Abstract
Aging of the unfertilized oocyte inevitably occurs following ovulation, limiting its fertilizable life-span. However, the mechanisms that regulate oocyte aging are still unclear. We hypothesize that reactive oxygen species such as superoxide (O2•−), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) are likely candidates that may initiate these changes in the oocyte. In order to test this hypothesis, we investigated direct effects of O2•− [hypoxanthine/xanthine oxidase system generating 0.12 (n=42) and 0.25 μM O2•−/min (n=45)], H2O2 (20 or 100 μM, n=60) and HOCl, (1, 10 and 100 μM, n=50) on freshly ovulated or relatively old mouse oocytes, while their sibling oocytes were fixed immediately or cultured under physiological conditions (n=96). The aging process was assessed by the zona pellucida dissolution time (ZPDT), ooplasm microtubule dynamics (OMD), and cortical granule (CG) status. The ZPDT increased 2-fold in relatively old, compared to young, untreated oocytes (P<0.0001). Exposure to O2•− increased it even further (P<0.0001). Similarly, more O2•− exposed oocytes exhibited increased OMD and major CG loss, with fewer having normal OMD and intact CG compared to untreated controls. Interestingly, young oocytes resisted “aging”, when exposed to 20 μM H2O2, while the same enhanced the aging phenomena in relatively old oocytes (P<0.05). Exposure to even very low levels of HOCl induced aging phenomena in young and relatively old oocytes, and higher concentrations of HOCl compromised oocyte viability. Overall, O2•−, H2O2 and HOCl each augment oocyte “aging”, more so in relatively old oocytes, suggesting compromised antioxidant capacity in aging oocytes.
Keywords: Cortical granules, hydrogen peroxide, hypochlorous acid, microtubule dynamics, oocyte aging, oxidative stress superoxide, oocyte temporal window, zona pellucida
INTRODUCTION
Reactive oxygen species (ROS) such as superoxide (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and hypohalous acids (HOX, where X = Cl−, Br−, I−, Br−, or SCN−) are molecules that are highly disruptive to cellular function [1–3]. Therefore, increase in the production of ROS contributes significantly to several diseases including those that may compromise reproduction and fertility [2,3]. In general, the major intracellular sources of O2•− are the electron transport chain in the mitochondria, which also generate H2O2, and the NADPH oxidase system in the cellular plasma membrane [1,2]. Similar to other systems, ROS may be overproduced in the oocyte microenvironment in response to several conditions, such as ongoing acute or chronic infections or inflammation, certain medications, radiation, and pollutants. Alternatively, a decrease in free radical scavengers may contribute to accumulation of ROS [4–6]. Likewise, compromise in the oocyte’s cellular mechanisms to combat or remove the ROS may also result in accumulation of ROS. Such compromise could occur either due to the deficiency of low molecular weight substances such as vitamin E, vitamin C, uric acid, glutathione, taurine, hypotaurine, and albumin, or a group of enzymes such as glutathione peroxidase, catalase, indolamine dioxygenase, and superoxide dismutase that help to scavenge oxygen radicals throughout the female reproductive tract [1–3,5–14].
Additionally, other follicular components, namely the cumulus cells that surround the oocytes, as well as the follicular fluid, may protect the oocytes from the damaging effects of the ROS [15–20]. Failure of one or more of these oocyte defenses could result in the development of oxidative stress with resultant oocyte damage.
Attempts have therefore been made to prevent deterioration in oocyte quality by supplementing the culture media with antioxidants, such as caffeine, vitamin C and reduced glutathione (GSH) [11,21]. These agents were protective against oocyte postovulatory aging [21,22]. Similar positive effects including activation of the cGMP pathway were noted by Goud et al. [23,24], when oocytes were treated with nitric oxide (NO). NO is a ubiquitous signaling molecule that plays essential bioregulatory roles in a wide range of processes and has the ability to react with hemoproteins at nearly diffusion-controlled rates. It promotes activation of guanylate cyclase and possibly inhibits many heme and non-heme proteins by interacting with their metal centers [25–29]. Hence, factors that affect rates of NO production and consumption are of significant interest.
A major pathway for NO removal is through near diffusion-controlled interaction with O2•− yielding peroxynitrite (ONOO−) [1,30]. Peroxynitrite is a powerful-oxidant that can react with tyrosine residues to form the stable adduct nitrotyrosine [31,32]. Its decay is responsible for an increase in the nitrite (NO2−)/nitrate (NO3−) ratio.
Recently, we have demonstrated that mammalian peroxidases (myeloperoxidase (MPO), lactoperoxidase (EPO), and eosinophil peroxidase (EPO)) may operate as alternative pathways for catalytic removal of NO [27–29,33]. These enzymes catalyze the reduction of H2O2 and halides or pseudo halides through a 2 e− pathway generating the corresponding hypohalous acid [34]. Hypohalous acids are potent cytotoxic oxidants, which directly oxidize reactive groups, including sulphydryls, iron-sulfur centers and hemes, or react with amines forming chloramines [1,35]. Similarly, mammalian peroxidases and various organic and inorganic compounds by two consecutive 1 e− transitions, can oxidize H2O2 yielding radical species [27,28,34–36].
Oocyte aging significantly contributes to fertilization and developmental abnormalities as well as chromosome aberrations in the ensuing embryo [22,37)]. The characteristics of oocyte aging include enhancement in ooplasmic microtubule dynamics (OMD), premature release of cortical granules (CG) and hardening of zona pellucida (ZP) with an increase in ZP dissolution time (ZPDT) [38,39]. The current study investigates the effect of O2•−, H2O2 and HOCl on these characteristics of oocyte aging.
MATERIALS AND METHODS
Materials
Hypoxanthine, xanthine oxidase, nicotanimide adenine dinucleotide phosphate hydrogen (NADPH), hydrogen peroxide (H2O2), and sodium hypochlorite (NaOCl) were purchased from Sigma (St. Louis, MO). Other chemicals and reagents were of the highest purity grades available and obtained from either Sigma or Aldrich (St. Louis, MO). Rhodamine conjugated lectin, lens culinaris agglutinin, and the mounting medium Vectashield® were obtained from Vector laboratories (Burlingame, CA).
Study Design
The study was approved by Wayne State University’s Animal Investigation Committee. The study involved the use of oocytes obtained from superovulated 6–10 week-old B6D2F1 mice [27,28], and consisted of the following three experimental sets: Set 1. Study of the effect of a O2•−-generating system; Set 2. Study of the effect of H2O2 on oocytes; Set 3. Study of the effect of hypocholrous acid. The end points of the experiments involved the assessment of zona pellucida dissolution time (ZPDT), ooplasmic microtubule dynamics (OMD) and cortical granule (CG) status. In experiment set 3, oocytes were retrieved at 13 (young) and 17.5 h (relatively old) following hCG, while the other two experiment sets involved freshly ovulated oocytes retrieved at 13 h post-hCG. In every experiment, sibling oocytes were assigned to treatment and control groups. Untreated control oocytes were assessed at two time points, namely immediately upon retrieval and after culturing without the presence of the agent studied. The treatments consisted of exposure to xanthine oxidase/hypoxanthine for releasing O2•− exposure to NaOCl to produce HOCl, while H2O2 exposure was direct (Table 1). All experiments included treatment of the oocytes with the microtubule enhancer, taxol, which enhances ooplasmic microtubules more in the older compared to the young oocytes [38]. Above mentioned age-related phenomena were finally compared in each experiment set between different groups using appropriate statistical tests.
Superovulation and Oocyte Retrieval
Six to ten week-old B6D2F1 mice were obtained from Jackson Laboratories (Bar Harbor, ME), and were adjusted to the 14 h light-10 h dark cycle for at least one week prior to superovulation with 7.5 IU each of pregnant mare’s serum gonadotropin (PMSG) and hCG (Sigma, St. Louis, MO), administered IP 48–52 h apart. Mice were sacrificed at 13 to 18 h after hCG injection according to appropriate experiment sets. Cumuli retrieved from the oviductal ampullae were treated with 0.1% hyaluronidase (w/v) in M2 medium (Sigma) for 2–3 minutes at 37 °C. Oocytes were subsequently denuded to remove all cumulus-corona cells with a narrow bore pulled glass Pasteur pipette, thoroughly rinsed in M2 medium, inspected to rule out abnormal morphology and were kept ready in M16 medium (Sigma) pre-equilibrated with 5% CO2 in air at 37 °C in a common pool before randomly assigning into test and control groups according to the experiment sets.
Taxol Treatment, ZP Dissolution, Tubulin and Cortical Granule
Taxol 1 mM stock solution was prepared in DMSO and stored at −20 °C. Just prior to experiments, it was diluted with M-2 medium containing 10% fetal bovine serum (FBS, Life Technologies) to a working concentration of 10 μM. Taxol treatment and tubulin staining was performed by the technique previously used by Goud et al. [38]. This process was followed by zona pellucida dissolution time determination, fluorescence immunocytochemistry for α-tubulin and cortical granule staining with rhodamine conjugated lens culinaris agglutinin [23,24,38]. The oocytes were thoroughly rinsed once again with the PBS TX 0.3% BSA solution prior to mounting in Vectashield with DAPI (Vector Laboratories), that contained 4′6 diamidino-2-phenylindole (DAPI), a fluorescent chromatin stain. The oocytes were stored in glass chambers in the mounting medium at 4° C until processing with confocal microscopy, image processing, and 3-D reconstructions (LSM 310; Carl Zeiss Inc., Thornwood, NY).
Confocal Microscopy, Assessment of Microtubules and Cortical Granules
The cortical granules were stained fluorescent red, which was distinct from the fluorescent green staining of the microtubules (MT) and fluorescent blue staining of chromosomes. Individual treated and control oocytes in each experiment set were closely examined for spindle/ooplasmic microtubules and cortical granule status. The ooplasmic MT dynamics in response to taxol were evaluated and graded into the following three categories of microtubule dynamics, minimal or negligible, moderate, and markedly increased using previously described criteria [23,24,38]. Similarly, cortical granule status in each oocyte was categorized as intact CG, minor CG loss, and major CG loss [23,24,38]. The categorization of oocytes based on MT and CG status was performed by an independent observer blinded to treatment group assignment, who used comprehensive evaluation of the individual optical sections and the 3-D reconstructed images.
Experiment Set 1: Effect of O2•− –Generating System on Oocyte Aging
The rate of O2•− production in the reactions was estimated by measuring the rate of SOD –inhibitible cytochrome C reduction. Briefly, HEPES buffer (40 mM. pH 7.4) containing 1.2 μM hypoxanthine and catalase (1300 U/ml) and 50 μM cytochrome C was incubated at 15 °C, and different amounts of xanthine were added to the start reaction, while reduction of the cytochrome C was monitored at 550 nm [40]. Reactions consisted of 1.2 mM hypoxanthine plus 0, 0.15, 0.3, 0.6 U/ml of xanthine oxidase. This generated 0, 0.12, 0.25 and 0.52 μM of O2•−/min respectively.
Oocytes retrieved at 13 h post-hCG from superovulated female B6D2F1 mice were exposed to 0.1 mM of hypoxanthine and 0.3 U/μl of xanthine oxidase (0.12 μM of O2•−, Group B1, young n = 42) and 0.15 U/μl of xanthine oxidase (0.25 μM of O2•−, group B2, n = 45) PBS buffer, (2 h, 37° C, 5% CO2), rinsed in M2 medium and treated with 10 μM taxol (Paclitaxel, Sigma) per our previously published protocol [23,24,38,39]. Oocytes were then rinsed again in M2 medium and subjected to acidified Tyrode’s solution, to assess the ZPDT [23,24]. Control sibling oocytes were either fixed immediately (group A, n = 10) or allowed to age in culture in M16 without exposure to the O2•−-generating system (group C, n=25). Oocytes from either group were then fixed in freshly prepared 4% paraformaldehyde at 37 °C following taxol treatment and the aging phenomena were assessed in each oocyte and compared between each group after staining for microtubules, chromatin, and cortical granules as above.
Experiment Set 2: Effect of Hydrogen Peroxide on Oocyte Aging
Young oocytes retrieved at 13 hours from group A, (n= 32), and those retrieved at 17.5 h from the relatively older oocytes group B (n=28) were exposed to 20 or 100 μM H2O2 in M16 medium for 2 h, 37° C, 5% CO2), rinsed in M2 medium and treated with 10 μM taxol (Paclitaxel, Sigma) per our previously published protocol [23,24,38,39]. Oocytes were then rinsed again in M2 medium and subjected to acidified Tyrode’s solution, to assess the ZPDT [39]. Sibling oocytes from Group C, young (n=16) and relatively old oocytes (n=18), were allowed to age in culture in M16 without the presence of H2O2.
Following the taxol treatment as above, oocytes from each group were then subjected to ZPDT and fixed in freshly prepared 4% paraformaldehyde at 37 °C, and subsequently, stained for tubulin, chromatin and cortical granules as described above.
Experiment Set 3: Effect of Hypochlorous Acid on Oocyte Aging
Young oocytes were retrieved at 13 h; a portion of them were fixed directly for confocal microscopy (Group A n=13) while the other part was subjected to sodium hypochlorite (NaOCl) treatment. A stock solution of the NaOCl was kept in the dark at 4 °C. Its concentration was determined at pH=12 using ε290=350M−1 [48]. It was diluted with 0.14 M to NaCl, 10 mM phosphate (pH 7.4), thereby, producing hypochlorous acid (HOCl) immediately prior to use. The oocytes were incubated with the NaOCL at 1 μM (Group B1, n=20), 10 μM (Group B2, n=20) or 100 μM (n=10) in M 16 medium for 2 h, 37 °C, 5% CO2) rinsed in M2 medium and treated with 10 μM taxol (Paclitaxel, Sigma) per our previously published protocol [23,24,38,39]. Oocytes were then rinsed again in M2 medium and subjected to acidified Tyrode’s solution, to assess the ZPDT [23,24]. Control sibling oocytes were allowed to age in culture in M16 without the presence of NaOCl (Group C, n=14). Oocytes from each group were then fixed in freshly prepared 4% paraformaldehyde at 37 °C following taxol treatment. Finally the oocytes were stained for tubulin, chromatin and cortical granules as described above.
Statistical Tests
Statistical analysis was performed using SPSS® version 14.0 (SPSS Inc., Chicago, IL). The frequency data in each test and control subgroup were analyzed using Chi Square tests. Frequencies of microtubule dynamics, CG status, as well as spindles in individual subgroups with various exposures within groups young and relatively old were compared to their respective sibling control oocyte subgroups using the Fisher’s exact test. The data on zona pellucida dissolution timings were compared between test and control subgroups using the Student’s unpaired t test or one way ANOVA and Student Newman-Keuls post-hoc test where appropriate. Data are expressed as mean ± SD.
RESULTS
Experiment Set 1: Effect of O2•− on Oocytes
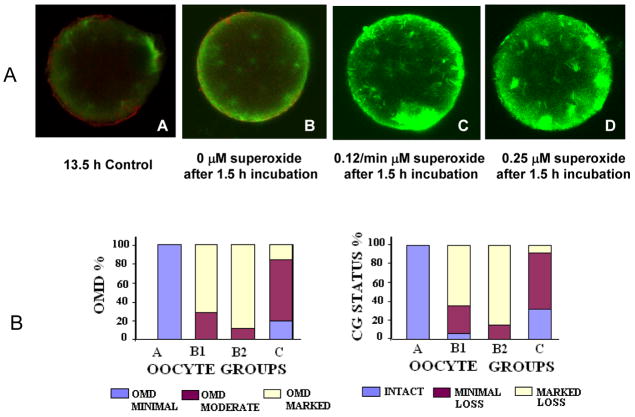
Although ZPDT increased with postovulatory age in group C (36.1 ± 6.2 seconds) compared to group A (17.7 ± 4.1 seconds), exposure to O2•− caused a further significant increase in ZPDT in groups B1 (47.9 ± 7.7 seconds) and B2 (57.5 ± 10.2 seconds) compared to their sibling unexposed oocytes (group C, P <0.0001). Similarly, O2•− exposure resulted in significantly more oocytes with increased OMD and major CG loss (B1: 71.4 and 64.3%; B2: 88.9 and 84.4%) and significantly fewer oocytes with minimal OMD and intact CG (B1: 0 and7.1%; B2: 0 and 0) compared to group C (increased OMD and major CG loss: 16.0 and 8%; minimal OMD and intact CG: 20.0 and 32%, respectively, P <0.0001 for all, Figs. 1A and 1B). Moreover, higher O2•− concentrations resulted in increased OMD and CG loss (P <0.05).
Fig. 1.
The effect of superoxide generating system on oocyte aging. Panel A shows Fluorescence photomicrographs of oocytes stained for α tubulin (FITC, green) and CG (rhodamine, red) after exposure to hypoxanthine/xanthine oxidase system generating 0.12 and 0.25 μM O2•−/min followed by taxol treatment. An increase in the ooplasmic microtubules is notable in the form of increased free microtubules and asters in panels C (0.12 μM O2•−/min) and D (0.25 μM O2•−/min) compared to the unexposed control oocytes (15 h post hCG, B) and the young controls (13.5 h post-hCG, A), also notable are the total absence of the CG’s in the O2 radical exposed oocytes (C and D) Original magnification: 400–600x; average oocyte diameter ~75–80 μm. Presented in panel B are bar charts showing OMD and CG status among oocytes exposed to the hypoxanthine/xanthine oxidase system generating 0.12 and 0.25 μM O2•−/min. A significantly increased OMD and marked loss in CG is evident in the groups B1 and B2 as compared to the controls A and C. Relatively old oocytes (17.5 h post-hCG) exposed to both 20 and 100 μM H2O2 also showed increased OMD and CG loss (not shown).
Experiment Set 2: Effect of H2O2 on Oocyte Aging
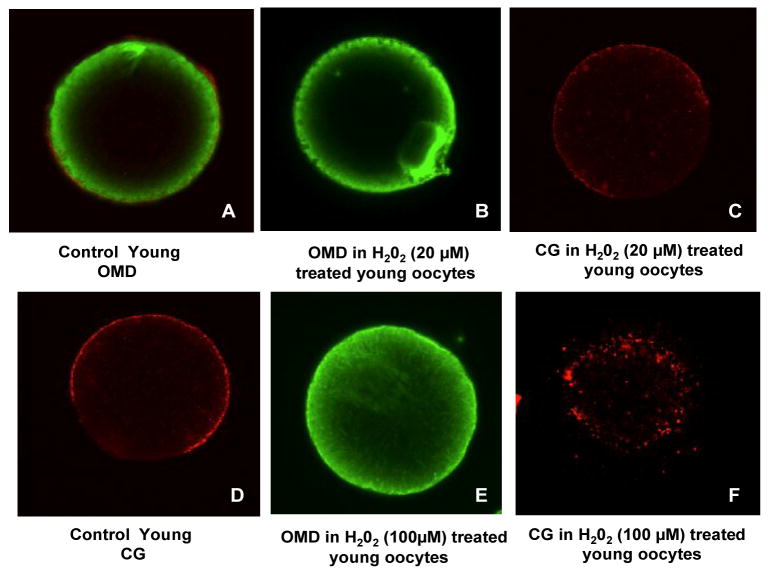
Similar to experimental set 1, the control oocytes in Groups A and B exhibited a post-ovulatory age dependent difference in aging phenomena. For instance, there was a significant increase in ZPDT in oocytes from group B compared to group A (P <0.05. Similarly, significantly more oocytes from group B had increased OMD and fewer oocytes had intact CG in group B compared to group A. Moreover, relatively old oocytes treated with H2O2 revealed a significantly higher ZPDT, with lower number of oocytes having minimal OMD and intact CG, and higher oocytes numbers with increased OMD and CG loss compared to the untreated relatively old oocytes (group B, P =0.001). On the other hand, despite treatment with 20 μM, the young oocytes showed no significant change in ZPDT, OMD or CG status. The young oocytes treated with 20μM H2O2 showed no effect in terms of age related change in OMD and CG as compared to the 100 μM H2O2 exposed oocytes in which OMD is significantly increased and CG is markedly lost as compared to young controls (Fig. 2). Overall, aging phenomena were observed in postovulatory old oocytes, with a further increase with H2O2 treatment, while young oocytes resisted aging phenomena when treated with 20 μM H2O2.
Fig. 2.
The effect of H2O2 on oocyte aging. Composite of micrographs shows young oocytes retrieved at 13.5 h exposed to 20 (A–C) or100 μM H2O2 (D–F). Oocytes were stained for α tubulin (FITC, green) and CG (rhodamine, red). An increase in the ooplasmic microtubules is notable in the form of increased free microtubules and asters in the ooplasm (E and F). Pictograph A depicts a young untreated control showing the markedly less OMD and intact CG (D). The young oocytes treated with 20μM H2O2 showed no effect in terms of age related change in OMD (B) and CG (C) as compared to the 100 μM H2O2 exposed oocytes in which OMD is significantly increased (E) and CG is markedly lost (F) as compared to young controls.
Experiment Set 3: Effect of HOCl on Oocytes
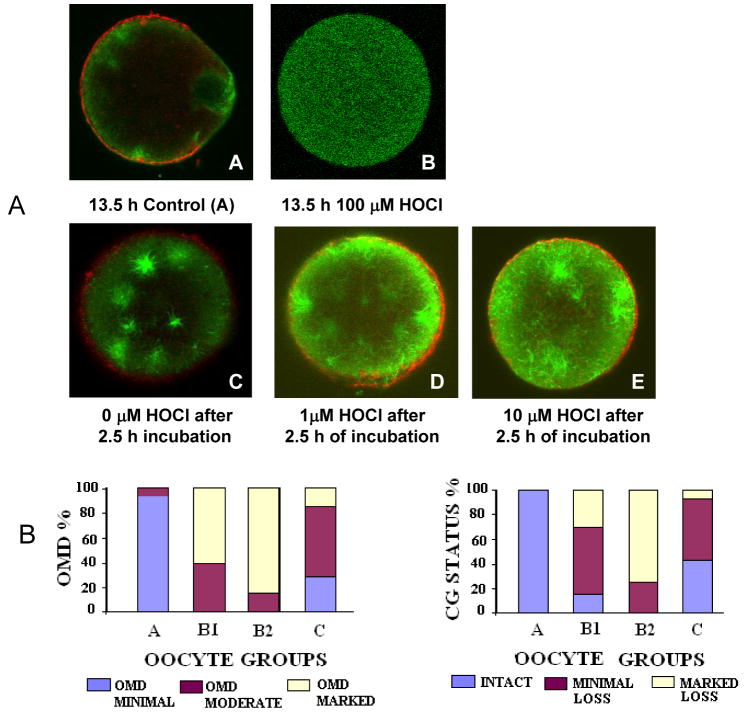
All oocytes treated with 100 μM HOCl lost viability as seen from dark ooplasm, and obvious signs of membrane damage or lysis (n=10) (Fig. 3A). Nonetheless, two oocytes were processed further for OMD and failed to show microtubules conforming a loss of viability. The oocytes treated with 1 and 10 μM HOCl on the other hand, showed significant enhancement in the aging phenomena of ZPDT, OMD and CG loss compared to control untreated oocytes cultured in medium for the same amount of time. The ZPDT showed a progressive increase with increasing concentrations of HOCl in both groups, B1 and B2 (P <0.05). Similarly, significantly fewer HOCl treated oocytes exhibited minimal OMD and intact CG (minimal OMD: 0% in oocytes treated with 1 and 10 μM HOCl versus 28.6% in the corresponding untreated control; intact CG: 15 and 0% in oocytes treated with 1 and 10 μM HOCl versus 42.9% in the corresponding untreated control oocytes, P <0.05 for all, Figs. 3A and 3B). On the other hand, the proportion of oocytes with increased OMD and CG loss were higher in these groups (increased OMD: 60 and 85% in oocytes treated with 1 and 10 μM HOCl versus 14.3% in the untreated control oocytes, P<0.001; major CG loss: 30 and 75% in oocytes treated with 1 and 10 μM HOCl versus 7.1% in controls, P <0.001, Figs. 3A and 3B). The hallmark of increased OMD was also seen in the form of increased size and numbers of microtubule arising from the microtubule organizing centers (MTOC, Fig. 3A). Overall, oocyte aging phenomena were enhanced with postovulatory age as well as both 1 and 10 μM concentrations of HOCl.
Fig. 3.
The effect of hypocholorous acid on oocyte aging. Panel A shows fluorescence photomicrographs of oocytes stained for α tubulin (FITC, green) and CG (rhodamine, red) after exposure to HOCL at 1 μM, 10 μM, 100 μM in PBS followed by taxol treatment. Micrograph A shows the minimal OMD and the intact CG in a young oocyte retrieved at 13.5 h, B shows a typically lysed oocyte fixed and stained immediately after exposure to HOCl (100μM). An increase in the ooplasmic microtubules is notable in the form of increased free microtubules and asters in micrographs D (1 μM) and E (10 μM) compared to the unexposed control oocytes (16.5 h post hCG, C) and the young controls (13.5 h post-hCG, A), also notable are the total absence of the CG’s in the O2•− radical exposed oocytes (C and D). Original magnification: 400–600x; average oocyte diameter ~75–80 μm. Panel B shows the effect of exposure to HOCl at 1 μM, 10 μM, and 100 μM in PBS the min on the OMD and the CG status in the oocytes. A significantly increased OMD and marked loss in CG was seen in the groups B1 and B2 as compared to the controls A and C (P < 0.05).
DISCUSSION
Reactive oxygen species (ROS) could play a prominent role in the development of disorders that significantly affect both male and female fertility [2,3,43]. Similarly, the ROS’s, themselves, may affect the integrity, viability and function of gametes and embryos, affecting the dynamic processes of gamete maturation, transportation through the reproductive tract and fertilization; as well as subsequent development and implantation of the preimplantation embryo. Reactive oxygen species may, therefore, be considered as mediators of the adverse influence exerted by disorders that affect reproduction.
In oocytes, as in other cells, ROS are important mediators of intracellular signaling responsible for numerous cellular functions under physiological conditions [44]. However, under pathological conditions, the ROS may contribute to oxidative stress, resulting in mutations, inactivation or loss of mitochondrial DNA, and synthesis and accumulation of abnormal or oxidized proteins. Similarly, oxidative stress could change the membrane lipid composition, decrease the concentrations of ascorbic acid, cause a drop in the GSH/GSSG ratio and increase cytosolic Ca2+ [45–48] Our current results clearly demonstrate that oocytes exposed to O2•−, H2O2 and HOCl enhance aging phenomena compared to their untreated sibling control oocytes. Thus, significant increases in ZPDT, OMD and CG loss occurred in oocytes exposed to these agents. These findings not only explain the ‘age-enhancing’ effects of ROS on the oocytes, but also support the theory of ROS in physiological regulation of the oocyte temporal window for optimal fertilization [22,49]. Moreover, this could also explain augmented oocyte aging and deteriorating oocyte quality known to occur with advancing age, diabetes mellitus, and a myriad of other clinical conditions, which significantly contribute to reproductive failure [24,43,50].
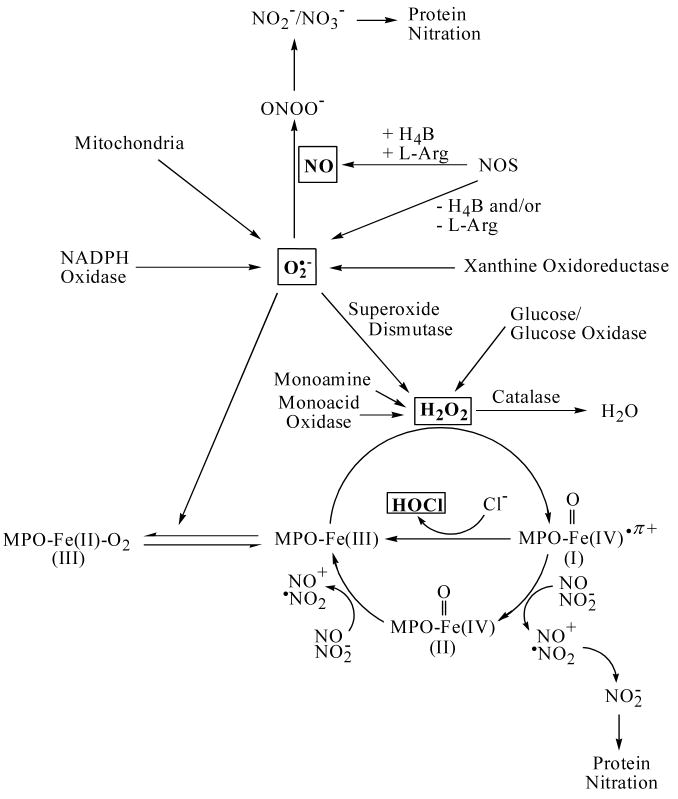
A scheme highlighting the pathways that modulate the bioavailability of O2•−, H2O2, and HOCl is shown in Fig. 4. Accordingly, mitochondria are the major intracellular sites of generation of O2•−, which is sequentially reduced to H2O2 and hydroxyl radical. However, a major source for the generation of microbicidal and/or pathological levels of O2•− is the enzyme NADPH oxidase, which is found in neutrophils, eosinophils, monocytes, and macrophages [1,51]. Alternatively, xanthine oxidoreductase (XOR), a major source of reactive oxygen species, converts hypoxanthine and xanthine to uric acid with simultaneous production of O2•− [6]. Imbalance between O2•− production and metabolism may mediate meiotic arrest and apoptotic cell death through activation of caspase-3 with DNA breaks and damage [52]. Similarly, compromised antioxidant machinery e.g., decreased GSH/GSSG ratio, could affect optimal chromatin decondensation at fertilization and consequently altered gene expression [53,54]. Similarly, DNA repair mechanisms could be altered as well, contributing further to DNA damage [53,54].
Fig. 4.
Model showing the factors that modulate the bioavailability of O2•−, H2O2 and HOCl.
Recently, we have demonstrated that supplementation with NO significantly prevented oocyte aging in both diabetic and non-diabetic animals [23,24]. Thus, the converse, namely decreased production of NO, may be an important contributor to enhanced aging under both physiological and pathological conditions. A decrease in the production of NO may occur due to substrate (L-arginine) or co-factor deficiency [55,56]. Under these conditions, the enzyme NOS may play a potential role in accelerating oocyte aging by serving as a O2•− generating system instead of producing NO, by undergoing steady-state catalysis of NADPH oxidation [55,56].
Enhancement in O2•− production may also significantly accelerate oocytes aging through its rapid reaction with NO yielding ONOO−. Peroxynitrite is a much more toxic reagent and attacks many cellular components, reacting with thiols and iron-sulfur centers, as well as initiating lipid peroxidation, which contributes up to 40% of the dry weight of the fully developed oocyte [57]. It also nitrates tyrosine by a reaction catalyzed by superoxide dismutase [32]. Nitrite, a major product of NO metabolism, can readily promote protein nitration via reactions with peroxidases [58,59]. Similar process may cause alterations in protein function in aged oocytes, thereby mediating the metabolism of several cytotoxic biological reactions.
Most of generated O2•− undergoes a nonenzymatic or superoxide dismutase (SOD)-catalyzed reaction generating H2O2 as an end product [1,60]. H2O2 is physiologically important among ROS due to the fact that its lifetime in the intracellular space is relatively long and that it is the precursor of the more toxic hydroxyl radical. H2O2 is freely diffusible through biological membranes, and its over production is extremely destructive to cells and tissues [1,60,61]. In addition to glucose/glucose oxidase, several oxidase enzymes, such as monoamine and amino acid oxidase, can also directly produce H2O2 [62] (Fig. 4).
Even though the reactivity of the H2O2 molecule makes it useful in many different biological applications, undesirable side reactions can occur. Interestingly, our current results demonstrate that physiological levels of H2O2 (20 μM), have modest effect on young oocytes in contrast to O2•− and HOCl. However, pathological concentration of H2O2 (100 μM) displays significant effect on accelerating oocytes aging. In contrast, relatively old oocytes exhibited exquisite sensitivity to even lower concentrations of H2O2 and exhibited the induction of the aging phenomena (Fig. 3). For example, H2O2 may react with O2•− generating toxic hydroxyl radicals.
Hydroxyl radicals may also be generated through Fenton-type chemistry, and Fe catalyzes its production from H2O2 [63]. Oxygen radicals and peroxides are highly destructive, damaging lipids, proteins and nucleic acids in the cell [64]. Hydroxyl radicals can also extract hydrogen atoms from DNA and RNA, causing mutations or cleavage of the phosphodiester backbone [65,66].
In the oocytes, H2O2 causes a significant change in the spaciotemporal characteristics of cytosolic Ca2+ release at fertilization [67]. Intracellular Ca2+ release occurring at fertilization is crucial to drive the post-fertilization activation of the oocyte and signaling the cell cycle to resume and complete meiosis and start embryonic development. Altered Ca2+ release patterns can affect development as seen from elegant experiments of Ozil et al. on parthenotes [68], as well as more recent studies on development [69,70]. Similarly, altered Ca2+ release patterns are also observed in fertilized aged oocytes [71]. Interestingly, exposure to H2O2 causes increased frequency and decreased amplitude of Ca2+ release similar to that observed in aged oocytes. Takahashi et al. [67] demonstrated that the aged and the H2O2 pretreated-fresh oocytes both showed significant alteration in Ca2+ oscillations and developmental deficiencies. Liu and Keefe [72] reported that exposure of mouse oocytes to higher H2O2 (200 μM) completely inhibited cleavage, and caused arrest of zygote at the 1-cell stage. Yang et al. [46] suggested a link between the concentration of endogenous H2O2 and the occurrence of apoptosis in human embryos. H2O2 may, thus, have a role in initiating or modulating oocyte aging. More importantly, increased sensitivity to H2O2 due to diminished cellular antioxidant defenses is likely to be a sentinel mechanism leading to oocyte aging.
Catalase is a major H2O2 scavenger and serves to protect cells from the toxic effects of H2O2 by catalyzing its decomposition into molecular oxygen and water. Ovoperoxidase, like catalase, degrades H2O2. This enzyme is one of several oocyte-specific proteins that are stored within sea urchin cortical granules, released during the cortical reaction, and incorporated into the newly formed fertilization envelope [73]. Ovoperoxidase plays a particularly important role in this process, crosslinking the envelope into a hardened matrix that is insensitive to biochemical and mechanical challenges, thereby providing a permanent block to polyspermy [74]. The sea urchin ovoperoxidase sequences conform to a profile shared by members of a heme-dependent animal peroxidase family, including the mammalian myelo-, lacto-, eosinophil, and thyroid peroxidases [74]. Mouse ZP may also be hardened by an ovoperoxidase (cross-links tyrosine residues) cytochemically identified in mouse CG and CG exudate [75].
Our results clearly demonstrate that HOCl produced by mammalian peroxidases is a much more powerful oxidant in accelerating oocyte aging than either O2•− or H2O2, and may easily be formed internally in oocytes or provided by the neighboring follicular cells through the reaction of MPO with H2O2 in the presence of chloride ions [34] (Fig. 4). Indeed, young oocytes exposed to lower concentrations (1–10 μM), accelerate aging phenomena, while higher concentration of HOCl (0.1–1mM) caused lysis of the cell membrane and death of the oocyte. In contrast, the older oocytes underwent lysis even on exposure to 1 μM HOCl. HOCl is known to contribute to tissue damage and may mediate oxidative modification/fragmentation of oocytes through its ability to undergo numerous reactions with biomolecules, including aromatic chlorination, double bond addition, chloramine formation, aldehyde generation, and oxidation of thiols [35]. Based on our current work, it is, therefore, perfectly conceivable to assume that HOCl and other ROS may take part in accelerating oocyte aging, fragmentation and eventual removal of unfertilized aged oocytes from the oviduct and uterus in animals.
Recently, we have demonstrated that mammalian peroxidases use NO as a physiological substrate, even in the presence of O2•−, thereby, limiting NO bioavailability and function [27–29]. In addition, the heme prosthetic groups of peroxidases accommodate a large variety of molecules as ligands of the Fe cation causes enzyme inhibition [27–29,34,76]. Thus, mammalian peroxidases may accelerate oocyte aging through more than one pathway: by generating HOX, consuming NO, or by mediating protein nitration. Under certain circumstances, therefore, mammalian peroxidase inhibition may display a beneficiary role in delaying oocyte aging.
Exogenously-mediated oxidative stress can be a consequence of perturbation in the oocyte microenvironment, such as decreased number or density of ovarian mural and cumulus granulosa cells [61]. This alteration in the oocyte surrounding could cause a decrease in oocyte/embryo viability resulting in the reduction in implantation and increase in embryo loss [46,77]. Reduced levels of antioxidants in follicular and oviductal fluid may also lead to compromised oocyte quality. Hence, increased oxidative stress with advancing age may be explained by changes in the oocyte microenvironment, making them more susceptible to ROS [72,78]. Thus, our current finding may also have implications for chronological aging, which is not only associated with increased production of ROS, but also with decreased number and viability of cumulus cells, as well as decreased levels of antioxidants such as GSH [79]. Understanding the mechanisms underlying the process of deteriorating oocyte quality is crucial to design strategies that could improve fertility and prevent reproductive losses in subjects with chronological aging as well as other similar conditions. Similar implications may also be relevant to regenerative medicine as several diseases are linked to the damaging effects of O2•−, H2O2 and hypohalous acids.
In conclusion, enhancement in ROS and subsequent oxidative stress are potential candidates responsible for the initiation of oocyte aging. Enhancement in ROS in oocytes is associated with a modification in proteins and other moieties crucial to maintain viability and integrity of various organelles and cytoskeleton, and thus may affect their activity, organization and distribution. It is also associated with significant decrease in NO bioavailability which, subsequently, disturbs the intracellular signal transduction pathways, especially the Ca2+-mediated pathway. On the other hand, supplementation with NO may offer options to correct this abnormality and improve the embryo outcome. Finally, O2•−, H2O2 and HOCl significantly enhanced oocyte aging with possible narrowing or abolition of the temporal window for optimal fertilization.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1 HL066367 (to H. M. AS), ASRM-Ortho Women’s Health Grant to (P. T. G), and the Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, MI, USA.
ABBREVIATIONS
- NO
nitric oxide (nitrogen monoxide)
- SNAP
S-Nitroso acetyl penicillamine
- OMD
ooplasmic microtubule dynamics
- CG
cortical granule
- DAPI
4′6 diamidino-2-phenylindole
- ZP
zona pellucida
- MPF
M-phase promoting factor
- ART
assisted reproductive technology
- IVF
in vitro fertilization
- ICSI
intracytoplasmic sperm injection
- MAPK
mitogen activated protein kinase
- NOS
nitric oxide synthase
- H4B
tetrahydrobiopterin
- L-NAME
Nω-nitro-L-arginine methyl ester
- 8-Br-cGMP
8-bromoguanosine 3′:5′-cyclic monophosphate
- ZP
zona pellucida
- ZPDT
zona pellucida dissolution time
- MTOC
microtubule organizing center
- PKI
protein kinase inhibitor
- CaM
calmodulin
Footnotes
The work has, so far, received eight national and international awards, including the prestigious Young Investigator Award at the Gordon Research Conference in Nitric Oxide, and a 1st Place Award at the Annual Junior Fellow Meeting of the American College of Obstetrics and Gynecology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC. In: Free radicals in biology and medicine. Halliwell B, Gutteridge JMC, editors. Oxford: Clarendon Press; 1989. pp. 1–20. [Google Scholar]
- 2.Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9:338–347. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online. 2005;11:641–650. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 4.Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Ferti l Steril. 2003;79:1288–1293. doi: 10.1016/s0015-0282(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77:861–870. doi: 10.1016/s0015-0282(02)02959-x. [DOI] [PubMed] [Google Scholar]
- 6.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod. 1993;49:228–232. doi: 10.1095/biolreprod49.2.228. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner CS, Salmen JJ, Brandt CJ, Stover SK. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol Reprod. 1998;59:431–436. doi: 10.1095/biolreprod59.2.431. [DOI] [PubMed] [Google Scholar]
- 9.Guyader-Joly C, Guerin P, Renard JP, Guillaud J, Ponchon S, Menezo Y. Precursors of taurine in female genital tract: effects on developmental capacity of bovine embryo produced in vitro. Amino Acids. 1998;15:27–42. doi: 10.1007/BF01345278. [DOI] [PubMed] [Google Scholar]
- 10.Pascoe GA, Fariss MW, Olafsdottir K, Reed DJ. A role of vitamin E in protection against cell injury. Maintenance of intracellular glutathione precursors and biosynthesis. Eur J Biochem. 1987;166:241–247. doi: 10.1111/j.1432-1033.1987.tb13508.x. [DOI] [PubMed] [Google Scholar]
- 11.Guerin P, Menezo Y. Hypotaurine and taurine in gamete and embryo environments: de novo synthesis via the cysteine sulfinic acid pathway in oviduct cells. Zygote. 1995;3:333–343. doi: 10.1017/s0967199400002768. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Foote RH, Simkin M. Development of rabbit zygotes cultured in protein-free medium with catalase, taurine, or superoxide dismutase. Biol Reprod. 1993;49:33–37. doi: 10.1095/biolreprod49.1.33. [DOI] [PubMed] [Google Scholar]
- 13.El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89:1–6. doi: 10.1016/s0301-2115(99)00169-4. [DOI] [PubMed] [Google Scholar]
- 14.Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays. 1993;15:531–538. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- 15.Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod. 1997;57:1413–1419. doi: 10.1095/biolreprod57.6.1413. [DOI] [PubMed] [Google Scholar]
- 16.Schweigert FJ, Zucker H. Concentrations of vitamin A, beta-carotene and vitamin E in individual bovine follicles of different quality. J Reprod Fertil. 1988;82:575–579. doi: 10.1530/jrf.0.0820575. [DOI] [PubMed] [Google Scholar]
- 17.Fatehi AN. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote. 2005;13:177–185. doi: 10.1017/s0967199405003126. [DOI] [PubMed] [Google Scholar]
- 18.Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakada T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71:1150–1157. doi: 10.1095/biolreprod.104.029264. [DOI] [PubMed] [Google Scholar]
- 19.Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod. 1998;58:747–753. doi: 10.1095/biolreprod58.3.747. [DOI] [PubMed] [Google Scholar]
- 20.Paszkowski T, Clarke RN. The Graafian follicle is a site of L-ascorbate accumulation. J Assist Reprod Genet. 1999;16:41–45. doi: 10.1023/A:1022597629622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000;63:715–722. doi: 10.1095/biolreprod63.3.715. [DOI] [PubMed] [Google Scholar]
- 22.Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124:745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- 23.Goud AP, Goud PT, Diamond MP, Abu-Soud HM. Nitric oxide delays oocyte aging. Biochemistry. 2005;44:11361–11368. doi: 10.1021/bi050711f. [DOI] [PubMed] [Google Scholar]
- 24.Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Activation of the cGMP signaling pathway is essential in delaying oocyte aging in diabetes mellitus. Biochemistry. 2006;45:11366–11378. doi: 10.1021/bi060910e. [DOI] [PubMed] [Google Scholar]
- 25.Cooper CE. Nitric oxide and iron proteins. Reactions of nitric oxide with metalloproteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Soud HM, Wu C, Ghosh DK, Stuehr DJ. Stopped-flow analysis of CO and NO binding to inducible nitric oxide synthase. Biochemistry. 1998;37:3777–3786. doi: 10.1021/bi972398q. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000a;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000b;275:5425–5430. doi: 10.1074/jbc.275.8.5425. [DOI] [PubMed] [Google Scholar]
- 29.Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci U S A. 2003;100:14766–14771. doi: 10.1073/pnas.2435008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 31.Akaike T, Suga M, Maeda H. Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. Proc Soc Exp Biol Med. 1998;217:64–73. doi: 10.3181/00379727-217-44206. [DOI] [PubMed] [Google Scholar]
- 32.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Soud HM, Khassawneh MY, Sohn JT, Murray P, Haxhiu MA, Hazen SL. Peroxidases inhibit nitric oxide (NO) dependent bronchodilation: development of a model describing NO-peroxidase interactions. Biochemistry. 2001;40:11866–11875. doi: 10.1021/bi011206v. [DOI] [PubMed] [Google Scholar]
- 34.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 35.Socker R, Kenney JF., Jr Role of oxidative modification in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Soud HM, Hazen SL. Interrogation of heme pocket environment of mammalian peroxidases with diatomic ligands. Biochemistry. 2001;40:10747–10755. doi: 10.1021/bi010478v. [DOI] [PubMed] [Google Scholar]
- 37.Goud P, Goud A, Van Oostveldt P, Van der Elst J, Dhont M. Fertilization abnormalities and pronucleus size asynchrony after intracytoplasmic sperm injection are related to oocytepostmaturity. Fertil Steril. 1999;72:245–252. doi: 10.1016/s0015-0282(99)00231-9. [DOI] [PubMed] [Google Scholar]
- 38.Goud AP, Goud PT, Van Oostveldt P, Diamond MP, Dhont M. Dynamic changes in microtubular cytoskeleton of human postmature oocytes revert after ooplasm transfer. Fertil Steril. 2004;81:323–331. doi: 10.1016/j.fertnstert.2003.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Goud AP, Goud PT, Van Oostveldt P, Diamond MP, Hughes MR. Microtubule turnover in ooplasm biopsy reflects aging related phenomena in the parent oocyte. Reprod BioMed. 2005;11:43–52. doi: 10.1016/s1472-6483(10)61297-7. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Soud HM, Ichimori K, Nakazawa H, Stuehr DJ. Regulation of inducible nitric oxide synthase by self-generated NO. Biochemistry. 2001;40:6876–6881. doi: 10.1021/bi010066m. [DOI] [PubMed] [Google Scholar]
- 41.Arnhold J, Panasenko OM, Schiller J, Vladimirov YA, Arnold K. The action of hypochlorous acid on phosphatidylcholine liposomes in dependence on the content of double bonds. Stoichiometry and NMR analysis. Chem Phys Lipids. 1995;78:55–64. doi: 10.1016/0009-3084(95)02484-z. [DOI] [PubMed] [Google Scholar]
- 42.Iborra A, Palacio JR, Martinez P. Oxidative stress and autoimmune response in the infertile woman. Chem Immunol Allergy. 2005;88:150–162. doi: 10.1159/000087832. [DOI] [PubMed] [Google Scholar]
- 43.Saito H, Seino T, Kaneko T, Nakahara K, Toya M, Kurachi H. Endometriosis and oocyte quality. Gynecol Obstet Invest. 2002;53:46–51. doi: 10.1159/000049424. [DOI] [PubMed] [Google Scholar]
- 44.Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991;113:551–60. doi: 10.1242/dev.113.2.551. [DOI] [PubMed] [Google Scholar]
- 45.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26:463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 46.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- 47.Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69:402–410. doi: 10.1002/mrd.20180. [DOI] [PubMed] [Google Scholar]
- 48.Gardiner CS, Salmen JJ, Brandt CJ, Stover SK. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol Reprod. 1998;59:431–436. doi: 10.1095/biolreprod59.2.431. [DOI] [PubMed] [Google Scholar]
- 49.Ducibella T. The cortical reaction and development ofactivation competence in mammalian oocytes. Hum Reprod Update. 1996;2:29–42. doi: 10.1093/humupd/2.1.29. [DOI] [PubMed] [Google Scholar]
- 50.Mills JL, Knopp RH, Simpson JL, Jovanovic-Peterson L, Metzger BE, Holmes LB, Aarons JH, Brown Z, Reed GF, Bieber FR, et al. Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organogenesis. NEJM. 1988;318:671–676. doi: 10.1056/NEJM198803173181104. [DOI] [PubMed] [Google Scholar]
- 51.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 53.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994;16:259–67. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 55.Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90:10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen GM, Tsai P, Weaver J, Porasuphatana S, Roman LJ, Starkov AA, Fiskum G, Pou S. The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem. 2002;277:40275–40280. doi: 10.1074/jbc.M200853200. [DOI] [PubMed] [Google Scholar]
- 57.Kawooya JK, Law JH. Role of lipophorin in lipid transport to the insect egg. J Biol Chem. 1988;263:8748–8753. [PubMed] [Google Scholar]
- 58.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 59.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 60.McCord JM. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 61.Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, Hibberts NA. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–96. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- 62.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 63.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc. 1984;65:899–910. [Google Scholar]
- 64.Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 65.Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res. 1999;443:37–52. doi: 10.1016/s1383-5742(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 66.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci U S A. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- 68.Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128:917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- 69.Rogers NT, Halet G, Piao Y, Carroll J, Ko MS, Swann K. The absence of a Ca(2+) signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction. 2006;132:45–57. doi: 10.1530/rep.1.01059. [DOI] [PubMed] [Google Scholar]
- 70.Campbell KD, Reed WA, White KL. Ability of integrins to mediate fertilization, intracellular calcium release, and parthenogenetic development in bovine oocytes. Biol Reprod. 2000;62:1702–1709. doi: 10.1095/biolreprod62.6.1702. [DOI] [PubMed] [Google Scholar]
- 71.Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72:1256–1261. doi: 10.1095/biolreprod.104.034926. [DOI] [PubMed] [Google Scholar]
- 72.Liu L, Keefe DL. Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol Reprod. 2000;62:1828–1834. doi: 10.1095/biolreprod62.6.1828. [DOI] [PubMed] [Google Scholar]
- 73.Deits T, Farrance M, Kay ES, Medill L, Turner EE, Weidman PJ, Shapiro BM. Purification and properties of ovoperoxidase, the enzyme responsible for hardening the fertilization membrane of the sea urchin egg. J Biol Chem. 1984;259:13525–11533. [PubMed] [Google Scholar]
- 74.LaFleur GJ, Jr, Horiuchi Y, Wessel GM. Sea urchin ovoperoxidase: oocyte-specific member of heme-dependent peroxidase superfamily that functions in the block to polyspermy. Mech Dev. 1998;70:77–89. doi: 10.1016/s0925-4773(97)00178-0. [DOI] [PubMed] [Google Scholar]
- 75.Hoodboy T, Talbot P. Mamalian cortical granules: contents, fate and function. Mol Reprod Dev. 1994;39:439–448. doi: 10.1002/mrd.1080390413. [DOI] [PubMed] [Google Scholar]
- 76.Abu-Soud HM, Raushel FM, Hazen SL. A novel multistep mechanism for oxygen binding to ferrous hemoproteins: rapid kinetic analysis of ferrous-dioxy myeloperoxidase (compound III) formation. Biochemistry. 2004;43:11589–11595. doi: 10.1021/bi049541h. [DOI] [PubMed] [Google Scholar]
- 77.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 78.Eichenlaub-Ritter U, Boll I. Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet Cell Genet. 1989;52:170–176. doi: 10.1159/000132871. [DOI] [PubMed] [Google Scholar]
- 79.De Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod. 1997;57:1420–1425. doi: 10.1095/biolreprod57.6.1420. [DOI] [PubMed] [Google Scholar]