Abstract
Background:
No formal guidelines exist to guide physicians caring for patients with sarcoidosis in their screening for management of patients with cardiac sarcoidosis. We conducted a modified Delphi study to investigate if a consensus could be reached on the best approaches for screening for and management of cardiac sarcoidosis.
Methods:
A modified Delphi study design with two rounds of questionnaires was used to investigate if a consensus existed among sarcoid experts in the United States on the best management approaches for cardiac sarcoidosis. Experts were identified based on their national reputation as sarcoid experts and by being actively involved in sarcoidosis clinics at their institutions.
Results:
Overall agreement was low to moderate. Agreement was reached on the role of history, physical examination, and 12-lead ECG in screening, echocardiogram, Holter monitor, myocardial fluorodeoxyglucose PET scan, and cardiac MRI in workup, and steroids in treatment. Agreement was not reached on the role of signal-averaged ECG in screening, optimum dose of prednisone, use of steroid-sparing agents, and duration of treatment. Several comments underscore the diverse approaches and uncertainty that exist in managing cardiac sarcoidosis.
Conclusions:
Our study highlights the dilemma that sarcoid experts face in their approach to cardiac sarcoidosis. It also highlights the lack of agreement among sarcoid experts on key aspects of diagnosis and management and stresses the importance of collaborative efforts to investigate the best strategies for screening for and management of cardiac sarcoidosis.
Sarcoidosis is a multisystem granulomatous disease.1 Only 5% of patients with sarcoidosis demonstrate cardiac involvement clinically,2 whereas it was detected in at least 27% of cases based on autopsy findings,3 strongly suggesting significant underdiagnosis. It is the second leading cause of death in patients with sarcoidosis (13%-25%).4,5 Guidelines for diagnosing cardiac sarcoidosis have been published by the Japanese Ministry of Health and Welfare.6 However, these guidelines have not been officially updated since 1993 and are based on the Japanese population that has a higher prevalence of cardiac sarcoidosis and a different genetic background than western populations.6
There are no formal guidelines in the United States that address screening methods, workup, diagnostic criteria, or management of cardiac sarcoidosis.7,8 Due to the rarity of cardiac sarcoidosis, prospective clinical trials are lacking and difficult to conduct. We conducted a Delphi study to investigate the approach of physicians in the United States with expertise in sarcoidosis and to investigate if a consensus could be reached on the best approach to screen, work up, and manage patients with sarcoidosis with potential cardiac involvement.
Materials and Methods
To learn about current clinical practices and attempt to see if consensus existed regarding these practices, a Delphi study design was used.9,10 The institutional review board at National Jewish Health approved the study under exempt status (HS#2513). Two rounds of questionnaires were e-mailed to sarcoidosis specialists in the United States. The questions were in the multiple choice format but also included an “other” choice for free-text comments.
The experts were chosen based on their known reputation as sarcoid experts and/or based on active involvement in sarcoidosis specialty clinics at their respective institutions. The experts were invited to participate via e-mail. The questionnaires were administered using the Web site www.surveymonkey.com. Physicians’ names and responses were kept anonymous. The first questionnaire consisted of 17 questions, with question 18 being a free-text option for comments. The questions addressed screening methods, workup, diagnostic modalities, management, and follow-up for cardiac sarcoidosis. The second questionnaire consisted of eight questions, with question number 9 being a free-text option. The second questionnaire was designed based on the responses from the first questionnaire and aimed at clarifying and resolving any conflicts in the first questionnaire. Participants who completed both questionnaires are listed in e-Appendix 1. Descriptive statistics were used to describe the data. Consensus was operationalized as 70% or higher agreement, whereas an agreement rate of 50% to 70% was moderate, and less than 50% was considered poor.
Results
Characteristics of the Study Participants
Forty-two sarcoid experts were invited to participate (36 pulmonologists, three cardiologists, three electrophysiologists, one rheumatologist). Thirty-one of the 42 (73.8%) responded to the first round of questions, and 27 of the 31 (87.1%) who responded to the first questionnaire responded to the second round of questions. Characteristics of the experts are shown in Table 1. The majority were pulmonologists and 64.5% have been practicing for > 10 years, and 87.1% (27/31) manage on average > 100 patients with sarcoidosis per year.
Table 1.
—Characteristics of Sarcoidosis Experts Who Participated in the Study
| Characteristic | No. of Respondents |
| Response rate, N = 42 | |
| Round 1 | 31/42 (73.8%) |
| Round 2 | 27/31 (87.1%) |
| Overall | 27/42 (64.3%) |
| Years of clinical experiencea | |
| < 10 | 35.5% |
| > 10 | 64.5% |
| Average No. of patients with sarcoidosis per yearb | |
| < 50 | 14.8% |
| 50-100 | 37.0% |
| > 100 | 48.1% |
| Specialty (n = 31) | |
| Pulmonary | 90.3% |
| Cardiology | 6.5% |
| Electrophysiology | 3.2% |
Question 16 in the first questionnaire.
Question 1 in the second questionnaire.
Screening and Diagnostic Testing for Cardiac Sarcoidosis
We sought the experts’ opinions on their approach to screen for, diagnose, and manage cardiac sarcoidosis based on their practice and understanding of existing literature (Table 2). For screening for cardiac sarcoidosis, there was consensus for clinical symptoms and physical examination and for use of 12-lead ECG (87.1%). A few suggested a role for MRI in screening. About three-quarters of the experts would not perform any further testing in an asymptomatic patient with normal cardiac examination and a normal 12-lead ECG. About one-half of the experts who responded to the first questionnaire would perform an echocardiogram and just over two-thirds an ambulatory ECG (Holter monitor). For suspected cardiac sarcoidosis, most would perform a 12-lead ECG, an echocardiogram, a cardiac MRI, and about two-thirds would perform a cardiac fluorodeoxyglucose (FDG) PET scan and Holter monitor. For 12-lead ECG changes, the very high agreement rates for changes indicative of potential cardiac sarcoidosis were found for bundle branch blocks, ventricular ectopies, and third- and second-degree atrioventricular blocks. About three-quarters of the experts do not use signal-averaged ECG (SAECG). For Holter monitor, runs of ventricular tachycardia (VT) and nonsustained VT had the highest agreement, followed by frequent isolated premature ventricular contractions; a few mentioned heart blocks and bundle branch blocks. Left ventricular (LV) dysfunction and wall motion abnormalities on an echocardiogram had the highest agreement for potential cardiac sarcoidosis among the experts, followed by right ventricular dysfunction. For cardiac FDG-PET scan patterns indicative of cardiac sarcoidosis, patchy uptake had very high agreement, with moderate agreement for patchy on diffuse uptake. Comments were made on the technical aspects of performing FDG-PET scan. For cardiac MRI findings, delayed enhancement had the highest agreement, whereas myocardial perfusion abnormalities and LV dysfunction had moderate agreement. Less than one-half of the experts refer their patients for an electrophysiology (EP) evaluation only if they had active arrhythmias, whereas a smaller percentage did for all their suspected patients. One expert commented on the ejection fraction as a reason for referral.
Table 2.
—Diagnostic Testing for Cardiac Sarcoidosis
| Topic | Procedure | No. (%) Endorsing |
| Routine screening (R1, Q1) | Clinical symptoms and physical examination | 30 (96.8) |
| 12-Lead ECG | 27 (87.1) | |
| Echocardiogram | 16 (51.6) | |
| SAECG | 4 (12.9) | |
| Ambulatory ECG (Holter or event monitor) | 12 (38.7) | |
| Other | 4 (12.9) | |
| Additional tests for asymptomatic patient with normal examination and 12-lead ECG (R2, Q2) | I would not perform any further screening tests | 19 (70.4) |
| SAECG | 2 (7.4) | |
| Ambulatory ECG (Holter or event monitor) | 4 (14.8) | |
| Echocardiogram | 5 (18.5) | |
| Brain natriuretic peptide | 0 (0) | |
| Cardiac PET scan | 2 (7.4) | |
| Cardiac MRI | 2 (7.4) | |
| Radionucleotide stress test | 0 (0) | |
| Other | 2 (7.4) | |
| Testing as part of workup for suspected cardiac sarcoidosis (R1, Q2) | 12-Lead ECG | 26 (83.9) |
| SAECG | 5 (16.1) | |
| Ambulatory ECG (Holter or event monitor) | 20 (64.5) | |
| Echocardiogram | 27 (87.1) | |
| Cardiac PET scan | 20 (64.5) | |
| Cardiac MRI | 24 (77.4) | |
| Brain natriuretic peptide | 2 (6.5) | |
| Troponin levels | 0 (0) | |
| Cardiac radionucleotide stress test | 2 (6.5) | |
| Cardiology consult | 9 (29.0) | |
| Coronary angiography | 4 (12.9) | |
| Endomyocardial biopsy | 1 (3.2) | |
| Electrophysiological evaluation | 6 (19.4) | |
| Other | 2 (6.5) | |
| ECG findings indicative of cardiac sarcoidosis (R1, Q3) | First-degree block | 17 (54.8) |
| Second-degree block | 24 (77.4) | |
| Third-degree block | 25 (80.6) | |
| Atrial arrhythmias (fibrillation/flutter) | 20 (64.5) | |
| Ventricular ectopy | 26 (83.9) | |
| Fragmented QRS | 10 (32.3) | |
| Bundle branch blocks | 27 (87.1) | |
| Other | 4 (12.9) | |
| SAECG findings indicative of cardiac sarcoidosis (R1, Q4) | 1/3 Domains abnormal | 4 (12.9) |
| 2/3 Domains abnormal | 2 (6.5) | |
| All domains abnormal | 1 (3.2) | |
| I do not use SAECG | 23 (74.2) | |
| Do not have access to SAECG | 2 (6.5) | |
| Other | 0 (0) | |
| Ambulatory ECG (Holter or event monitor) findings indicative of cardiac sarcoidosis (R1, Q5) | Frequent isolated PVCs | 19 (61.3) |
| Frequent PACs | 10 (32.3) | |
| Nonsustained VT | 30 (96.8) | |
| Runs of VT | 30 (96.8) | |
| Bradycardia | 14 (45.2) | |
| Do not use ambulatory ECG testing | 1 (3.2) | |
| Do not have access to ambulatory ECG testing | 0 (0) | |
| Other | 3 (9.7) | |
| Echocardiographic findings indicative of cardiac sarcoidosis (R1, Q6) | LV dysfunction | 28 (90.3) |
| RV dysfunction | 20 (64.5) | |
| Wall motion abnormalities | 27 (87.1) | |
| Elevated right ventricular systolic pressure | 8 (25.8) | |
| Diastolic dysfunction | 11 (35.5) | |
| Do not use echocardiogram | 0 (0) | |
| Do not have access to echocardiogram | 0 (0) | |
| Other | 4 (12.9) | |
| Cardiac PET scan findings indicative of cardiac sarcoidosis (R1, Q7) | Diffuse uptake | 6 (19.4) |
| Patchy uptake | 24 (77.4) | |
| Patchy on diffuse uptake | 16 (51.6 | |
| LV lateral wall uptake | 9 (29.0) | |
| No uptake | 1 (3.2) | |
| Do not use cardiac FDG-PET scan | 3 (9.7) | |
| Do not have access to cardiac FDG-PET scan | 0 (0) | |
| Other | 5 (16.1) | |
| Cardiac MRI findings indicative of cardiac sarcoidosis (R1, Q8) | Delayed enhancement | 25 (80.6) |
| Edema | 10 (32.3) | |
| RV dysfunction | 10 (32.3) | |
| LV dysfunction | 17 (54.8) | |
| Myocardial perfusion abnormalities | 18 (58.1) | |
| Do not use cardiac MRI | 1 (3.2) | |
| Do not have access to cardiac MRI | 0 (0) | |
| Other | 2 (6.5) | |
| EP evaluation as part of workup for suspected cardiac sarcoidosis (R1, Q9) | Yes | 12 (38.7) |
| Only if they have active arrhythmias | 13 (41.9) | |
| Do not refer for EP evaluation | 3 (9.7) | |
| Do not have access to an electrophysiologist | 0 (0) | |
| Other | 5 (16.1) |
Round 1 responses rate, 31 of 42; Round 2 response rate, 27 of 31. Bold indicates agreement ≥ 70%; italic font indicates agreement ≥ 50% but < 70%. EP = electrophysiologist; FDG-PET = fluorodeoxyglucose PET; LV = left ventricular; PAC = premature atrial contraction; PVC = premature ventricular contractions; Q = question No.; R = questionnaire round; RV = right ventricle; SAECG = signal-averaged ECG; VT = ventricular tachycardia.
Management of Cardiac Sarcoidosis
Immunomodulatory therapy is generally used to manage active or progressive sarcoidosis in the various involved organs (Table 3).1 More than three-quarters of the experts would initiate immunomodulatory therapy for cardiac sarcoidosis based on the presence of ventricular arrhythmias, hypermetabolic activity on a cardiac FDG-PET scan, and/or LV dysfunction, whereas many would in the presence of conduction defects or in the presence of delayed enhancement on cardiac MRI. Comments included presence of other abnormalities with an abnormal FDG-PET scan. No consensus was reached on the role of immunomodulatory therapy in a clinical scenario that was presented describing an asymptomatic patient with a normal cardiac examination and workup except for a positive cardiac FDG-PET scan. Comments included close monitoring for arrhythmias, EP evaluation, extent of FDG-PET scan, would not have ordered a FDG-PET scan in the first place, and discussions with the patient. No agreement was reached in a similar clinical scenario with an asymptomatic patient with a normal cardiac examination and workup except for a positive cardiac MRI scan. Comments included EP evaluation, would not have ordered an MRI in the first place, discuss with patient, and close monitoring. Prednisone was nearly universally the drug of choice when initiating immunosuppressive (IS) therapy, but no agreement was reached on the optimum starting dose of prednisone, although 58.1% of the experts chose a dose ≤ 40 mg. The majority of the experts use the same dosing regimen regardless of the indication to initiate therapy (arrhythmias vs cardiomyopathy). The experts were divided on initiating a steroid-sparing agent at the same time they initiate corticosteroids. No agreement was reached on the optimum duration of immunosuppressive therapy, and comments were made on the lack of evidence to guide us and the role of the clinical course and other testing in making a decision. There was moderate agreement that the duration of therapy was not affected by the indication for therapy (arrhythmias vs cardiomyopathy), although several comments were made indicating that the degree of cardiomyopathy or arrhythmias influenced the duration. When assessing and following response to therapy, more than one-half of the experts use echocardiogram and cardiac FDG-PET scan and/or cardiac MRI scans. Several experts mentioned they follow using the abnormalities that led to the diagnosis and symptoms. The role of automated implantable cardiac defibrillators (AICDs) in cardiac sarcoidosis remains unknown. Solid agreement was reached that AICD placement should be considered in patients with a positive EP study and after survival from a sudden cardiac death event. Some experts commented on LV dysfunction as another factor. No agreement was reached on the role of a lone pacemaker in cardiac sarcoidosis. A few deferred the decision to a cardiologist.
Table 3.
—Management of Cardiac Sarcoidosis
| Topic | Procedure |
| Indications for initiation of immunomodulatory therapy (R1, Q12) | Hypermetabolic activity on a cardiac FDG-PET scan |
| Delayed enhancement on cardiac MRI | |
| Conduction defects | |
| LV dysfunction | |
| RV dysfunction in the absence of pulmonary hypertension | |
| Ventricular arrhythmias | |
| Atrial arrhythmias | |
| Other | |
| Clinical scenario: asymptomatic patient with only a positive PET scan (R2, Q3) | No |
| Yes | |
| Other | |
| Clinical scenario: asymptomatic patient with only a positive MRI scan (R2, Q4) | No |
| Yes | |
| Other | |
| Immunosuppressive therapies used in treating cardiac sarcoidosis (R1, Q13) | Prednisone |
| Methotrexate | |
| Azathioprine | |
| Mycophenolate mofetil | |
| Anti-TNF agent | |
| Other | |
| Dose of prednisone used (R1, Q14) | 20 mg |
| 30-40 mg | |
| 60 mg | |
| 0.5 mg/kg/ideal body weight | |
| 1 mg/kg/ideal body weight | |
| I do not use prednisone | |
| Other | |
| Initiation of steroid-sparing agent with corticosteroids (R2, Q5) | No |
| Yes | |
| Other | |
| Does the initial dosage of steroids differ depending on indication (active arrhythmias vs cardiomyopathy)? (R2, Q6) | No, I use the same dosing range no matter what the indication |
| Yes, I choose a higher dose for arrhythmias | |
| Yes, I choose a higher dose for cardiomyopathy | |
| Other | |
| Duration of immunosuppressive therapy (R2, Q7) | 1 y |
| 2 y | |
| Indefinitely | |
| Other | |
| Does the duration of IS therapy differ depending on indication (active arrhythmias vs cardiomyopathy)? (R2, Q8) | No |
| Yes | |
| Other | |
| Evaluation of response to IS therapy (R1, Q15) | 12-Lead ECG |
| SAECG | |
| Ambulatory ECG (Holter or event monitor) | |
| Echocardiogram | |
| Cardiac FDG-PET scan | |
| Cardiac MRI | |
| Brain natriuretic peptide | |
| Troponin levels | |
| Cardiac radionuclide stress test | |
| Other | |
| Consideration for placement of an AICD (R1, Q10) | Survival from a sudden cardiac death event |
| Positive EP study | |
| Presence of arrhythmias on ambulatory ECG (Holter or event monitor) | |
| Abnormal cardiac MRI | |
| Abnormal cardiac PET scan | |
| Do not have access to EP | |
| I do not advocate implanting AICDs | |
| Other | |
| Consideration for placement of a lone pacemaker (R1, Q11) | Low-grade conduction blocks |
| High-grade conduction blocks | |
| Do not have access to EP | |
| I do not advocate implanting pacemakers | |
| Other |
Round 1 responses rate, 31 of 42; round 2 response rate, 27 of 31. Bold indicates agreement ≥ 70%; italic font indicates agreement ≥ 50% but < 70%. AICD = automated implantable cardiac defibrillator; IS = immunosuppressive; TNF = tumor necrosis factor. See Table 2 legend for expansion of the other abbreviations.
Discussion
In an editorial titled “Cardiac sarcoidosis: there is no instant replay,” Dr Marc Judson8 exemplified the anguish physicians have to deal with when caring for patients with sarcoidosis. Detected clinically in 5% of cases2 but most likely present in at least 40%,11 sarcoid physicians struggle to narrow this gap. There is a significant lack of prospective studies that can guide physicians on the best screening, diagnostic, and management strategies. In addition, the lack of a gold standard for the diagnosis of cardiac sarcoidosis poses a challenge when conducting and interpreting studies and managing patients.
We used a modified Delphi method to examine the practices of expert sarcoid physicians in the United States to determine if consensus could be reached on the best screening, diagnostic, and management strategies. Response rate was acceptable. Overall, although evidence of significant agreement emerged suggestive of “best practices,” in most areas consensus was not achieved. For screening for cardiac sarcoidosis, most respondents were satisfied with cardiac symptoms, cardiac examination, and 12-lead ECG. These three elements earned the highest endorsement as best screening modalities, and most would not perform any further testing in an asymptomatic patient with normal testing. In a prospective study investigating the usefulness of baseline testing consisting of cardiac symptoms, cardiac examination, 12-lead ECG, echocardiogram, and Holter monitor, Mehta et al11 found that the presence of at least one abnormal screening test had a 100% sensitivity and 87% specificity in detecting cardiac sarcoidosis, with history/examination, echocardiogram, and Holter monitor being the most predictive components. Previous studies reported a sensitivity of 33% to 58% and a specificity of 22% to 71% for ECG to detect cardiac sarcoidosis. The wide range was due to differences in the gold standard used in each study.12,13 In one previous study, 24-Holter monitor had a sensitivity of 67% and a specificity of 80% in detecting cardiac sarcoidosis,14 and in the study by Mehta et al11 it had a sensitivity of 50% and specificity of 97%.11 The role of SAECG in cardiac sarcoidosis is yet unknown, and the majority of the experts do not use it, although a recent study reported a sensitivity of 52% and specificity of 82% for SAECG as a screening modality in cardiac sarcoidosis.15 Further research investigating the role of SAECG in cardiac sarcoidosis is still needed.
There was a moderate to high agreement on the use of echocardiogram, Holter monitor, FDG-PET scan, and cardiac MRI in the workup of cardiac sarcoidosis. In the study by Mehta et al11, echocardiogram had a sensitivity of 25% and specificity of 95% in detecting cardiac sarcoidosis.11 Cardiac FDG-PET scan and/or MRI scans are becoming the preferred imaging modalities in cardiac sarcoidosis.11,16‐21 The experts considered a patchy uptake pattern on cardiac FDG-PET scan the finding most likely indicative of cardiac sarcoidosis, followed by patchy on diffuse uptake pattern. Previous studies reported an 85% to 100% sensitivity and 38.5% to 90.9% specificity for cardiac FDG-PET scan to detect cardiac sarcoidosis.18,19,22 Delayed enhancement on a cardiac MRI was considered indicative of cardiac sarcoidosis by the majority of the experts. Previous studies reported a sensitivity of 75% to 100% and specificity of 76.9% to 78% for cardiac MRI.18,23
The experts were less clear about the role of IS therapy in cardiac sarcoidosis. Most would initiate IS therapy for ventricular arrhythmias, hypermetabolic activity on a FDG-PET scan, conduction defects, and/or delayed enhancement on a cardiac MRI. Although more than three-quarters of the experts would initiate IS therapy for a positive FDG-PET scan, less than one-quarter would initiate therapy in an asymptomatic patient with only an abnormal FDG-PET scan. Most stated that they would not order an FDG-PET scan in an asymptomatic patient. Other findings suggestive of possible cardiac sarcoidosis raised by individual experts included pericardial effusions, myocardial nodules detected on MRI, and perfusion-metabolism mismatches on FDG-PET scan. Corticosteroids were the treatment of choice for nearly all the experts, and most started prednisone at a dose ≤ 40 mg daily, but there was little agreement on the optimum duration of therapy in a patient who exhibits an excellent response to IS therapy. One retrospective Japanese study demonstrated that immunosuppressive therapy had an impact on the morbidity and mortality of patients with cardiac sarcoidosis and that a dose ≤ 30 mg daily was as good as a dose ≥ 40 mg daily.24 There have been numerous case reports on improved LV function, rhythm disturbances, and hypermetabolic activity25 with the use of immunosuppressive therapy.
The role of AICD in cardiac sarcoidosis is unclear. AICDs have been effective in reducing sudden cardiac death in other cardiac conditions. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines states that “ICD implantation is reasonable for patients with cardiac sarcoidosis,” giving it a class IIa indication rating with a level of evidence C.26
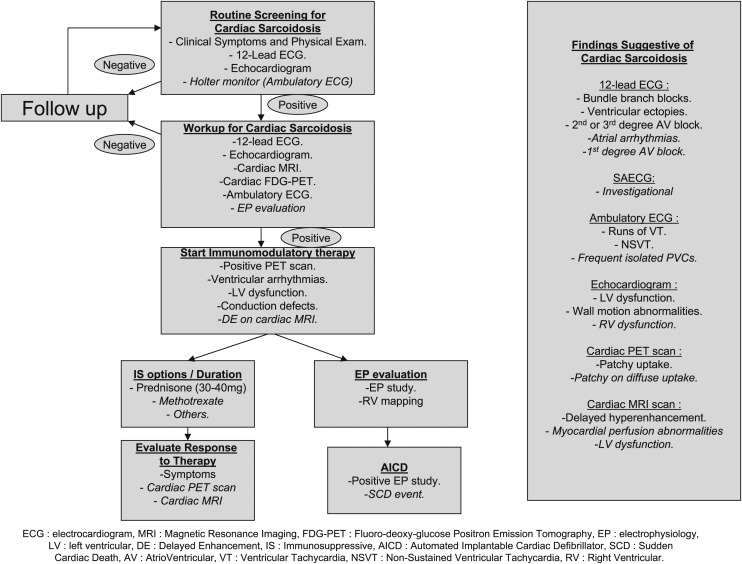
Figure 1 represents an algorithm for proposed “best practices” for the diagnosis and management of cardiac sarcoidosis based on the observed responses. Given that consensus among “experts” does not always indicate correct answers or approaches to a problem, further research is needed to produce actual guidelines.
Figure 1.
Proposed “best practices” for the diagnosis and management of cardiac sarcoidosis. Roman font indicates strong agreement ( > 70%); italic font indicates moderate agreement (50%-70%). SAECG = signal-averaged ECG.
Our study has a number of limitations; the response rate was adequate, but not optimum, in spite of an introductory e-mail followed by three e-mail reminders to complete the questionnaires. Cardiologists and electrophysiologists were underrepresented. We limited the number of questionnaires to two for several reasons. Consensus was not strengthening through the use of questions designed to increase clarity between the two questionnaires. This was interpreted as reflecting difference of opinion based on uncertainty in the existing evidence base. Indeed, a number of experts mentioned that various factors influenced their decision and choice of answers, and also the lack of evidence or guidelines made their choices difficult. In addition, the percentage of experts responding was decreasing, which limited our ability to determine consensus
In summary, our study provides an insight on the approach of sarcoid experts toward evaluating and managing cardiac sarcoidosis. It highlights some areas of agreement that do exist, potentially suggesting best practices. More importantly, though, it highlights areas of controversy that need to be addressed in future research to guide the experts and patients when faced with the potential involvement of the myocardium with sarcoidosis. Due to the rarity, but significance, of cardiac sarcoidosis, a very broad-based research effort, including ongoing efforts like the current Delphi study, as well as, whenever possible, collaborative, multicenter efforts, such as registry or database initiatives, opportunistic observational studies, and small, targeted clinical trials, will be needed to address areas of controversies in the management of cardiac sarcoidosis.
Acknowledgments
Authors contributions: Dr Hamzeh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Hamzeh: contributed to the study concept, design, and implementation; data analysis and interpretation; and the writing of the manuscript.
Dr Wamboldt: contributed to the study concept and reviewed and critiqued the manuscript.
Dr Weinberger: contributed to the study design and data analysis and reviewed and critiqued the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was done at National Jewish Health, Denver, CO.
Additional information: The e-Appendix can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/1/154/suppl/DC1.
Abbreviations
- AICD
automated implantable cardiac defibrillator
- EP
electrophysiology
- FDG
fluorodeoxyglucose
- IS
immunosuppressive
- LV
left ventricular
- SAECG
signal-averaged ECG
- VT
ventricular tachycardia
Footnotes
Funding/Support: Supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154. Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736-755 [DOI] [PubMed] [Google Scholar]
- 2.Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103(1):253-258 [DOI] [PubMed] [Google Scholar]
- 3.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204-1211 [DOI] [PubMed] [Google Scholar]
- 4.Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979-1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100(4):423-427 [DOI] [PubMed] [Google Scholar]
- 6.Hiraga Kyamhea H. Guideline for Diagnosis of Cardiac Sarcoidosis: Study Report on Diffuse Pulmonary Disease From the Japanese Ministry of Health and Welfare. Tokyo, Japan: Japanese Ministry of Health and Welfare; 1993:23-24 [Google Scholar]
- 7.Sharma OP. Myocardial sarcoidosis. A wolf in sheep’s clothing. Chest. 1994;106(4):988-990 [DOI] [PubMed] [Google Scholar]
- 8.Judson MA. Cardiac sarcoidosis: there is no instant replay. Chest. 2005;128(1):3-6 [DOI] [PubMed] [Google Scholar]
- 9.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008-1015 [PubMed] [Google Scholar]
- 10.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426-1435 [DOI] [PubMed] [Google Scholar]
- 12.Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004;83(6):315-334 [DOI] [PubMed] [Google Scholar]
- 13.Okayama K, Kurata C, Tawarahara K, Wakabayashi Y, Chida K, Sato A. Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis. Chest. 1995;107(2):330-334 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Kanda T, Kubota S, Imai S, Murata K. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106(4):1021-1024 [DOI] [PubMed] [Google Scholar]
- 15.Schuller JL, Lowery CM, Zipse M, et al. Diagnostic utility of signal-averaged electrocardiography for detection of cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16(1):70-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhôte R, Vignaux O, Blanche P, et al. Value of MRI for the diagnosis of cardiac involvement in sarcoidosis [in French]. Rev Med Interne. 2003;24(3):151-157 [DOI] [PubMed] [Google Scholar]
- 17.Györik S, Ceriani L, Menafoglio A, Gallino A, Wyttenbach R. 18F-FDG PET scan as follow-up tool for sarcoidosis with symptomatic cardiac conduction disturbances requiring a pacemaker. Thorax. 2007;62(6):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35(5):933-941 [DOI] [PubMed] [Google Scholar]
- 19.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45(12):1989-1998 [PubMed] [Google Scholar]
- 20.Smedema J-P, Snoep G, van Kroonenburgh MPG, et al. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128(3):1629-1637 [DOI] [PubMed] [Google Scholar]
- 21.Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol. 2005;185(1):110-115 [DOI] [PubMed] [Google Scholar]
- 22.Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18F-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16(5):801-810 [DOI] [PubMed] [Google Scholar]
- 23.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45(10):1683-1690 [DOI] [PubMed] [Google Scholar]
- 24.Yazaki Y, Isobe M, Hiroe M, et al. ; Central Japan Heart Study Group Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010 [DOI] [PubMed] [Google Scholar]
- 25.Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44(7):1030-1036 [PubMed] [Google Scholar]
- 26.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):2820-2840 [DOI] [PubMed] [Google Scholar]