Abstract
Purpose.
We characterized the ocular pharmacokinetics of bevacizumab in vitrectomized eyes with silicone oil tamponade.
Methods.
A total of 18 pigmented rabbits underwent vitrectomy and silicone oil tamponade before an intra-silicone oil injection of 1.25 mg bevacizumab. At post-injection days 1, 7, 14, 28, 42, and 56, 3 rabbits were sacrificed and enucleated, and bevacizumab concentrations were measured in various ocular tissues and plasma.
Results.
The bevacizumab peak concentration was reached at day 14 in the aqueous humor (4030.70 ng/mL), retina (42,171.7 ng/g), and choroid (56,243.33 ng/g). In the iris/ciliary body and plasma, the peak concentration was reached at day 7 with 52,648.30 ng/g and 197.70 ng/mL, respectively. The choroid had the maximum exposure to bevacizumab with an area under the curve calculated from time zero to the last observed time (AUClast) of 1,151,633.40 ng/day/g and the aqueous humor had the minimum exposure (AUClast = 74,611.28 ng/day/g) among the ocular tissues, while the drug exposure to the plasma was the smallest of all tissues studied (AUClast = 3795.17 ng/day/g). The terminal half-lives and the mean residence time of bevacizumab in the ocular tissues ranged from 3–5 and 10–13 days, respectively.
Conclusions.
The peak concentration of bevacizumab in various ocular tissues and plasma was delayed and lower than that found in normal rabbit eyes; however, the terminal half-lives were similar to those found in the eyes with native vitreous following an intravitreal injection. Oil may have impacted the distribution of bevacizumab and led to an altered profile of drug level in the ocular tissues.
Intra-silicone oil injection of bevacizumab led to a delayed, lower peak concentration with sustained drug levels in the ocular tissues, while with very little change in terminal half-lives when compared to the intravitreal injection.
Introduction
The use of bevacizumab (Avastin) has experienced a rapid proliferation since its introduction into the ophthalmologic community in 2005.1 Many studies have demonstrated its effectiveness in the treatment of vitreoretinal diseases following intravitreal injections, including choroidal neovascularizations (CNVs) associated with various macular degenerations, retinal vein occlusion, and diabetic retinopathy, including proliferative vitreoretinopathy, which recently is being managed by vitrectomy and a presurgical intravitreal injection of bevacizumab as a surgical adjunct.2–4 An intravitreal bevacizumab injection is administrated on a monthly basis, based on earlier preclinical ocular pharmacokinetic studies of bevacizumab and ranibizumab (Lucentis).5–7 Lucentis is an FDA-approved monthly intravitreal injection treatment for the wet form of age-related macular degeneration (AMD). A recent clinical trial has shown that a monthly intravitreal injection of bevacizumab has the equivalent therapeutic effect on the wet form of AMD as a monthly intravitreal injection of Lucentis.8 The vitreous half-life of bevacizumab in rabbit eyes was reported to be 4.32 or 5.95 days following a 1.25 mg intravitreal injection and the drug elimination followed the first order kinetics.6,7,9 These half-lives were shorter than the bevacizumab half-life found in human vitreous, which was reported to be 6.7 days.10
In medical retinal practice, there are times that neovascularization recurs on the retina or iris even after an aggressive vitrectomy combined with an endolaser retinopexy and silicone oil infusion.11 Recently, a study demonstrated that an intra-silicone injection of bevacizumab is effective in the treatment of patients with iris neovascularization (INV) and neovascularization glaucoma (NVG) following vitrectomy for advanced proliferative diabetic retinopathy.12 Silicone oil is an important adjunct in the management of complex vitreoretinal surgical procedures. In the presence of a silicone oil tamponade, drug injected into the posterior segment of the eye could behave significantly different compared to a vitreous-filled eye or an eye following vitrectomy with fluid infusion.13 Due to the increasing popularity of the intraocular bevacizumab application for various chorioretinal diseases, there is an unmet need to understand the ocular kinetics of bevacizumab in silicone oil-filled eyes. Our current study was designed to investigate the ocular pharmacokinetics of bevacizumab following an intra-silicone oil injection. The study would provide valuable information for the application of bevacizumab in eyes with a silicone oil infusion.
Methods
Experimental Animals
An approval of animal use was granted by the Animal Care and Use Committee at WenZhou Medical College. All animal management followed the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research (ARVO Animal Policy). We used for this study 18 adult chinchilla pigmented rabbits, with a mean body weight of 2.65 ± 0.42 kg. Only the right eye of each rabbit was used for the vitrectomy procedure and the later bevacizumab intra-silicone injection, while the left eye was left untouched and served as an internal control. All animals underwent a baseline examination using slit-lamp, indirect ophthalmoscopy, and tonometer (Tono-Pen XL, Medtronic Solan). All the right eyes were operated on by an experienced retinal surgeon (YSY) for a standard three-port vitrectomy and silicone oil infusion (Tono-Pen XL; Medtronic Solan, Jacksonville, FL).
Vitrectomy Procedure
Rabbits were anesthetized using an intramuscular injection of ketamine (21 mg/kg) and xylazine (5.25 mg/kg). The surgical field was draped for sterilization following a topical application of proparacaine 0.5%. The eye surface and conjunctival sacs were prepared with 5% povidone iodine. A standard three-port pars plana vitrectomy was done using a Midlab 20-gauge cutter. Following a core vitrectomy, the posterior hyaloid was removed using indocyanine green for visualization. The air-fluid exchange was done when most of the vitreous was removed. Then, air-silicone oil exchange was performed using 1.2–1.5 mL of silicon oil (Acri. Sil-ol 5000, Acri. Tec GmbH, Germany). Following the vitrectomy, the rabbit eyes were ensconced with a tobramycin and dexamethasone ophthalmic suspension (Qilu Pharmaceutical Co. LTD, Shandong, China) and ofloxacin eye ointment (Sinqi Pharmaceutical Co. LTD, Liaoning, China) for the duration of the recovery period from the anesthesia.
Bevacizumab Administration
After the surgery, the rabbit eyes were monitored weekly using slit-lamp, indirect ophthalmoscope, and tonometer. Two rabbits showed retinal detachment from surgery, and were excluded and replaced with rabbits that had undergone successful operations. A minimum 4 weeks of observation was performed before an intraocular injection of bevacizumab using a criterion of no circulating cells in the aqueous humor. We reasoned that the ocular pharmacokinetics of a drug would be distorted by surgery-related inflammation if the drug was administered immediately following vitrectomy. The purpose of our study was to model the pharmacokinetics of bevacizumab in a silicone-filled eye, in which retinal or choroidal neovascularization might have developed following successful vitrectomy. Retinal regularity was confirmed using indirect ophthalmoscopy. The rabbits received a single intravitreal injection of 1.25 mg/0.05 mL bevacizumab (Genentech, San Francisco, CA; packaged and distributed by Franck's Compounding Lab, Ocala, FL) into the right eye under general anesthesia. After the drug injection, rabbits were monitored daily for three days, then weekly using slit-lamp, indirect ophthalmoscopy, and tonometer until the scheduled sacrifice.
After an intra-silicone injection of bevacizumab, migration of the bevacizumab fluid droplet in the silicone oil was documented using indirect ophthalmoscopy and then recorded by fundus photography on some rabbit eyes. Three rabbits were sacrificed at each of the 6 predetermined time points (1 day, and 1, 2, 4, 6, and 8 weeks) following the bevacizumab intra-silicone injection. Before sacrifice, 1 mL blood samples were collected into vials containing 250 μL 3.8% citrate. Blood samples were centrifuged for 10 minutes at 1258 × g (Centrifuge 5810R, Eppendorf, Germany) at room temperature, and the plasma was collected and stored under −80°C for analysis. After sacrifice, both eye globes of each rabbit were enucleated. The left eye was snap frozen and dissected into aqueous humor, iris/ciliary body, vitreous body, retina, and choroid as described previously.14,15 The ocular samples were collected in individual vials and kept at −80°C until a drug analysis was performed. For the right eye, the aqueous was sampled first through a 30-gauge needle, and then the silicone oil was collected through an air-oil exchange. The globe then was cut into two halves along the medullary ray, which divides the retina into superior and inferior portions. The superior and inferior retina was collected separately into individual vials. In addition, the iris/ciliary body and choroid were dissected and collected into individual vials for further drug analysis. All samples were kept under −80°C until drug analysis.
ELISA for Bevacizumab
The iris/ciliary body, retina, choroid, and collected silicon oil/vitreous first were individually homogenized with deionized water and then centrifuged for 10 minutes at 20,817g (Centrifuge 5810R, Eppendorf, Germany) at 4°C. The supernatant then was collected from all samples with the exception of the oil/vitreous. For the oil/vitreous sample, the fluid phase at the bottom was collected and sampled for further analysis. All samples were diluted as necessary in 1% bovine serum albumin (BSA; Roche, Switzerland) in 1× tris-buffered saline (TBS; Dycent Biotech, Shanghai, China) before the immunoassay, and all samples were analyzed in triplicate. Bevacizumab concentrations were measured using ELISA. The minimum detection limit of the assay is 0.1 ng/ml. Human recombinant VEGF-165 (Sino Biological Inc, Beijing, China) was diluted to a concentration of 1 μg/mL in 1× Dulbecco's phosphate buffered saline (DPBS; Gibco, Invitrogen, Carlsbad, CA), and 50 μL were aliquoted into 96-well high-binding ELISA plates (Costar). The plates were incubated in a light-blocking plastic box at 4°C, using wet paper towels to maintain proper humidity overnight. The plates were washed with 1× PBS and blocked for 4 hours with 1% BSA in 1× TBS. The plates were washed again with 1× PBS and the study samples were aliquotted into plates with a volume of 50 μL/well. For each individual assay, a standard curve was constructed using bevacizumab of known concentrations, ranging from 0.1–500 ng/ml. After 1 hour of incubation at room temperature, the plates were washed 5 times with 1× PBS. Goat anti-human IgG-Fc-HRP (Chemicon, Millipore) was diluted to 1 μg/mL in 1% BSA in 1× TBS and 50 μL were aliquotted into each well. After 1 hour of incubation at room temperature, the plates were washed with 1× PBS 5 times. A 50 μL supersignal ELISA Pico chemiluminescent substrate (Thermo, IL) was aliquotted onto the plates at 50 μL/well. Once aliquoting was complete, plates were covered with a lid and shaken for 1 minute. The plates then were read in a microplate reader (SpectraMax M5, Molecular Devices) at 425 nm.
Pharmacokinetic Analysis
For pharmacokinetic data analysis, the bevacizumab concentration-time data in various ocular tissues and plasma were analyzed using Phoenix WinNonlin 6.2 (Pharsight - A Certara Company, St. Louis, MO) by fitting the data to the extravascular input model, and the non-compartmental parameters of the bevacizumab elimination were estimated and reported.
Results
Clinical Observation
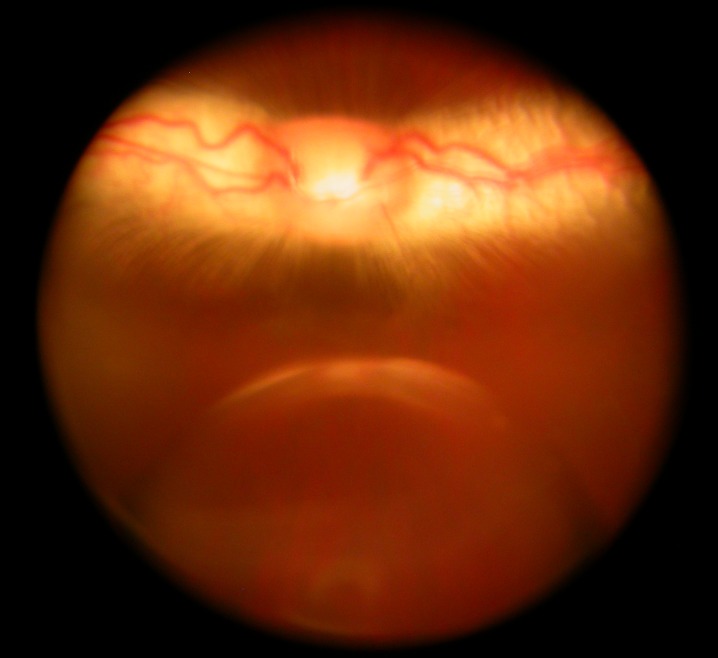
Before vitrectomy, the mean difference of intraocular pressure between the right and left eyes was 0.33 mm Hg, and the difference was 0.30 mm Hg before sacrifice. There was no significant difference between the study eyes and their fellow eyes at each time point, or between pre-vitrectomy and pre-sacrifice (P = 0.95, paired t-test). Following an intra-silicone oil injection of 0.05 mL bevacizumab, a fluid droplet or multiple droplets were formed in the oil (Fig. 1). The droplet or droplets were observed in all eyes during the first 24 hours, in 25% of eyes during the second day, and in only 8.3% of eyes on the third day. On the fourth day, no fluid droplet was observed using indirect ophthalmoscopy.
Figure 1. .
Fundus photograph demonstrates a bevacizumab droplet in a silicone oil-filled vitreous cavity, taken 18 hours after an intra-silicone oil injection. The droplet was floating in the vitreous cavity, and the optic nerve head and medullary ray show normal appearance. The droplet looked larger than its actual size because of eye refraction and camera focusing.
Bevacizumab Levels and Clearance in Various Ocular Tissues
The Injected Eyes.
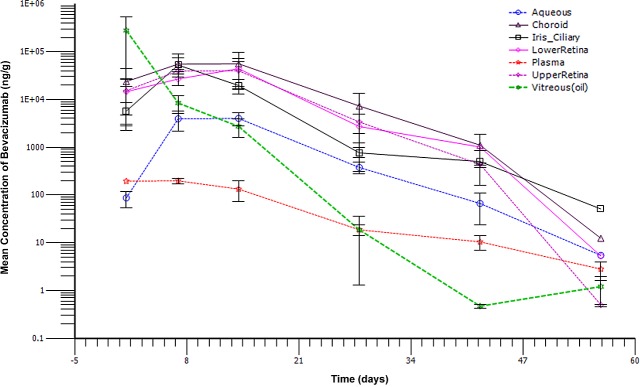
The bevacizumab peak concentration was reached at day 14 in the aqueous humor, retina, and choroid with concentrations of 4030.7 ng/mL, 42,171.7 ng/g, and 56,243.33 ng/g, respectively. In the iris/ciliary body and plasma, peak concentration was reached at day 7 with 52,648.30 ng/g and 197.70 ng/mL, respectively. Bevacizumab clearance from the oil of the injected eyes followed a first-order kinetics with a half-life of 3.3 days and a maximum concentration observed (Cmax) of 280 μg/mL at a time when the maximum concentration is reached (Tmax) of 1 day, which was the first sampling time point. Bevacizumab in plasma was cleared much slower with a longer half-life of 8 days (see Table). The other ocular tissues, including the retina, choroid, and aqueous, demonstrated an absorption phase in the first week, with a sustained drug level in the second week followed by a first-order elimination course (Fig. 2). For the iris-ciliary body, the first week absorption was followed by a first-order elimination, without a sustained drug level in the second week (Fig. 2). The ocular tissue with the maximum drug exposure was the choroid, with an area under the curve calculated from time zero to the last observed time (AUClast) of 1,151,633.4 ng/day/g while the aqueous humor had the lowest exposure (AUClast = 74,611.28 ng/day/g) among the ocular tissues (see Table); systemic exposure (plasma) to bevacizumab was minimal (AUClast = 3795.17 ng/day/g, see Table). The pharmacokinetic parameters from the upper and lower retina as well as the concentration-time profiles were similar during the first 4 weeks, then the drug concentrations in the upper retina at weeks 6 and 8 were lower (Fig. 2, see Table). The mean residence time of bevacizumab in the ocular tissues and plasma ranged from 10.09–12.90 days (see Table).
Figure 2. .
Individual bevacizumab concentration-time profile for plasma as well as the aqueous humor, iris/ciliary body, vitreous humor (oil), retina, and choroids of the right eyes following a 1.25 mg/eye intra-silicone oil administration in the right eyes.
Table. .
Pharmacokinetic Parameters of Bevacizumab in Right Eyes and Plasma
|
Sample |
Rsq Adjusted |
Terminal Half-Life (days) |
Tmax (days) |
Cmax (ng/g) |
Auclast (ng·day/g) |
Mrtlast (days) |
| Plasma | 0.94 | 7.98 | 7.00 | 197.70 | 3,795.17 | 11.54 |
| Aqueous | 0.98 | 4.50 | 14.00 | 4,030.70 | 74,611.28 | 12.90 |
| Iris ciliary | 0.94 | 5.02 | 7.00 | 52,648.30 | 587,558.32 | 10.09 |
| Vitreous (oil) | 0.83 | 3.35 | 1.00 | 280,015.37 | 1,063,956.50 | 1.65 |
| Upper retina | 0.87 | 2.70 | 14.00 | 40,063.57 | 786,518.40 | 11.87 |
| Lower retina | 0.88 | 3.45 | 14.00 | 44,279.83 | 743,948.37 | 12.70 |
| Choroid | 0.93 | 3.58 | 14.00 | 56,246.33 | 1,151,633.40 | 12.50 |
Rsq adjusted, R square adjusted; MRTlast, mean residence time calculated to the last observed time.
The Fellow Eyes.
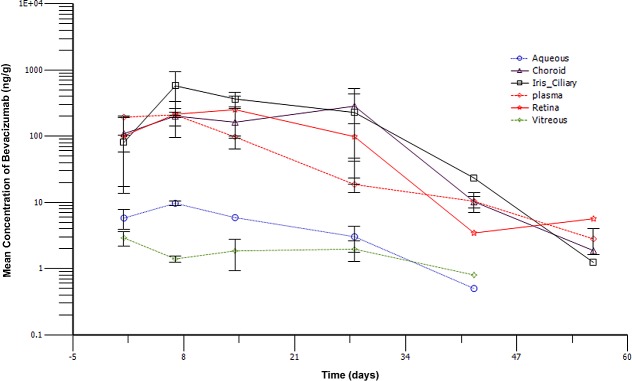
In the non-injected left eyes, bevacizumab levels in the solid tissues (iris-ciliary body, retina, and choroid) demonstrated a similar concentration-time profile to that of the plasma, with the drug levels being 100–500 ng/g during the first 4 weeks before an elimination phase began (Fig. 3). In contrast, bevacizumab levels in the aqueous and vitreous were much lower, ranging from 1–10 ng/mL (Fig. 3).
Figure 3. .
Individual bevacizumab concentration-time profile for plasma as well as the aqueous humor, iris/ciliary body, vitreous humor, retina, and choroids of the left eyes following a 1.25 mg/eye intra-silicone oil administration in the right eyes.
Discussion
To our knowledge, there is no previous study characterizing the ocular pharmacokinetics of bevacizumab in silicone oil-filled eyes. In two prior reports of intravitreal bevacizumab pharmacokinetics in rabbit eyes, Bakri et al. studied the bevacizumab levels in the vitreous, aqueous, and plasma;6 and additionally, Nomoto et al. studied the drug level in retina/choroid and iris/ciliary body.9 In our study, the vitreous cavity was filled with at least 1.2 mL of silicone oil. From clinical observation, during the first 48 hours following an intra-silicone oil injection, the bevacizumab fluid droplet disappeared from the oil phase in over 90% of the eyes. The bevacizumab migration in the oil phase and its gradual integration into the vitreous fluid phase could be a complex process. There may be emulsification at the interface of vitreoretinal surface,16 which provided a slower penetration of bevacizumab into the ocular tissues. However, once bevacizumab reaches an ocular tissue, its clearance is expected to be the same as that following an intravitreal injection.
In our study, the bevacizumab half-life in the various ocular tissues and the plasma was very similar to that reported from an intravitreal injection. The half-life of bevacizumab in the oil/vitreous fluid mixture in our study was 3.35 days and in the study of Bakri et al. it was 4.32 days.6 For the aqueous and plasma, the half-life in our study versus the Bakri et al study6 was 4.5 vs. 4.88 and 7.98 vs. 6.86 days, respectively. Comparing our study to that of Nomoto et al., which also evaluated the bevacizumab level in iris/ciliary and retina/choroid,9 the drug terminal half-life in the iris/ciliary also was comparable (5.74 vs. 5.02 days). We studied the retina and choroid separately, while Nomoto et al. studied a mixture of retina and choroid.9 The terminal half-life is a better parameter for comparison of drug handling systems because, to a certain extent, the half-life is independent of dosing or systemic error of drug detecting, which often is present from one study to the next. The similarity of bevacizumab half-life in various ocular tissues between our study and the previous studies is expected because once bevacizumab migrates from oil/vitreous fluid to the ocular tissues, its rate of clearance should be the same.
Bevacizumab pharmacokinetics also was studied in human aqueous and vitreous following an intravitreal injection of 1.25 mg.10,17,18 The reported vitreous half-life was 6.7 days10 and the half-life in the aqueous humor was 8.2–11.17 days.17,18 From the perspective of species scaling (eye scaling), the drug half-life should be shorter in a rabbit than in a human,19 which was the case when reviewing studies involving the rabbit and human eye, including our current study, in which the bevacizumab half-lives in ocular tissues were 5 days or less.6,10,17,18
There also was some marked difference between our study (intra-silicone oil injection of bevacizumab) and previously reported pharmacokinetic studies using intravitreal injection.6,9 In our study, Tmax was delayed in all kinds of ocular tissues except for in the oil/vitreous fluid mixture. For example, the Tmax for the aqueous in the study of Bakri et al. was day 3 (second sampling time point),6 while it was day 14 (third sampling time point) in our study. In the Nomoto et al study, the Tmax for the aqueous, iris/ciliary body, and retina/choroid all came in the first sampling, which was one day after the drug administration.9 In contrast, the Tmax for the iris/ciliary body and retina/choroid in our study was days 7 and 14, respectively. These delayed peak concentrations may stem from an initial gradual integration of the droplets of bevacizumab solution in the oil phase into the fluid phase in the vitreous cavity. This assumption also is supported with a lower Cmax of various ocular tissues and plasma in our study than the Cmax reported in the other comparable studies. For instance, the Cmax for aqueous and plasma in the study of Bakri et al was 37,700 and 3330 ng/mL, respectively,6 compared to 4031 and 198 ng/mL, respectively, in our study. Lower concentrations of bevacizumab in plasma may induce less systemic physiologic changes and minimize possible systemic side effects. The Cmax of the iris/ciliary body and the retina/choroid in the study of Nomoto et al. was 109,192 and 93,990 ng/g, respectively,9 but the Cmax for those two types of eye tissues in our study was 52,648 and 46,863 ng/g, respectively, which is roughly 50% lower. These differences suggest that the silicone oil slowed down the initial drug distribution to the ocular tissues, though the terminal half-life in these tissues was not affected significantly. In addition, it also is interesting to note that in our study, bevacizumab was found in the contralateral eyes at a level that may induce a physiologic change. Indeed, it has been reported that regression of rubeosis or reduced VEGF levels in the contralateral eye was observed when bevacizumab was injected intravitreally.20,21
In our study, the retina in the right eye was divided into upper and lower portions from the medullary ray, and sampled separately. We hypothesized that after the bevacizumab enters the limited vitreous fluid between the oil and retina, the gravity could lead to a higher drug concentration in the inferior fluid body and inferior retina. This hypothesis is supported by the current data at the later (6–8 weeks) pharmacokinetic process of the study. It is expected that the difference that originated from an uneven distribution of drug in the vitreous fluid would become prominent when the total drug level became low. Perkins et al. found that the vitreous ganciclovir levels at days 21 and 42 were similar in the saline-filled and silicone oil-filled eyes, but that at day 70 the drug levels in the saline-filled eyes were significantly lower than in the silicone-filled eyes.22
In summary, following an intra-silicone oil injection of bevacizumab, the visible bevacizumab droplets migrated in silicone oil for 24–72 hours before integrating into the vitreous fluid existing between the silicone oil body and retina surface. This integration process could be gradual, which would slow down the distribution of bevacizumab into various ocular tissues, resulting in a longer Tmax, smaller Cmax, and relatively sustained bevacizumab levels in the ocular tissues. The process of integrating from oil into vitreous fluid, then into solid ocular tissues could be complex due to the possibility of oil emulsification at the interface of vitreoretinal surface16 and the details must be studied further. It is possible that an increased exchange of drug solution or crystals from oil phase into fluid phase on the retina could lead to retinal toxicity due to high drug concentration in the reduced volume of vitreal fluid or due to direct contact between the drug crystals and retina.13,23 The toxicity will depend on the drug and the dose used. Hegzy et al. demonstrated that a previously determined nontoxic dose of ganciclovir in normal rabbit eyes was toxic in oil-filled rabbit eyes, and that 25% of the dose was not toxic.13 In contrast, 2.5 mg bevacizumab were injected into human eyes, which later had INV under a silicone oil tamponade, and all five eyes had improved visual acuity and a regression of INV without retinal toxicity.24 In the current ocular pharmacokinetic study, the rabbit eye was used as a model, and we acknowledge that the rabbit eye differs from the human eye. However, a preclinical study of the ocular pharmacokinetics of bevacizumab was conducted in rabbit eyes,6 and subsequent studies in nonhuman primates and in human eyes did reveal a considerable similarity.17,25 Our study suggested that an intra-silicone oil injection of bevacizumab can be an alternative treatment modality for those eyes in which new neovascularization or neovascular glaucoma develops under a silicone oil tamponade.
Acknowledgments
Authors contributed to the design of the study (LC, YX, HC), conducting the study (YX, YY, WD, CZ, JL, JM), analysis of the study (LC, YX, YY, WD, CZ, JL, JM), writing the article (LC, YX, YY, WD, CZ, JL, JM), and obtaining funding (LC, HC, YX).
Footnotes
Supported by National Natural Science Foundation of China 30870631, NIH EY 020617-01A1, and Zhejiang Province Foundation for Innovative Team in Ophthalmology 2011R09039-12.
Disclosure: Y. Xu, None; Y. You, None; W. Du, None; C. Zhao, None; J. Li, None; J. Mao, None; H. Chen, None; L. Cheng, None
References
- 1.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335 [PubMed] [Google Scholar]
- 2.Smit DP, Meyer D. Intravitreal bevacizumab: an analysis of the evidence. Clin Ophthalmol. 2007;1:273–284 [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95:1216–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–938 [DOI] [PubMed] [Google Scholar]
- 5.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhufabv2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733 [DOI] [PubMed] [Google Scholar]
- 6.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855–859 [DOI] [PubMed] [Google Scholar]
- 7.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–2182 [DOI] [PubMed] [Google Scholar]
- 8.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813 [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Ziemssen F, Henke-Fahle S, et al. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755 [DOI] [PubMed] [Google Scholar]
- 11.Yeh PT, Yang CM, Lin YC, Chen MS, Yang CH. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina. 2009;29:768–774 [DOI] [PubMed] [Google Scholar]
- 12.Falavarjani KG, Modarres M, Nazari H. Therapeutic effect of bevacizumab injected into the silicone oil in eyes with neovascular glaucoma after vitrectomy for advanced diabetic retinopathy. Eye. 2010;24:717–719 [DOI] [PubMed] [Google Scholar]
- 13.Hegazy HM, Kivilcim M, Peyman GA, et al. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina. 1999;19:553–557 [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Hostetler KY, Lee J, et al. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrugs of ganciclovir and cyclic cidofovir. Invest Ophthalmol Vis Sci. 2004;45:4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nan K, Sun S, Li Y, et al. Characterisation of systemic and ocular drug level of triamcinolone acetonide following a single sub-Tenon injection. Br J Ophthalmol. 2010;94:654–658 [DOI] [PubMed] [Google Scholar]
- 16.Heidenkummer HP, Kampik A, Thierfelder S. Emulsification of silicone oils with specific physicochemical characteristics. Graefes Arch Clin Exp Ophthalmol. 1991;229:88–94 [DOI] [PubMed] [Google Scholar]
- 17.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Amer J Ophthalmol. 2008;146:508–512 [DOI] [PubMed] [Google Scholar]
- 18.Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877–1884 [DOI] [PubMed] [Google Scholar]
- 19.Riviere JE. Comparative Pharmacokinetics. Iowa City, IA: Iowa State Press; 1999:296–307 [Google Scholar]
- 20.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705 [DOI] [PubMed] [Google Scholar]
- 21.Matsuyama K, Ogata N, Matsuoka M, Wada M, Nishimura T, Takahashi K. Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharmacol Ther. 2011;27:379–383 [DOI] [PubMed] [Google Scholar]
- 22.Perkins SL, Yang CH, Ashton PA, Jaffe GJ. Pharmacokinetics of the ganciclovir implant in the silicone-filled eye. Retina. 2001;21:10–14 [DOI] [PubMed] [Google Scholar]
- 23.Spitzer MS, Kaczmarek RT, Yoeruek E, et al. The distribution, release kinetics, and biocompatibility of triamcinolone injected and dispersed in silicone oil. Invest Ophthalmol Vis Sci. 2009;50:2337–2343 [DOI] [PubMed] [Google Scholar]
- 24.Falavarjani KG, Modarres M, Nazari H. Therapeutic effect of bevacizumab injected into the silicone oil in eyes with neovascular glaucoma after vitrectomy for advanced diabetic retinopathy. Eye. 2010;24:717–719 [DOI] [PubMed] [Google Scholar]
- 25.Miyake T, Sawada O, Kakinoki M, et al. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Invest Ophthalmol Vis Sci. 2010;51:1606–1608 [DOI] [PubMed] [Google Scholar]