preferably a biodegradable one, to mimic the functions and structure of the naturally existing ECM. In constructing an engineered tissue, the cells are initially isolated from the donor tissue and cultured under in vitro conditions (Figure 1). A polymeric scaffold is designed by means of various processing methods such as solvent casting, salt leaching, phase separation, self-assembly, gas foaming and electrospinning [26]. The cells are then seeded and cultured on this scaffold (or cell carrier). In order to imitate the natural environment of cells, the above steps are performed in either static culture conditions or dynamic bioreactor systems [26]. The cells that are cultured on a scaffold should have a similar behaviour as they would have in the body without a synthetic scaffold. Healing occurs as the cells proliferate, migrate, differentiate and remodel the scaffold and the surrounding tissue.
Figure 1.
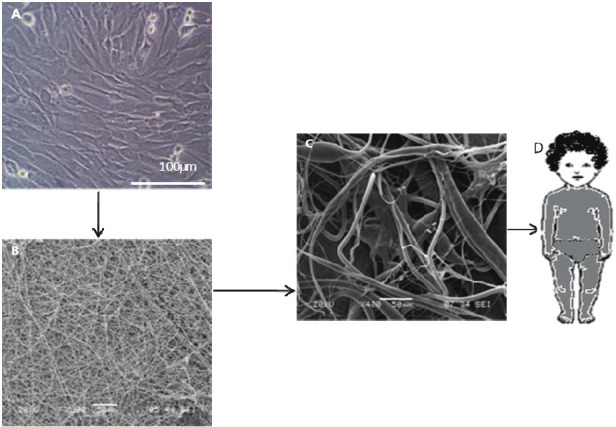
Schematic presentation of tissue engineering methodology (A) cell culture (B) biogradable nanofiber scaffold (C) cell seeded on scaffold (D) tissue grown on bioreactor.
Scaffold materials
Since most of the cells are dependent on the scaffold characteristics, biomaterial choice for scaffold design is of great importance for proper adhesion, spreading etc. Polymers are the most suitable scaffold materials due to their flexibility and controllable functional properties. Depending on the requirements of the target tissue, the material is chosen to be either a naturally derived polymer (collagen [27], cellulose [28], chitin [29], starch [30], hyaluronic acid [31], silk fibroin [29] or a synthetic one (poly (lactic acid) [32], poly (glycolic acid) [32,33], poly (lactide-co-caprolactone) [34] poly (ethylene glycol) and their combinations (Table 1)[35].
Biodegradable materials are preferred as tissue engineering scaffolds since they degrade while the new tissue forms. Another requirement is that the carrier material and the degradation products should be biocompatible so that no adverse body reactions occur when the material degrades. Copolymers have also been utilized in the manufacture of scaffolds since polymers with varying degrees of crystallinity, and thus with a range of properties, are available. These copolymers still crystallize but have the ability to melt at lower temperatures, thus making processing easier. Copolymers have the advantage that they degrade in vivo to D-3-hydroxybutyrate, which is a normal constituent of human blood, and to 3-hydroxyvalerate. Their in vitro biodegradability [36] and biocompatibility in the presence of various cell lines [37,38] are reported in the literature. Further, it was demonstrated that even the form of the scaffold affects the results [19]. Higher biodegradability was observed with nonwoven, fibrous PHBV8 structures in comparison to films of the same material. Furthermore, the suitability of this non-woven, fibrous material as a scaffold for tissue engineering [37-40] and it’s in vivo biocompatibility [41] has been demonstrated previously.
Types scaffold
As mentioned above, the first step of tissue engineering is the design of a 3-D scaffold that mimics the ECM. Therefore, when designing the scaffolds there are some very important points that should be considered. One of the most crucial is that both the carrier material and its degradation products should have proven biodegradability and biocompatibility. Moreover, the scaffold should possess appropriate porosity and permeability to allow the transfer of nutrients necessary for the cells and wastes produced by the cells. A surface chemistry that enables attachment and spreading of cells is also required. The material should have an appropriate degradation rate and mechanical properties so that the artificial material is eliminated in time and different stresses that may develop during new tissue formation can be handled [24,42]. Finally, the technique that is used to fabricate the scaffolds should not affect the biocompatibility of the material used [19]. To date, scaffolds have been produced and tested for their suitability for tissue engineering in different forms such as films [32,43], foams [44] and fibers [63] with widely differing chemistries and a variety of studies ranging from in situ to clinical have been carried out. Foam and film types proved most popular, initially, but there has fibrous scaffolds have been seeing an increase in use recently.
Some recent studies have shown that cells attach, grow and organize well on nanofibrous structures even though the fiber diameter is smaller than that of the cells [45]. High porosity that allows rapid transfer of nutrients and wastes and the large surface areas that provide sites for the cells to attach are the requirements of a successful scaffold. Furthermore, micro and nanofeatured scaffolds with controlled pore size, geometry, dimension and spatial orientation are being intensely investigated. Since fibrous scaffolds with diameters of fibers on a nanometer scale have been found to be satisfactory for tissue engineering extensive research towards developing processes for the fabrication of these fibrous structures is being pursued.
Commercial skin substitutes
Fibroblasts also play an important role in skin substitutes in addition to the epithelial nature of components (cultured epithelial autografts) of certain constructs (Figure 2). The dermal component improves critical communication pathways (i.e.) between the epidermis and the dermis) (Figure 2) and enhances the formation of more mature basement membrane. Moreover, in both fetal development and adult wound repair, the formation of a more complete basement membrane requires epidermal mesenchymal interactions. Advances in tissue culture and molecular biology began to merge in the early 1970’s to render possible the recombinant techniques for producing growth factors and the cell biology required for tissue-engineering attempts aimed at the reconstitution of injured tissues and organs. Rheinwald and Green [46] developed techniques for growing human epidermal keratinocytes from small patient biopsy samples by providing mesenchymal cells in the form of irradiated 3T3 fibroblasts. Subsequently, the field advanced to clinical applications with further developments in basic science. Examples of these developments are the use of cultured epithelia for burn victims [47], epidermal coverage [48] for stimulation of new (neodermis) connective tissue [68], and increased graft take when combined with cadaver dermis [49-51]. Admittedly, scarring and wound contraction remain significant problems [52]. These findings may be attributable to dermal factors influencing epithelial migration, differentiation, attachment, and growth [53,54]. Decreased immunogenicity of human cadaver allografts has been partially circumvented, for example, by the use of acellular dermal matrix with an intact basement membrane to aid the take and healing of ultrathin autografts (AlloDerm) [55]. In other studies, investigators have redirected granulation tissue formation through the use of scaffolds and living cells. For example, Yannas et al. [56] designed a collagen -glycosaminogly can sponge to serve as a scaffold or template for dermal extracellular matrix. A commercial version of this material composed of bovine collagen and chondroitin sulfate, with a silicone membrane covering (Integra), is approved for use in burns [57-59]. Slowly, resorption of the dermal layers occurs and followed by the eventual removal of the silicon membrane and its replacement buy a thin autograft. The ability of the matrix to survive long enough to redirect formation of tissue must be balanced with effects of the matrix on inflammation. One way to accomplish this is to form a biological tissue that is recognized as living tissue, not a foreign substance. At least partial success with this approach has been seen in the use of human neonatal fibroblasts grown on biodegradable mesh (Dermagraft) [60]. In another approach, a nylon mesh coated with porcine collagen and layered with a nonpermeable silicone membrane (Smith & Nephew, Hull, United Kingdom) was used as a podium for deposition of human matrix proteins and associated factors by the human dermal fibroblasts [61]. In ongoing series of experiments, Boyce et al. [62] have modified the approach first proposed by Yannas et al. [11] to form a bilayered composite skin made using a modified collagen-glycosaminoglycan substrate seeded with fibroblasts and overlaid with epidermal keratinocytes [62,63]. Several challenges face the use of cultured epithelial autografts, including variable results on full-thickness wounds and time delays for culture cells to confluence in preparation for clinical application. Various schemes have been adopted by researchers to reduce these problems and include the use of dermal allografts and vascularized collagen-glycosaminoglycan matrices (i.e., Integra) [64].
Figure 2.
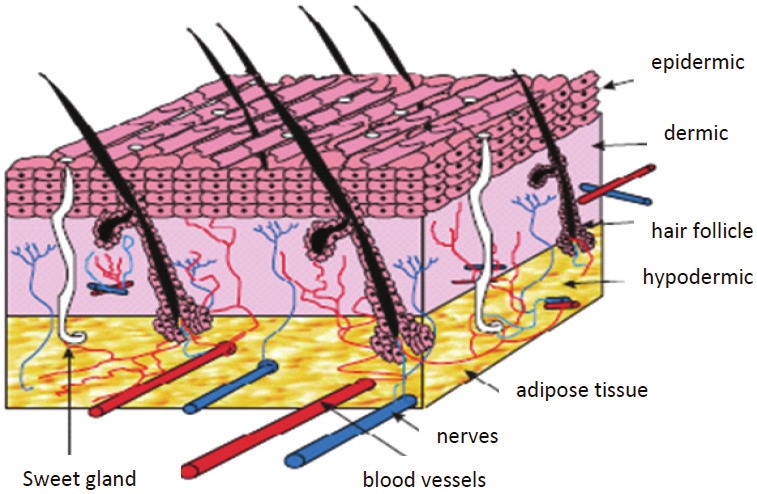
Schematic of skin structure [69].
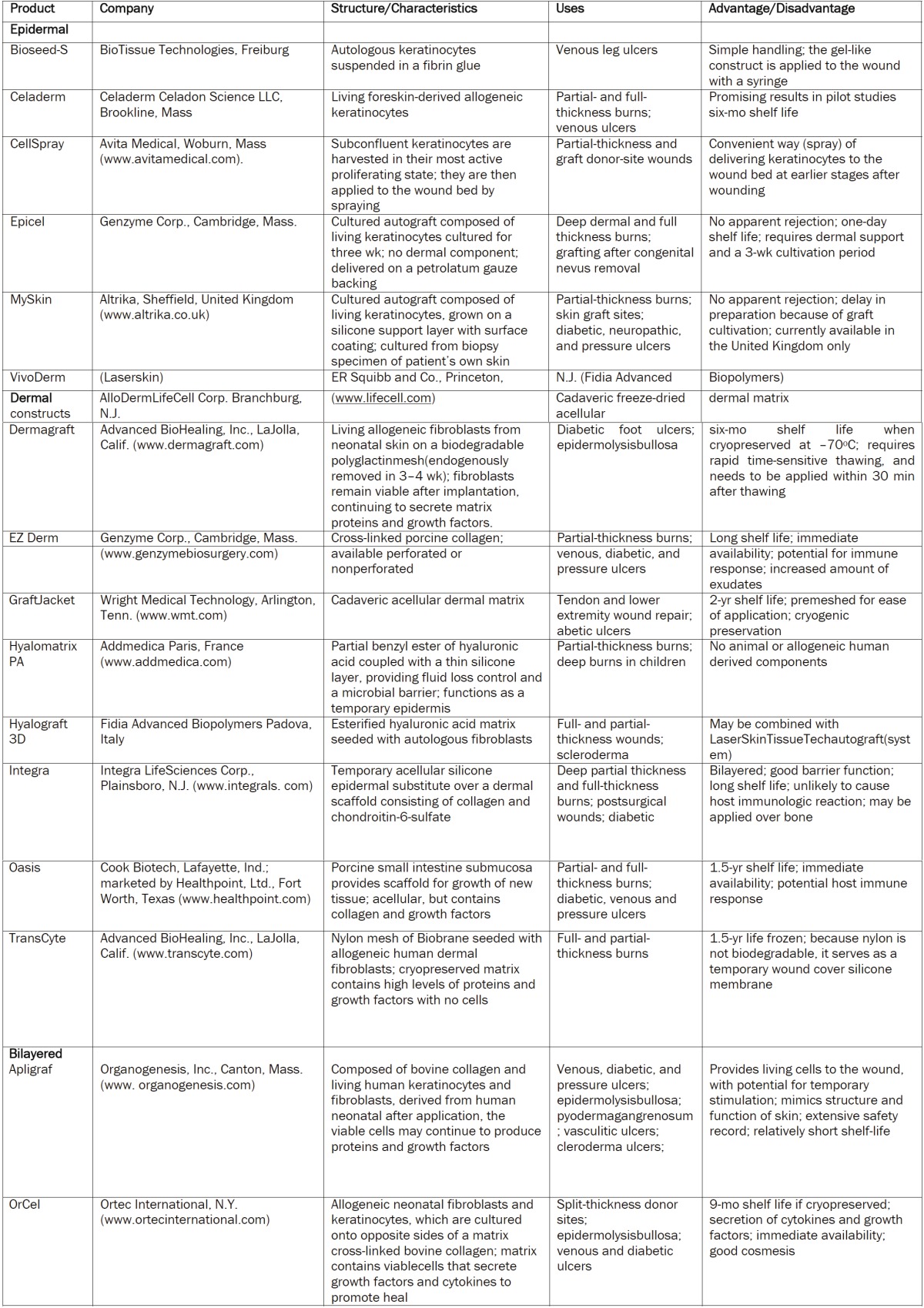
Vascularized collagen-glycosaminoglycan matrices were shown to produce a favourable substrate for cultured epithelial autografts and may improve the take of cultured epithelial autografts in burn patients. Another advance in skin bioengineering is the combination of seeded keratinocytes and tissue-engineered dermal matrices. Compton et al. [65] observed in vivo regeneration of organized skin structure in a month from a biodegradable collagen-glycosaminoglycan matrix impregnated with a dilute suspension of freshly isolated autologous keratinocytes [65]. This was the first time that an acellular matrix was shown to lead to true dermal regeneration. In this and other applications, epidermal- mesenchymal interaction is critical for exchange of information between keratinocytes and fibroblasts in skin morphogenesis during development, and in maintaining skin structure in adults. Epidermal-mesenchymal interaction has led to the concept of double paracrine signaling, whereby keratinocytes initiate growth factors in fibroblasts, which then stimulate keratinocyte proliferation. Moreover, fibroblasts can acquire a myofibroblast phenotype under the control of keratinocytes [66]. Other experiments have shown the cellular origin of basement membrane and extracellular matrix components in epidermal dermal co-cultures [67]. Therefore, epithelial-mesenchymal co-cultures provide an environment where the epidermal stem cell phenotype and keratinocyte proliferation are supported by a synergistic mix of growth factors, extracellular matrix components, and direct cell-to-cell contacts [66], epidermal, dermal, or composite; bilayered or dermo epidermal) (Table 2); (1) duration of the cover (temporary, semipermanent, or permanent); (2) cellular composition (cellular or acellular); and (3) material type [biological (consisting of autologous, allogeneic, or xenogeneic cells/materials) or synthetic (biodegradable or nonbiodegradable)]. It should be noted that Table 2 is an attempt to provide information on many different types of products. Some of the products may not be easily commercially available; they have nevertheless played a role in the advancement and evolution of the field. Initially, especially with such constructs as cultured epidermis and living bilayered skin constructs (Graftskin/Apligraf), investigators hypothesized that there could be some degree of permanent engraftment. However, it is now clear that such constructs act primarily not as tissue or cell replacement, but rather as a temporary stimulus for tissue repair and/or as pharmacologic agents capable of delivering critical signals to the wound. In fact, it is important to understand that a key role of nanotechnology tissue may be to deliver growth factors, extracellular matrix proteins and possibly to attract differentiated cells (e.g., fibroblasts, endothelial cells) or stem cells to the wound. Therefore, these nanotechnology skin tissue products should not be regarded as being the same as autografts. There is generally no true take, although investigative and manufacturing efforts are being made to allow some degree of take. The skin constructs usually do not stay in the wound for more than a few weeks, as shown by biochemical markers and DNA evidence. Instead, the constructs serve to stimulate and augment the wound’s intrinsic healing process and wound bed preparation [68].
Table 2.
Commercially created skin substitutes, skin products and representative properties
|
Summary and future outlook
Skin tissue engineering's primary goal is to enable the rapid formation of a construct that will support and enable the complete regeneration of functional skin with all the skin appendages, the various layers (epidermis, dermit, fatty subcutis), and a fully functioning and scar free integration of the vascular and nerve network with the host tissue.
There has been progress in skin tissue engineering research such that more use is being made of biomaterials and nanotechnology for tissue engineering applications and it is anticipated that further novel skin tissue biomaterials using nanotechnology are coming.
Currently, the success of tissue engineering skin is very dependent on the skilful use of surgical techniques and preparation of the wound bed and so the hope is that future technologies will be less dependent upon these factors and hence increase the success of tissue replacement and the increase the rate of recovery.
Acknowledgement
The authors would like to thank Dr. Moorehouse Fellowship, Manitoba Diabetes Foundation, NSERC Discovery, Manitoba Children’s Foundation and Manitoba Institute of Child Health.
References
- 1.Liu WaYC. Application of scaffold materials in tissue reconstruction in immunecompetent mammals: Our experience and future requirements. Biomaterials. 2007;28:5078–5086. doi: 10.1016/j.biomaterials.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–39. doi: 10.22203/ecm.v005a03. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z-M, Zhang YZ, Ramakrishna S, Lim CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer. 2004;45:5361–5368. [Google Scholar]
- 4.Matthews JA, Boland ED, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen type II: A feasibility study. Journal of Bioactive and Compatible Polymers. 2003;18:125–134. [Google Scholar]
- 5.Zambuzzi WF, et al. Biological behavior of pre-osteoblasts on natural hydroxyapatite: A study of signaling molecules from attachment to differentiation. Journal of Biomedical Materials Research Part A. 2011;97:193–200. doi: 10.1002/jbm.a.32933. [DOI] [PubMed] [Google Scholar]
- 6.Nijweide PJ, van der Plas A, Olthof AA. Osteoblastic differentiation. CIBA Foundation Symposia. 1988;136:61–77. doi: 10.1002/9780470513637.ch5. [DOI] [PubMed] [Google Scholar]
- 7.Li S, et al. Removal of Cationic Dye from Aqueous Solution by a Macroporous Hydrophobically Modified Poly(acrylic Acid-acrylamide) Hydrogel with Enhanced Swelling and Adsorption Properties. Soil, Air, Water. 2010;38:378–386. [Google Scholar]
- 8.Lee WJ, Lee TG, Koh WG. Grafting of Poly (acrylic acid) on the Poly(ethylene glycol) Hydrogel Using Surface-initiated Photopolymerization for Covalent Immobilization of Collagen. Journal of Industrial and Engineering Chemistry. 2007;13:1195–1200. [Google Scholar]
- 9.Liu C-B, et al. A new multifunctional polymer: Synthesis and characterization of mPEG-b-PAA-grafted chitosan copolymer. Journal of Central South University of Technology. 2010;17:936–942. [Google Scholar]
- 10.Wu HH, Thomas JA, Momand J. p53 protein oxidation in cultured cells in response to pyrrolidine dithiocarbamate: a novel method for relating the amount of p53 oxidation in vivo to the regulation of p53-responsive genes. Biochem J. 2000;351:87–93. doi: 10.1042/0264-6021:3510087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yannas I. Tissue and organ regeneration in adults. Springer. 2001;10 [Google Scholar]
- 12.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. International Journal of Pharmaceutics. 2001;221:1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 13.Nam J, et al. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomaterialia. 2011;7:1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minton AP. Confinement as a determinant of macromolecular structure and reactivity. Biophysical Journal. 1992;63:1090–1100. doi: 10.1016/S0006-3495(92)81663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatahet F, et al. Disruption of reducing pathways is not essential for efficient disulfide bond formation in the cytoplasm of E. coli. Microbial Cell Factories. 2010;9:67. doi: 10.1186/1475-2859-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilie C, et al. The influence of nonionic surfactants on the carbopol-peg interpolymer complexes. Revue Roumaine De Chimie. 2010;55:409–417. [Google Scholar]
- 17.Herrmann JM, Köhl R. Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. Journal of Cell Biology. 2007;176:559–563. doi: 10.1083/jcb.200611060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collet JF, Bardwell JC. Oxidative protein folding in bacteria. Molecular Microbiology. 2002;44:1–8. doi: 10.1046/j.1365-2958.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 19.Derman A, et al. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993;262:1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- 20.Prinz W, et al. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. Journal of Biological Chemistry. 1997;272:15661–15667. doi: 10.1074/jbc.272.25.15661. [DOI] [PubMed] [Google Scholar]
- 21.Stewart EJ, Aslund F, Beckwith J. Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J. 1998;17:5543–5550. doi: 10.1093/emboj/17.19.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable Self-Assembling Peptide Nanofibers Create Intramyocardial Microenvironments for Endothelial Cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis-Behnke RG, Liang Y-X, Tay DKC, Kau PWF, Schneider GE, Zhang S, Wu W, So K-F. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine: Nanotechnology, Biology and Medicine. 2006;2:207–215. doi: 10.1016/j.nano.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Gomes ME, Godinho JS, Tchalamov D, Cunha AM, Reis RL. Alternative tissue engineering scaffolds based on starch: processing methodologies, morphology, degradation and mechanical properties. Materials Science and Engineering: C. 2002;20:19–26. [Google Scholar]
- 25.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 26. {Radisic, 2004 #2246}
- 27.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan G, Murugesan S, Pushparaj V, Nalamasu O, Ajayan PM, Linhardt RJ. Preparation of Biopolymer Fibers by Electrospinning from Room Temperature Ionic Liquids. Biomacromolecules. 2006;7:415–418. doi: 10.1021/bm050837s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byung-Moo Mina, Sung WL, Jung NL, et al. Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers. Polymer. 2004;45:7137–7142. [Google Scholar]
- 30.Tuzlakoglu K, Bolgen N, Salgado A, Gomes M, Piskin E, Reis R. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. Journal of Materials Science: Materials in Medicine. 2005;16:1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 31.El Backly RM, Cancedda R. Bioreactor Systems for Tissue Engineering Ii: Strategies for the Expanison and Directed Differentiation of Stem Cells. Berlin: Springer-Verlag Berlin; 2010. Bone Marrow Stem Cells in Clinical Application: Harnessing Paracrine Roles and Niche Mechanisms; pp. 265–292. [DOI] [PubMed] [Google Scholar]
- 32.Zong X, Kim K, Fang D, Ran S, Hsiao B, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–4412. [Google Scholar]
- 33.Kim K, Yu M, Zong X, Chiu J, Fang D, Seo Y-S, Hsiao BS, Chu B, Hadjiargyrou M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(,-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977–4985. doi: 10.1016/s0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 34.Son WK, Youk JH, Lee TS, Park WH. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly (ethylene oxide) fibers. Polymer. 2004;45:2959–2966. [Google Scholar]
- 35.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. Journal of Biomedical Science. 2009;16:108–109. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JS, Lee SW, Jeong L, Bae S-H, Min BC, Youk JH, Park WH. Effect of organosoluble salts on the nanofibrous structure of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) International Journal of Biological Macromolecules. 2004;34:249–256. doi: 10.1016/j.ijbiomac.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Chen G-Q, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Köse GT, Kenar H, HasIrcI N, HasIrcI V. Macroporous poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for bone tissue engineering. Biomaterials. 2003;24:1949–1958. doi: 10.1016/s0142-9612(02)00613-0. [DOI] [PubMed] [Google Scholar]
- 39.Köse GT, Korkusuz F, Özkul A, Soysal Y, Özdemir T, Yildiz C, Hasirci V. Tissue engineered cartilage on collagen and PHBV matrices. Biomaterials. 2005;26:5187–5197. doi: 10.1016/j.biomaterials.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 40.Köse GT, Korkusuz F, Korkusuz P, Purali N, Özkul A, HasIrcI V. Bone generation on PHBV matrices: an in vitro study. Biomaterials. 2003;24:4999–5007. doi: 10.1016/s0142-9612(03)00417-4. [DOI] [PubMed] [Google Scholar]
- 41.Hohman M, Rutledge G, Brenner MP. Electrospinning and electrically forced jets. I. Stability theory. Physics of Fluids. 2001 [Google Scholar]
- 42.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 43.Keun Kwon I, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929–3939. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, Vescovi A, Gelain F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnology. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K, Luu YK, Chang C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactideco-glycolide)-based electrospun nanofibrous scaffolds. Journal of Controlled Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 47.Gallico GG, O'Connor NE, Compton CC, Kehinde O, Green H. Permanent Coverage of Large Burn Wounds with Autologous Cultured Human Epithelium. New England Journal of Medicine. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 48.Compton CC, Hickerson W, Nadire K, Press W. Acceleration of Skin Regeneration From Cultured Epithelial Autografts by Transplantation to Homograft Dermis. Journal of Burn Care and Rehabilitation. 1993;14:653–662. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Compton CC, Gill JM, Bradford DA, Regauer S, Gallico GG, Oconnor NE. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting - a light, electron-microscopic and immunohistochemical study. Laboratory Investigation. 1989;60:600–612. [PubMed] [Google Scholar]
- 50.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. The Lancet. 1986;327:1123–1124. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 51.Langdon RC, Cuono CB, Birchall N, Madri JA, Kuklinska E, McGuire J, Moellmann GE. Reconstitution of structure and cell-function in human-skin grafts derived from cryopreserved allogeneic dermis and autologous cultured keratinocytes. Journal of Investigative Dermatology. 1988;91:478–485. doi: 10.1111/1523-1747.ep12476623. [DOI] [PubMed] [Google Scholar]
- 52.Sheridan RL, Tompkins RG. Cultured Autologous Epithelium in Patients with Burns of Ninety Percent or More of the Body Surface. The Journal of Trauma. 1995;38:48–50. doi: 10.1097/00005373-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Cuono CB, Langdon R, Birchall N, Barttelbort S, McGuire J. Composite autologous-allogeneic skin replacement-development and clinical-application. Plastic and Reconstructive Surgery. 1987;80:626–635. doi: 10.1097/00006534-198710000-00029. [DOI] [PubMed] [Google Scholar]
- 54.Phillips TJ, Bhawan J, Leigh IM, Baum HJ, Gilchrest BA. Cultured epidermal autografts and allografts: a study of differentiation and allograft survival. Journal of the American Academy of Dermatology. 1990;23:189–198. doi: 10.1016/0190-9622(90)70197-p. [DOI] [PubMed] [Google Scholar]
- 55.Lattari V, Jones LM, Varcelotti JR, Latenser BA, Sherman HF, Barrette RR. The use of a permanent dermal allograft in full-thickness burns of the hand and foot: A report of three cases. Journal of Burn Care and Rehabilitation. 1997;18:147–155. doi: 10.1097/00004630-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Yannas I, Burke J, Orgill D, Skrabut E. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 57.Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Annals of Surgery. 1981;194:413–428. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, Jordan M, McManus W, Solem L, Warden G, Zawacki B. Artificial dermis for major burns. A multi-center randomized clinical trial. Annals of Surgery. 1988;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorenz C, Petracic A, Hohl HP, Wessel L, Waag KL. Early wound closure and early reconstruction. Experience with a dermal substitute in a child with 60 per cent surface area burn. Burns. 1997;23:505–508. doi: 10.1016/s0305-4179(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 60.Hansbrough JF, Dore C, Hansbrough WB. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. Journal of Burn Care and Rehabilitation. 1992;13:519–529. doi: 10.1097/00004630-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Hansbrough J. Dermagraft-TC for partial-thickness burns: A clinical evaluation. Journal of Burn Care and Rehabilitation. 1997;18:S25–S28. doi: 10.1097/00004630-199701001-00011. [DOI] [PubMed] [Google Scholar]
- 62.Boyce ST, Hansbrough JF. Biologic attachment, growth, and differentiation of cultured human epidermal-keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery. 1988;103:421–431. [PubMed] [Google Scholar]
- 63.Hansbrough JF, Boyce ST, Cooper ML, Foreman TJ. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. Jama-Journal of the American Medical Association. 1989;262:2125–2130. [PubMed] [Google Scholar]
- 64.Orgill DP, Butler C, Regan JF, Barlow MS, Yannas IV, Compton CC. Vascularized collagen-glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plastic and Reconstructive Surgery. 1998;102:423–429. doi: 10.1097/00006534-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Compton CC, Butler CE, Yannas IV, Warland G, Orgill DP. Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. Journal of Investigative Dermatology. 1998;110:908–916. doi: 10.1046/j.1523-1747.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 66.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. Journal of Investigative Dermatology. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 67.Marinkovich MP. The molecular genetics of basement membrane diseases. Archives of Dermatology. 1993;129:1557–1565. [PubMed] [Google Scholar]
- 68.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair and Regeneration. 2003;11:S1–S28. doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 69.Metcalfe AD FM. Tissue engineering of replacement skin: the cross roads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–437. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikkola L, Seppälä J, Harlin A, Ndreu A, Ashammakhi N. Electrospun multifunctional diclofenac sodium releasing nanoscaffold. Stevenson Ranch, CA, Etats-Units: American Scientific Publishers; 2006. [DOI] [PubMed] [Google Scholar]
- 71.Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. Poly(vinyl alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules. 2005;6:1484–1488. doi: 10.1021/bm0492576. [DOI] [PubMed] [Google Scholar]
- 72.Kenawy E-R, Bowlin GL, Mansfield K, et al. Release of tetracycline hydrochloride from electrospun poly(ethyleneco-vinylacetate), poly(lactic acid), and a blend. Journal of Controlled Release. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 73.Chua K-N, Lim W-S, Zhang P, Lu H, Wen J, Ramakrishna S, Leong KW, Mao H-Q. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–2547. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 74.Chew S, Mi R, Hoke A, Leong K. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653–661. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–2589. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 76.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verreck G CI, Rosenblatt J, Peeters J, Dijck A, Mensch J, Noppe M, Brewster M. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J Control Release. 2003;92:349–360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 78.Rho KS, Jeong L, Lee G, Seo B-M, Park YJ, Hong S-D, Roh S, Cho JJ, Park WH, Min B-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Geng X, Kwon O-H, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26:5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 80.Kim CH, Khil MS, Kim HY, Lee HU, Jahng KY. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;78B:283–290. doi: 10.1002/jbm.b.30484. [DOI] [PubMed] [Google Scholar]
- 81.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 82.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takigami S, Takigami M, Phillips GO. Hydration characteristics of the cross-linked hyaluronan derivative hylan. Carbohydrate Polymers. 1993;22:153–160. [Google Scholar]
- 84.Jha AK, Xu X, Duncan RL, Jia X. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32:2466–2478. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreil G. Hyaluronidases — a group of neglected enzymes. Protein Science. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee F, Chung JE, Kurisawa M. An injectable enzymatically crosslinked hyaluronic acidtyramine hydrogel system with independent tuning of mechanical strength and gelation rate. Soft Matter. 2008;4:880–887. doi: 10.1039/b719557e. [DOI] [PubMed] [Google Scholar]
- 87.Mironov V. Fabrication of tubular tissue constructs by centrifugal casting of cells suspended in an in situ crosslinkable hyaluronangelatin hydrogel. The FASEB Journal. 2006;20:A436. doi: 10.1016/j.biomaterials.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 88.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide Cross-Linked Hyaluronan Hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]


