Abstract
Introduction
Coagulopathy can occur after hemorrhage, trauma and resuscitation, and has been associated with dilution of coagulation factors and hypothermia. Recombinant activated Factor VII (rFVIIa) has been used, often as a last resort, to improve hemostasis in trauma/hemorrhage patients with coagulopathy. The aim of this study was to further characterize the effects of rFVIIa on various coagulation parameters and the influence of temperature and hemodilution.
Methods
Whole blood from healthy human volunteers was incubated in a combination of three conditions: undiluted or diluted 40% with either lactated Ringer’s solution or Hextend, at 37°C or 34°C, and with and without rFVIIa (1.26 μg/ml, final concentration). Blood or plasma, as appropriate, was measured for coagulation by thrombin generation, thromboelastography (TEG), prothrombin Time (PT) and activated partial thromboplastin (aPTT).
Results
Incubation of plasma at 34°C significantly elevated thrombin generation, and prolonged PT and aPTT. Dilution of blood or plasma with 40% Hextend, but not lactated Ringer’s, had a significant effect on TEG parameters, and prolonged PT and aPTT. In control conditions (37°C, 0 dilution), the addition of rFVIIa to human plasma or whole blood led to a significant change in all TEG parameters, and Lagtime for thrombin generation, but not to PT or aPTT.
Conclusion
Theses data show that thrombin generation is affected by hypothermia, but not 40% dilution. TEG is affected by 40% dilution with Hextend, but not by hypothermia. PT and aPTT are significantly affected by both hypothermia and dilution. Recombinant FVIIa caused a greater change in thrombin generation at 34°C as compared to 37°C, and a greater change in PT at 40% dilution, suggesting that the effect of rFVIIa on coagulation is both temperature and dilution dependant.
Keywords: TEG, thrombin generation, PT, aPTT
Introduction
Coagulopathy is a clinical condition defined as a disorder of hemostasis. It can be manifest as a hypo- or hyper-coagulation state. Coagulopathy has been well documented in hemorrhage/trauma patients as a consequence of acidosis, hypothermia, dilution from resuscitation. In addition, a trauma-induced coagulopathy has been observed and is associated with a poor predictable outcome in trauma patients [1-4]. Restoration and maintenance of circulating blood volume, as well as correction of the acidosis, hypothermia and impaired coagulation are critical steps in the successful resuscitation of severely injured patients [5-10].
Fluid resuscitation has been a long standing therapy for trauma and hemorrhagic shock to restore blood volume, cardiac output and flow to the microcirculation [11-16]. Lactated Ringer’s (LR) and Hextend (HX) are the primary fluids used by many trauma units and the US Army for pre-hospital resuscitation [17]. HX, a hetastarch-based product in a balanced electrolyte solution with glucose, was selected for the battlefield resuscitation of casualties in shock by the Tactical Combat Casualty Care committee, for its volume sparing effects as compared to LR. In patients with severe hemorrhage, large volume infusions of crystalloids or colloids have led to significant hemodilution and coagulopathy [4,18,19]. This can be experimentally induced in animals [14,20] and allows for the study of this pathophysiology.
Over the past several years, rFVIIa has received much attention as a novel hemostatic agent for treating coagulopathy associated with hemorrhage and trauma [15,17-24]. Infusion of rFVIIa results in an enhancement of thrombin generation on the platelet surface at the site of injury independent of the presence of Factor VIII/Factor IX [21,25,26]. Thrombin generation reflects the action of the initial hemostatic mechanism and is a reliable test that could be used experimentally to determine the effects of fluid resuscitation and temperature on coagulation.
The aims of this study were to measure thrombin generation and other measures of coagulation, in human blood and plasma in vitro 1) with and without rFVIIa, 2) under normal or 40% hemodilution conditions (with either HX or LR), and 3) under normal- or hypothermic temperature conditions (37°C-34°C). Acidosis was not addressed in this study.
Materials and methods
Blood Collection
This study was conducted under a protocol reviewed and approved by the Brooke Army Medical Center Institutional Review Board. Subjects were screened for any known coagulation disorders, any drug prescription or over-the-counter medication or supplement that could have an effect on coagulation function, and pregnancy. Any subject meeting any of these exclusion conditions was not used for this study. After informed consent was acquired, blood specimens were collected from 9 normal healthy volunteers via venipuncture into sodium citrate vacutainers according to standard clinical operating procedures. Blood samples were split and half were centrifugation at 2000g for 10 minutes, plasma separated (platelet poor) and kept at -80°C until testing for thrombin generation, prothrombin time (PT) and activated partial thromboplastin time (aPTT).
PT and aPTT were measured in duplicate by standard clinical methods (BCS Coagulation Analyzer, Dade Behring, Deerfield, IL).
Thrombin generation assay
HX or LR was added to plasma to achieve 40% hemodilution, then incubated at 34°C or 37°C for 15 min in a water bath. Activated recombinant FVII was added to the plasma (1.26 μg/ml, approximately equivalent to a 90 μg/kg clinical dose [14]. Thrombin generation was studied according to the assay of Hemker et al. [25,27,28]. Briefly, thrombin generation was triggered by adding a solution containing Tissue Factor (TF), phosphatidyl choline/phosphatidyl serine (PCPS;Hemotologic Technologies), CaCl2 and Fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC HCl (Bachem) to 80μL platelet poor plasma. Samples were run in at least duplicate. The increase in fluorescence intensity, which is proportional to the concentration of the generated thrombin, was monitored every 30 sec for 120 min at 34°C or 37°C using a Microplate Fluorescence Reader SPECTRA max M2 (Molecular Devices, Sunnyvale, CA) with an excitation/emission wavelength of 360nm/460nm. A curve was generated that reflected the conversion of fluorogenic substrate to fluorescent product. The fluorescence data were not corrected for the inner filter effect or substrate consumption. The 1st derivative was calculated (Figure 1) to produce the thrombin generation curve. Thrombin generation was expressed using four parameters that described the curve; Lagtime (the initial reaction time when the thrombogram curve reaches 20 RFU/min), Peak (the peak or maximum thrombin generated), ttPeak (the time to reach the peak height of thrombin generated) and endogenous thrombin potential (ETP) or area under the curve (integrated thrombin generation between time 0-50min).
Figure 1.
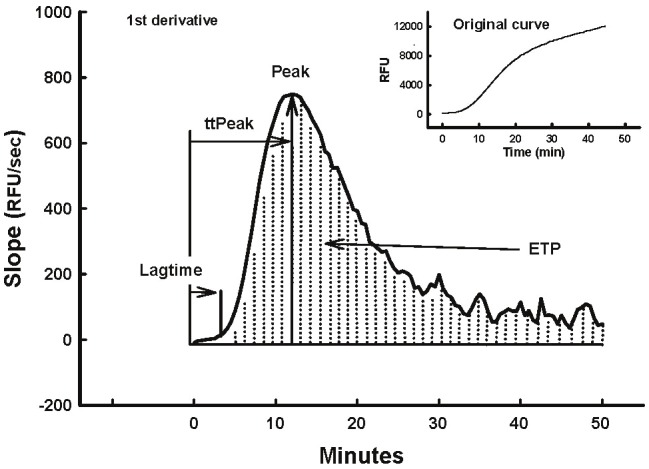
The thrombin generation curve was derived as the 1st derivative of the appearance of the fluorescent product over time (inset). Lagtime, ttPeak, Peak and ETP are illustrated.
Thromboelastography (TEG)
All samples were run in triplicate on a Haemoscope Model 5000, (Skokie, IL) at 34°C and 37°C, with and without rFVIIa (1.29 μg/ml Novo Nordisk, Denmark, equivalent to 90 μg/kg in vivo dose). The machine was calibrated daily. Before the blood was added, 10μl of tissue factor (Innovin, diluted 1:500), 20 μl of 0.2 M CaCl2, and 4.3 μl of 19.2 μg/ml Corn Trypsin Inhibitor (CTI) were added to each cup and allowed to equilibrate. Citrated blood (340 μl) was then added to each cup and the TEGs were started immediately. The tests were terminated 30 minutes after maximum amplitude (MA) was reached.
The parameters that were measured included R, (min, the time it takes for the initial detection of fibrin formation), K (min, the time for formation of the clot); α angle (degree, the kinetics of clot development), and MA (mm, the maximum amplitude (strength) of the developed clot).
Data analysis
Data was analyzed by 2-way and 3-Way ANOVA (rFVIIa, Temperature and dilution were the variables) followed by multiple comparison analysis using the Tukey’s or Sidac-Holms Methods. Statistics were performed by SAS or Sigmastat®. P < 0.05 was considered significant. Change was calculated as the difference between rFVIIa and control (no rFVIIa), and % change was calculated as the change/control x100% for Table 1. The data are expressed as the mean ± standard error of the mean.
Table 1.
Effect of temperature and dilution on the change (%change) of various coagulation parameters to rFVIIa
| 0 dilution | 40% LR | 40% Hex | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 37°C | 34°C | 37°C | 34°C | 37°C | 34°C | |
| Thrombin Generation | ||||||
| Lagtime (minutes) | -1.8±0.1 (-53±10) | -2.4±0.6 (-82±6) | -2.6±0.4 (-65±7) | -1.9±0.4 (-78±7) | -2.5±0.4 (-75±8) | -2.0±0.3 (-84±5) |
| ttPeak (minutes) | -2.9±0.7 (-20±4) | -6.1±1.2 (-42±6) | -3.9±0.7 (-25±4) | -3.8±0.6 (-33±3) | -3.9±0.8 (-28±2) | -4.6±0.5 (-40±3) |
| Peak (RFU/min) | 150±31 (21±5) | 579±74* (85±20)* | 49.5±22.2 (10±4) | 288±66* (26±7) | 105.5±32.7 (15±6) | 360±46* (35±7) |
| ETP | 1297±388 (10±3) | 3777±496* (25±5)* | 181±278 (2±2) | 1196±496 (5±2) | 1315±385 (9±3) | 2207±173 (11±1) |
| TEG | ||||||
| R (minutes) | -3.6±0.9 (-69±11) | -5.3±1.9 (-88±23) | -2.0±0.5 (-45±6) | -3.6±1.0 (-59±9) | -2.9±1.0 (-51±12) | -4.9±1.9 (-70±21) |
| K (minutes) | -1.2±0.1 (-69±5) | -1.1±0.7 (-64±24) | -0.2±0.3 (-15±11) | -0.6±0.3 (-25±11) | -2.2±0.7 (-84±36) | -2.0±1.3 (-55±35) |
| α angle (degrees) | 12.3±1.0 (19±2) | 12.7±3.7 (20±7) | 3.6±2.5 (5±5) | 6.1±0.6 (11±4) | 11.3±3.6 (22±6) | 7.7±3.6 (15±8) |
| MA (mm) | 6.8±1.2 (11±2) | 4.5±2.1 (7±4) | 2.2±1.1 (4±2) | 1.9±0.6 (4±1) | 7.8±2.5 (16±4) | 2.2±1.9 (4±4) |
| PT (seconds) | -0.2±0.3 (-2±3) | -1.4±0.5 (-12±4) | -2.3±1.1# (-15±10) | -3.7±0.3 (-27±1) | -2.5±0.4# (-20±2) | -2.5±0.3 (-20±2) |
| aPTT (seconds) | -1.7±0.8 (-6±3) | -4.1±1.3 (-12±4) | -2.9±1.5 (-9±5) | -4.8±0.5 (-14±1) | -4.1±0.4 (-13±1) | -3.8±0.3 (-12±1) |
Values represent Mean±SEM of the change to the addition of rFVIIa;
=P<0.05 as compared to 37°C at that dilution;
=P<0.05 as compared to 37°C, 0 dilution.
Results
Several coagulation parameters were measured in plasma or whole blood with and without rFVIIa, at 37 and 34°C, and diluted with 0 or 40% Hextend or lactated Ringer’s.
Thrombin generation
In undiluted blood, thrombin generation was not significantly affected by temperature (Figures 2 and 3). Furthermore, thrombin generation was not affected by dilution with Hextend or LR at 37°C. However, thrombin generation Peak and ETP were significantly elevated at 34°C as compared to 37°C in both 40% diluted groups, whereas lagtime and time to peak were significantly shortened in 34°C plasma diluted with LR as compared to 37°C diluted plasma (Figures 2 and 3). Incubation of plasma with rFVIIa under control conditions (37°C, 0 dilution), significantly reduced thrombin generation Lagtime, but had no significant effect on ttPeak, Peak or ETP (Figures 2 and 3). However, in undiluted plasma at 34°C, rFVIIa significantly shortened lagtime and ttPeak and elevated Peak and ETP (Figures 2 and 3). Similar results were seen in both 40% dilution groups at either temperature (Figures 2 and 3). Generally speaking, temperature had a greater effect on thrombin generation than dilution.
Figure 2.
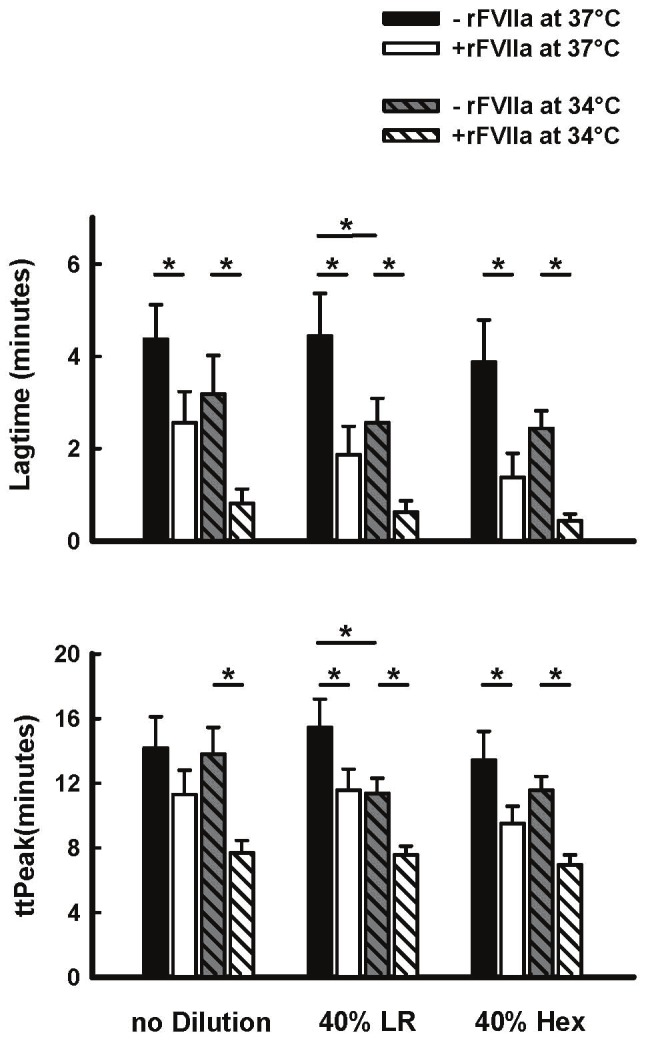
Effect of rFVIIa (1.26μg/ml, final concentration in PPP), temperature (34°C and 37°C), and 40% blood hemodilution (Hextend [HX] or lactated ringers [LR]) on Lagtime and ttPeak in human plasma in vitro. Values represent Mean±SEM. *=P<0.05 between or among groups spanned by the horizontal bars
Figure 3.

Effect of rFVIIa (1.26μg/ml), temperature (34°C and 37°C), and 40% blood hemodilution (Hextend [HX] or lactated ringers [LR]) on Peak and ETP on human plasma in vitro. Values represent Mean±SEM. *=P<0.05.
Thromboelastography
In undiluted blood, there were no significant changes in any TEG parameter at 34°C compared to 37°C. These non-significant responses to temperature were also observed in both 40% dilution groups (Figures 4 and 5). With respect to 40% dilution, only the Hextend group showed significant prolongation of K time and reduced α angle and MA in comparison to undiluted blood (Figures 4 and 5).
Figure 4.
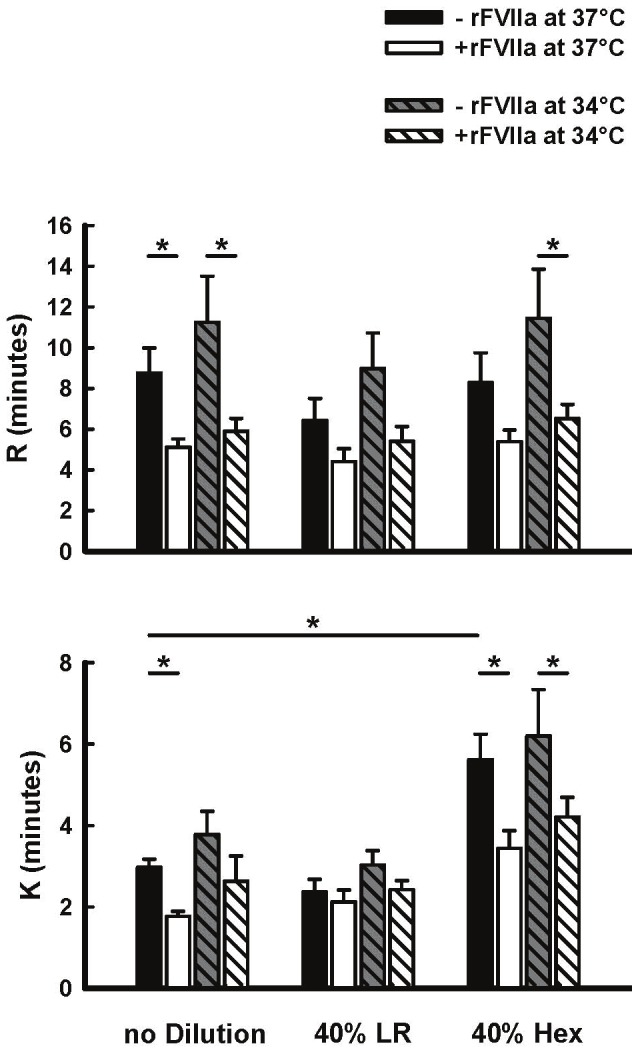
Effect of rFVIIa (1.26μg/ml), temperature (34°C and 37°C), and 40% blood hemodilution (Hextend [HX] or lactated ringers [LR]) on R and K in human blood in vitro. Values represent Mean±SEM. *=P<0.05.
Figure 5.
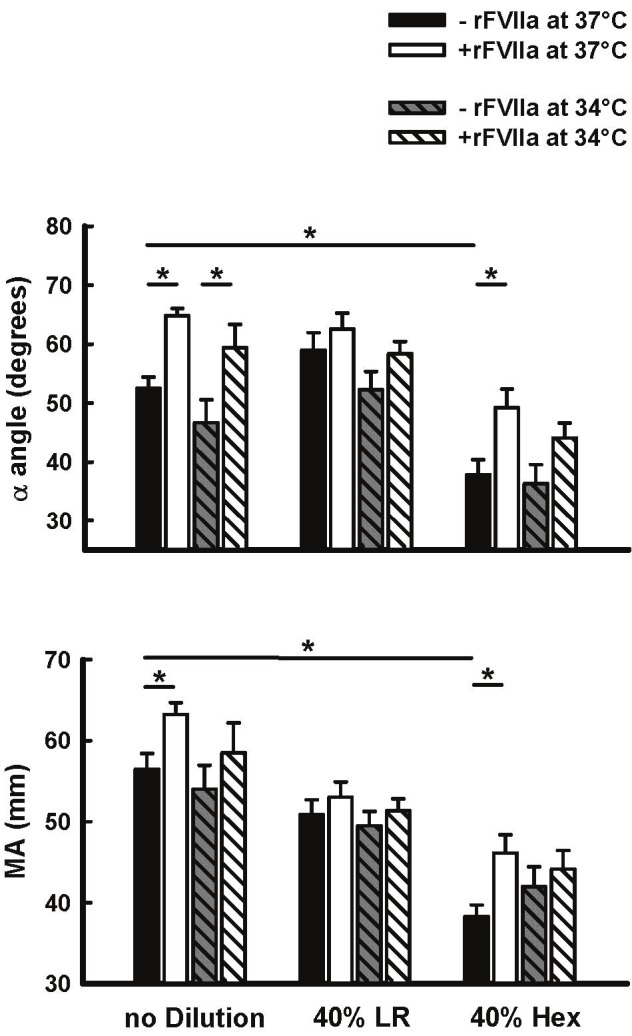
Effect of rFVIIa (1.26μg/ml), temperature (34°C and 37°C), and 40% blood hemodilution (Hextend [HX] or lactated ringers [LR]) on α angle and MA in human blood in vitro. Values represent Mean±SEM. *=P<0.05.
Incubation of undiluted blood with rFVIIa at 37°C significantly shortened R and K times, and increased α angle and MA (Figures 4 and 5). In undiluted blood at 34°C, addition of rFVIIa was still able to significantly reduce R-time and increase α angle. rFVIIa had no significant effect on any TEG parameter in blood diluted 40% with LR at either temperature, whereas the response in blood diluted 40% with Hextend was different (Figures 4 and 5). In this group rFVIIa shortened K-time and increased α angle and MA at 37°C, but only shortened R and K times, with no significant effects on α angle and MA at 34°C.
PT and aPTT
In undiluted blood, PT and aPTT were significantly prolonged in response to the colder temperature, and as expected were prolonged in response to 40% dilution with either fluid in comparison to undiluted blood (Figure 6).
Figure 6.

Effect of rFVIIa (1.26μg/ml), temperature (34°C and 37°C), and 40% blood hemodilution (Hextend [Hex] or lactated Ringers [LR]) on PT and aPTT in human plasma in vitro. Values represent Mean±SEM. *=P<0.05.
Incubation of undiluted plasma with rFVIIa at 37°C had no effect on PT or aPTT, but did significantly decrease PT and aPTT after incubation at 34°C (Figure 6). In plasma diluted 40% with either LR or Hextend and at either 37°C or 34°C, addition of rFVIIa also significantly reduced PT and aPTT compared to the respective plasma samples without rFVIIa (Figure 6).
Discussion
The purpose of this study was to evaluate the effects of temperature (37°C vs 34°C) and dilution (0 or 40% LR or Hextend) on the ability of rFVIIa to improve coagulation in human plasma as measured by thrombin generation and TEG, as well as standard coagulation measures such as PT and aPTT. In general, rFVIIa has been known to induce thrombin generation as part of its mechanism of action [29] and combining thrombin generation with TEG provides data on the stability of the fibrin clot, as well [30].
In the present study, temperature, rather than dilution, had the greater affect on thrombin generation. Specifically, the lower incubation temperature of 34°C significantly elevated Peak and ETP as compared to 37°C. Also, the change in Peak thrombin concentration in response to rFVIIa was greater in plasma incubated at the lower temperature. This enhancement of thrombin generation by lower temperatures has been described by others. For example, an elevated ETP at lower temperature was described by Tchaikovski et al., [31] after characterization of a calibrated automated thrombography in mouse plasma. An explanation for the effect of temperature was proposed by Hemker et al [32]. They proposed that low temperature affects thrombin inactivation (Thrombin/Antithrombin) more so than thrombin activation (Prothrombin to Thrombin). Because the total amount of thrombin is dependent on the rate it is produced vs. the rate it is bound to antithrombin and inactivated, lowering the incubation temperature would attenuate thrombin inactivation more than thrombin activation, and explain the higher Peak. Several studies, both in vitro and in vivo have reported that rFVIIa can improve coagulation and even reduce bleeding time under hypothermic conditions [33-35].
In contrast to the effects on thrombin generation, dilution with 40% Hextend had the most effect on TEG parameters as compared to 40% lactated Ringer’s, or incubation at 34°C. Dilution with 40% Hextend significantly prolonged K-time, and decreased α angle and MA, as compared to control (37°C, 0 Dilution), similar to our previous study [36] Incubation of whole blood at 34°C had little to no effect on TEG parameters. These data are in agreement with other laboratories showing that 20, 40 and 60% dilution of whole blood with HES 130 significantly reduced α angle and MA [35], and that incubation of whole blood at 32°C had little effect on TEG parameters. Dilution of human blood 40-60% with 5% albumin, isotonic saline, lactated Ringer’s or dextran significantly prolong clotting time, and reduced mean clotting firmness as measured by rotational thromboelastometry [37-39]. In the present study, rFVIIa had little effect on PT and aPTT in non-diluted plasma incubated at 37°C. Incubation of plasma at 34°C, or hemodilution, significantly prolonged both PT and aPTT as has been described by others [39,40]. However, rFVIIa significantly shortened PT in the diluted samples to levels not different from control suggesting that rFVIIa can return PT toward normal levels after dilution of 40%. These observations are consistent with the present observations that rFVIIa had the most pronounced effects on R- or K-time in undiluted or diluted blood at both temperatures. Although rFVIIa had a similar effect on aPTT, it did not return it to control levels. The present results are consistent with other findings that rFVIIa was still effective on TEG parameters or reducing bleeding time in hemodiluted rabbits and pigs [20; 37-43].
In conclusion, we have shown that low temperature had a more significant effect on thrombin generation than did 40% hemodilution. On the other hand, hemodilution, rather than temperature, had a significant effect on TEG. Both temperature and dilution affected PT and aPTT. Furthermore, rFVIIa caused a greater change in thrombin generation at 34°C as compared to 37°C, and a greater change in PT at 40% dilution, suggesting that rFVIIa can be effective at temperatures and levels of hemodilution that may be encountered in a surviving trauma patient.
Acknowledgment
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or United States Government. This work was performed during Dr. Kremenevskiy’s tenure as a research fellow under the National Research Council Research Associateship Award program.
Conflict of Interest Statement
None of the authors have any conflicts of interest regarding the contents of this manuscript or the compounds that were examined.
References
- 1.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 2.Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. 1999;10:85–94. [PubMed] [Google Scholar]
- 3.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11:590–597. doi: 10.1097/01.ccx.0000186374.49320.ab. [DOI] [PubMed] [Google Scholar]
- 4.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 5.Darlington DN, Jones RO, Magnuson TA, Gann DS. Role of intestinal fluid in restitution of blood volume and plasma protein after hemorrhage in awake rats. Am J Physiol. 1995;268:R715–722. doi: 10.1152/ajpregu.1995.268.3.R715. [DOI] [PubMed] [Google Scholar]
- 6.Darlington DN, Jones RO, Marzella L, Gann DS. Changes in regional vascular resistance and blood volume after hemorrhage in fed and fasted awake rats. J Appl Physiol. 1995;78:2025–2032. doi: 10.1152/jappl.1995.78.6.2025. [DOI] [PubMed] [Google Scholar]
- 7.Fried SJ, Satiani B, Zeeb P. Normothermic rapid volume replacement for hypovolemic shock: an in vivo and in vitro study utilizing a new technique. J Trauma. 1986;26:183–188. doi: 10.1097/00005373-198602000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima Y, Wang P, Cioffi WG, Bland KI, Chaudry IH. Restoration of body temperature to normothermia during resuscitation following trauma-hemorrhage improves the depressed cardiovascular and hepatocellular functions. Arch Surg. 2000;135:175–181. doi: 10.1001/archsurg.135.2.175. [DOI] [PubMed] [Google Scholar]
- 9.O'Benar JD, Bruttig SP, Wade CE, Dubick MA. Hemodynamic and metabolic responses to repeated hemorrhage and resuscitation with hypertonic saline dextran in conscious swine. Shock. 1998;10:223–228. doi: 10.1097/00024382-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Wettstein R, Erni D, Intaglietta M, Tsai AG. Rapid restoration of microcirculatory blood flow with hyperviscous and hyperoncotic solutions lowers the transfusion trigger in resuscitation from hemorrhagic shock. Shock. 2006;25:641–646. doi: 10.1097/01.shk.0000209532.15317.15. [DOI] [PubMed] [Google Scholar]
- 11.Dailey SE, Dysart CB, Langan DR, Slye MJ, Nuttall GA, Schrader LM, Williams BA, Oliver WC. An in vitro study comparing the effects of Hextend, Hespan, normal saline, and lactated ringer's solution on thrombelastography and the activated partial thromboplastin time. J Cardiothorac Vasc Anesth. 2005;19:358–361. doi: 10.1053/j.jvca.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Handrigan MT, Bentley TB, Oliver JD, Tabaku LS, Burge JR, Atkins JL. Choice of fluid influences outcome in prolonged hypotensive resuscitation after hemorrhage in awake rats. Shock. 2005;23:337–343. doi: 10.1097/01.shk.0000156667.04628.1f. [DOI] [PubMed] [Google Scholar]
- 13.Rafie AD, Rath PA, Michell MW, Kirschner RA, Deyo DJ, Prough DS, Grady JJ, Kramer GC. Hypotensive resuscitation of multiple hemorrhages using crystalloid and colloids. Shock. 2004;22:262–269. doi: 10.1097/01.shk.0000135255.59817.8c. [DOI] [PubMed] [Google Scholar]
- 14.Sapsford W, Watts S, Cooper G, Kirkman E. Recombinant activated factor VII increases survival time in a model of incompressible arterial hemorrhage in the anesthetized pig. J Trauma. 2007;62:868–879. doi: 10.1097/ta.0b013e318034204b. [DOI] [PubMed] [Google Scholar]
- 15.Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, Salinas J, Mehta S, Wade CE, Holcomb JB. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–293. doi: 10.1097/TA.0b013e318162759f. discussion 293-284. [DOI] [PubMed] [Google Scholar]
- 16.Todd SR, Malinoski D, Muller PJ, Schreiber MA. Hextend attenuates hypercoagulability after severe liver injury in swine. J Trauma. 2005;59:589–593. discussion 593-584. [PubMed] [Google Scholar]
- 17.Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am. 2007;87:55–72. vi. doi: 10.1016/j.suc.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 19.Hardy JF, De Moerloose P, Samama M. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2004;51:293–310. doi: 10.1007/BF03018233. [DOI] [PubMed] [Google Scholar]
- 20.Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, Lynn M. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001;51:431–438. doi: 10.1097/00005373-200109000-00002. discussion 438-439. [DOI] [PubMed] [Google Scholar]
- 21.Allen GA, Hoffman M, Roberts HR, Monroe DM 3rd. Recombinant activated factor VII: its mechanism of action and role in the control of hemorrhage. Can J Anaesth. 2002;49:S7–14. [PubMed] [Google Scholar]
- 22.Franchini M, Manzato F, Salvagno GL, Lippi G. Potential role of recombinant activated factor VII for the treatment of severe bleeding associated with disseminated intravascular coagulation: a systematic review. Blood Coagul Fibrinolysis. 2007;18:589–593. doi: 10.1097/MBC.0b013e32822d2a3c. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman M, Monroe DM rd, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S61–65. [PubMed] [Google Scholar]
- 24.Szlam F, Taketomi T, Sheppard CA, Kempton CL, Levy JH, Tanaka KA. Antithrombin affects hemostatic response to recombinant activated factor VII in factor VIII deficient plasma. Anesth Analg. 2008;106:719–724. table of contents. doi: 10.1213/ane.0b013e3181618702. [DOI] [PubMed] [Google Scholar]
- 25.Hemker HC, Al Dieri R, Béguin S. Thrombin generation assays: accruing clinical relevance. Curr Opin Hematol. 2004;11:170–175. doi: 10.1097/01.moh.0000130314.33410.d7. [DOI] [PubMed] [Google Scholar]
- 26.Korte WC, Moor S. Near fatal hemorrhage in traumatic bilateral leg amputation with coagulopathy, acidosis, and hypothermia and salvage therapy with recombinant factor VIIa. J Trauma. 2007;63:E1–4. doi: 10.1097/01.ta.0000246956.72328.6c. [DOI] [PubMed] [Google Scholar]
- 27.Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–561. [PubMed] [Google Scholar]
- 28.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Béguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 29.Hedner U. Mechanism of action of factor VIIa in the treatment of coagulopathies. Semin Thromb Hemost. 2006;32:77–85. doi: 10.1055/s-2006-939557. [DOI] [PubMed] [Google Scholar]
- 30.Dargaud Y, Prevost C, Lienhart A, Claude Bordet J, Negrier C. Evaluation of the overall haemostatic effect of recombinant factor VIIa by measuring thrombin generation and stability of fibrin clots. Haemophilia. 2011 doi: 10.1111/j.1365-2516.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- 31.Tchaikovski SN, VAN Vlijmen BJ, Rosing J, Tans G. Development of a calibrated automated thrombography based thrombin generation test in mouse plasma. J Thromb Haemost. 2007;5:2079–2086. doi: 10.1111/j.1538-7836.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 32.Hemker HC, E DES, Hemker PW. During coagulation, thrombin generation shifts from chemical to diffusional control. J Thromb Haemost. 2005;3:2399–2400. doi: 10.1111/j.1538-7836.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 33.Kheirabadi BS, Delgado AV, Dubick MA, Scherer MR, Fedyk CG, Holcomb JB, Pusateri AE. In vitro effect of activated recombinant factor VII (rFVIIa) on coagulation properties of human blood at hypothermic temperatures. J Trauma. 2007;63:1079–86. doi: 10.1097/TA.0b013e31815885f1. [DOI] [PubMed] [Google Scholar]
- 34.Godier A, Mazoyer E, Cymbalista F, Cupa M, Samama CM. Recombinant activated factor VII efficacy and safety in a model of bleeding and thrombosis in hypothermic rabbits: a blind study. J Thromb Haemost. 2007;5:244–9. doi: 10.1111/j.1538-7836.2007.02320.x. [DOI] [PubMed] [Google Scholar]
- 35.Viuff D, Lauritzen B, Pusateri AE, Andersen S, Rojkjaer R, Johansson PI. Effect of haemodilution, acidosis, and hypothermia on the activity of recombinant factor VIIa (NovoSeven) Br J Anaesth. 2008;101:324–331. doi: 10.1093/bja/aen175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darlington DN, Delgado AV, Kheirabadi BS, Fedyk CG, Scherer MR, Pusateri AE, Wade CE, Cap AP, Holcomb JB, Dubick MA. Effect of hemodilution on coagulation and recombinant factor VIIa efficacy in human blood in vitro. J Trauma. 2011;71:1152–63. doi: 10.1097/TA.0b013e318215178c. [DOI] [PubMed] [Google Scholar]
- 37.Bladbjerg EM, Jespersen J. Activity of recombinant factor VIIa under different conditions in vitro: effect of temperature, pH, and haemodilution. Blood Coagul Fibrinolysis. 2008;19:369–374. doi: 10.1097/MBC.0b013e328304b602. [DOI] [PubMed] [Google Scholar]
- 38.Fenger-Eriksen C, Tonnesen E, Ingerslev J, Sørensen B. Mechanisms of hydroxyethyl starch-induced dilutional coagulopathy. J Thromb Haemost. 2009;7:1099–1105. doi: 10.1111/j.1538-7836.2009.03460.x. [DOI] [PubMed] [Google Scholar]
- 39.Ganter MT, Schmuck S, Hamiel CR, Wischmeyer PE, Heule D, Zollinger A, Hofer CK. Monitoring recombinant factor VIIa treatment: efficacy depends on high levels of fibrinogen in a model of severe dilutional coagulopathy. J Cardiothorac Vascular Anesthesia. 2008;22:675–680. doi: 10.1053/j.jvca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Kheirabadi BS, Crissey JM, Deguzman R, Holcomb JB. In vivo bleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy than prothrombin time. J Trauma. 2007;62:1352–1359. doi: 10.1097/TA.0b013e318047b805. discussion 1359-1361. [DOI] [PubMed] [Google Scholar]
- 41.Klemcke HG, Delgado A, Holcomb JB, Ryan KL, Burke A, DeGuzman R, Scherer M, Cortez D, Uscilowicz J, Macaitis JM, Bliss J, Wojtaszczyk J, Christensen S, Currier H, Pusateri AE. Effect of recombinant FVIIa in hypothermic, coagulopathic pigs with liver injuries. J Trauma. 2005;59:155–161. doi: 10.1097/01.ta.0000174557.89804.a2. discussion 161. [DOI] [PubMed] [Google Scholar]
- 42.Lauritzen BP, Viuff DP, Tranholm MP, Ezban M. rFVIIa and NN1731 reduce bleeding in hydroxyethyl starch hemodiluted rabbits. J Trauma. 2010;69:1196–1202. doi: 10.1097/TA.0b013e3181c6619d. [DOI] [PubMed] [Google Scholar]
- 43.Dickneite G, Dorr B, Kaspereit F, Tanaka KA. Prothrombin Complex Concentrate Versus Recombinant Factor VIIa for Reversal of Hemodilutional Coagulopathy in a Porcine Trauma Model. J Trauma. 2010;68:1151–1157. doi: 10.1097/TA.0b013e3181b06364. [DOI] [PubMed] [Google Scholar]


