Abstract
The formal syntheses of N-methylwelwitindolinone C isothiocyanate, N-methylwelwitindolinone C isonitrile, N-methylwelwitindolinone D isonitrile, 3-hydroxy-N-methylwelwitindolinone C isothiocyanate, and 3-hydroxy-N-methylwelwitindolinone C isonitrile is reported. The synthesis features several novel processes, including a Lewis acid mediated coupling between a benzylic-type heteroaromatic alcohol and a highly functionalized silyl ketene acetal, an intramolecular enolate arylation, and a regioselective, Pd(0)-catalyzed π-allylic cyclization of a γ-benzoyloxy enone moiety that is revealed by unmasking a furan ring.
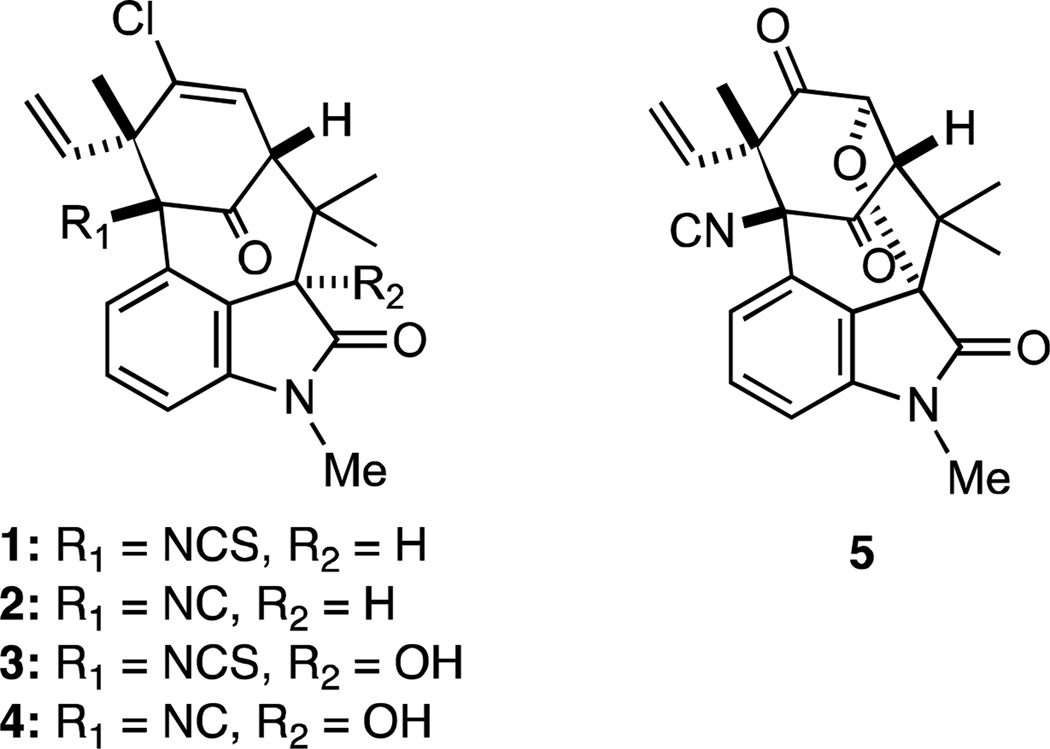
Several novel indole alkaloids, including N-methylwelwitindolinone C isothiocyanate (1) and N-methylwelwitindolinone C isonitrile (2), were isolated by Moore and coworkers from the extracts of the blue-green cyanobacteria Hapalosiphon wetwitschii and Westiella intracta in 1994 (Figure 1).1 These compounds, which were collectively named welwitindolinones and possess a unique bicyclo[4.3.1]decane ring system, were isolated together with the related fisherindoles and hapalindoles, and a putative biogenetic relationship among these alkaloids was proposed.1 The related alkaloids 3-hydroxy-N-methylwelwitindolinone C isothiocyanate (3), 3-hydroxy-N-methylwelwitindolinone C isonitrile (4), and N-methylwelwitindolinone D isonitrile (5) with the same skeletal framework were later isolated from the cyanophytes Fischerella muscicola and Fischerella major.2 These natural products exhibit a number of useful biological properties, including antifungal and larvicidal activities, microtubule depolymerization, and the ability to reverse P-glycoprotein-mediated multidrug drug resistance (MDR) in human cancer cells.3
Figure 1.
Representative welwitindolinone alkaloids
Owing to their novel structures and exciting biological activities, the welwitindolinone family of natural products has captured the attention of many in the synthetic community, and these efforts have been chronicled in numerous publications over the past 15 years.4 Despite these extensive efforts, 1,5,6 2,6,7 3,6,7 4,6,7 and 58 succumbed to total synthesis only very recently. We now report the details of some of our work in the area that has culminated in the formal syntheses of the naturally occurring welwitindolinones 1–5.
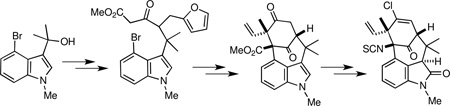
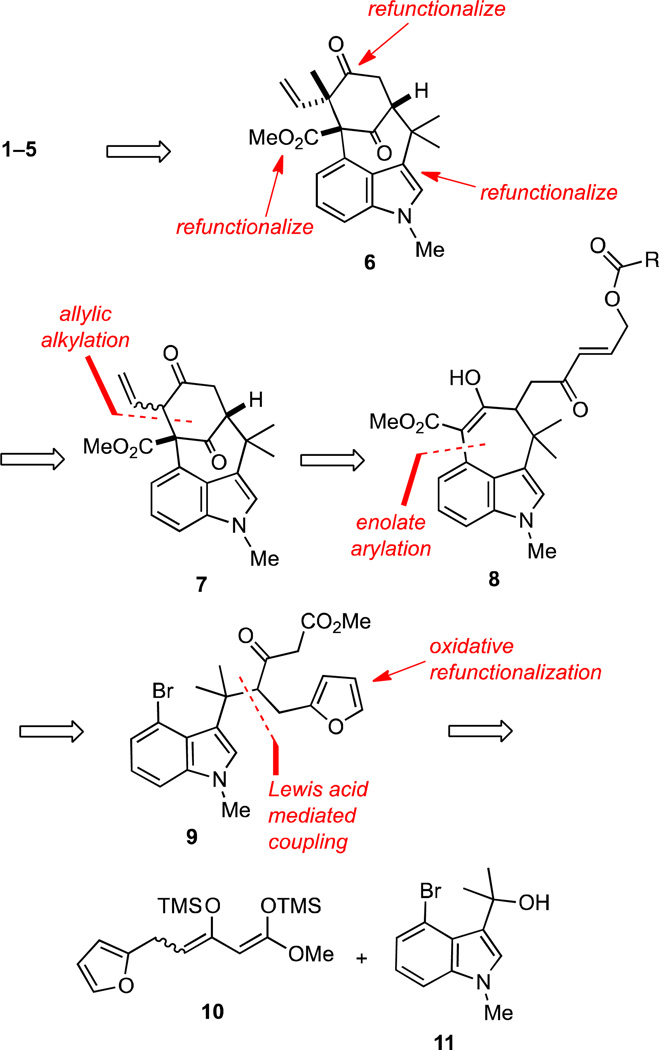
Our approach to the welwitindolinones 1–5 is outlined in retrosynthetic format in Scheme 1 and features refunctionalization of the advanced common intermediate 6, which is accessed by the stereoselective methylation of the dienolate derived from 7. We envisioned that the tetracycle 7 could be formed via a novel allylic alkylation of enone 8. Although there are a plethora of examples of π-allylic alkylations,9 we are aware of no precedent for a Pd(0)-catalyzed π-allylic alkylation of a γ-acyloxy enone moiety as found in 8 that proceeds alpha to the carbonyl group. This construction would thus represent a novel advance that could prove to be of broader utility. The synthesis of 8 from 9 features formation of the tricyclic core of the welwitindolinones by an enolate arylation, which is precedented in our earlier work in the area,4v and the oxidative unmasking of a furan ring to reveal the latent γ-acyloxy enone subunit. Access to 9 then requires trapping of the carbocation generated from the known alcohol 114v with the π-nucleophile 10 via a vinylogous Mannich-like reaction10 utilizing methodology that we previously developed specifically for this bond forming step.11
Scheme 1.
Retrosynthetic approach to welwitindolinone alkaloids
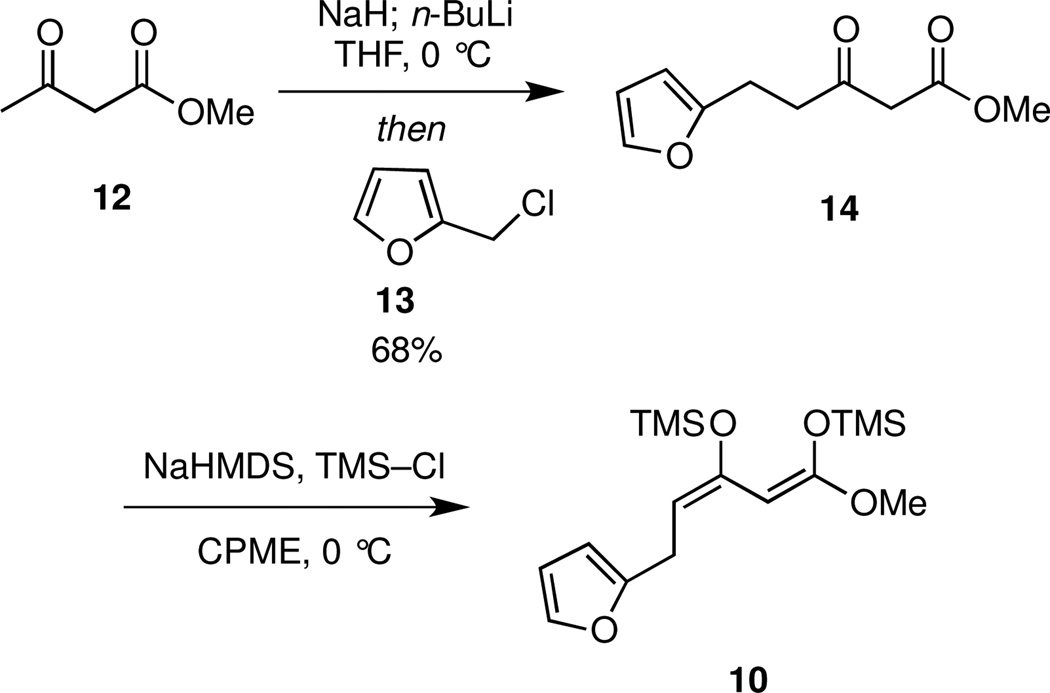
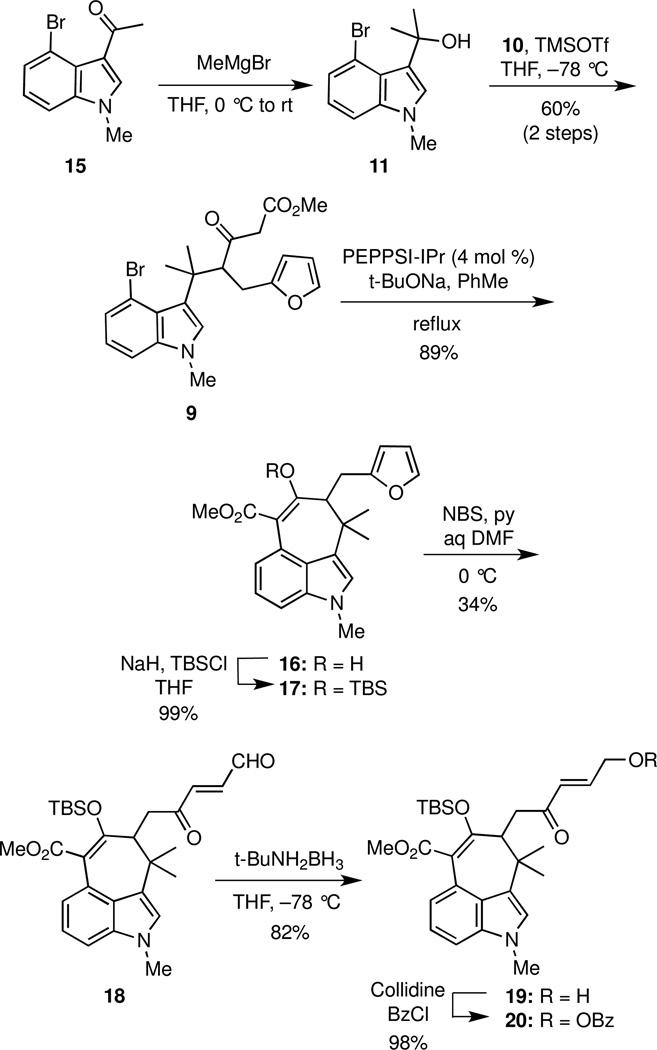
The first task required the synthesis of the silyl ketene acetal 10. In the event, γ-alkylation of the dianion of methyl acetoacetate (12)12 with furfuryl chloride (13)13 furnished ketoester 14 (Scheme 2). Subsequent deprotonation of 14 with NaHMDS followed by trapping with TMSCl gave 10 as a single isomer,14 which was used directly without purification.
Scheme 2.
Synthesis silyl ketene acetal 10
The methyl ketone 15, which was prepared from commercially available 4-bromoindole in two steps,4v was treated with methylmagnesium bromide to give the somewhat labile alcohol 11, which was treated immediately with silyl ketene acetal 10 in the presence of TMSOTf to deliver ketoester 9 as a mixture (ca 4:1) of keto and enol tautomers in 60% yield over two steps. (Scheme 3). Construction of the seven-membered carbocyclic ring was then achieved to give 16 in 89% yield via a Pd(0)-catalyzed intramolecular enolate arylation that was optimally promoted with PEPPSI-IPr15 in the presence of NaOt-Bu. Initial attempts to selectively oxidize the furan ring in 16 were unsuccessful because the enol moiety in 16 cyclized onto the intermediate unsaturated keto aldehyde, thus leading to unwanted byproducts. This troublesome side reaction was avoided simply by protecting 16 as its tert-butyldimethylsilyl enol ether 17. In practice, the enolate arylation and enol protection steps were performed without purification of the intermediate 16.
Scheme 3.
Synthesis of benzoate 20
The selective oxidative cleavage of the furan moiety of 17 proved to be considerably more challenging than originally anticipated, primarily because of competing halogenation of the indole ring. Numerous reagents and conditions were screened. Br2 and dibromohydantoin in aqueous solvents gave low yields of 18 along with significant amounts of overbrominated products in which bromine atoms had been introduced onto the indole ring. Use of N-chlorosuccinimide (NCS) in aqueous solvents led only to chlorination of the furan and indole rings; no aldehyde was detected in the reaction mixtures. MeReO3/urea hydrogen peroxide oxidized the indole moiety while leaving the furan ring intact.16 Dimethyldioxirane (DMDO) and magnesium monoperoxyphthalate (MMPP) gave mixtures of products, whereas use of singlet oxygen produced the Z-isomer of 18 in only about 20% yield.
We eventually discovered that treating 17 with N-bromosuccinimide (NBS) in aqueous DMF gave aldehyde 18 in 34% yield, together with 7% of a brominated derivative of 18 and 6% of a brominated derivative of 17. Selective reduction of the aldehyde moiety in 18 was initially somewhat problematic because mixtures of 1,2-and 1,4-reduction products were formed. However, we discovered that tert-BuNH2BH317 reduced 18 to give alcohol 19 in 82% yield, and no overreduction of the ketone moiety was observed. O-Benzoylation of alcohol 19 with BzCl gave 20, thereby setting the stage for the pivotal intramolecular π-allylic alkylation. Although the acetate analog of 20 was also prepared, it was found to be less stable and gave lower yields in the cyclization.
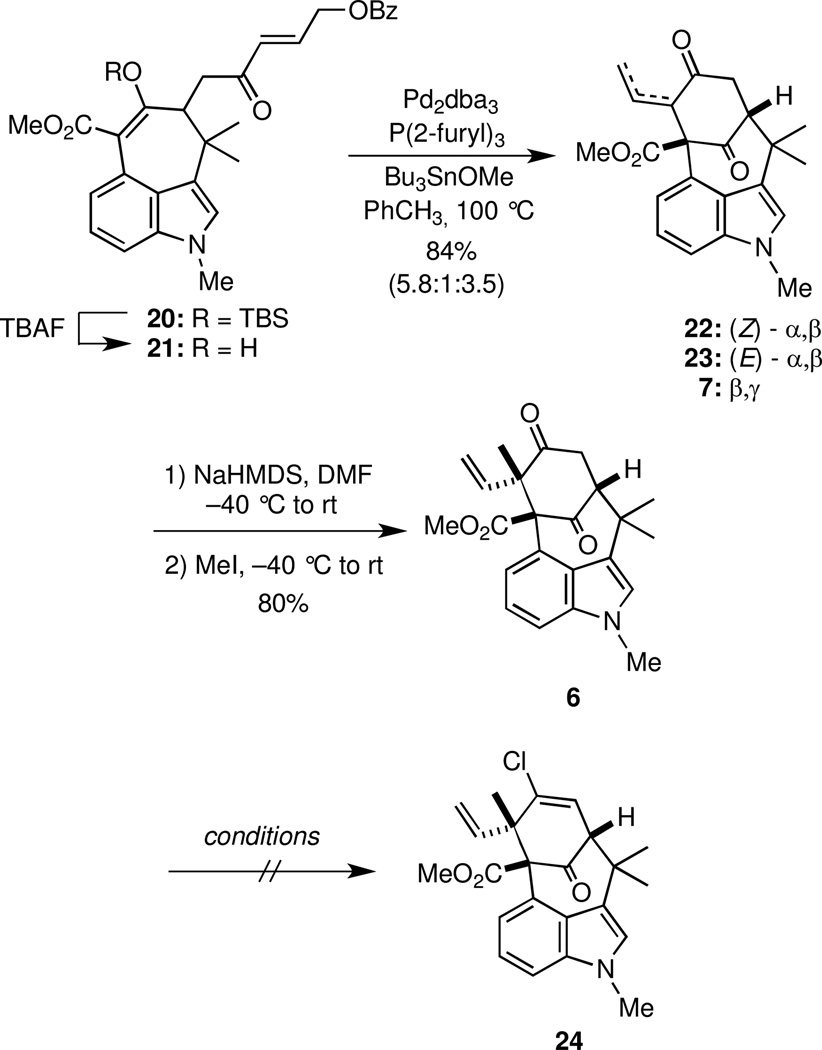
Deprotection of the silyl enol ether moiety in 20 with TBAF provided the enol 21, which was treated directly with Bu3SnOMe18 and Pd2dba3 in the presence of trifurylphosphine to deliver a mixture (5.8:1:3.5) of enones 22 and 23 and vinyl ketone 7 (Scheme 4). When this mixture of 22, 23, and 7 was deprotonated with freshly prepared NaHMDS under equilibrating conditions, the dienolate thus generated was methylated with MeI to furnish 6 as a single diastereomer. The structure of 6 was unequivocally established by X-ray crystallography. At this stage, the entire carbon skeleton of the welwitindolinones 1–5 had been assembled, but several functional group manipulations were needed.
Scheme 4.
Side chain elaboration
The primary challenge at this juncture was the conversion of the ketone functional group into the vinyl chloride 24, and a number of one-step methods are known. However, in a number of preliminary experiments, we found that treating ketone 6 with PCl519 WCl6,20 PPh3/CCl4,21 P(OPh)3/Cl2,22 and Sc(OTf)3/AcCl23 under a variety of established conditions and modifications thereof did not furnish any of the desired vinyl chloride 24. Other protocols for transforming ketones into vinyl chlorides were also examined. For example, the reaction of hydrazones with NCS is known to generate vinyl chlorides.24 Initial efforts to prepare the hydrazone of 6 led solely to extensive reduction of the pendant vinyl group, which presumably occurred because dimide was generated in situ. However, even when 6 was treated with hydrazine under rigorous exclusion of oxygen and trace metals, no hydrazone formation was observed, even under forcing conditions that led to the formation of uncharacterizable mixtures. Because this protocol was used successfully by Rawal to prepare the vinyl chloride of a closely related ketone, additional work is perhaps indicated.6 The ketone 6 was also converted into its vinyl triflate by sequential reaction with LiHMDS and Comins’ reagent, but preliminary attempts to convert this triflate to a vinyl stannane following a protocol used by Garg5 for a related substrate were unsuccessful. Rawal observed an unusual rearrangement for a closely related enol triflate having a bridgehead aldehyde instead of an ester,4z although we observed no such reaction.
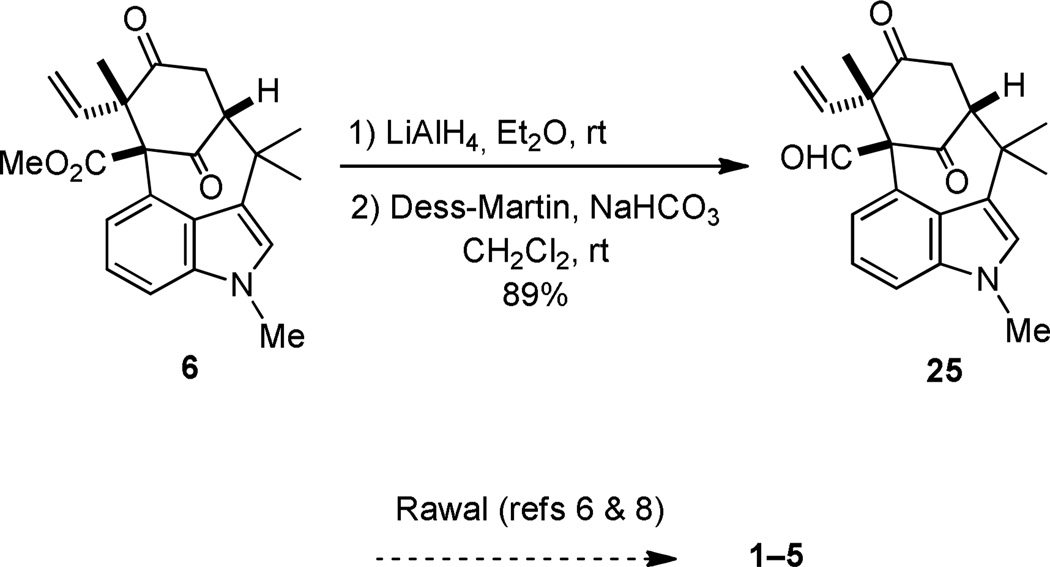
We continue to explore methods to effect the conversion of ketone 6 into the vinyl chloride 24 in order to complete a total synthesis of N-methylwelwitindolinone C isothiocyanate (1). However, we decided to exploit an opportunity to complete the formal syntheses of the welwitindolinones 1–5 by intercepting a pivotal intermediate that Rawal has converted into these natural products. In the event, global reduction of 6 with LiAlH4 gave an intermediate triol that underwent facile oxidation with Dess-Martin periodinane to furnish aldehyde 25 in 89% yield over two steps (Scheme 5). Spectral data (1H and 13C NMR) for the synthetic 25 thus obtained are consistent with those reported.8 Inasmuch as Rawal and coworkers have converted 25 into welwitindolinones 1–5,6,8 its preparation by the route outlined in this account constitutes a formal synthesis of these alkaloids.
Scheme 5.
Completion of Formal Synthesis
In summary, the formal syntheses of welwitindolinones 1–5 were successfully completed by the independent synthesis of the aldehyde 25, a key intermediate in Rawal's syntheses of these alkaloids.6,8 The synthesis features a number of interesting steps. The first of these is a Lewis acid mediated coupling that involves a variant of a vinylogous Mannich reaction in which a benzylic-type carbocation is trapped by a highly functionalized π-nucleophile. Other highlights include an intramolecular enolate arylation to form a seven-membered ring and a novel, regioselective π-allylic alkylation of a γ-acyloxy enone moiety to construct the tetracyclic core of the welwitindolinones.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM 25439) the Robert A. Welch Foundation (F-0652) for their generous support of this research. We are also grateful to Vince Lynch (The University of Texas) for X-ray crystallography and Steve Sorey (The University of Texas) for NMR spectroscopy.
Footnotes
Supporting Information Available: Experimental procedures and 1H NMR spectra for all new compounds, plus CIF files representing X-ray coordinates for compound 6 are included. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]
- 2.Jimenez JI, Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1999;62:569–572. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]
- 3.(a) Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]; (b) Zhang XQ, Smith CD. Mol. Pharmacol. 1996;49:288–294. [PubMed] [Google Scholar]
- 4.For reviews, see: Avendaño C, Menéndez JC. Curr. Org. Synth. 2004;1:65–82. Brown LE, Konopelski JP. Org. Prep. Proc. Intl. 2008;40:411–445. Huters AD, Styduhar ED, Garg NK. Angew. Chem. Int. Ed. 2012;51:3758–3765. doi: 10.1002/anie.201107567. Wood JL. Nature Chem. 2012;4:341–343. doi: 10.1038/nchem.1335. See also: Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107. Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J. Am. Chem. Soc. 1999;121:6326–6327. Deng H, Konopelski JP. Org. Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r. Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838. López-Alvarado P, García-Granda S, Álvarez-Rúa C, Avendaño C. Eur. J. Org. Chem. 2002:1702–1707. MacKay JA, Bishop RL, Rawal VH. Org. Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. Baudoux J, Blake AJ, Simpkins NS. Org. Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t. Greshock TJ, Funk RL. Org. Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799. Lauchli R, Shea KJ. Org. Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747. Xia J, Brown LE, Konopelski JP. J. Org. Chem. 2007;72:6885–6890. doi: 10.1021/jo071156l. Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas TS, Pohjakallio A, Baran PS. J. Am. Chem. Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k. Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem. Commun. 2009:1398–1400. doi: 10.1039/b820674k. Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286. Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684. Trost BM, McDougall PJ. Org. Lett. 2009;11:3782–3785. doi: 10.1021/ol901499b. Brailsford JA, Lauchli R, Shea KJ. Org. Lett. 2009;11:5330–5333. doi: 10.1021/ol902173g. Freeman DB, Holubec AA, Weiss MW, Dixon JA, Kakefuda A, Ohtsuka M, Inoue M, Vaswani RG, Ohki H, Doan BD, Reisman SE, Stoltz BM, Day JJ, Tao RN, Dieterich NA, Wood JL. Tetrahedron. 2010;66:6647–6655. doi: 10.1016/j.tet.2010.04.131. Heidebrecht RW, Gulledge B, Martin SF. Org. Lett. 2010;12:2492–2495. doi: 10.1021/ol1006373. Ruiz M, Lopez-Alvarado P, Menendez JC. Org. Biomol. Chem. 2010;8:4521–4523. doi: 10.1039/c0ob00382d. Bhat V, Rawal VH. Chem. Commun. 2011;47:9705–9707. doi: 10.1039/c1cc13498a. Bhat V, MacKay JA, Rawal VH. Org. Lett. 2011;13:3214–3217. doi: 10.1021/ol201122f. Bhat V, MacKay JA, Rawal VH. Tetrahedron. 2011;67:10097–10104. doi: 10.1016/j.tet.2011.09.088.
- 5.Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J. Am. Chem. Soc. 2011;133:15797–15799. doi: 10.1021/ja206538k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan KM, Kobayashi K, Rawal VH. J. Am. Chem. Soc. 2012;134:1392–1395. doi: 10.1021/ja210793x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quasdorf KW, Huters AD, Lodewyk MW, Tantillo DJ, Garg NK. J. Am. Chem. Soc. 2012;134:1396–1399. doi: 10.1021/ja210837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat V, Allan KM, Rawal VH. J. Am. Chem. Soc. 2011;133:5798–5801. doi: 10.1021/ja201834u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Trost BM, van Vranken DL. Chem. Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Lee C. Asymmetric Allylic Alkylation Reactions. In: Oijima I, editor. Catalytic Asymmetric Synthesis. 2nd ed. New York: Wiley-VCH; 2000. p. 593. [Google Scholar]; (c) Lu Z, Ma S. Angew. Chem. Int. Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]
- 10.For a review of the vinylogous Mannich reaction, see: Martin SF. Acc. Chem. Res. 2002;35:895–904. doi: 10.1021/ar950230w.
- 11.Fu T-h, Bonaparte A, Martin SF. Tetrahedron Lett. 2009;50:3253–3257. doi: 10.1016/j.tetlet.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huckin SN, Weiler L. J. Am. Chem. Soc. 1974;96:1082–1087. [Google Scholar]
- 13.(a) Chaudhari SS, Akamanchi KG. Synlett. 1999:1763–1765. [Google Scholar]; (b) Kirner WR. J. Am. Chem. Soc. 1928;50:1955–1961. [Google Scholar]
- 14.Okabayashi T, Iida A, Takai K, Nawate Y, Misaki T, Tanabe Y. J. Org. Chem. 2007;72:8142–8145. doi: 10.1021/jo701456t. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien CJ, Kantchev EAB, Valente C, Hadei N, Chass GA, Lough A, Hopkinson AC, Organ MG. Chem. Eur. J. 2006;12:4743–4748. doi: 10.1002/chem.200600251. [DOI] [PubMed] [Google Scholar]
- 16.Finlay J, McKervey M, Gunaratne H. Tetrahedron Lett. 1998;39:5651–5654. [Google Scholar]
- 17.Andrews GC. Tetrahedron Lett. 1980;21:697–700. [Google Scholar]
- 18.Johns DM, Mori M, Williams RM. Org. Lett. 2006;8:4051–4054. doi: 10.1021/ol061524s. [DOI] [PubMed] [Google Scholar]
- 19.Braude EA, Bruun T, Weedon BCL, Woods RJ. J. Chem. Soc. 1952:1419–1425. [Google Scholar]
- 20.Jung ME, Wasserman JI. Tetrahedron Lett. 2003;44:7273–7275. [Google Scholar]
- 21.Isaacs NS, Kirkpatrick D. J. Chem. Soc. Chem. Comm. 1972:443–444. [Google Scholar]
- 22.Spaggiari A, Vaccari D, Davoli P, Torre G, Prati F. J. Org. Chem. 2007;72:2216–2219. doi: 10.1021/jo061346g. [DOI] [PubMed] [Google Scholar]
- 23.Su W, Jin C. Org. Lett. 2007;9:993–996. doi: 10.1021/ol062991c. [DOI] [PubMed] [Google Scholar]
- 24.Mori H, Tsuneda K. Chem. Pharm. Bull. 1963;11:1413–1417. doi: 10.1248/cpb.11.1413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.