Abstract
Objectives. We evaluated changes in colorectal cancer (CRC) incidence and mortality by anatomic site to assess the possible impact of CRC screening.
Methods. Using data from 9 Surveillance, Epidemiology, and End Results cancer registries, we estimated trends in 1975–2007 CRC incidence and 1985–2007 incidence-based mortality. We evaluated trends separately for proximal and distal CRC, overall and by stage, tumor site, and race.
Results. Between 1975 and 2007, 323 237 adults in the study area were diagnosed with CRC. For most tumor and population subgroups, incidence rates increased between 1975 and 1985 and subsequently declined markedly. Declines were most rapid between 1999 and 2007 and were greater for distal than proximal CRC. Declines in incidence were greater for White than Black adults and greatest for regional-stage disease. There was little difference in trends across subsites within the proximal and distal colorectum. Declines in incidence-based mortality mirrored those for incidence.
Conclusions. Recent declines in CRC incidence and mortality are greater for distal than proximal CRC. Differing trends across populations may reflect variations in screening prevalence; distinct trends by tumor characteristics likely reflect differences in screening efficacy.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality in the United States.1 Recent reports indicate a decline in the burden associated with CRC in the United States on the basis of changes in incidence2–6 and mortality2–4 rates over time. However, such patterns of decline have been shown to differ across population groups and according to tumor characteristics.6–8 In particular, unequal declines in CRC mortality rates by race have resulted in increased racial disparities in CRC mortality,7 and observed declines in incidence rates have been more pronounced with respect to distant stage than local stage disease.6,8 The utilization of screening for CRC is likely a key contributing factor for these observed trends as well as for differences in trends across populations.4,9–17 However, the benefits of certain CRC screening modalities have been reported to differ for tumors arising in different sites in the colon and rectum.9–15,18,19 In particular, the sensitivity of fecal occult blood testing (FOBT) is higher for distal and rectal CRC than for CRC arising in the proximal colon,19 sigmoidoscopy does not allow visualization of the proximal colon, and there is some suggestion that colonoscopy of the entire large bowel is more strongly associated with a reduction in risk of incidence and mortality for distal than for proximal CRC.11,12,14 Thus, to the extent that screening is responsible for observed declines in CRC incidence and mortality, temporal trends in CRC may be expected to differ across populations and tumor subgroups.
We have characterized temporal patterns of CRC incidence and incidence-based mortality (IBM) across anatomic locations and tumor stage and according to population characteristics, with the underlying goal of characterizing the impact that screening may have had on these rates.
METHODS
We conducted a descriptive epidemiological study using data from 9 population-based cancer registries contributing to the Surveillance, Epidemiology, and End Results (SEER) cancer registry network from 1975 to 2007 (Atlanta, Detroit, San Francisco–Oakland, the Seattle–Puget Sound region, Connecticut, Hawaii, Iowa, New Mexico, and Utah). We calculated annual incidence rates for invasive CRC, overall and by tumor site, with age adjustment to the 2000 United States Standard Population.20 Tumors located in the cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure were grouped together as proximal CRC (International Classification of Diseases for Oncology, third revision codes C180, C182, C183, C184, C185).21 Tumors arising in the descending colon (C186), sigmoid colon (C187), rectosigmoid junction (C199), and rectum (C209) were grouped together as distal CRC. We also estimated incidence rates for invasive proximal and distal CRC within strata defined by stage at diagnosis (SEER historic stage: localized, regional, or distant), race (White or Black), gender, and age (50–59, 60–69, 70–84 years). In light of screening recommendations, we restricted our analysis to the population aged 50–84 years.
We estimated annual percentage changes (APC) in incidence rates between 1975 and 2007 using Joinpoint Regression Program software version 3.4.3 (National Cancer Institute, Bethesda, MD)22; we also used this program to estimate the average annual percentage change (AAPC) over the last 10 years of the study period. This software estimates piecewise linear regression models of cancer incidence trends. The points at which contiguous linear sections meet are known as “joinpoints.” We determined the number and placement of joinpoints across observed data via a Monte Carlo permutation method, as described elsewhere.23
In addition to analyses of incidence rates over time, we calculated IBM rates24 using SEER*Stat version 6.6.2 (National Cancer Institute, Bethesda, MD) and assessed trends in these rates over time using Joinpoint Regression Program software. IBM is equivalent to the cross-sectional mortality rate in the population as a whole for a given tumor type (e.g., distal CRC) diagnosed within a specified calendar period. We restricted our calculation of IBM to invasive CRC diagnosed within 10 years of the year of death; for this reason, our calculation of IBM rates is limited to years 1985 through 2007. As an example, in calculating the IBM for localized distal CRC in 1997, the numerator is equal to the number of deaths that occurred in 1997 among adults diagnosed with localized distal CRC between 1988 and 1997; the denominator is derived from the midyear population count in the catchment area of the 9 SEER registries in 1997. As with incidence analyses, we identified joinpoints and APC patterns across the study period overall and by tumor site within strata defined by stage at diagnosis and race; we also calculated AAPC over the most recent 10 years within these strata as well as strata defined by gender and age. In all analyses, we excluded cases of in situ disease from rate numerators.
RESULTS
From 1975 to 2007, 323 237 individuals aged 50 to 84 years were diagnosed with invasive CRC within the SEER 9 ascertainment area (Table 1); approximately 59.2% (n = 191 477) of those cancers were distal. The proportion of proximal CRC increased over the study period from 36.7% in 1975–1979 to 44.8% in 2005–2007 and increased across categories of age at diagnosis (from 31.6% among those aged 50–59 years at diagnosis to 50.6% among those aged 80–84 years). The proportion of CRC that was proximal was greater in women than in men (45.2% vs 36.6%), was greater for regional and distant stage disease than for localized (45.2% vs 35.7%), and differed somewhat by race (40.9% vs 46.9% of CRC among White and Black individuals, respectively).
TABLE 1—
Frequency and Distribution of Incident Colorectal Cancer Among Patients Aged 50–84 Years: Surveillance, Epidemiology, and End Results 9, 1975–2007
| Total (n = 323 237), No. | Distal (n = 191 477), % | Proximal (n = 131 760), % | |
| Year of diagnosis | |||
| 1975–1979 | 41 928 | 63.3 | 36.7 |
| 1980–1984 | 47 784 | 62.7 | 37.3 |
| 1985–1989 | 51 439 | 61.6 | 38.4 |
| 1990–1994 | 50 524 | 58.7 | 41.3 |
| 1995–1999 | 51 315 | 56.8 | 43.2 |
| 2000–2004 | 51 274 | 55.6 | 44.4 |
| 2005–2007 | 28 973 | 55.2 | 44.8 |
| Age at diagnosis, y | |||
| 50–59 | 54 482 | 68.4 | 31.6 |
| 60–69 | 97 268 | 63.6 | 36.4 |
| 70–79 | 122 173 | 55.6 | 44.4 |
| 80–84 | 49 314 | 49.4 | 50.6 |
| Gender | |||
| Men | 168 342 | 63.4 | 36.6 |
| Women | 154 895 | 54.8 | 45.2 |
| Race | |||
| White | 275 381 | 59.1 | 40.9 |
| Black | 26 702 | 53.1 | 46.9 |
| Other | 20 419 | 68.7 | 31.3 |
| Unknown | 735 | 73.3 | 26.7 |
| SEER registry | |||
| Atlanta (metropolitan) | 20 191 | 57.0 | 43.0 |
| Connecticut | 56 236 | 60.7 | 39.3 |
| Detroit (metropolitan) | 58 036 | 58.8 | 41.2 |
| Hawaii | 14 656 | 66.2 | 33.8 |
| Iowa | 51 879 | 55.9 | 44.1 |
| New Mexico | 15 133 | 61.9 | 38.1 |
| San Francisco-Oakland SMSA | 50 329 | 59.7 | 40.3 |
| Seattle (Puget Sound) | 42 798 | 58.5 | 41.5 |
| Utah | 13 979 | 61.2 | 38.8 |
| Stage at diagnosis | |||
| Localized | 129 393 | 64.3 | 35.7 |
| Regional | 120 633 | 54.7 | 45.3 |
| Distant | 59 048 | 54.9 | 45.1 |
| Unknown | 14 163 | 69.5 | 30.5 |
| Subsite within the colorectum | |||
| Cecum | 53 685 | … | 16.6 |
| Ascending colon | 35 269 | … | 10.9 |
| Hepatic flexure | 11 599 | … | 3.6 |
| Transverse colon | 22 070 | … | 6.7 |
| Splenic flexure | 9137 | … | 2.8 |
| Descending colon | 16 609 | 5.1 | … |
| Sigmoid colon | 79 172 | 24.5 | … |
| Rectosigmoid junction | 31 410 | 9.7 | … |
| Rectum | 64 286 | 19.9 | … |
Note. SEER = surveillance, epidemiology, and end results; SMSA = standard metropolitan statistical area.
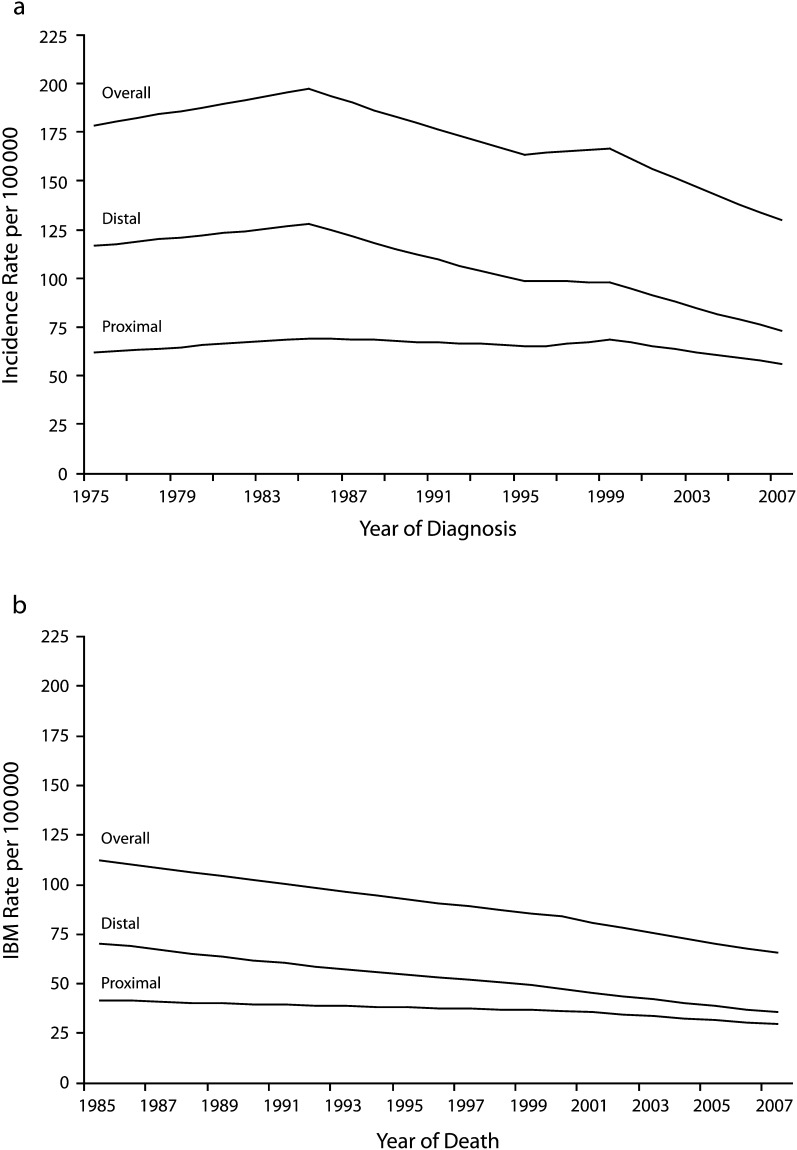
Annual CRC incidence rates, both overall and by tumor site, are illustrated in Figure 1 with fitted joinpoints. Overall, CRC incidence declined over this period, although this decline was largely restricted to distal CRC. From 1975 to 1985, overall CRC incidence rates increased by 0.7% per year, with similar increases noted for proximal and distal disease. Rates then fell sharply by 1.9% per year overall; however, when stratified by tumor site, it was evident that this decline was far more pronounced for distal than for proximal disease (−2.9% vs −0.8% per year through 1995 and 1996, respectively). After some stabilization in the later 1990s, incidence rates resumed a steady decline in 2000–2007, with the differences between distal and proximal declines somewhat less pronounced. Estimated 10-year AAPCs similarly indicated a significant decline in incidence rates in the later portion of the study period (Table 2), particularly for distal CRC. Incidence rates for invasive CRC at all subsites within the distal side of the colon and rectum exhibited significant decline (Table 2); declines in incidence rates for proximal CRC were more modest for cancers in the ascending colon (AAPC = −0.6%) and transverse colon (AAPC = −1.1%).
FIGURE 1—
Age-adjusted (a) incidence and (b) incidence-based mortality (IBM) rates of colorectal cancer among patients aged 50–84 years: Surveillance, Epidemiology, and End Results 9, 1975–2007.
TABLE 2—
Average Annual Percentage Change in Colorectal Cancer Incidence and Incidence-Based Mortality Rates Among Patients Aged 50–84 Years, by Population and Tumor Characteristics: Surveillance, Epidemiology, and End Results 9, 1998–2007
| Incidence Rates |
Incidence-Based Mortality Rates |
|||||
| Total AAPC (95% CI) | Distal AAPC (95% CI) | Proximal AAPC (95% CI) | Total AAPC (95% CI) | Distal AAPC (95% CI) | Proximal AAPC (95% CI) | |
| Overall | −2.7 (−3.1, −2.3) | −3.2 (−3.7, −2.7) | −2.0 (−2.5, −1.4) | −3.1 (−3.5, −2.7) | −3.7 (−4.2, −3.3) | −2.4 (−3.1, −1.6) |
| Race | ||||||
| White | −2.9 (−3.3, −2.5) | −3.5 (−4.0, −2.9) | −2.4 (−2.8, −2.0) | −2.9 (−3.3, −2.5) | −3.9 (−4.4, −3.4) | −2.5 (−3.2, −1.9) |
| Black | −1.9 (−3.2, −0.5) | −1.4 (−1.8, −1.0) | 0.1 (−0.3, 0.4) | −1.9 (−3.2, −0.5) | −2.9 (−4.6, −1.3) | −0.5 (−0.9, −0.1) |
| Stage | ||||||
| Localized | −1.9 (−2.7, −1.1) | −2.9 (−3.9, −1.9) | −0.7 (−1.4, 0.1) | −2.4 (−3.4, −1.4) | −3.5 (−4.5, −2.5) | 0.0 (−0.3, 0.3) |
| Regional | −3.6 (−4.3, −2.9) | −4.0 (−4.8, −3.1) | −3.5 (−4.2, −2.7) | −3.9 (−4.5, −3.3) | −3.5 (−3.6, −3.3) | −3.2 (−4.5, −2.0) |
| Distant | −2.2 (−2.4, −1.9) | −2.2 (−2.3, −2.0) | −2.1 (−2.8, −1.5) | −2.2 (−2.4, −2.0) | −2.7 (−3.0, −2.4) | −2.6 (−3.3, −1.9) |
| Gender | ||||||
| Men | −3.1 (−3.5, −2.6) | −3.4 (−4.0, −2.8) | −2.4 (−3.2, −1.6) | −3.6 (−4.1, −3.1) | −4.3 (−4.9, −3.6) | −2.6 (−3.3, −1.9) |
| Women | −2.6 (−3.0, −2.2) | −3.1 (−3.6, −2.6) | −1.8 (−2.7, −0.8) | −2.9 (−3.7, −2.1) | −2.9 (−3.1, −2.8) | −2.4 (−3.3, −1.5) |
| Age, y | ||||||
| 50–59 | −0.3 (−0.8, 0.1) | −0.2 (−0.7, 0.4) | −0.7 (−1.1, −0.4) | −2.5 (−2.8, −2.2) | −2.6 (−3.0, −2.2) | −2.3 (−2.6, −1.9) |
| 60–69 | −3.3 (−3.8, −2.8) | −4.0 (−4.7, −3.2) | −2.6 (−3.3, −1.9) | −4.2 (−4.9, −3.5) | −4.6 (−5.4, −3.8) | −3.5 (−5.4, −1.7) |
| 70–84 | −3.2 (–3.5, −2.8) | −3.7 (−4.4, −3.0) | −2.1 (–2.9, −1.2) | −3.0 (−3.5, −2.6) | −3.8 (−4.4, −3.3) | −2.1 (−2.9, −1.3) |
| Subsite | ||||||
| Cecum | … | −2.5 (−3.4, −1.7) | … | −2.7 (−3.5, −2.0) | ||
| Ascending colon | … | −0.6 (−1.7, 0.5) | … | −0.1 (−0.4, 0.3) | ||
| Hepatic flexure | … | −3.3 (−4.9, −1.6) | … | −4.0 (−5.8, −2.3) | ||
| Transverse colon | … | −1.1 (−1.3, −0.9) | … | −2.0 (−2.3, −1.8) | ||
| Splenic flexure | … | −3.2 (−4.0, −2.5) | … | −3.1 (−3.6, −2.5) | ||
| Descending colon | −2.8 (−3.0, −2.5) | … | −3.3 (−3.7, −2.9) | … | ||
| Sigmoid colon | −3.7 (−4.7, −2.7) | … | −3.2 (−3.5, −3.0) | … | ||
| Rectosigmoid junction | −3.6 (−4.0, −3.2) | … | −5.1 (−5.8, −4.4) | … | ||
| Rectum | −2.4 (−3.0, −1.8) | … | −2.2 (−2.4, −2.0) | … | ||
Note. AAPC = average annual percentage change; CI = confidence interval.
Recent declines in invasive CRC incidence, both overall and by tumor site, were closely matched by declines in IBM (Figure 1; Table 2). Again, relative (and absolute) declines were most pronounced with respect to distal CRC (AAPCDistal = −3.7%; AAPCProximal = −2.4%). In contrast to temporal incidence patterns, IBM rates declined throughout the full study period, with more rapid declines beginning in 1999–2000.
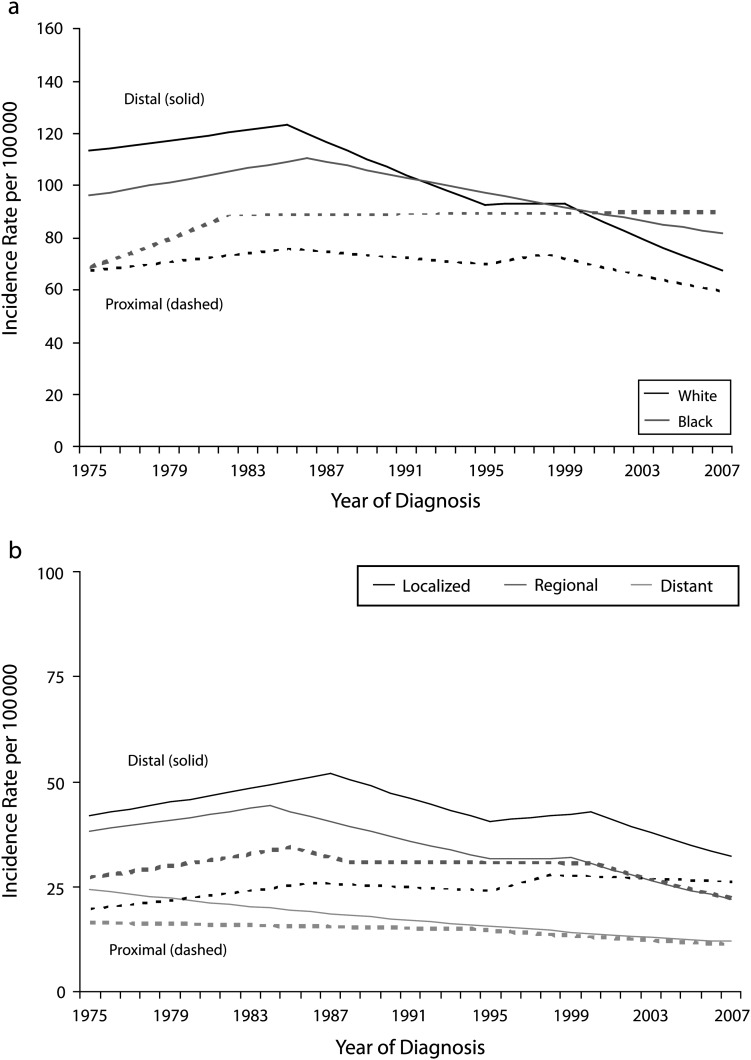
When stratified by race, several differences in incidence and IBM patterns for invasive distal and proximal CRC became evident (Figure 2). Among Whites and Blacks, incidence rates of distal CRC increased over the early part of the observation period and subsequently declined. However, the extent of decline in distal CRC incidence differed by race, such that incidence rates of distal CRC among Whites were lower than among Blacks in 2007 (67.4 and 78.4 per 100 000, respectively) even though they had been higher in Whites than in Blacks in 1975 (112.1 and 93.4 per 100 000, respectively). IBM rates for distal CRC in Blacks remained higher than did rates in Whites throughout the observation period and declined to a lesser extent (Table 2). With respect to proximal disease, incidence and IBM rates in Whites declined modestly but significantly over the study period. In contrast, incidence rates of proximal CRC in Blacks increased from 74.5 per 100 000 in 1975 to 78.6 per 100 000 in 2007, with no evidence of decline in recent years; the 10-year AAPC for IBM within this group was similarly flat.
FIGURE 2—
Age-adjusted incidence rates of colorectal cancer by (a) tumor location and race and (b) stage at diagnosis among patients aged 50–84 years: Surveillance, Epidemiology, and End Results 9, 1975–2007.
Differences in temporal patterns of site-specific invasive CRC incidence and IBM rates were also evident by stage at diagnosis (Figure 2). Incidence rates for distal localized and, in particular, regional-stage CRC declined significantly across the study period, with only a brief period of stabilization in the late 1990s. These decreases in incidence were matched by a steady decline in IBM rates. Declines in incidence and IBM rates for proximal regional-stage CRC were more gradual overall but were considerably accelerated between 2000 and 2007. Rates for proximal localized disease, however, increased slightly over the span of the study period: IBM rates increased consistently at an APC of 0.1% (95% confidence interval [CI] = −0.3%, 0.4%), and incidence rates climbed from 18.3 to 23.4 per 100 000 between 1975 and 2007, despite a recent decline (AAPC = −0.4%). Incidence rates for distant stage CRC consistently declined over the study period, regardless of tumor site. The same was true with respect to IBM rates for distant stage disease.
In evaluating 10-year AAPC in incidence and IBM rates by gender, age, and subsite within the colorectum, several other differences emerged. Declines in incidence and IBM rates between 1998 and 2007 were slightly more pronounced in men than in women, regardless of tumor site. Across all ages, declines in IBM rates were greater for distal CRC than for proximal CRC. With respect to incidence rates, declines were similar in those aged 60 to 69 years and those aged 70 to 84 years; however, declines in CRC incidence rates among adults aged 50 to 59 years at diagnosis were much more modest. Across subsites within the proximal colon, declines in incidence and IBM rates were greatest for cancers arising in the hepatic flexure and weakest for cancers arising in the ascending colon. The most modest declines for distal CRC were observed for cancers arising in the rectum.
DISCUSSION
In this descriptive analysis of temporal patterns in invasive CRC incidence and IBM rates, it was evident that the burden of CRC in the United States has declined considerably since 1975, with more rapid declines between 2000 and 2007. However, these results also indicate that observed declines in incidence and IBM rates for CRC vary by tumor site, stage, race, age, and gender. In particular, decreases in incidence and IBM rates since 2000 have been greater for distal than for proximal disease and greater for White individuals than for Black individuals, and they have been most pronounced with respect to regional-stage disease. When considering tumor subsites within the proximal and distal colorectum, observed reductions were most pronounced with respect to cancers arising in the sigmoid colon (incidence) and rectosigmoid junction (IBM); declines in incidence and IBM rates were lowest with respect to cancers arising in the ascending colon and transverse colon. Differences in observed declines between population groups most likely reflect differences in the uptake of available screening modalities, whereas differences by tumor characteristics likely reflect differences in the efficacy of screening.
These findings should be interpreted in the context of study limitations. In particular, no individual-level data were available to link screening information with CRC incidence or mortality. Thus, although it is likely that observed temporal changes in CRC incidence and IBM reflect changes in CRC screening utilization over time, the attribution of observed trends to changes in screening patterns is speculative. In the absence of individual-level data, we were also unable to adjust for potential confounders or assess sources of heterogeneity other than basic demographic factors and tumor characteristics. Lastly, although the SEER registries included in this analysis cover an extensive and demographically diverse ascertainment area, it is plausible that incidence and IBM patterns with respect to more finely characterized tumor subgroups or within certain population groups are not captured by these data.
Despite these limitations, there are several important strengths to our analysis. The utilization of high quality, standardized, population-based SEER data allowed us to assess trends in CRC incidence and IBM rates spanning 33 years. The large size of this study population also allowed us to assess trends specific to distal and proximal CRC and, within strata defined by tumor location, to assess additional heterogeneity by relevant patient and tumor characteristics.
Our results expand on previously published descriptive studies assessing temporal trends in CRC incidence and mortality. In particular, the observed overall decline in CRC incidence, starting in the mid-1980s, has been described by numerous prior studies.2–5 Consistent with our results, past reports noted an increase in CRC incidence over the mid-1970s to mid-1980s followed by marked declines,2,4 with a brief stabilization of incidence rates during the mid to late 1990s.4 Other studies, focusing on more recent, short-term trends in CRC incidence, have similarly suggested that declines in CRC incidence rates have been greatest among non-Hispanic Whites6,7 and more pronounced among men than women.6
To the best of our knowledge, prior studies have not evaluated trends in IBM rates with respect to CRC. The use of IBM methods allows the calculation of mortality rates specific to subtypes of CRC defined by tumor characteristics. Our IBM-based results are consistent with previous reports of recent gains in stage-specific CRC 5-year relative survival.4 These results further contribute to this existing literature, providing evidence that declines in CRC mortality rates differ by tumor site. Reductions in mortality have been greatest with respect to distal disease, especially CRC arising in the rectosigmoid junction, and less pronounced with respect to proximal disease, especially for CRC arising in the ascending colon.
Several biological factors distinguish CRC arising in the proximal versus distal colorectum. In particular, proximal colorectal tumors are more likely than are distal tumors to exhibit microsatellite instability and CpG island methylation,25–27 are more likely to be mucinous,28 and tend to exhibit a higher grade.28–30 Proximal and distal colorectal tumors have also been shown to differ with respect to the distribution of specific somatic mutations31,32 and allelic imbalances25 thus suggesting possible differences in etiology. Fundamental differences are further supported by observations that proximal CRC, as compared with distal CRC, tends to be diagnosed at a later age and is more common among women.28–30,32,33
To the extent that proximal and distal CRC are associated with different etiologies, differences in temporal trends for these 2 classes of CRC may reflect changes in risk factors in the population over time. The most likely contributing factor to these differences in temporal trends, however, is screening for the primary prevention and early detection of CRC. The prevalence of screening for CRC in US adults has increased considerably in recent decades, and over this same period there has been a shift toward more sensitive screening modalities.17,34 The American Cancer Society has long recommended that average-risk adults aged 50 years and older receive either FOBT every year (recommended since 1980), sigmoidoscopy every 5 years (recommended since 1989), or colonoscopy every 10 years (recommended since 1997).35 The prevalence of recent CRC screening consistent with these guidelines, derived from data from adults participating in the National Health Interview Survey, was approximately 23% in 1987 compared with 47% in 2005 and 53% in 2008.17,34
Gains in screening rates primarily reflect increases in screening by sigmoidoscopy and colonoscopy and have been accompanied by declines in FOBT. FOBT screening has been associated with a modest reduced risk of CRC mortality in clinical trials9; however, the lower sensitivity of this screening modality, particularly for guaiac FOBT but also for immunochemical FOBT,19,36 and the need for follow-up colonoscopy in the event of a positive test result have lead to declines in the use of FOBT.17,34 Randomized clinical trials10,37 have found that sigmoidoscopy is significantly associated with a reduced risk of CRC incidence and mortality, with estimated effects greater than those observed in trials of FOBT: sigmoidoscopy has been associated with a 27%–31% reduced risk of CRC mortality in 2 trials10,37 and a 23% reduced risk of CRC incidence.10 However, results from these trials and from observational studies13,15 highlight the limitation that sigmoidoscopy does not allow visualization of the proximal colon. That is, benefits of sigmoidoscopy with respect to incidence and mortality are largely limited to CRC arising in the distal colon and rectum. Because of the limitations of FOBT and sigmoidoscopy, colonoscopy is increasingly becoming the predominant tool for CRC screening.34 Although colonoscopy is more invasive and expensive than are FOBT and sigmoidoscopy, 2 recent studies have suggested that colonoscopy is more strongly associated with a reduced risk of CRC incidence (relative risk [RR] = 0.52, 0.71)12,14; another recent study reported a 37% reduced risk of CRC mortality associated with colonoscopy.11 However, although colonoscopy offers a more complete view of the full length of the colon and rectum, these studies have also suggested that the association between colonoscopy and reduced risk of CRC incidence and mortality is stronger for distal CRC.11,12,14 Thus, the more modest declines in incidence and IBM noted here with respect to proximal versus distal CRC likely reflect a shift toward more sensitive screening modalities that offer a more complete view of the colon and rectum as well as the general tendency of distal CRC to be more sensitive to screening than is proximal CRC.
Differences in rates and temporal trends in incidence and IBM by stage at diagnosis further support the role of screening. Marked declines in the incidence of regional and distant stage disease, accompanied by greater stability in the incidence of localized stage CRC, suggest stage shifting. The fact that incidence and IBM rates for localized distal CRC have been in significant decline since 1999–2000 may reflect the contribution of screening to the primary prevention of CRC through the detection of precancerous adenomas. Adenomas, and possibly cancers, in the proximal colon are more likely to be sessile or flat than are adenomas in the distal colon and rectum and, thus, are more likely to be missed at colonoscopy.11,38
Similarly, differences in rates and temporal trends in incidence and IBM by race, age, and gender are consistent with differences in the prevalence of screening. Data from the 2008 National Health Interview Survey indicated a considerable disparity in the prevalence of CRC screening by race: only 48.9% of Black adults surveyed reported having received FOBT within the past year or endoscopy screening in the past 10 years, compared with 56.0% of White respondents.34,39 National data also indicate that the prevalence of recent CRC screening (defined as FOBT in the past year or sigmoidoscopy or colonoscopy in the past 5 years) is greatest among adults aged 60 to 79 years (57%–60% vs 40% in adults aged 50–59 years and 50% in adults aged ≥ 80 years) and is slightly greater in men than in women (51.7% vs 48.7%).39 Thus, to the extent that screening is responsible for changing patterns of CRC incidence and IBM, greater declines in rates may be expected in men, in adults aged 60 to 79 years, and in adults of White race. These differences in patterns of decline, particularly by race, may also reflect differences in access to treatment after diagnosis and socioeconomic factors.40 Consistent with such confounding, a recent report from the Women's Health Initiative indicated that, although CRC risk was significantly greater in Black women than in non-Hispanic White women after adjusting for age, screening history, history of polyp removal, and family history of CRC (hazard ratio [HR] = 1.19; 95% CI = 1.01, 1.40), no such difference was evident after further adjusting for education, diabetes, body mass index, physical activity, smoking, alcohol consumption, use of nonsteroidal antiinflammatory drugs, fiber intake, calcium intake, red meat consumption, fruit and vegetable consumption, and duration of prior hormone therapy use (HR = 0.99; 95% CI = 0.82, 1.20).41
Overall, the temporal trends in CRC incidence and IBM rates observed here indicate considerable reductions in the burden of CRC in the United States in recent years but with differing impact on specific populations and subtypes of disease. These observed trends are consistent with changes in screening patterns over time and support the hypothesis that CRC screening contributes to reductions in CRC incidence and mortality. Differences in these reductions between tumor sites, particularly for proximal versus distal CRC, likely reflect differences in tumor biology and screening efficacy. Differences in CRC incidence and IBM rate reductions across population groups, particularly for Black versus White adults, reflect growing disparities of public health concern.
Acknowledgments
This article was supported in part by the National Cancer Institute, National Institutes of Health (grants R25-CA94880, R25-CA92408, and K05-CA152715).
Note. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Human Participant Protection
No protocol approval was necessary because there were no human participants directly engaged in this work.
References
- 1.American Cancer Society Colorectal Cancer Facts & Figures 2008–2010. Atlanta: American Cancer Society; 2008 [Google Scholar]
- 2.Chu KC, Tarone RE, Chow WH, Alexander GA. Colorectal cancer trends by race and anatomic subsites, 1975 to 1991. Arch Fam Med. 1995;4(10):849–856 [DOI] [PubMed] [Google Scholar]
- 3.Ries LA, Wingo PA, Miller DSet al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–2424 [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BAet al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85(8):1670–1676 [PubMed] [Google Scholar]
- 6.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001. Cancer. 2006;107(suppl 5):1142–1152 [DOI] [PubMed] [Google Scholar]
- 7.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Public Health. 2010;100(10):1912–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo AL, Thiagalingam A, Pan Het al. Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res. 2005;11(7):2466–2470 [DOI] [PubMed] [Google Scholar]
- 9.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549 [DOI] [PubMed] [Google Scholar]
- 10.Atkin WS, Edwards R, Kralj-Hans Iet al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–1633 [DOI] [PubMed] [Google Scholar]
- 11.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8 [DOI] [PubMed] [Google Scholar]
- 12.Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010;105(3):663–673, quiz 674 [DOI] [PubMed] [Google Scholar]
- 13.Newcomb PA, Storer BE, Morimoto LM, Templeton A, Potter JD. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst. 2003;95(8):622–625 [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102(2):89–95 [DOI] [PubMed] [Google Scholar]
- 15.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–657 [DOI] [PubMed] [Google Scholar]
- 16.Shavers VL, Jackson MC, Sheppard VB. Racial/ethnic patterns of uptake of colorectal screening, National Health Interview Survey 2000–2008. J Natl Med Assoc. 2010;102(7):621–635 [DOI] [PubMed] [Google Scholar]
- 17.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–1713 [DOI] [PubMed] [Google Scholar]
- 18.Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150(3):162–169 [DOI] [PubMed] [Google Scholar]
- 19.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129(2):422–428 [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute Surveillance Epidemiology and End Results. Available at: www.seer.cancer.gov. Accessed September 21, 2011
- 21.World Health Organization International Classification of Diseases for Oncology. 3rd ed. Geneva; 2000 [Google Scholar]
- 22.Joinpoint Regression Program, Version 3.4.3. [computer program] Bethesda, MD: Statistical Research Applications Branch, National Cancer Institute; April 2010 [Google Scholar]
- 23.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351 [DOI] [PubMed] [Google Scholar]
- 24.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461 [DOI] [PubMed] [Google Scholar]
- 25.Sugai T, Habano W, Jiao YFet al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn. 2006;8(2):193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649 [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Lee HS, Choe G, Chung JH, Kim WH. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch. 2006;449(1):40–47 [DOI] [PubMed] [Google Scholar]
- 28.Snaebjornsson P, Jonasson L, Jonsson T, Moller PH, Theodors A, Jonasson JG. Colon cancer in Iceland—a nationwide comparative study on various pathology parameters with respect to right and left tumor location and patients age. Int J Cancer. 2010;127(11):2645–2653 [DOI] [PubMed] [Google Scholar]
- 29.Pappas AV, Lagoudianakis EE, Dallianoudis IGet al. Differences in colorectal cancer patterns between right and left sided colorectal cancer lesions. J BUON. 2010;15(3):509–513 [PubMed] [Google Scholar]
- 30.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64 [DOI] [PubMed] [Google Scholar]
- 31.Breivik J, Lothe RA, Meling GI, Rognum TO, Borresen-Dale AL, Gaudernack G. Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer. 1997;74(6):664–669 [DOI] [PubMed] [Google Scholar]
- 32.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–408 [DOI] [PubMed] [Google Scholar]
- 33.Hemminki K, Santi I, Weires M, Thomsen H, Sundquist J, Bermejo JL. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BMC Cancer. 2010;10:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith RA, Cokkinides V, Brooks D, Saslow D, Shah M, Brawley OW. Cancer screening in the United States 2011a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2011;61(1):8–30 [DOI] [PubMed] [Google Scholar]
- 35.Levin B, Lieberman DA, McFarland Bet al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160 [DOI] [PubMed] [Google Scholar]
- 36.Kahi CJ, Rex DK. Current and future trends in colorectal cancer screening. Cancer Metastasis Rev. 2004;23(1–2):137–144 [DOI] [PubMed] [Google Scholar]
- 37.Hoff G, Grotmol T, Skovlund E, Bretthauer M; Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heresbach D, Barrioz T, Lapalus MGet al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40(4):284–290 [DOI] [PubMed] [Google Scholar]
- 39.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1623–1630 [DOI] [PubMed] [Google Scholar]
- 40.Yan B, Noone AM, Yee C, Banerjee M, Schwartz K, Simon MS. Racial differences in colorectal cancer survival in the Detroit metropolitan area. Cancer. 2009;115(16):3791–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon MS, Thomson CA, Pettijohn Eet al. Racial differences in colorectal cancer incidence and mortality in the Women's Health Initiative. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1368–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]