Abstract
Trauma, often accompanied by hemorrhage, is a leading cause of death worldwide, often leading to inflammation-related late complications that include sepsis and multiple organ failure. These secondary complications are a manifestation of the complexity of biological responses elicited by trauma/hemorrhage, responses that span most, if not all, cell types, tissues, and organ systems. This daunting complexity at the patient level is manifest by the near total dearth of available therapeutics, and we suggest that this dire condition is due in large part to the lack of a rational, systems-oriented framework for drug development, clinical trial design, in-hospital diagnostics, and post-hospital care. We have further suggested that mechanistic computational modeling can form the basis of such a rational framework, given the maturity of systems biology/computational biology. Herein, we briefly summarize the state of the art of these approaches, and highlight the biological insights and novel hypotheses derived from these approaches. We propose a rational framework for transitioning through the currently fragmented process from identification of biological networks that are potential therapeutic targets, through clinical trial design, to personalized diagnosis and care. Insights derived from systems and computational biology in trauma and sepsis include the centrality of Damage-Associated Molecular Pattern molecules as drivers of both beneficial and detrimental inflammation, along with a novel view of multiple organ dysfunction as a cascade of containment failures with distinct implications for therapy. Finally, we suggest how these insights might be best implemented to drive transformational change in the fields of trauma and sepsis.
Keywords: Trauma, sespsis, systems biology, computational biology, mathematical modeling
Introduction
Trauma, either from violence or traffic accidents, is a leading cause of death worldwide among young people [1,2]. The societal disruption caused by trauma is even greater, by affecting the segment of the population of greatest future productivity. With increasing prosperity comes improved prevention and initial care for trauma victims; however, the consequence of those improvements has been a vast increase in the number of individuals experiencing late complications that include sepsis and multisystem organ failure [3]. This in increase in secondary complications is a manifestation of the complexity of biological responses elicited by trauma/hemorrhage, responses that span most, if not all, cell types, tissues, and organ systems [4,5]. Even when examined at the cellular level, trauma/hemorrhage elicits a majority of transcriptional pathways in circulating inflammatory cells [6]. It should not be surprising, then, that survival from the initial insult results in wide-ranging physiological derangements that require high-intensity care in specialized critical care units.
This daunting complexity at the patient level is manifest by the near total dearth of available therapeutics for acute inflammatory indications. A decade ago, the field of sepsis received with both hope and skepticism the approval of recombinant human activated protein C (Eli Lilly & Co.’s Xigris™) by the US. Food and Drug Administration. However, following a mandated repeat of the Phase III clinical trial in Europe, in 2011 the drug was found to offer no benefit over standard of care, and was subsequently removed from the market [7,8]. As disappointing as this event was, it pales against the state of therapeutics intended to affect and beneficially modulate biological mechanisms present in the systemic response to trauma: i.e. none.
Why so little, this late in the game? There are clearly many reasons for this unfortunate state of events discussed extensively over the past 20-plus years, including but not limited to: imperfect selection of drug targets, inadequate pre-clinical models, and numerous issues surrounding the design of clinical trials. We have suggested that the thread binding these factors is the lack of a rational framework for drug development, clinical trial design, in-hospital diagnostics, and post-hospital care. We have further suggested that mechanistic computational modeling can form the basis of such a rational framework, given the maturity of systems biology/computational biology [5,9-15]. Below, we briefly summarize the state of the art of these approaches, and highlight the biological insights and novel hypotheses derived from these approaches. Finally, we suggest how these insights might be best implemented to drive transformational change in the fields of trauma and sepsis.
“A systems approach”: many meanings, many nuances
The advent of high-dimensional genomics, proteomics, metabolomics, physiomics, and “next-omics” has resulted in both a deluge of data and a promise that in those data would be found the key to therapy of complex diseases such as trauma- and sepsis-induced multiple organ dysfunction [4,16]. There have been notable successes in this approach, which has led to the possibility of better defining the dynamic patient state. Various studies have shed mechanistic insights into the biology of trauma and sepsis based on DNA microarray (including the landmark first study from the Trauma and the Host Response to Injury “Glue” grant and studies in several countries identifying signature responses of sepsis, trauma, and burn patients) [6,17-21]; plasma proteomics in similar patients [22,23]; and the use of signal processing techniques, multivariate dynamic clustering, and machine-learning algorithms based on physiologic measurements and inflammation biomarkers [24-28]. Furthermore, “omics” studies and data-driven computational analyses in animal models of trauma/hemorrhage, burns, and sepsis have both verified the importance of known biological pathways and suggested some novel ones [29-31].
However, this field has not been spared the socalled “curse of dimensionality”: i.e. more data leads to more possible explanations for those data. Moreover, trauma/sepsis research has been affected by the “curse of practicality”: i.e. technical, practical, and economic challenges to implementation as a robust clinical diagnostic methodology. Finally, excessive reliance on these “omics” techniques may lead to the “curse of forgetting-that-correlation-does-notequal-causality.” Pattern-oriented “omics” data coupled with increasingly sophisticated bioinformatics tools may directly point out important genotype/phenotype associations in the settings of trauma and sepsis [6,17]. However, an unfortunate methodological fact is that the identification of valid causal mechanisms - which are needed to develop therapeutic modalities - requires investigators to make intuitive leaps beyond these correlative analyses themselves, based on their own insights, hypotheses, and experience. It is here that the overt complexity of the response to injury renders that intuition insufficient, and has resulted in the disappointing current situation.
From high-dimensional data to computational models
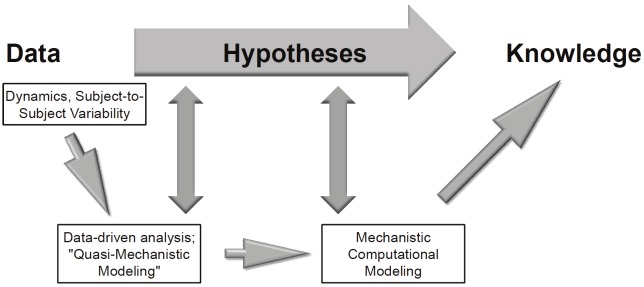
We suggest that in order to break through the bottleneck of too much data accompanied by too few therapeutically relevant insights, there is a need to close the scientific loop between the acquisition of high-dimensional, dynamic data and the derivation of useful mechanistic knowledge [32]. This process involves the generation of mechanistic computational simulation (using equation-, agent-, or rules-based models) of the biological processes inferred from data-driven analyses (including Principal Component Analysis, dynamic network analyses, and related methods; Figure 1) [5,9-15,33]. Though this intermediate step might initially appear to slow the progress from data to therapeutically-relevant knowledge, the mechanistic modeling step can in fact both accelerate the discovery of biological knowledge and streamline the process of generating novel drug candidates based on that knowledge. How so? Though the first step of data-driven modeling based on high-dimensional data may yield testable hypotheses on its own (Figure 1), mechanistic computational models are likely required to string together these hypotheses into a larger framework (Figure 1). In essence, mechanistic computational models are in silico instantiations of multiple hypotheses [32,34], and therefore simulated experiments carried out using such models can suggest non-intuitive behaviors of complex systems such as the inflammatory response. Thus, generating mechanistic models can allow investigators to glean actionable knowledge (Figure 1) at every stage of healthcare delivery - from basic scientists attempting to suggest novel biological pathways to modulate therapeutically [35,36], to scientists in industry seeking to determine which of a plethora of drug candidates to follow through to pre-clinical and clinical studies [14], to designers of clinical trials seeking to optimize clinical trial design (including patient sub-groups to target as well as optimal timing and dosage of drug administration) [37-39], and finally to the delivery of personalized diagnosis and care [13,40,41]. While the majority of mechanistic simulations of inflammation have been based on, calibrated, and validated with data on inflammatory mediators at the protein level (or end product level [e.g. NO2 -/NO3 -]), there is a need to link “omics” data - and associated bioinformatics - with mechanistic computational models. This is an area of study that has seen recent encouraging developments, in studies in which transcriptomic data were used as indirect surrogates for pro- and anti-inflammatory pathways [42-46].
Figure 1.
The process from data to knowledge, needed to drive novel therapies for sepsis and trauma. In order to obtain mechanistic, therapeutically-relevant knowledge from high-content data, the data need to describe the dynamics of the biological process, as well as accounting for subject-to-subject variability. Such data are amenable to data-driven analyses such as Principal Component Analysis and Dynamic Network Analysis, methods that can give quasi-mechanistic insights into the biological process and hence may suggest testable hypotheses. In order to increase the likelihood of deriving true therapeutically actionable knowledge from high-content data, insights from data-driven analysis and modeling techniques should be used to create mechanistic computational simulations that, in turn, will yield more refined hypotheses.
From data to models to knowledge: insights from systems approaches to trauma and sepsis
A key hope of most investigators using systems and computational biology approaches is the generation of major new insights into complex biological phenomena [47]. Prior studies of trauma and sepsis in both animals and humans have suggested that an appropriately robust inflammatory response is necessary for appropriate resolution of the insult, with dysregulated inflammation being the hallmark of morbidity and mortality [48,49]. This adaptive responsiveness to stress can be observed both at the physiological and inflammatory levels, which reinforces the concept that these processes are interlinked [50]. Recent “omics” studies have supported this paradigm [6]. The responses to injury and infection involve a cycle of which is initially driven by chemokines and classical proinflammatory cytokines such as TNF-α and IL-1β [31]. Activation of TNF-α and IL-1β in part mediated by cytokines such as IFN-γ - leads to the production of DAMP’s such as HMGB1 [51]. In turn, DAMP’s cause the release of cytokines such as TNF-α [52], setting in motion a feedforward mechanism of inflammation à damage/dysfunction à inflammation [5,10].
Interestingly, data-driven modeling approaches suggest that when IL-1β is elevated in the absence of TNF-α in experimental surgical trauma, a process driven by the chemokine IP-10 (CXCL10), the predominant outcome is a well-coordinated inflammatory response that leads to resolution [31]. In contrast, more severe trauma/hemorrhage lead to disconnected elevation of cytokines such as IL-6 in a manner apparently driven by the chemokine MIG (CXCL9), and leading to elevated production of TNF-α [31]. Genomic signatures of this process can be seen in the activation of multiple signaling pathways, key among them the NF-κB pathway as well as signatures of DAMP-triggered pathways [6].
As modeled computationally, this inflammatory cycle is dampened by the influences of antiinflammatory/pro-healing mediators, chief among them being catecholamine-induced IL-10 [35,36,53] in a manner apparently also driven by chemokines such as IP-10 and MIG (Azhar and Vodovotz, unpublished). This anti-inflammatory response, which is induced nearly simultaneously with the pro-inflammatory response described above, results in resolution of inflammation response and - at least partial - restoration of tissue integrity and consequent organ function. However, if the DAMP-driven positive feedback loop is induced to a high degree, or remains active beyond the point that anti-inflammatory mediators can suppress it, then the well-known “cytokine storm” [48] (and its accompanying “genomic storm” [6]) is observed. A hallmark of this process is the long-known elevation of both pro- and anti-inflammatory mediators, key among them being IL-6 [49,54,55]. An equally detrimental alternative is an overly-damped inflammatory response, which is apparently connected to lack of physiological responsiveness [49,50]. We suggest that this is driven by an over-exuberant production catecholamines and the attendant over-production of IL-10. We hypothesize that, in addition to the well-studied stimulation of inflammation by DAMP’s, the anti-inflammatory response to injury or infection is also indirectly driven by DAMP’s. This hypothesis is supported by studies showing that DAMP’s can stimulate the expression of anti-inflammatory cytokines, and that wound healing-related processes are stimulated by DAMP’s. In further support of this hypothesis, we have found that the prototypical DAMP, HMGB1, correlated with distinct sets of chemokines and cytokines in trauma survivors vs. non-survivors (Namas et al, unpublished), suggesting that DAMP’s stimulate predominantly different spectra of inflammation that lead to resolution vs. augmentation or persistence of detrimental inflammation.
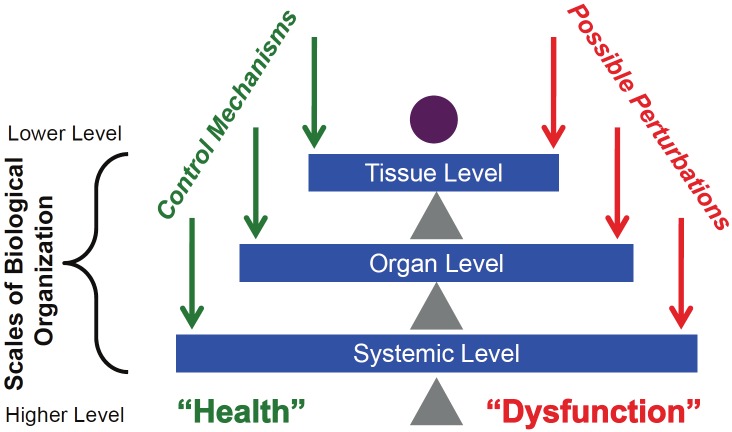
Inflammation and its attendant impact on physiological processes is a prototypical multiscale process [15]. Inflammatory responses, while being ubiquitous, are functionally manifest in specific tissue and organ compartments in which overall clinical physiology is driven by distinct but interconnected organ systems. Death following trauma or sepsis occurs largely through multiple organ failure, in a process catalyzed and maintained by inflammation. Accordingly, mechanistic computational models of trauma and sepsis have needed to link inflammation and physiology via multiscale - and likely multi-compartment - computational models in order to make both qualitative and quantitative predictions. We have previously described such multiscale, multi-compartment, mechanistic computational models that suggest how failure in a given compartment is communicated via inflammatory mediators to cause multi-organ failure [41,56]. Based on insights derived from such computational models, we hypothesize that inflammation proceeds at a given “nested” level (or scale; Figure 2), for example at the local cellular level, until positive feedback ramps up the system towards a tipping point, that, when passed, results in a phase transition to dysfunction at the higher biological scale (e.g. from cellular, to tissue/organ, to multiple organs, to the animal as a whole; Figure 3). This process can be viewed as cascading systems failure, in which scale-dependent control mechanisms reach the limits of their capability, and disorder propagates across components, such as in extension of damage to adjacent cells or tissue within an organ, and levels of components, such as seen in the effect of one organ’s dysfunction on the function of other organs (i.e. gut ischemia leading to acute respiratory distress syndrome).
Figure 2.
Multi-scale control structure of inflammation. This figure demonstrates the tiered scales of biological organization. Control mechanisms (such as inflammation) attempt to balance insults/perturbations that threaten the health state (abstractly represented as the purple circle). Balance occurs at multiple tiers, and the multi-scale nature of the control mechanisms allow for considerable robustness of the system. Note that the control mechanisms themselves have a complex structure that can shift the balance as well, but these mechanisms have been abstracted for clarity.
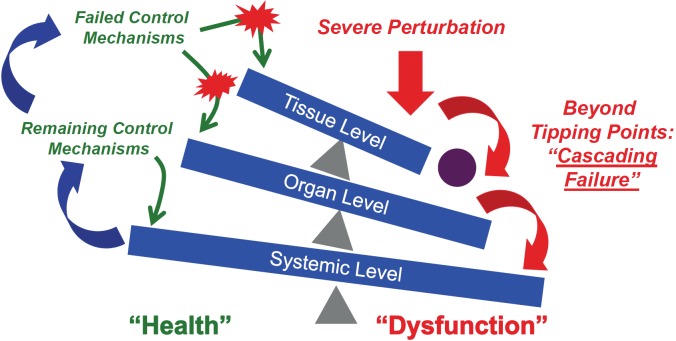
Figure 3.
Tipping Points and Cascading Systems Failure. If there is control failure at one component level of the schematic illustrated in Figure 2 due to the feed-forward loop of inflammation à damage à inflammation, the “tipping” of that system past a point of no return can lead to an override of control mechanisms at the higher level, leading to a cascading effect that culminates in systemic dysfunction (Red Arrows). Importantly, spillover to the next component not only affects that component, but also affects the prior one, propagating the feed-forward behavior of inflammation back across components and compartments (Blue Arrows). Note that these components can be defined by scale of organization, but also by the spatial containment inherent to an organ’s anatomical structure and relationship to other organ systems.
We hypothesize that for as long as inflammation remains effectively controlled and confined to a given scale/compartment, the process will affect only the physiology characteristic of that scale. If the perturbation and its dynamic consequences can be reversed within that scale, the component remains acting within its functional tolerances and limits the possibility of impacting higher scales. A global, system-level insult such as severe injury and hemorrhagic shock can lead to trans-compartment, systemic activation of inflammation due to the feed-forward nature of inflammation discussed above. This containment failure leads to the presence of inflammatory mediators throughout the circulation. However, the subsequent actuators of inflammation remain local ones, and are propagated via compartmental behavior in conjunction with influences from subsequent components, thereby propagating the feed-forward loop of inflammation → damage → inflammation in another dimension. In essence, the initial insult may affect a range of organ systems, but it is only after the individual organ dynamics exceed their inflammatory tolerances that they contribute to the sequence of generalized system failure (Figure 3).
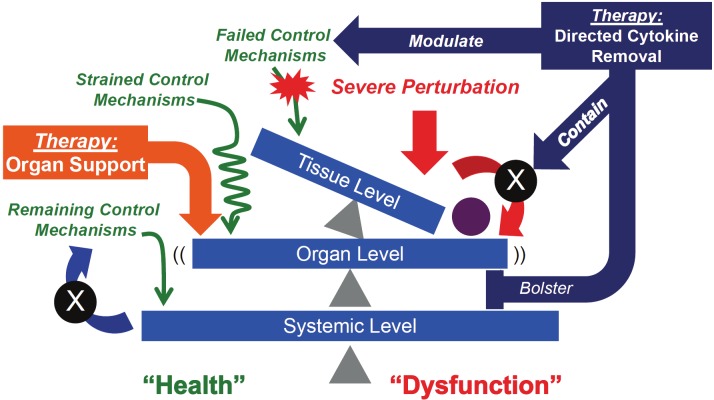
It is clear that at this point (the classical clinical manifestation of septic shock and multiple organ failure), that attempts to modulate the lowest level of control (i.e. at the cellular level via cytokine manipulation) will be “too late”: we do not currently have the degree of mechanistic knowledge of cellular and molecular control to effectively manipulate those systems with enough precision to provide benefit. Therefore, current therapeutic strategies focus on organ support at the physiological level, thereby supporting the function of a particular organ vis-àvis that organ’s relationship to the system as a whole (Figure 4). This approach could, in theory, temporize higher-level system failures and allow lower-level control systems to “come back online.” We clearly recognize that these therapies themselves carry detrimental potential (i.e. ventilator-associated barotraumas or hemodynamic instability associated with hemodialysis). In fact, currently there appears to be a disconnect (at best) or a detriment (at worst) with respect to such higher-level strategies helping the lower level components regain their homeostatic control; it is this disconnect that we seek to counter and address through the recognition of this pattern of cascading system dysfunction.
Figure 4.
Effects of current and potential therapeutic interventions for trauma/hemorrhage and sepsis. Current therapies for trauma/hemorrhage and sepsis are based on organ-level physiological support (Orange Block Arrows). These strategies temporize the tipping effects on the higher-level system (e.g. organism-wide consequences), but currently do not feedback on the dysfunction at the lower level. The result is a fragile “meta-stable” state, in which organ support attempts to prevent global system failure while waiting for the lower-level systems to “come back on line”. The difficulty with this approach, however, lies in the known potentially detrimental consequences of organ support (such as barotrauma from ventilators or hemodynamic instability from hemodialysis), which can delay or compromise the restitution of cellular-molecular homeostasis. Alternatively, we propose that modulation strategies directed at lower level control structures (i.e. cytokines) at an appropriate point in the failure sequence (i.e. prior to complete lost of containment and tipping to the next level) may help reset the underlying control mechanisms, limit spill-over effects and bolster maintenance of compartmental containment (Purple Block Arrows). All these points of interdiction can forestall the propagation of cascading systems failure if the intervention can be applied at the appropriate time, and due to the feed-forward behavior of the inflammatory response.
This hypothesis leads to the suggestion that using drugs targeting disordered inflammation at a cellular-molecular level (such targets as chemokines or TNF-α) operating within a given compartment/space (e.g. the peritoneum) prior to the loss of control of containment may be efficacious in forestalling the “tip-over” (Figure 4), but that such drugs would lose efficacy (or even become detrimental) once containment is lost and systemic or lymphatic spillover occurs. We hypothesize that in its most efficacious form, directed cytokine removal would modulate inflammation at the cellular level, help contain inflammation (and infection in the case of sepsis) by limiting spill-over to the next compartment, and also bolster organ function. The feedforward nature of inflammation would help this type of therapy rather than hindering it, since inhibiting the feed-forward loop in both local and distal compartments/dimension should ramp inflammation down fairly quickly. In support of this hypothesis, we have recently found in a rat model of sepsis that interventions such as hemoadsorption appear to re-compartmentalize inflammation and simultaneously result in both reduced organ dysfunction and improved clearance of bacteria (Namas, Kellum, and Vodovotz, unpublished). Furthermore, interventions targeting the mesenteric lymph may prevent subsequent lung injury including the acute respiratory distress syndrome (ARDS) in a clinically-relevant porcine model of sepsis [57]. In addition, our group has shown that removal of ascites from the peritoneal cavity prevents lung injury in the same porcine model of ARDS [58].
Computational and systems biology and the future of trauma research
We have learned much from reductionist research over the past several centuries. As in many other biomedical research fields, clinicians in combination with basic researchers have largely driven the pace of new knowledge (though few therapies) for trauma and sepsis. Over the past decade, bioinformatics specialists have joined this team, lending their expertise in mining the reams of “omics” data facilitated by ever-more-rapid technological development. We suggest that computational modelers need to join this interdisciplinary team in order to drive the next generation of insights into the pathobiology of trauma and sepsis, with an eye toward practical application of these methods to drug development [14], clinical trial design [5,14], and personalized diagnostics and therapy [13].
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01GM67240, P50GM53789, R33HL089082, R01HL080926, R01AI080799, R01HL76157, R01DC008290, and UO1 DK072146; National Institute on Disability and Rehabilitation Research grant H133E070024; National Science Foundation grant 0830-370-V601; a Shared University Research Award from IBM, Inc.; and grants from the Commonwealth of Pennsylvania, the Pittsburgh Lifesciences Greenhouse, and the Pittsburgh Tissue Engineering Initiative/Department of Defense.
Abbreviations
- DAMP
damage-associated molecular pattern molecule
- IL
interleukin
- IP-10
interferon-gamma inducible protein of 10 kDa
- MIG
monokine inducible by gamma interferon
- TNF-α
tumor necrosis factor-α
References
- 1.Patton GC, Coffey C, Sawyer SM, Viner RM, Haller DM, Bose K, Vos T, Ferguson J, Mathers CD. Global patterns of mortality in young people: a systematic analysis of population health data. Lancet. 2009;374:881–892. doi: 10.1016/S0140-6736(09)60741-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization report: Young People: Health Risks and Solutions. 2011 [Google Scholar]
- 3.Neunaber C, Zeckey C, Andruszkow H, Frink M, Mommsen P, Krettek C, Hildebrand F. Immunomodulation in polytrauma and polymicrobial sepsis - where do we stand? Recent Pat Inflamm Allergy Drug Discov. 2011;5:17–25. doi: 10.2174/187221311794474892. [DOI] [PubMed] [Google Scholar]
- 4.Cobb JP, Suffredini AF, Danner RL. The Fourth National Institutes of Health Symposium on the Functional Genomics of Critical Injury: Surviving stress from organ systems to molecules. Crit Care Med. 2008;36:2905–2911. doi: 10.1097/ccm.0b013e318186a720. [DOI] [PubMed] [Google Scholar]
- 5.Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:1–6. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitka M. Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA. 2011;306:2439–2440. doi: 10.1001/jama.2011.1755. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 9.Vodovotz Y, Constantine G, Rubin J, Csete M, Voit EO, An G. Mechanistic simulations of inflammation: Current state and future prospects. Math Biosci. 2009;217:1–10. doi: 10.1016/j.mbs.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodovotz Y, An G. Systems Biology and Inflammation. Methods Mol Biol. 2010;662:181–201. doi: 10.1007/978-1-60761-800-3_9. [DOI] [PubMed] [Google Scholar]
- 11.Vodovotz Y, Constantine G, Faeder J, Mi Q, Rubin J, Sarkar J, Squires R, Okonkwo DO, Gerlach J, Zamora R, Luckhart S, Ermentrout B, An G. Translational systems approaches to the biology of inflammation and healing. Immunopharmacol Immunotoxicol. 2010;32:181–195. doi: 10.3109/08923970903369867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vodovotz Y. Translational systems biology of inflammation and healing. Wound Repair Regen. 2010;18:3–7. doi: 10.1111/j.1524-475X.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi Q, Li NYK, Ziraldo C, Ghuma A, Mikheev M, Squires R, Okonkwo DO, Verdolini Abbott K, Constantine G, An G, Vodovotz Y. Translational systems biology of inflammation: Potential applications to personalized medicine. Personalized Medicine. 2010;7:549–559. doi: 10.2217/pme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An G, Bartels J, Vodovotz Y. In silico augmentation of the drug development pipeline: Examples from the study of acute inflammation. Drug Dev Res. 2010;72:1–14. doi: 10.1002/ddr.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Chang S, Billiar TR, Kellum JA, Angus DC, Vodovotz Y. Sepsis: Something old, something new, and a systems view. J Crit Care. 2011 doi: 10.1016/j.jcrc.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb JP, O'Keefe GE. Injury research in the genomic era. Lancet. 2004;363:2076–2083. doi: 10.1016/S0140-6736(04)16460-X. [DOI] [PubMed] [Google Scholar]
- 17.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF Large Scale Collab Res Program. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 18.McDunn JE, Husain KD, Polpitiya AD, Burykin A, Ruan J, Li Q, Schierding W, Lin N, Dixon D, Zhang W, Coopersmith CM, Dunne WM, Colonna M, Ghosh BK, Cobb JP. Plasticity of the systemic inflammatory response to acute infection during critical illness: development of the riboleukogram. PLoS ONE. 2008;3:e1564. doi: 10.1371/journal.pone.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, Freishtat RJ, Anas N, Meyer K, Checchia PA, Monaco M, Odom K, Shanley TP. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B, Xu W, Herndon D, Tompkins R, Davis R, Xiao W, Wong WH, Toner M, Warren HS, Schoenfeld DA, Rahme L, McDonald-Smith GP, Hayden D, Mason P, Fagan S, Yu YM, Cobb JP, Remick DG, Mannick JA, Lederer JA, Gamelli RL, Silver GM, West MA, Shapiro MB, Smith R, Camp DG, Qian W, Storey J, Mindrinos M, Tibshirani R, Lowry S, Calvano S, Chaudry I, West MA, Cohen M, Moore EE, Johnson J, Moldawer LL, Baker HV, Efron PA, Balis UG, Billiar TR, Ochoa JB, Sperry JL, Miller-Graziano CL, De AK, Bankey PE, Finnerty CC, Jeschke MG, Minei JP, Arnoldo BD, Hunt JL, Horton J, Cobb JP, Brownstein B, Freeman B, Maier RV, Nathens AB, Cuschieri J, Gibran N, Klein M, O'keefe G. Analysis of factorial time-course microarrays with application to a clinical study of burn injury. Proc Natl Acad Sci USA. 2010;107:9923–9928. doi: 10.1073/pnas.1002757107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland A, Thomas M, Brandon RA, Brandon RB, Lipman J, Tang B, McLean A, Pascoe R, Price G, Nguyen T, Stone G, Venter D. Development and validation of a novel molecular biomarker diagnostic test for the early detection of sepsis. Crit Care. 2011;15:R149. doi: 10.1186/cc10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Qian WJ, Gritsenko MA, Xiao W, Moldawer LL, Kaushal A, Monroe ME, Varnum SM, Moore RJ, Purvine SO, Maier RV, Davis RW, Tompkins RG, Camp DG, Smith RD. High dynamic range characterization of the trauma patient plasma proteome. Mol Cell Proteomics. 2006;5:1899–1913. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian WJ, Petritis BO, Kaushal A, Finnerty CC, Jeschke MG, Monroe ME, Moore RJ, Schepmoes AA, Xiao W, Moldawer LL, Davis RW, Tompkins RG, Herndon DN, Camp DG, Smith RD. Plasma proteome response to severe burn injury revealed by 18O-labeled "universal" reference-based quantitative proteomics. J Proteome Res. 2010;9:4779–4789. doi: 10.1021/pr1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gang Y, Malik M. Heart rate variability in critical care medicine. Curr Opin Crit Care. 2002;8:371–375. doi: 10.1097/00075198-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8:R367–R384. doi: 10.1186/cc2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancio LC, Batchinsky AI, Salinas J, Kuusela T, Convertino VA, Wade CE, Holcomb JB. Heart-rate complexity for prediction of prehospital lifesaving interventions in trauma patients. J Trauma. 2008;65:813–819. doi: 10.1097/TA.0b013e3181848241. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MJ, Grossman AD, Morabito D, Knudson MM, Butte AJ, Manley GT. Identification of complex metabolic states in critically injured patients using bioinformatic cluster analysis. Crit Care. 2010;14:R10. doi: 10.1186/cc8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Convertino VA, Moulton SL, Grudic GZ, Rickards CA, Hinojosa-Laborde C, Gerhardt RT, Blackbourne LH, Ryan KL. Use of advanced machine-learning techniques for noninvasive monitoring of hemorrhage. J Trauma. 2011;71:S25–S32. doi: 10.1097/TA.0b013e3182211601. [DOI] [PubMed] [Google Scholar]
- 29.Cobb JP, Laramie JM, Stormo GD, Morrissey JJ, Shannon WD, Qiu Y, Karl IE, Buchman TG, Hotchkiss RS. Sepsis gene expression profiling: murine splenic compared with hepatic responses determined by using complementary DNA microarrays. Crit Care Med. 2002;30:2711–2721. doi: 10.1097/00003246-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Dasu MR, Cobb JP, Laramie JM, Chung TP, Spies M, Barrow RE. Gene expression profiles of livers from thermally injured rats. Gene. 2004;327:51–60. doi: 10.1016/j.gene.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, Vodovotz Y. A dynamic view of trauma/hemorrhage-induced inflammation in mice: Principal drivers and networks. PLoS ONE. 2011;6:e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An G. Closing the scientific loop: Bridging correlation and causality in the petaflop age. Science Translational Med. 2010;2:41ps34. doi: 10.1126/scitranslmed.3000390. [DOI] [PubMed] [Google Scholar]
- 33.An G, Faeder J, Vodovotz Y. Translational systems biology: Introduction of an engineering approach to the pathophysiology of the burn patient. J Burn Care Res. 2008;29:277–285. doi: 10.1097/BCR.0b013e31816677c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An G. Translational systems biology using an agent-based approach for dynamic knowledge representation: An evolutionary paradigm for biomedical research. Wound Rep Reg. 2010;18:8–12. doi: 10.1111/j.1524-475X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 35.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock states. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 36.Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, Namas R, Constantine G, Zamora R, Puyana JC, Vodovotz Y. Mathematical modeling of post-hemorrhage inflammation in mice: Studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock. 2009;32:172–178. doi: 10.1097/SHK.0b013e318193cc2b. [DOI] [PubMed] [Google Scholar]
- 37.Clermont G, Bartels J, Kumar R, Constantine G, Vodovotz Y, Chow C. In silico design of clinical trials: a method coming of age. Crit Care Med. 2004;32:2061–2070. doi: 10.1097/01.ccm.0000142394.28791.c3. [DOI] [PubMed] [Google Scholar]
- 38.An G. In-silico experiments of existing and hypothetical cytokine-directed clinical trials using agent based modeling. Crit Care Med. 2004;32:2050–2060. doi: 10.1097/01.ccm.0000139707.13729.7d. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Chow CC, Bartels J, Clermont G, Vodovotz Y. A mathematical simulation of the inflammatory response to anthrax infection. Shock. 2008;29:104–111. doi: 10.1097/SHK.0b013e318067da56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li NYK, Verdolini K, Clermont G, Mi Q, Hebda PA, Vodovotz Y. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS ONE. 2008;3:e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieman K, Brown D, Sarkar J, Kubiak B, Ziraldo C, Vieau C, Barclay D, Gatto L, Maier K, Zamora R, Mi Q, Chang S, Vodovotz Y. Endotoxin-induced acute inflammation in swine: Insights from combined data-driven and mechanistic modeling. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e31823e986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Modeling endotoxin-induced systemic inflammation using an indirect response approach. Math Biosci. 2009;217:27–42. doi: 10.1016/j.mbs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. In silico simulation of corticosteroids effect on an NFkB- dependent physicochemical model of systemic inflammation. PLoS ONE. 2009;4:e4706. doi: 10.1371/journal.pone.0004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong X, Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Agent-Based Modeling of Endotoxin-Induced Acute Inflammatory Response in Human Blood Leukocytes. PLoS ONE. 2010;5:e9249. doi: 10.1371/journal.pone.0009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Multiscale model for the assessment of autonomic dysfunction in human endotoxemia. Physiol Genomics. 2010;42:5–19. doi: 10.1152/physiolgenomics.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264:1068–1076. doi: 10.1016/j.jtbi.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Mesarovic MD, Sreenath SN, Keene JD. Search for organising principles: understanding in systems biology. Syst Biol (Stevenage ) 2004;1:19–27. doi: 10.1049/sb:20045010. [DOI] [PubMed] [Google Scholar]
- 48.Pinsky MR. Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib Nephrol. 2001;(132):354–366. doi: 10.1159/000060100. [DOI] [PubMed] [Google Scholar]
- 49.Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, Gordon L, Zenker S, Kim HK, Hermus L, Zamora R, Rosengart MR, Clermont G, Peitzman A, Billiar TR, Ochoa J, Pinsky MR, Puyana JC, Vodovotz Y. An adequately robust early TNF-α response is a hallmark of survival following trauma/hemorrhage. PLoS ONE. 2009;4:e8406. doi: 10.1371/journal.pone.0008406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez H, Mesquida J, Hermus L, Polanco P, Kim HK, Zenker S, Torres A, Namas R, Gordon L, Severyn D, Vodovotz Y, Clermont G, Puyana JC, Pinsky MR. Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: Can irreversibility be anticipated? J Surg Res. 2012 doi: 10.1016/j.jss.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, Wang H. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 52.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Platzer C, Docke W, Volk H, Prosch S. Catecholamines trigger IL-10 release in acute systemic stress reaction by direct stimulation of its promoter/enhancer activity in monocytic cells. J Neuroimmunol. 2000;105:31–38. doi: 10.1016/s0165-5728(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 54.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, Harbrecht BG, Peitzman AB, Billiar TR, Maier RV, Remick DG, Minei JP. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- 55.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An G. Introduction of a agent based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theor Biol Med Model. 2008;5:11. doi: 10.1186/1742-4682-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207:E103–E111. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 58.Kubiak BD, Albert SP, Gatto LA, Snyder KP, Maier KG, Vieau CJ, Roy S, Nieman GF. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34:525–534. doi: 10.1097/SHK.0b013e3181e14cd2. [DOI] [PubMed] [Google Scholar]