Abstract
Background:
At the onset of acute hypoxic respiratory failure, critically ill patients with acute lung injury (ALI) may be difficult to distinguish from those with cardiogenic pulmonary edema (CPE). No single clinical parameter provides satisfying prediction. We hypothesized that a combination of those will facilitate early differential diagnosis.
Methods:
In a population-based retrospective development cohort, validated electronic surveillance identified critically ill adult patients with acute pulmonary edema. Recursive partitioning and logistic regression were used to develop a decision support tool based on routine clinical information to differentiate ALI from CPE. Performance of the score was validated in an independent cohort of referral patients. Blinded post hoc expert review served as gold standard.
Results:
Of 332 patients in a development cohort, expert reviewers (κ, 0.86) classified 156 as having ALI and 176 as having CPE. The validation cohort had 161 patients (ALI = 113, CPE = 48). The score was based on risk factors for ALI and CPE, age, alcohol abuse, chemotherapy, and peripheral oxygen saturation/Fio2 ratio. It demonstrated good discrimination (area under curve [AUC] = 0.81; 95% CI, 0.77-0.86) and calibration (Hosmer-Lemeshow [HL] P = .16). Similar performance was obtained in the validation cohort (AUC = 0.80; 95% CI, 0.72-0.88; HL P = .13).
Conclusions:
A simple decision support tool accurately classifies acute pulmonary edema, reserving advanced testing for a subset of patients in whom satisfying prediction cannot be made. This novel tool may facilitate early inclusion of patients with ALI and CPE into research studies as well as improve and rationalize clinical management and resource use.
The finding of acute hypoxemia and bilateral lung infiltrates on frontal chest radiograph is common in the ICU setting, and differentiation between cardiogenic pulmonary edema (CPE) and noncardiogenic pulmonary edema (acute lung injury [ALI]) is difficult and challenging in the early stages of illness.1,2 Left atrial hypertension (LAH) as a principal cause of acute pulmonary edema must be excluded before making a diagnosis of ALI and its more severe form, ARDS.3 Conversely, to exclude ALI, one needs not only the evidence of LAH but also the absence of significant ALI risk factors. Traditionally, a pulmonary artery occlusion pressure (PAOP) > 18 mm Hg has been used as a surrogate marker of LAH. It is rarely used in current clinical practice because it is invasive, the efficacy of pulmonary artery catheter-guided therapy in critically ill patients has not been proven,4 and some studies have suggested increased morbidity and mortality associated with its use.5
Alternative markers of LAH, such as B-type natriuretic peptide (BNP),6,7 central venous pressure (CVP),1 and echocardiographic evidence of systolic and diastolic dysfunction (ejection fraction, ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity [E/E′])8 are commonly used to estimate LAH. However, no single variable provides sufficient diagnostic accuracy. In both practice and research, clinical judgment is the most common diagnostic tool.1 Retrospective review of medical records by experienced providers taking into account all available clinical information and the course of disease has demonstrated reliable distinction between ALI and CPE and is currently the best available reference standard.6,9 Early in the course of illness, however, clinical judgment is limited by its subjectiveness and substantial interobserver variability. Indeed, clinical judgment is often inferior to more objective formal clinical decision tools, which mostly allow restriction of advanced testing to a smaller and more focused share of patients.10‐13 Our aim was to develop a decision support tool helping with early differential diagnosis in patients with acute pulmonary edema, which may improve diagnostic and therapeutic management, improve resource use, and facilitate pharmacologic and other interventional research in early stages of ALI or CPE.
Materials and Methods
For the development of the decision support tool we used a two-step approach: A model was created using a population-based development cohort (DC) and was validated in an independent validation cohort (VC) of referral patients. This retrospective, observational study was performed at a tertiary care hospital in Rochester, Minnesota. The target population was adult patients admitted to medical, cardiac, and mixed medical-surgical ICUs at the study institution who developed acute hypoxic respiratory failure and had chest radiographic evidence of bilateral pulmonary infiltrates consistent with acute pulmonary edema. In the DC we used a population-based design, including only Olmsted County (Minnesota) residents admitted to ICUs between January 2006 and September 2009. The VC was based on a cohort of referral patients (ie, non-Olmsted County residents) admitted to ICUs July through November 2006.
A previously validated electronic surveillance system (ALI Sniffer) identified for both cohorts patients with a Pao2/Fio2 < 300 on arterial blood gas analysis and the presence of bilateral infiltrates and/or edema on the radiologist report of portable digital chest radiographs.9 Positively screened patients were reviewed post hoc by critical care experts who ascertained the final diagnosis of ALI, CPE, both, or other (gold standard). Excluded were patients classified as Other. This category included also patients who had chest radiograph findings not consistent with ALI14,15: unilateral infiltrates, lower zone opacities only, difficult-to-interpret chest radiograph, chronic only, or no infiltrates. We excluded patients who were on mechanical ventilation prior to development of acute pulmonary edema and those who had insufficient routine clinical data available (ie, patients who expired within 6 h of onset of acute pulmonary edema or lacked timely documentation). Patients subjected to high-risk surgery or severe trauma (definitions in e-Appendix 1) were excluded due to diagnostic difficulties in the ascertainment of bilateral pulmonary infiltrates (postoperative atelectasis) and poor model performance based on preliminary data. Patients who previously denied use of their medical records for research (∼ 5%) were omitted from electronic screening automatically. Mayo Clinic institutional review board approved the use of medical records for research and waived the requirement for written informed consent (IRB# 06-005244).
Expert reviewers blinded to the model prediction classified patients as ALI vs CPE using all available information at the time of hospital discharge, including the course of illness and response to therapeutic intervention. ALI was defined according to the American-European Consensus Conference (AECC) statement3 as acute ( < 24 h) hypoxemia (Pao2/Fio2 ratio < 300) with bilateral lung infiltrates consistent with pulmonary edema on frontal chest radiograph without any evidence of LAH. LAH was excluded by echocardiographic findings (E/E′ < 15), BNP levels (BNP < 250 pg/mL in the absence of renal failure), and venous filling pressures (PAOP ≤ 18 or CVP ≤ 12 mm Hg in the absence of pulmonary hypertension). CPE was defined by a combination of clinical signs (jugular venous distension, systolic hypertension); radiographic (cardiothoracic ratio of > 0.53 and vascular pedicle width of > 65 mm), ECG (new ST-segment and T-wave changes), laboratory (elevated troponin T level > 0.1 ng/mL), and hemodynamic findings (PAOP > 18 mm Hg, CVP > 12 mm Hg, decreased ejection fraction < 45%, E/E′ > 15, presence of severe left-sided valvular heart disease [aortic or mitral stenosis or regurgitation]); and a brisk response (hours) to appropriate therapy (preload/afterload reduction, treatment of ischemia or inotropic agents).6 Cases with mixed criteria were counted as Both (ALI + CPE) and in analysis treated as ALI cases. Interobserver variability was calculated and discordant results resolved by consensus. Additional details of post hoc expert assessment are provided in e-Appendix 2.
Predictor variables available within 6 h after onset of acute pulmonary edema were collected from electronic medical records by investigators blinded to expert diagnosis. These included: (1) clinical data: age, sex, previous medical history of heart disease, presence of risk factors of ALI, transfusion of blood products, chemotherapy, alcohol abuse; (2) examination findings: temperature and oxygen saturation (Spo2); (3) blood tests: leukocytes, bicarbonate, lactate, troponin T, BNP, and creatinine levels; (4) EKG findings: new > 1 mm ST segment elevation or depression in two contiguous leads or new left bundle branch block (LBBB). A list of all variables and the applied definitions are shown in e-Appendix 1.
Statistical Analysis
In both cohorts, data were summarized as median (interquartile range [IQR]) or percent (number) for each group (ALI vs CPE). Univariate statistical analysis was carried out using the Wilcoxon rank sum test for continuous variables and the χ2 or Fisher exact test for categorical data, respectively.
Using univariate logistic regression analysis, variables associated with ALI vs CPE were identified. For this and all further analyses, continuous predictor variables were dichotomized applying standard thresholds (yes = 1, no = 0).16 Using recursive partitioning, a first outline of the model was created and possible interactions between variables explored. A scoring model was created by stepwise logistic regression: Starting from a full model, colinear variables and those with least impact on predictability (area under curve [AUC]) of the model were eliminated using clinical judgement.17 Several clinically suspected interactions were introduced and examined for statistical significance. The estimates from the model were transformed into a simple scoring scale. The discrimination and calibration properties of this (translated) score were assessed using the area under the receiver operating characteristic curve and the Hosmer-Lemeshow (HL) goodness-of-fit test. Sensitivity and specificity (with 95% CI) were determined for different cutoff values.
The final model derived from the DC was applied onto the VC and its performance judged by the resulting AUC and HL test. Missing data were treated as follows: For logistic regression a single imputation method was used. For variables including 0 in their “normal” reference range this value was used (eg, if someone had no EKG, we assumed that no ST changes were present); if not, the overall median was chosen. Recursive partitioning dealt with missing data by assigning these cases randomly to one or the other category.
Although patients with both conditions were treated as ALI cases in all steps of modeling, a sensitivity analysis excluded these patients. JMP (version 7.0, SAS) and SAS (version 8.0, SAS) statistical software were used for all data analysis with the traditional P value of .05 used as cutoff to judge statistical significance.
Results
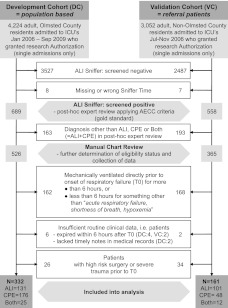
Of 4,224 adult Olmsted County residents admitted to ICUs during the study period, 689 were screened positive for acute pulmonary edema (ALI Sniffer positive), with 332 eventually meeting all eligibility criteria of the DC. For the VC, 3,052 patients (non-Olmsted County residents) were screened for acute pulmonary edema. Of 558 positively screened patients, 161 met all inclusion and exclusion criteria (Fig 1).
Figure 1.
Study flowchart. AECC = American European Consensus Conference; ALI = acute lung injury; CPE = cardiogenic pulmonary edema; T0 = onset of acute pulmonary edema.
There was very good agreement between post hoc reviewers regarding gold standard diagnosis (κ, 0.86). A summary of all collected information is shown for each cohort separately in Table 1. Recursive partitioning revealed a positive interaction between ALI risk factors and alcohol abuse and led to the outline of a four-step model (e-Figure 1).
Table 1.
—General Characteristics of Both Cohorts: The Results of Univariate Analyses
| Development Cohort |
Validation Cohort |
|||||||
| Characteristic | No. | ALI/ALI + CPE (n = 156) | CPE (n = 176) | P Value | No. | ALI/ALI + CPE (n = 113) | CPE (n = 48) | P Value |
| General information | ||||||||
| Age, y | 71.5 (57.6-81.3) | 78.2 (66.8-85.7) | < .001 | 65.2 (46.6-77.8) | 72.5 (63.3-85.7) | .004 | ||
| Female sex | 47 (74) | 57 (100) | .09 | 47 (53) | 50 (24) | .72 | ||
| BMI | 331 | 26.4 (23.3-30.5) | 28.5 (23.7-33.1) | .02 | 159 | 26.5 (22.1-32.3) | 28.4 (24.7-32.7) | .12 |
| NIV | 19 (30) | 30 (53) | .02 | 33 (37) | 44 (21) | .18 | ||
| IMV | 64 (100) | 39 (68) | < .001 | 67 (76) | 56 (27) | .18 | ||
| ALI risk factors | ||||||||
| Sepsis | 51 (80) | 15 (26) | < .001 | 40 (45) | 13 (6) | .001 | ||
| Pancreatitis | 1 (2) | 0 (0) | .13 | 2 (2) | 0 (0) | 1 | ||
| Shock | 35 (54) | 20 (35) | .003 | 35 (39) | 23 (11) | .15 | ||
| Pneumonia | 44 (68) | 23 (41) | < .001 | 58 (66) | 27 (13) | < .001 | ||
| Aspiration | 14 (22) | 7 (12) | .03 | 30 (34) | 8 (4) | .002 | ||
| CPE risk factors | ||||||||
| Hx of CAD | 30 (46) | 51 (90) | < .001 | 18 (20) | 44 (21) | .001 | ||
| Hx of heart failure | 21 (32) | 40 (71) | < .001 | 22 (25) | 56 (27) | < .001 | ||
| Hx of valvular disease | 13 (20) | 17 (30) | .28 | 14 (16) | 23 (11) | .17 | ||
| New ST changes/LBBB | 235 | 14 (14) | 29 (38) | .005 | 117 | 5 (4) | 27 (11) | .002 |
| Laboratory results and vital sign measurements | ||||||||
| BNP, pg/mL | 74 | 708 (160-1007) | 749 (309-1591) | .18 | 41 | 690 (181-1,302) | 1,610 (380-3,080) | .03 |
| Bicarbonate, mmol/L | 317 | 23 (19-27) | 24 (21-28) | .003 | … | … | … | |
| Creatinine, mg/dL | 312 | 1.1 (0.8-1.8) | 1.2 (0.9-1.8) | .27 | … | … | … | |
| Lactate, mmol/L | 142 | 1.9 (1.3-2.8) | 1.6 (1-2.8) | .31 | … | … | … | |
| Leukocytes, count/nL | 311 | 12.9 (8.5-17.9) | 12.2 (9.3-15.8) | .72 | … | … | … | |
| Troponin T, ng/mL | 213 | 0.02 (0.01-0.10) | 0.05 (0.02-0.21) | .01 | 0.02 (0-0.13) | 0.105 (0.03-0.39) | .004 | |
| Max temperature, °C | 268 | 37.2 (36.7-38.1) | 37.3 (36.7-37.9) | .69 | … | … | … | |
| Spo2/Fio2 ratio at 6 ha | 324 | 179 (132-247) | 238 (167-329) | < .001 | 110 | 297 (160-443) | 443 (190-462) | .02 |
| Risk modifiers | ||||||||
| Chemotherapy | 10 (16) | 1 (2) | < .001 | 12 (13) | 6 (3) | .40 | ||
| Alcohol abuse ( > 2 drinks/d) | 12 (19) | 4 (7) | .006 | 9 (10) | 10 (5) | .75 | ||
| Smoking (active/ > 20 PY) | 46 (72) | 44 (77) | .66 | 32 (36) | 33 (16) | .86 | ||
| Diabetes mellitus | 23 (36) | 31 (55) | .1 | 21 (24) | 38 (18) | .03 | ||
| ILD | 5 (7) | 5 (8) | .98 | … | … | … | ||
| Transfusions (any within 24 h) | ||||||||
| Platelets | 4 (6) | 1 (2) | .15 | … | … | … | ||
| RBCs | 13 (20) | 11 (20) | .68 | … | … | … | ||
| Fresh frozen plasma | 3 (4) | 6 (11) | .12 | … | … | … | ||
Data are presented as median (IQR) or % (No.). ALI = acute lung injury; BNP = brain natriuretic peptide; CAD = coronary artery disease; CPE = cardiogenic pulmonary edema; Hx = history; ILD = interstitial lung disease; IMV = invasive mechanical ventilation (includes patients with both NIV + IMV); IQR = interquartile range; LBBB = left bundle branch block; NIV = noninvasive ventilation (excludes patients who received also IMV during hospitalization); PY = pack years; Spo2 = peripheral oxygen saturation.
Spo2/Fio2 ratio at 6 h after the onset of acute pulmonary edema.
In the DC, 13 of 23 potential predictor variables were found to be significantly associated with ALI in univariate analysis (e-Table 1). The final model comprised 11 predictor variables (Table 2).
Table 2.
—Logistic Regression Model for Prediction of ALI/ALI + CPE vs CPE
| Wald χ2 Test |
|||||
| Predictor | Estimate | (95% CI) | SE | χ2 | P Value |
| Intercept | −0.82 | (−1.37 to −0.30) | 0.27 | 9.1 | .003 |
| Age < 45 y | 2.26 | (0.81 to 4.21) | 0.83 | 7.4 | .007 |
| Hx of heart failure | −0.66 | (−1.25 to −0.07) | 0.30 | 4.8 | .03 |
| Hx of CAD | −0.38 | (−0.93 to 0.17) | 0.28 | 1.8 | .18 |
| New ST changes/LBBB | −0.76 | (−1.55 to −0.03) | 0.38 | 3.9 | .05 |
| Sepsis or pancreatitis | 1.29 | (0.70 to 1.89) | 0.30 | 18.0 | < .001 |
| Pneumonia | 0.54 | (−0.07 to 1.14) | 0.31 | 3.0 | .08 |
| Aspiration | 0.24 | (−0.71 to 1.2) | 0.48 | 0.3 | .62 |
| Alcohol abuse × ALI RFa | 2.10 | (0.39 to 5.03) | 1.07 | 3.8 | .05 |
| Chemotherapy | 2.07 | (0.71 to 3.96) | 0.79 | 6.9 | .009 |
| Spo2/Fio2 ratio at 6 h < 235b | 0.51 | (−0.02 to 1.05) | 0.27 | 3.5 | .06 |
| AUC = 0.81 | |||||
A higher estimate indicates a higher probability for ALI/ALI + CPE vs CPE and vice versa. AUC = area under curve; RF = risk factor. See Table 1 legend for expansion of other abbreviations.
Variable was 1 if patient had any of the following: sepsis, pancreatitis, pneumonia, aspiration; all else, 0.
Spo2/Fio2 ratio at 6 h after onset of acute pulmonary edema.
To facilitate clinical usage of the model, the calculated estimates were translated into a simple score shown in Table 3: Estimates were doubled and then rounded to the closest 0.5. This score demonstrated good discrimination (AUC = 0.81; 95% CI, 0.77-0.86) and was well calibrated (HL P = .16).
Table 3.
—Score Derived From Logistic Regression Model
| Predictor | Estimates Translated Into Score |
| Demographic | |
| Age < 45 yr | 4.5 |
| CPE risk factors | |
| Hx of heart failure | −1.5 |
| Hx of CAD | −1 |
| New ST changes/LBBB | −1.5 |
| ALI risk factors | |
| Sepsis or pancreatitis | 2.5 |
| Pneumonia | 1 |
| Aspiration | 0.5 |
| ALI risk modifier (only counted if any ALI RF) | |
| Alcohol abuse | 4 |
| Miscellaneous | |
| Chemotherapy | 4 |
| Persistent hypoxemia = Spo2/Fio2 ratio at 6 h < 235 | 1 |
The predicted probability of ALI vs CPE (or vice versa) for a single patient with a given score sum is shown in Table 4. This table also shows how many patients of the development cohort fall into each category (distribution of score sums among patients with ALI and CPE, respectively, is shown in e-Figure 2). Sensitivity and specificity of the score range each from approximately 50% to 90%, depending on which cutoff value is chosen (Table 5).
Table 4.
—Probability of ALI/ALI + CPE vs CPE (or Vice Versa) for a Given Score Sum
| Calculated Score | % | No. | % ALI | % CPE |
| ≤ −2 | 13 | 44 | 11 | 89 |
| −1.5 to −0.5 | 20 | 67 | 19 | 81 |
| 0 to 0.5 | 13 | 44 | 34 | 66 |
| 1 to 1.5 | 17 | 55 | 51 | 49 |
| 2 to 3 | 11 | 35 | 63 | 37 |
| 3.5 to 4.5 | 12 | 38 | 71 | 29 |
| 5 to 6 | 5 | 16 | 88 | 13 |
| ≥ 6.5 | 10 | 33 | 97 | 3 |
See Table 1 legend for expansion of abbreviations.
Table 5.
—Performance of the Score at Different Cutoff Values
| Cutoff Point | Sensitivity | 95% CI | Specificity | 95% CI |
| > −1.5 | 94 | 88-97 | 36 | 29-44 |
| > −0.5 | 89 | 82-93 | 53 | 45-60 |
| > 0 | 81 | 74-87 | 68 | 61-75 |
| > 1 | 64 | 55-71 | 82 | 75-87 |
| > 2 | 51 | 43-59 | 87 | 81-91 |
| > 3 | 47 | 39-55 | 92 | 87-95 |
When applied to the VC, the model showed similar performance (AUC = 0.80; 95% CI, 0.72-0.88) and calibration (HL P = .13). Model performance (in DC) was the same when excluding patients with both conditions (ALI + CPE, according to expert review) from logistic regression analysis (AUC = 0.83; 95% CI, 0.78-0.88). An excel calculator is provided in e-Appendix 3. (Disclaimer: this calculator is only preliminary, not yet validated externally and must not be used for patient care.)
Discussion
We present a simple prediction score for the early differential diagnosis of ALI vs CPE based on readily available clinical data. The results of univariate analysis concur with the previous findings demonstrating that no single clinical variable can predict the diagnosis accurately in a substantial portion of patients.1,6‐8 A formal combination of clinical variables may provide accurate diagnosis early in the course of acute hypoxic respiratory failure more objectively than the common approach of using clinical judgment.1,12 The possibility of easily calculating specific pretest probabilities allows an objective appreciation of the risk-benefit ratio of advanced procedures.18 If successfully validated, this simple prediction tool may be used to improve diagnostic assessment of patients with acute pulmonary edema early in the course of illness and to facilitate timely enrollment into clinical research studies.
For a long time, pulmonary artery catheter-based assessment was considered the gold standard, but as shown multiple times, its accuracy in routine clinical practice is limited.19‐21 The Fluids and Catheters Treatment Trial (FACTT) showed that about one-third of the patients with ALI in the pulmonary artery catheter portion of the trial had a PAOP > 18 mm Hg at the time of catheter insertion. This is a substantial proportion, highlighting the inadequacy of the pulmonary artery catheter in differentiating between the two conditions as well as the possibility that both may coexist.22 We approached this issue by treating patients with both conditions as ALI cases to account for the more severe prognosis and to concur with previous practice.22,23 The majority of these patients had intermediate-range scores (data not shown) suggesting further testing. In the post hoc sensitivity analysis, those cases were excluded without substantially changing the performance of the score.
BNP is sometimes used in clinical practice to distinguish between ALI and CPE. However, common occurrence of shock and acute kidney injury limit its usefulness in the ICU setting.6 In a post hoc analysis of a subgroup of patients who had this biomarker measured (e-Appendix 4), addition of BNP marginally improved the prediction, suggesting that this test may be used electively in uncertain cases.
Recently, Ware et al24 documented that acute pulmonary edema can be categorized into ALI vs CPE on the basis of edema fluid to plasma protein ratio. They reported very good sensitivity (81%) and specificity (81%). Unfortunately, edema fluid is available in a minority of patients and requires the presence of an endotracheal tube. Similarly, physiologic studies that used lung uptake of membrane-impermeable radionuclide tracers have a limited value outside the research setting.25 Ultimately, the discovery of reliable biomarkers of alveolar-capillary membrane injury26,27 and wider availability and improved accuracy of bedside ICU ultrasonography28 may be helpful to more precisely identify the cause of the pulmonary edema early in the course of the illness.
The major limitation of our study is one that plagues the field: the lack of a good gold standard. By definition, each new test can only be as good as its reference standard. Following previous practice,6,9,29 we used a post hoc expert review to compare our model. Although the dependence of reviewers on information from medical records means some loss of data as compared with bedside assessment, this is more than compensated by the fact that all testing and clinical data (including the course of disease) become overt. The good agreement among expert reviewers (κ, 0.86) indicates that this process allowed reliable differentiation between ALI and CPE. Although the κ statistic does not allow judgment of the accuracy of this process (ie, how often the experts agreed on the true diagnosis), we assume that it was good due to the high level of reviewers’ experience and training. The reliance of the model on information commonly used for clinical assessment1 and its validation in an independent second cohort improve the generalizability of our findings. However, before general adoption for clinical practice, prospective validation and impact analysis are necessary.30
Another important limitation of our approach is intrinsic to the clinical nature of ALI or CPE in the sense that the conditions can coexist. The AECC definition3 of ALI calls for exclusion of left atrial hypertension, but this does not account for existence of both as discussed previously in the context of FACTT trial.22 Obviously, this will affect the accurate differentiation and performance of any diagnostic test that aims to differentiate between these two conditions. Our score reflects this fact by assigning an intermediate score value to the majority of this patient group. Although more extreme score values provide a strong and objective rationale for omitting further testing, advanced investigation may be warranted in patients with intermediate results. In our study, only 17% of patients had an undetermined score (ie, 1-1.5); the remaining 83% fell either below or above this gray zone. This suggests that the presented tool will be helpful in a substantial fraction of patients.
A third limitation may be the dependence of the screening tool (ALI Sniffer) on the availability of arterial blood gas measurements. The aim of our study, however, was to provide help with the differentiation of ALI vs CPE in patients in whom this may be challenging. Patients lacking arterial blood gas measurements most likely did not have severe enough acute respiratory failure to entertain the diagnosis of ALI and were, therefore, not our target population.
Other issues are that due to the shape of the oxyhemoglobin dissociation curve, the Spo2/Fio2 ratio may not be reliable in cases in which the Spo2 was ≥ 97%. In a preliminary study, Spo2/ Fio2 ratios of patients with ALI compared with those with CPE were significantly lower at 6 h after onset of acute pulmonary edema, suggesting that this ratio may be nonetheless a good marker for the differentiation of both conditions.31 Also, we did not compare the accuracy of our model against the clinical judgment by bedside providers. Although previous studies suggest that health-care providers fail to recognize ALI in a majority of patients,9,32 the clinical impact of our tool needs to be evaluated in a prospective validation.
Conclusions
We present a decision support tool for differential diagnosis of ALI vs CPE based on readily available clinical information. Once externally validated, our simple, quick, cheap, and noninvasive tool may be useful not only for early enrollment into research studies but also to help acute care providers at bedside, especially those who are at an earlier stage in their career. In addition, this tool can be easily programmed within the new generation of electronic medical records.
Supplementary Material
Acknowledgments
Author contributions: Mr Schmickl had full access to the data and vouches for the integrity of the data analysis.
Mr Schmickl: contributed to study design, conduct, and manuscript writing.
Dr Shahjehan: contributed to study design, conduct, and manuscript writing.
Dr Li: contributed to the conduct of the study and preparation of the manuscript.
Dr Dhokarh: contributed to the conduct of the study and preparation of the manuscript.
Dr Kashyap: contributed to the conduct of the study and revision of the manuscript.
Mr Janish: contributed to the conduct of the study and revision of the manuscript.
Dr Alsara: contributed to the conduct of the study and revision of the manuscript.
Dr Jaffe: contributed to preparation of the manuscript.
Dr Hubmayr: contributed to preparation of the manuscript.
Dr Gajic: contributed to supervision and was involved as senior author in all critical parts of the study.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Jaffe is a consultant to many diagnostic companies, including Roche, Beckman, Siemens, Ortho, Alere, Abbott, Critical Diagnostics, Pfizer, and Amgen. He has received honoraria for talks from Siemens, Roche and Abbott. Messrs Schmickl and Janish and Drs Shahjehan, Li, Dhokarh, Kashyap, Alsara, Hubmayr, and Gajic have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: Mayo Clinic Critical Care Reseach Committee and the National Library of Medicine were neither involved in the design or conduct of the study nor in the preparation of the manuscript.
Other contributions: We thank Michael Malinchoc, MSc, and Andrew Henson, MSc, for their help with statistical analysis; Rodrigo Cartin-Ceba, MD, and Daryl Kor, MD, for critically reviewing the manuscript; Osama Alsara, MD, for helping with data collection; Vitaly Herasevich, MD, PhD, and Chinmay Manohar, MS, for helping with the creation of the online calculator; and all members of the METRIC group for constant and constructive feedback. The study was performed at the Mayo Clinic, Rochester, MN.
Additional information: The e-Appendices, e-Figures, and e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/1/43/suppl/DC1.
Abbreviations
- AECC
American European Consensus Conference
- ALI
acute lung injury
- AUC
area under curve
- BNP
brain natriuretic peptide
- CAD
coronary artery disease
- CPE
cardiogenic pulmonary edema
- CVP
central venous pressure
- DC
development cohort
- E/E′
ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E′)
- HL
Hosmer-Lemeshow test
- IQR
interquartile range
- LAH
left atrial hypertension
- LBBB
left bundle branch block
- PAOP
pulmonary occlusion pressure
- Spo2
peripheral oxygen saturation
- VC
validation cohort
Footnotes
Mr Schmickl and Dr Shahjehan contributed equally to this study.
Funding/Support: This work was supported in part by Mayo Clinic Critical Care Research Committee and the National Library of Medicine [Grant RC1 LM10468].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 2.Fein AM, Goldberg SK, Walkenstein MD, Dershaw B, Braitman L, Lippmann ML. Is pulmonary artery catheterization necessary for the diagnosis of pulmonary edema? Am Rev Respir Dis. 1984;129(6):1006–1009. doi: 10.1164/arrd.1984.129.6.1006. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 4.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 5.Connors AF, Jr, Speroff T, Dawson NV, et al. SUPPORT Investigators The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA. 1996;276(11):889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 6.Rana R, Vlahakis NE, Daniels CE, et al. B-type natriuretic peptide in the assessment of acute lung injury and cardiogenic pulmonary edema. Crit Care Med. 2006;34(7):1941–1946. doi: 10.1097/01.CCM.0000220492.15645.47. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Daniels CE, Kojicic M, et al. The accuracy of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic) in the differentiation between transfusion-related acute lung injury and transfusion-related circulatory overload in the critically ill. Transfusion. 2009;49(1):13–20. doi: 10.1111/j.1537-2995.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 9.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845–850. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 11.Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269(9):1127–1132. doi: 10.1001/jama.269.9.1127. [DOI] [PubMed] [Google Scholar]
- 12.Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129(12):997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286(15):1841–1848. doi: 10.1001/jama.286.15.1841. [DOI] [PubMed] [Google Scholar]
- 14.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5):1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 15.Criteria for chest x-ray findings consistent with acute lung injury. University of Washington Web site. http://depts.washington.edu/kclip/about.shtml. Accessed July 20, 2010.
- 16.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. for the National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Richardson WS, Wilson MC, Guyatt GH, Cook DJ, Nishikawa J. Evidence-Based Medicine Working Group Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. JAMA. 1999;281(13):1214–1219. doi: 10.1001/jama.281.13.1214. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28(8):1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 20.Jacka MJ, Cohen MM, To T, Devitt JH, Byrick R. Pulmonary artery occlusion pressure estimation: how confident are anesthesiologists? Crit Care Med. 2002;30(6):1197–1203. doi: 10.1097/00003246-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Komadina KH, Schenk DA, LaVeau P, Duncan CA, Chambers SL. Interobserver variability in the interpretation of pulmonary artery catheter pressure tracings. Chest. 1991;100(6):1647–1654. doi: 10.1378/chest.100.6.1647. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler AP, Bernard GR, Thompson BT, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 23.Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 24.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35(2):331–337. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster DP, Stark T, Stephenson J, Royal H. Detecting lung injury in patients with pulmonary edema. Intensive Care Med. 2002;28(9):1246–1253. doi: 10.1007/s00134-002-1414-3. [DOI] [PubMed] [Google Scholar]
- 26.Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(5 suppl):S109–S113. doi: 10.1097/01.CCM.0000214311.56231.23. [DOI] [PubMed] [Google Scholar]
- 27.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 28.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Pérez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133(5):1113–1119. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Evidence-Based Medicine Working Group Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 31.Schmickl CN, Shahjehan K, Pickering B, et al. Comparison of serial vital sign measurements around the time of presentation of either acute lung injury or cardiogenic pulmonary edema [abstract]. Presented at the Society of Critical Care Medicine’s 39th Critical Care Congress; January 9-13, 2010; Miami Beach, Florida. [Google Scholar]
- 32.Cedeño A, Galera A, Torres A, Rodríguez-Cintrón W. Acute lung injury/acute respiratory distress syndrome: a need for education. P R Health Sci J. 2002;21(4):305–308. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.