Abstract
Objective
Statins, among the most commonly prescribed of medications, are associated with a wide range of musculoskeletal side effects. These include a progressive autoimmune myopathy with anti-HMGCR antibodies that requires immunosuppression. However, it remains unknown whether these antibodies are found in statin users with and without self-limited musculoskeletal side effects; this limits their diagnostic utility. The current work assesses the prevalence of anti-HMGCR antibodies in these groups of statin users.
Methods
We determined the prevalence of anti-HMGCR antibodies in (a) 1966 participants (including 763 current statin users) in a sub-study of the community-based Atherosclerosis Risk in Communities (ARIC) Study and (b) 98 French Canadian subjects with familial hypercholesterolemia, including 51 with documented statin intolerance.
Results
No participant in the ARIC sub-study, including those with past or current statin exposure at the time of sample collection, had anti-HMGCR antibodies. Similarly, none of 51 patients with self-limited statin intolerance or 47 statin-tolerant patients on maximal statin therapy were anti-HMGCR positive.
Conclusions
The vast majority patients with and without statin exposure, including those with self-limited statin intolerance, do not develop anti-HMGCR antibodies. Thus, anti-HMGCR antibodies are highly specific for those with an autoimmune myopathy.
INTRODUCTION
Lipid lowering agents are among the most frequently used medications, with nearly 30 million Americans prescribed a statin in 2005 (1). Mild musculoskeletal complaints such as myalgias are common, occurring in as many as 20% of statin users (2), but typically resolve within weeks to months of discontinuing the offending medication. In contrast, recent reports have established that statins can also be associated with development of an immune-mediated myopathy requiring immunosuppressive therapy to control (3–6). Since patients with both self-limited and autoimmune statin-associated myopathy may initially present with myalgias, weakness, and/or elevated creatine kinase (CK) levels (5), a laboratory test to help determine whether a statin-treated patient with musculoskeletal complications has a self-limited condition or will likely require treatment for an autoimmune process would be clinically valuable.
Autoantibodies recognizing 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), the pharmacologic target of statins, have been identified in patients with statin-associated autoimmune myopathy (6). However, the prevalence of these antibodies in a large population of statin-exposed subjects has not been determined. Furthermore, it is not known whether patients with self-limited statin-associated myotoxicity produce anti-HMGCR antibodies. These gaps in knowledge limit the diagnostic utility of anti-HMGCR testing. We performed this study to determine whether statin-exposed subjects with and without self-limited statin intolerance also develop anti-HMGCR autoantibodies.
PATIENTS AND METHODS
Study Populations
The Atherosclerosis Risk in Communities (ARIC) Study is an on-going community-based prospective cohort study of 15,792 middle-aged adults who were enrolled from 1987–1989. In 2004–2005, 2,006 participants from the original cohort were recruited into the Carotid MRI (CARMRI) sub-study. This sub-study has been described in detail elsewhere (7). The present study included 1,966 ARIC CARMRI participants with sufficient sera for measurement of anti-HMGCR antibodies and non-missing information on current statin use. We also obtained measurements on plasma samples from 98 patients affected by familial hypercholesterolemia (FH) due to LDLR gene mutations evaluated at the Chicoutimi Hospital Lipid Clinic and ECOGENE-21 Clinical Research Center (Chicoutimi, Quebec, Canada), 51 of which presented with signs and symptoms of muscular intolerance to statins. The degree of myalgias and muscular weakness was self-reported by subjects as part of a detailed questionnaire. The clinical evaluation also included plasma creatine phosphokinase (CK) and myoglobinuria assessment. IRB and/or ethics review board approval and participants’ written informed consent was obtained from each participant.
Antibody testing
We used a previously described anti-HMGCR ELISA test as an initial screening tool (6). Based on quality control analyses comparing anti-HMGCR ELISA titers obtained from plasma and serum samples collected simultaneously from anti-HMGCR positive patients, raw plasma ELISA titers were multiplied by a correction factor of 1.24. Specimens with anti-HMGCR titers greater than 3 standard deviations above the mean of all ARIC CAMRI participants on repeated testing were confirmed by using these samples to immunoprecipitate full-length 35S-methionine–labeled in vitro transcription/translated (IVTT) HMGCR protein as described elsewhere (6).
RESULTS
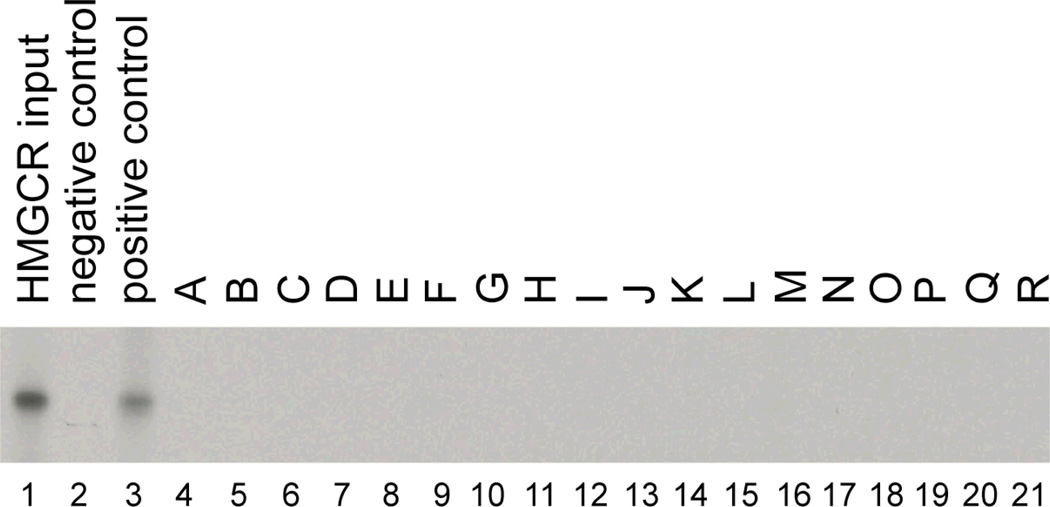
At the time of blood collection from the ARIC CARMRI study participants, 763 were currently taking a statin drug, 322 other subjects reported using a cholesterol-lowering medication at some point previously during follow-up, and 881 never reported using a cholesterol-lowering medication. Anti-HMGCR titers were determined by ELISA for all ARIC CARMRI participants. The mean titer and standard deviation of the mean titer were 0.109 and 0.086 normalized absorbance units (NAU), respectively. Among all ARIC CARMRI participants, 14 (0.7%) had anti-HMGCR titers greater than 3 standard deviations above the mean (0.367 NAU), including 8 without prior statin exposure. None of these 14 serum samples immunoprecipitated the full-length 35S-labeled HMGCR protein (figure 1). There was no significant difference in the mean anti-HMGCR antibody titers comparing current statin users (0.106 normalized absorbance units) to never users (0.111 normalized absorbance units, p-value =0.300) and to past users (0.098 normalized absorbance units, p-value =0.097).
Figure 1. ELISA positive sera from ARIC subjects do not immunoprecipitate HMGCR.
Immunoprecipitations were performed using full length 35S-methionine labeled HMGCR. Lane 1 shows the input IVTT product. Immunoprecipitations using serum from anti-HMGCR negative (#485) and positive (#9190) controls are shown in lanes 2 and 3, respectively. Lanes 4–21 show immunoprecipitations using serum from those patients in the ARIC cohort with the highest anti-HMGCR titers by ELISA.
Plasma samples were obtained from 98 FH patients including 47 statin-tolerant patients on maximal statin therapy and 51 patients with documented muscular signs and symptoms of statin-intolerance having required the cessation of treatment. The clinical characteristics of the statin-intolerant patients are detailed in Table 1. 31 patients experienced myalgias, 17 patients became weak, and 9 patients had CK elevations or myoglobinuria that resolved once statin exposure was discontinued. None of these 98 patients had anti-HMGCR titers greater than 3 standard deviations above the mean of the ARIC CARMRI cohort. The statin-intolerant patients tended to have higher mean anti-HMGCR antibody titers than subjects without statin-intolerance, but this did not reach statistical significance (0.101±0.069 vs. 0.073±0.076 NAU, respectively, p=0.062).
Table 1.
|
FH Subjects’ characteristics | |||
|---|---|---|---|
| Statin-tolerants (n=47) |
Statin-intolerants (n=51) |
p-value | |
| Gender (M/F) | 26/21 | 29/22 | 0.878 |
| Age (years) | 57.8±11.1 | 56.5±10.2 | 0.56 |
| CK (U/L) | 105.09±47.34 | 131.44±71.07 | 0.038 |
| Distribution of drug tolerance markers among statin-intolerant FH subjects (n=51) | |
|---|---|
| Muscular weakness | |
| Low | 8 (15.7%) |
| Moderate | 9 (17.6%) |
| Myalgia | |
| Low | 6 (11.8%) |
| Moderate | 19 (37.3%) |
| Severe | 6 (11.8%) |
| CK > 300 U/L | 3 (6.0%) |
| Myoglobinuria | 6 (12.0%) |
| Rhabdomyolysis | 1 (2.0%) |
Data are mean ± SD
In our initial description of the anti-HMGCR ELISA, we defined a cutoff for normal as greater than 3 standard deviations above the mean value of samples from just 20 healthy subjects (0.215 NAU) (6). Having screened 1966 subjects from ARIC CARMRI, a community-based cohort, we now define a new cutoff value of 0.367 NAU, which represents 3 standard deviations above the mean for this group. To determine the sensitivity and specificity of the ELISA using this cutoff relative to our gold standard, immunoprecipitation of radiolabeled HMGCR, we compared the ELISA and immunoprecipitation results from 307 consecutive subjects enrolled at the Johns Hopkins Myositis Center as described in our previous publication (6) (Table 2). To summarize, 18 sera immunoprecipitated HMGCR protein and 17 of these sera were positive by ELISA. Conversely, among the 19 sera positive by ELISA, 17 were positive by immunoprecipitation. Thus, the sensitivity and specificity of the anti-HMGCR ELISA are 94.4% and 99.3%, respectively.
Table 2.
Anti-HMGCR ELISA vs. Immunoprecipitation two by two table.
| IP positive | IP negative | |
|---|---|---|
| ELISA positive | 17 | 2 |
| ELISA negative | 1 | 287 |
Sensitivity of ELISA = 17/18 = 0.944 (confidence interval: 0.706 to 0.997)
Specificity of ELISA = 287/289 = 0.993 (confidence interval: 0.972 to 0.999)
DISCUSSION
In this study, we screened for anti-HMGCR antibodies by ELISA and, in those with titers greater than 3 standard deviations above the mean of the ARIC CARMRI participants, confirmed their presence by the gold standard: immunoprecipitation of full-length HMGCR protein. Definitive anti-HMGCR antibodies were not found in any of the 1966 ARIC CARMRI study participants, including 763 with current statin-exposure and an additional 322 who reported prior exposure to a cholesterol-lowering medication. This demonstrates a very low background prevalence of anti-HMGCR autoimmunity, not only in statin-naïve subjects, but also in those with statin exposure. Similarly, in contrast to patients we have previously described with statin-associated autoimmune myopathy, none of 51 FH subjects with documented self-limited statin myotoxicity were anti-HMGCR positive by ELISA. This indicates that anti-HMGCR autoantibodies are rarely, if ever, found in the general population; this includes the large number who may experience self-limited statin intolerance, such as FH patients, who are generally treated with maximal statin dosage and/or combination therapy.
Although not statistically significant, those FH subjects with statin intolerance tended to have higher anti-HMGCR titers than those who were statin tolerant. The significance of this is uncertain, especially since none of the subjects had titers exceeding the cut-off for normal. Future longitudinal studies will be required to determine whether anti-HMGCR titers increase, albeit within the normal range, in statin-intolerant subjects during the course of statin treatment.
A limitation of our previous study describing the anti-HMGCR ELISA was the small number of controls used to define a normal value for this test (6). We have now screened a much larger population, the ARIC CARMRI cohort, and have been able to better define the normal range of anti-HMGCR titers. Given the new cutoff values, we determined that the sensitivity and specificity of the anti-HMGCR ELISA are 94.4% and 99.3%, respectively. Assuming that anti-HMGCR autoimmune myopathy is rare, with a prevalence of only 1 in 100,000, the negative predictive value of the ELISA test in an unselected population is greater than 0.999. Indeed, even in our specialized myositis clinic where the prevalence of anti-HMGCR autoimmune myopathy is 7 in 100, the ELISA has a negative predictive value of 0.996. Consequently, regardless of the clinical setting, a negative HMGCR ELISA result makes it highly unlikely that a patient has anti-HMGCR autoimmune myopathy that would require immunosuppressive therapy. In contrast, the positive predictive value of the HMGCR ELISA would be only 0.910 in our clinic and 0.001 in an unselected population. This underscores the need, in any setting, to verify a positive ELISA result by performing a confirmatory HMGCR immunoprecipitation assay.
Two major limitations should be considered in the interpretation of these data. First, we do not have sufficient clinical information to exclude the possibility that one or more of the 1966 ARIC subjects had anti-HMGCR autoimmune myopathy. However, this is unlikely given that the approximate prevalence of autoimmune myopathy is 22 per 100,000 (8) and anti-HMGCR antibodies are found in less than 10% of these (6). Second, blood samples from the statin-intolerant patients in the clinic sample may have been collected in some cases after muscle symptoms and laboratory abnormalities had resolved. This raises the possibility that these subjects could have had transient elevations in anti-HMGCR titers that subsequently resolved. Again, this seems unlikely given (a) the known durability of the antibody response in general and (b) our finding that anti-HMGCR antibody titers remain elevated even in well-treated patients with anti-HMGCR myopathy (unpublished result).
In summary, this study demonstrates that anti-HMGCR antibodies are not found in the vast majority of statin-exposed individuals, including those with self-limited statin myopathy. Taken together with our prior studies (5, 6), this indicates that statin-exposed patients who present with myalgias, muscle weakness, and elevated CK levels that test positive for anti-HMGCR antibodies most likely have an autoimmune myopathy requiring immunosuppression. Furthermore, our finding that anti-HMGCR antibodies are not found in those with self-limited statin-associated musculoskeletal side-effects suggests that this represents a distinct pathologic process from statin-associated autoimmune myopathy.
Significance and Innovation.
Anti-HMGCR antibodies are not found in the vast majority of statin-treated subjects, including those with self-limited statin-associated myopathy.
The presence of anti-HMGCR antibodies is highly specific for subjects with an autoimmune myopathy requiring immunosuppression.
Acknowledgments
This work was supported by NIH grant K08-AR-054783 (A.M.). These studies were also supported by the Ira Fine Discovery Fund, the Dorothy and Donald Stabler Foundation and by ECOGENE-21, the Canadian Institutes of Health Research (CIHR team in community genetics (grant #CTP-82941)). D.G. is the chairholder of the Canada Research Chair in preventive genetics and community genomics (www.chairs.gc.ca). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) with the ARIC carotid MRI examination funded by U01HL075572-01. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosures: Dr. Andrew L. Mammen has a provisional patent on an anti-HMGCR assay; this has not been licensed. No other disclosures.
REFERENCES
- 1.Trends in statins utilization and expenditures for the U.S. civilian noninstitutionalized population, 2000 and 2005.[homepage on the Internet] Rockdale (MD): Agency for Healthcare Research and Quality; 2008. May, Statistical brief #205. Available from: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st205/stat205.pdf. [PubMed] [Google Scholar]
- 2.Franc S, Dejager S, Bruckert E, Chauvenet M, Giral P, Turpin G. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther. 2003;17:459–465. doi: 10.1023/b:card.0000015861.26111.ab. [DOI] [PubMed] [Google Scholar]
- 3.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007;17:194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 5.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62:2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, et al. Correlates of carotid plaque presence and composition as measured by MRI: The atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2009;2:314–322. doi: 10.1161/CIRCIMAGING.108.823922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernatsky S, Joseph L, Pineau CA, Belisle P, Boivin JF, Banerjee D, et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: Age, sex and regional differences. Ann Rheum Dis. 2009;68:1192–1196. doi: 10.1136/ard.2008.093161. [DOI] [PubMed] [Google Scholar]