Abstract
Progressive familial intrahepatic cholestasis, type 2 (PFIC2), characterized by cholestasis in infancy that may progress to cirrhosis, is caused by mutation in ABCB11, which encodes bile salt export pump (BSEP). We correlated histopathologic, immunohistochemical, and ultrastructural features in PFIC2 with specific mutations and clinical course. Twelve patients with clinical PFIC2 and ABCB11 mutations were identified, and 22 liver biopsy and explant specimens were assessed. All had hepatocellular cholestasis; most had canalicular bile plugs. At least 1 specimen from every patient had centrizonal/sinusoidal fibrosis, often with periportal fibrosis. Neonatal hepatitis-like features (inflammation, giant cells, necrosis) varied. In 2 of the 5 patients with paired specimens obtained > 6 months apart, lobular and portal fibrosis worsened. Transmission electron microscopy (EM) in all 9 patients studied showed canalicular dilatation, microvilli loss, abnormal mitochondrial internal structure, and varying intra-canalicular accumulation of finely granular bile. Canalicular staining for BSEP was absent in 10 patients and present in 2 patients, 1 of whom had intermittent symptoms. ABCB11 sequencing of all patients identified 6 novel and 10 previously described mutations, with nonsense, missense, and/or noncoding mutations in the 10 patients without immunohistochemically demonstrable BSEP. Missense and/or noncoding mutations were identified in the 2 patients with demonstrable BSEP, whose clinical course was more indolent. Mutations ending ABCB11 transcription appear linked, through hepatocellular necrosis and fibrosis, to worse outcome. In conclusion, light microscopy and electron microscopy findings in clinical PFIC2 can support diagnosis, but are variable and nonspecific. Therefore, no correlation between specific mutations and histopathology is yet possible.
Keywords: PFIC2, progressive familial intrahepatic cholestasis, bile salt export pump, BSEP, ABCB11, neonatal hepatitis
Progressive familial intrahepatic cholestasis, type 2 (PFIC2) is a rare form of cholestasis in infancy that results from mutations in ABCB11, which encodes bile salt export pump (BSEP). Specific mutations in ABCB11 are related to BSEP expression and clinical features. The classically described histology of early PFIC2 includes neonatal hepatitis-like changes such as giant cell transformation, inflammation, and canalicular cholestasis.3 Later changes include canalicular cholestasis, ductular reaction or proliferation, and fibrosis.5
In this study, we correlate morphologic and ultra-structural features of the liver with clinical and genetic findings in patients with genetically confirmed PFIC2. We also describe the histologic progression of PFIC2 liver disease in a subset of patients who had multiple liver tissue evaluations.
MATERIALS AND METHODS
Twelve patients (7 boys, 5 girls) with clinical PFIC2 and mutations in ABCB11 were identified by review of hospital records. All patients were infants at the time of symptom onset, and had normal or slightly elevated serum γ-glutamyltransferase values at presentation despite nonremitting conjugated hyperbilirubinemia. All patients had mutation(s) in ABCB11. Coding sequence, including intron-exon junctions, was evaluated by polymerase chain reaction amplification and sequencing of the exons and flanking intron regions of the gene, performed commercially for patients 9–11 (Baylor College of Medicine, Houston, TX) or by customized cholestasis chip analysis.11,13 In patient 12, whose sister had been diagnosed with PFIC2, only regions abnormal in his sister were sequenced; in all other patients, the entire gene was examined. Liver biopsy and/or explant specimens, obtained between 1995 and 2010, were drawn from the files of Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, Texas Children’s Hospital, Houston, TX, and University of California, San Francisco, CA. Specimens from all patients were retrospectively assessed by GK and KE. Hematoxylin/eosin-stained and trichrome-stained sections of formalin-fixed, paraffin-embedded tissue were reviewed.
The extent of lobular mononuclear leukocyte inflammation was scored as absent, rare (<1 focus per lobule), focal (≥ 1 focus per lobule), or diffuse (generalized, present in most lobules). The extent of hepatocellular necrosis was scored as absent/rare (≤2 necrotic hepatocytes per sample), few (>2 scattered necrotic hepatocytes per sample but ≤2 per lobule), or many (>2 scattered necrotic hepatocytes per lobule). Giant cells, defined as hepatocytes with 3 or more nuclei independent of cell size, were scored as absent, rare (1 or 2 per sample), present (giant cells occupying up to 10% of lobular area), common (giant cells occupying 10% to 49% of lobular area), or prominent (giant cells occupying ≥ 50% of lobular area). Hepatocellular swelling, defined as cellular enlargement with rarefaction of cytoplasm in the absence of distinct vacuoles, was scored as absent, rare (<5% of lobular area), present (5% to <50% of lobular area), or prominent (50% or more of lobular area). Pseudorosette formation, defined as a dilated canaliculus bordered by >2 hepatocytes, was scored as absent, present (<1 per lobule), or prominent (at least 1 in every lobule). Lobular sinusoidal/centrizonal fibrosis was graded as absent, focal (<50% of lobular area), or prominent (≥ 50% of lobular area), and if present, was assessed for extension beyond zone 3.
The intensity of portal mononuclear leukocyte inflammation was scored as absent, focal (<50% of portal areas), or generalized (≥ 50% of portal areas), and if present, was graded as mild or moderate/marked. Interface ductular reaction was scored as absent, focal (< 50% of portal areas), or generalized (≥50% of portal areas), and if present, was graded as mild or moderate to severe. The distribution of bile plugs, if present, was scored as canalicular and/or duct/ductular. Bile ducts were examined for injury (nuclear size variation, vacuolated cytoplasm, and/or apoptosis). Portal fibrosis was scored by the Scheuer criteria,18 with stage 0 indicating no fibrosis, stage 1 indicating fibrous portal tract expansion, stage 2 indicating periportal or portal-portal septa with an intact architecture, stage 3 indicating bridging fibrosis with distorted architecture, and stage 4 indicating probable or definite cirrhosis.
Immunostaining for BSEP, with a homologous canalicular transporter control, multidrug resistance-associated protein 2 (MRP2, ABCC2), was performed as reported7 for clinical diagnostic purposes on at least 1 liver sample from all patients. The polyclonal anti-BSEP antibody used, raised in rabbit, was a generous gift from Dr B. Stieger. Sections were heated for 10 minutes in ethylenediamine tetraacetic acid-Tris buffer (pH 9); the antibody was used at 1:500 dilution of the original stock. Immunostaining for BSEP was scored as present, defined as positive canalicular staining, or absent. Transmission electron microscopy (EM) was performed and evaluated in 9 patients (9 samples). Clinical and laboratory findings were studied by examination of medical records. This study was approved by the Institutional Review Board of the University of California San Francisco, Cincinnati Children’s Hospital, and Texas Children’s Hospital.
RESULTS
Clinical and Laboratory Features
Patients ranged from 1 to 8 months of age at sign or symptom onset; initial signs and symptoms included jaundice, pruritus, poor weight gain, bruising, and bleeding (Table 1). Patients ranged from 6 weeks to 7 years of age on first evaluation at our institution. In 11 cases, this was the patient’s first comprehensive evaluation for liver disease. Patient 9, the older brother of patient 10, had been previously evaluated in Saudi Arabia, and these early records were not available for our study. Serum transaminase activity and γ-glutamyltransferase activity were elevated and normal or mildly elevated, respectively, in all patients. Almost all patients presented with pruritus and/or jaundice; patient 11 presented with intracranial bleeding, and patient 12 came to clinical attention because of an affected older sister (patient 2). Clinical illness was intermittent in 1 patient (patient 7).
TABLE 1.
Clinical and Laboratory Features
| Patient | Age at Onset of Signs or Symptoms | Initial Symptoms and Signs | Age at Presentation for Medical Care | Symptoms and Signs at Presentation for Medical Care | Clinical-laboratory Results at Presentation*
|
Procedure(s); age | Indication | Age at the Last Follow-up | |
|---|---|---|---|---|---|---|---|---|---|
| GGT | ALT | ||||||||
| 1 | 16 wk | Jaundice, hepatomegaly, bruising | 5 mo | Pruritus, hepatomegaly | 14 | 236 | Biliary diversion, 17 mo | Pruritus | 41 mo |
| 2 | 6 mo | Jaundice, pruritus | 17 mo | Pruritus | 14 | 145 | Ileal exclusion, 13 mo Transplant, 28 mo |
Pruritus Continued pruritus |
86 mo |
| 3 | ND | ND | 9 mo | Jaundice, pruritus | 14 | 93 | None | NA | 30 mo |
| 4 | 8 wk | Jaundice | 8 wk | Jaundice, scleral icterus | 39 | 569 | Transplant, 20 mo | Cirrhosis, pruritus | 92 mo |
| 5 | ND | ND | 30 mo | Jaundice, diarrhea | 58 | 199 | Transplant, 37 mo | ND | 37 mo |
| 6† | 1 mo | Jaundice | 6 mo | Jaundice, pruritus, failure to thrive, hepatomegaly, scleral icterus | 35 | 959 | Biliary diversion, 11 mo Transplant, 16 mo |
Pruritus Portal hypertension, coagulopathy, ascites |
3 y |
| 7 | 6 wk | Poor weight gain | 13 mo | Intermittent jaundice, intermittent pruritus, hepatosplenomegaly | 35 | 60 | Ileal exclusion, 9 y | Pruritus | 15 y |
| 8 | 3 mo | Pruritus | 8 mo | Jaundice, pruritus, hepatomegaly, scleral icterus | 29 | 254 | Ileal exclusion, 15 mo | Pruritus | 65 mo |
| 9 | 2 mo | Jaundice, scleral icterus | 7 y | Pruritus, poor growth, intermittent abdominal pain and distention | 19 | 130 | Transplant, 88 mo | Cirrhosis | 92 mo |
| 10 | 8 mo | Jaundice | 21 mo | Jaundice, pruritus, poor growth, hepatosplenomegaly | 24 | 164 | Biliary diversion, 22 mo | Pruritus | 23 mo |
| 11 | 4 mo | Intracranial hemorrhage | 4 mo | Coagulation abnormalities, mild hepatomegaly | 19 | 285 | Transplant, 62 mo | Pruritus | 70 mo |
| 12 | 3 mo | Jaundice, pruritus | 6 wk | None (affected sibling) | 33 | 259 | Transplant, 17 mo | Pruritus | 18 mo |
Expected ranges for these analytes are not provided because they were measured at different institutions and at different times.
Patient 6 was included in a recent report of gene mutations in children with cholestasis.13
ALT indicates alanine transaminase; GGT, γ-glutamyltransferase; NA indicates not applicable; ND, not determined (unknown).
Three patients were treated for pruritus with ileal exclusion at the ages of 13 months, 15 months, and 9 years. The youngest of these patients experienced continued pruritus and was transplanted at the age of 18 months (Table 1). The other 2 patients responded to treatment. Three patients were treated for pruritus with external biliary diversion at ages of 11 months, 17 months, and 22 months. The youngest of these patients was later transplanted at the age of 16 months because of progressive coagulopathy, portal hypertension, and ascites. The other 2 patients responded to treatment.
In addition to the 2 patients who received liver transplants after failing to respond to surgical intervention, 5 other patients were transplanted, at ages ranging from 17 months to 7 years. Five patients (42%) survived with their native liver with a median follow-up of 41 months (range, 23 to 180 mo). One patient had not undergone any surgical intervention by the last follow-up (age, 30 mo). All patients were alive at the last follow-up.
Histopathologic Features
Fifteen liver biopsy specimens and 7 explanted livers were available for study (22 samples total). Lobular and portal tract changes are summarized in Tables 2 and 3. The amount of lobular inflammation varied greatly, ranging from absent or rare (2 and 7 samples, respectively) to diffuse (5 samples) (Table 3, Figs. 1A, C, E and Figs. 2A, C). Similarly, the number of necrotic hepatocytes varied. They were absent/rare in 14 samples, few in 4 samples, and many in 4 samples, all of which were from patients who underwent transplantation (patients 4, 6, and 12). Portal tract inflammation also varied. Giant cell transformation was present at least rarely in all 22 samples, and was prominent in 4 of 6 samples obtained during infancy (<12 mo of age).
TABLE 2.
Histopathologic Features
| Patient | Age | Type | Lobular Inflammation | Necrotic Hepatocytes | Giant Cells | Swelling | Pseudorosettes | Sinusoidal Fibrosis* | Portal-tract Inflammation | Ductular Reaction | Bile Plugs | Portal Fibrosis† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 mo | B | Rare | Few | Common | Present | Prominent | 1 | Focal, mild | Absent | Canalicular | 1 |
| 2 | 18 mo | E | Rare | Absent/rare | Present | Rare | Present | 2 | Focal, mild | Absent | Canalicular | 2 |
| 3 | 12 mo | B | Rare | Absent/rare | Rare | Absent | Present | 1 | Focal, mild | Absent | Absent | 1 |
| 4 | 8 wk 20 mo |
B E |
Focal Focal |
Absent/rare Many |
Prominent Present |
Prominent Prominent |
Absent Present |
ND 4 |
Generalized, moderate to severe Generalized, moderate to severe |
Absent Generalized, mild |
Absent Canalicular and ductal/ductular |
ND 4 |
| 5 | 37 mo | E | Absent | Few | Rare | Prominent | Present | 4 | Focal, mild | Generalized, mild | Canalicular and ductal/ductular | 4 |
| 6 | 2 mo 6 mo 11 mo 16 mo |
B B B E |
Focal Absent Diffuse Focal |
Many Absent/rare Many Absent/rare |
Prominent Prominent Prominent Prominent |
Prominent Prominent Prominent Prominent |
Prominent Prominent Prominent Prominent |
0 4 4 4 |
Generalized, mild Focal, mild Generalized, mild Generalized, mild |
Absent Absent Absent Absent |
Canalicular Canalicular Canalicular Absent |
0 2 3 4 |
| 7 | 13 mo 17 mo 28 mo 9 y |
B B B B |
Focal Focal Rare Focal |
Absent/rare Absent/rare Few Absent/rare |
Present Rare Rare Rare |
Present Prominent Rare Present |
Present Prominent Absent Present |
3 3 3 4 |
Absent Generalized, moderate to severe Generalized, mild Generalized, mild |
Focal, mild Focal, mild Generalized, mild Absent |
Canalicular Canalicular Canalicular Canalicular |
2 1 1 1 |
| 8 | 7 mo 15 mo |
B B |
Rare Rare |
Absent/rare Few |
Present Present |
Prominent Rare |
Prominent Present |
3 4 |
Focal, mild Absent |
Focal, mild Absent |
Canalicular Canalicular |
2 3 |
| 9 | 7 y 7 y |
B E |
Rare Diffuse |
Absent/rare Absent/rare |
Rare Present |
Rare Rare |
Prominent Present |
2 4 |
Focal, mild Generalized, moderate to severe |
Absent Focal, mild |
Canalicular Canalicular |
4 4 |
| 10 | 21 mo | B | Diffuse | Absent/rare | Present | Rare | Present | 4 | Generalized, mild | Absent | Canalicular | 1 |
| 11 | 3 y 5 y |
B E |
Diffuse Diffuse |
Absent/rare Absent/rare |
Present Present |
Absent Absent |
Prominent Prominent |
4 4 |
Generalized, mild Generalized, mild |
Focal, mild Absent |
Canalicular Canalicular |
3 3 |
| 12 | 17 mo | E | Focal | Many | Present | Present | Prominent | 4 | Focal, mild | Focal, mild | Canalicular | 2 |
Sinusoidal fibrosis: 0, absent; 1, focal, confined to zone 3; 2, focal, extends beyond zone 3; 3, prominent, confined to zone 3; 4, prominent, extends beyond zone 3; ND, not determined (trichrome-stained section not available).
Portal fibrosis: Staged by Scheuer criteria.
B indicates biopsy specimen; E, explanted liver.
TABLE 3.
Frequency of Major Morphologic Features
| Feature | No. (%) | |
|---|---|---|
| Lobular sinusoidal fibrosis | Prominent, extends beyond zone 3 | 12 (57) |
| Prominent, confined to zone 3 | 4 (19) | |
| Focal, extends beyond zone 3 | 2 (10) | |
| Focal, confined to zone 3 | 2 (10) | |
| Absent | 1 (5) | |
| Cannot assess | 1 | |
| Portal fibrosis (Scheuer criteria) | Stage 4 | 5 (24) |
| Stage 3 | 4 (19) | |
| Stage 2 | 5 (24) | |
| Stage 1 | 6 (29) | |
| Stage 0 | 1 (5) | |
| Cannot assess | 1 | |
| Bile plugs | Canalicular and ductal/ductular | 2 (9) |
| Canalicular only | 17 (77) | |
| Ductal/ductular only | 0 (0) | |
| Absent | 3 (14) | |
| Pseudorosette formation | Prominent | 11 (50) |
| Present | 9 (41) | |
| Absent | 2 (9) | |
| Lobular mononuclear leukocyte inflammation | Diffuse | 5 (23) |
| Focal | 8 (36) | |
| Rare | 7 (32) | |
| Absent | 2 (9) | |
| Portal inflammation | Generalized, moderate/marked | 4 (18) |
| Generalized, mild | 8 (36) | |
| Focal, mild | 8 (36) | |
| Absent | 2 (9) | |
| Hepatocellular necrosis | Many | 4 (18) |
| Few | 4 (18) | |
| Absent/rare | 14 (64) | |
| Giant cell transformation | Prominent | 5 (23) |
| Common | 1 (5) | |
| Present | 10 (45) | |
| Rare | 6 (27) | |
| Hepatocellular swelling | Prominent | 9 (41) |
| Present | 4 (18) | |
| Rare | 6 (27) | |
| Absent | 3 (14) |
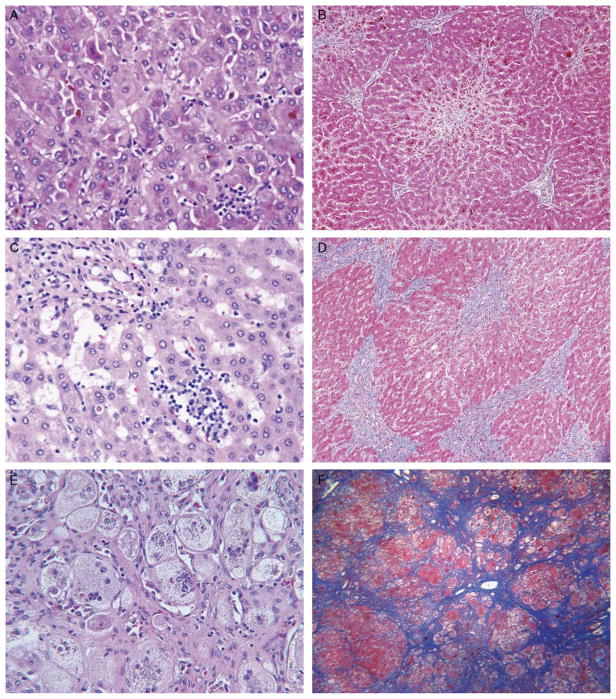
FIGURE 1.
Light microscopy findings in PFIC2 include varying lobular inflammation, portal inflammation, giant cell transformation, hepatocellular necrosis, hepatocellular swelling, portal fibrosis, and centrizonal/sinusoidal fibrosis. A and B, Explanted liver of patient 12 at the age of 17 months shows cholestasis, focal lobular inflammation, hepatocellular necrosis (including necrotic giant cells), prominent centrizonal fibrosis, and stage 2 portal fibrosis. Hematoxylin and eosin (H&E; × 400) and trichrome (× 100) stains. C and D, Explanted liver of patient 9 at the age of 7 years shows more prominent, diffuse lobular inflammation and areas of bridging fibrosis (shown) and nodule formation (not shown) with architectural distortion. H&E (× 400) and trichrome (× 100) stains. E and F, Explanted liver of patient 6 at the age of 16 months shows giant cells, hepatocyte swelling, and centrizonal/sinusoidal fibrosis, all prominent, and cirrhosis. Patchy intense lobular inflammation and pervasive bands of vascularized intralobular replacement fibrosis are seen. Central veins and portal-to-portal scars are not discernible; most of the fibrosis is pericellular. H&E (× 160) and trichrome (× 100) stains.
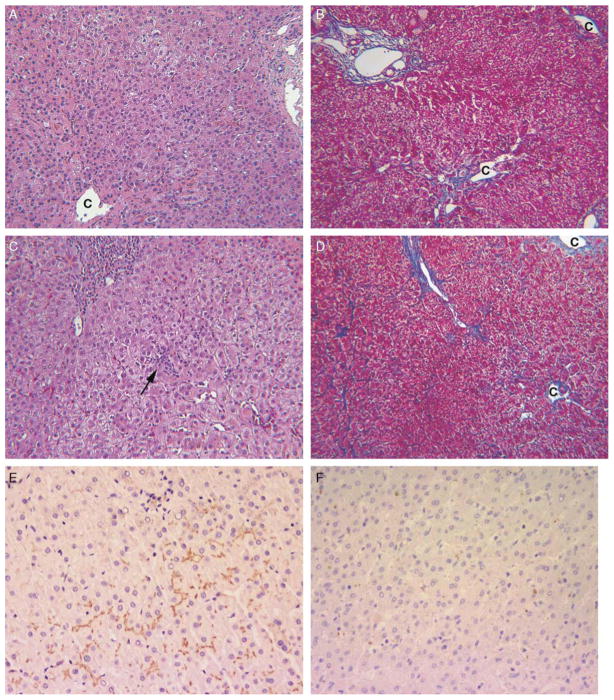
FIGURE 2.
Patient 7 had early-onset, intermittent pruritus and a long course (present age, 15 y), and his liver tissue showed intact canalicular BSEP immunostaining. Multiple liver biopsy specimens (A to D) showed stable liver disease with exacerbations of lobular cholestasis and hepatitis. A and B, Minimal cholestasis, occasional multinucleate giant hepatocytes, absent portal tract inflammation, and centrilobular fibrosis confined to zone 3 at the age of 13 months. C indicates central vein. Hematoxylin and eosin (H&E) and trichrome stains (each × 100). C and D, Mild portal and focal lobular inflammation (arrow) accompany lobular cholestasis and mild cell swelling at the age of 9 years. Fibrosis is mild. H&E and trichrome stains (each × 100) C indicates central vein. E, Immunostain for BSEP (hematoxylin counterstain) performed on liver biopsy tissue of patient 7 at the age of 9 years shows normal intensity and distribution of canalicular reactivity (× 200). F, In contrast, liver biopsy of patient 10 at the age of 21 months shows no canalicular reactivity. Immunostain for BSEP, hematoxylin counterstain (× 200).
All samples showed hepatocellular cholestasis. Most samples (19 samples) had bile plugs in canaliculi; 2 of these also had bile plugs in ductules and, rarely, in small ducts. Pseudorosette formation was either present (9 samples) or prominent (11 samples) in most specimens. Hepatocellular swelling was variable, but 19 samples showed at least some swelling, and prominent swelling was observed in 9 samples. Interlobular bile duct injury was noted in only 1 needle biopsy specimen and was mild. No sample had mononuclear inflammatory cells infiltrating the biliary epithelium or within the duct lumina. No portal tract edema, periductal fibrosis, or acute cholangitis was observed in any sample.
All patients showed centrizonal/sinusoidal fibrosis in at least 1 sample (Table 2, Figs. 1B, D, F and Figs. 2B, D). This pericellular fibrosis was usually prominent (16 samples) and often extended beyond zone 3 (14 samples) (Fig. 1F). Similarly, portal fibrosis was common and often prominent, particularly in the 7 patients (patients 2, 4, 5, 6, 9, 11, and 12) who required liver transplantation (Table 2, Fig. 1D). Of the 9 samples from the patients who survived with their native livers (patients 1, 3, 7, 8, and 10), only 1 showed more than stage 2 portal fibrosis.
After their initial biopsies, 5 patients underwent at least 1 additional procedure (biopsy or transplant) >6 months later. Two of these patients (patients 6 and 8) showed progression of both lobular and portal fibrosis over time. No trichrome-stained section of the initial biopsy specimen from patient 4 was available; thus, the progression of fibrosis could not be assessed. Patient 11 had advanced fibrosis by his initial biopsy at the age of 3 years, and his explant at the age of 5 years showed the same stage of fibrosis. In patient 7, multiple liver biopsies showed stable liver disease with exacerbations of lobular cholestasis and hepatitis (Figs. 2A–D).
Immunohistochemical Staining
Most patients (10 of 12) lacked detectable BSEP (Fig. 2F, Table 4). Canalicular staining for BSEP was observed in patients 3 and 7; the latter is our oldest patient (age 15 years at the last follow-up) (Fig. 2E). Canalicular expression of MRP2 (not shown) was present in all patients, indicating that when BSEP marking was lost, this was a specific disease-related phenomenon.
TABLE 4.
Immunohistochemical Findings and Genetic Abnormalities
| Patient | BSEP | Mutation | Type of Mutation(s) |
|---|---|---|---|
| 1 | Absent | c.890A>G (p.E297G)* | Missense5,7,10,13,16,19,20 |
| 2 | Absent | c.1723C>T (p.R575X) | Nonsense7,19,20 |
| c.2178+1G>T | Noncoding region20 | ||
| 3 | Present | c.1708G>A (p.A570T) | Missense20 |
| c.3634G>T (p.V1212F) | Missense, predicted deleterious | ||
| 4 | Absent | c.3164T>C (p.L1055P)* | Missense, predicted deleterious |
| 5 | Absent | c.3692G>A (p.R1231Q) | Missense20 |
| c.2296G>A (p.G766R) | Missense20 | ||
| 6 | Absent | c.2782C>T (p.R928X) | Nonsense13 |
| c.3268C>T (p.R1090X) | Nonsense5,7,13 | ||
| 7 | Present | c.3347G>A (p.G1116E) | Missense, predicted deleterious |
| IVS 23-8 G-A | Noncoding region | ||
| 8 | Absent | IVS 16-8 T>G† | Noncoding region10 |
| 9 | Absent | c.2944G>A (p.G982R) | Missense5,7,19,20 |
| c.2296G>A (p.G766R) | Missense20 | ||
| 10 | Absent | c.2944G>A (p.G982R) | Missense5,7,19,20 |
| c.2296G>A (p.G766R) | Missense20 | ||
| 11 | Absent | c.319T>C (p.C107R) | Missense, predicted deleterious |
| c.611+4A>G | Noncoding region | ||
| 12 | Absent | c.1723C>T (p.R575X) | Nonsense7,19,20 |
| c.2178+1G>T | Noncoding region20 |
Homozygous.
Monoallelic mutation.
Transmission EM
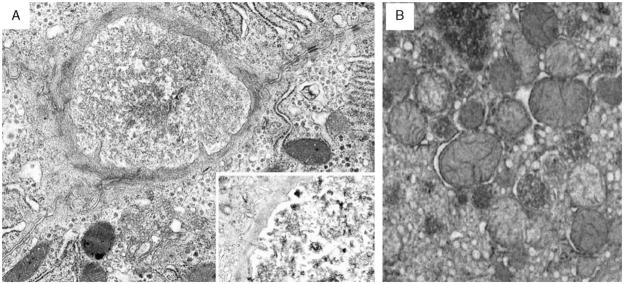
Ultrastructural abnormalities in the 9 patients studied were similar to each other and to those reported in patients with PFIC2,3 serving to confirm the changes observed by light microscopy and differing among each other only in degree. The major findings were nonspecific features of lobular cholestasis such as increased dense polymorphous secondary lysosomes, canalicular dilatation with effacement of microvilli, variable accumulation of finely granular bile in canalicular lumina (Fig. 3A), and subtle nonspecific abnormalities of mitochondrial internal structure (Fig. 3B). In this series of patients, the bile never resembled the coarsely particulate bile characteristic of PFIC1.3 A circumferential band of intermediate filaments, presumed to be actin, was often visible around dilated canaliculi and was present as early as 12 months of age in patient 3.
FIGURE 3.
Ultrastructural abnormalities of liver tissue were similar in the 9 patients studied. A, Liver biopsy from patient 7 at the age of 13 months shows canalicular dilatation, subtotal effacement of microvilli, a pericanalicular collar of microfilaments, and intact desmosomes. Canalicular bile is finely granular. Inset: Another severely dilated canaliculus in this patient contains occasional coarse aggregates mixed with the finely granular bile. Osmium tetroxide/uranyl acetate/lead citrate stain (× 3150). B, Liver biopsy from patient 11 at the age of 3 years shows mild mitochondrial pleomorphism, with alterations in size, shape, and matrix density. Osmium tetroxide/uranyl acetate/lead citrate stain (× 2000).
Genetics
Sequencing showed 12 different single nucleotide substitutions in the coding region and 4 in the noncoding region of ABCB11 in the 12 patients. Of the former, 3 variants are predicted to result in nonsense mutations and 9 in missense mutations. Ten mutations have been described before5,7,10,13,16,19,20; the new variants include 4 sequence alterations predicted to cause deleterious mis-sense mutations (Table 4). For 11 patients, mutations in ABCB11 were identified on both alleles. Only 1 mutation was identified in patient 8; this mutation was most likely heterozygous due to a lack of a history of consanguinity. (The other allele in this patient may contain a mutation located outside the sequenced region or a microdeletion.) Nonsense, missense, or noncoding region mutations were identified in the 10 patients without immunohistochemically demonstrable BSEP. Missense and/or noncoding region mutations were identified in the 2 patients with immunohistochemically demonstrable BSEP.
DISCUSSION
Here we describe the morphologic, immunohistochemical, and ultrastructural features in 12 patients with genetically confirmed PFIC2. All liver samples showed lobular cholestasis, and most had bile plugs in canaliculi. Other common features included lobular and/or portal inflammation, giant cell transformation, hepatocellular necrosis, and hepatocellular swelling. This neonatal hepatitis-like pattern has been described in PFIC2, particularly in early biopsy specimens.1,4,6,12,14,15,21 A comprehensive histopathologic examination of 84 liver biopsy specimens in the setting of PFIC identified similar features in some specimens, including hepatocellular and canalicular cholestasis, ballooned hepatocytes, and giant cell transformation, and prominent bile duct loss and injury,2 but this study was carried out before the genetic basis of PFIC2 was known.19 Thus, it may have included cases of PFIC1, a related form of cholestatic liver disease caused by mutations in familial intrahepatic cholestasis, type 1 (FIC1)/ATP8B1 that clinically can resemble PFIC2.9 The bile duct loss and injury reported2 might have been observed in patients with PFIC1, as these changes were not characteristic in our patients with PFIC2.
A comparison of histopathologic differences between PFIC1 and PFIC2 reported portal and lobular fibrosis, many giant hepatocytes, and occasional hepatocyte necrosis in PFIC2, just as we have observed.5 Chart review of patients with PFIC1 and patients with PFIC2 also identified giant or multinucleate hepatocytes in most patients with PFIC2,16 whereas giant hepatocytes and necrosis were rare in PFIC1.5,16 In our study, giant cells were present at least rarely in all samples, and were prominent in most samples obtained during infancy (<12 mo of age). Thus, our observations are in keeping with the major histologic findings of these 2 recent studies, and support the proposition that giant cells (even in patients older than 1 y), portal and lobular fibrosis, and occasional hepatocyte necrosis characterize PFIC2.
Our study included 5 patients with multiple liver samples taken at least 6 months apart, which let us examine changes in histopathologic features of PFIC2 over time (Table 2). Interestingly, the prominence of neonatal hepatitis-like findings varied greatly among patients and within samples from individual patients, and we observed no clear trends. For example, in 3 patients, the frequency of giant cells remained approximately the same over time, whereas in 2 patients the frequency decreased. Lobular inflammation fluctuated in 2 patients and remained similar in 3 patients. Hepatocellular swelling fluctuated in 1 patient, remained similar in 3 patients, and decreased in 1 patient. However, our finding that giant cells and lobular inflammation were either rare or absent in some biopsy specimens indicates that the absence of classic neonatal hepatitis-like features on light microscopy does not exclude the diagnosis of PFIC2. Importantly, biopsy specimens from 3 of our oldest patients (patients 7, 9, and 11; ages 9, 7, and 5 y, respectively) continued to show at least some neonatal hepatitis-like features, including giant cells, hepatocyte swelling, and inflammation. This finding indicates that regardless of age, patients with PFIC2 are likely to show at least some neonatal hepatitis-like changes on biopsy.
All patients had some portal-based fibrosis in at least 1 sample. Variation in portal fibrosis, including progression to micronodular cirrhosis, has been reported in PFIC2.4,5,10,12,14–17,20,21 All patients in this study had sinusoidal/centrizonal fibrosis in at least 1 sample. Sinusoidal fibrosis was usually prominent, and often extended beyond zone 3. The pronounced lobular fibrosis in PFIC2 is distinct from portal-based or post-necrotic fibrosis. Lobular fibrosis, together with lobular inflammation, giant cells, and cell swelling, may be helpful clues to the possibility of PFIC2 on initial light microscopic examination in the appropriate clinical setting, and should perhaps prompt immunohistochemical and genetic studies.
Mutations in ABCB11 are typically associated with loss of immunohistochemically demonstrable BSEP expression, indicating that immunohistochemical staining is a useful diagnostic tool in PFIC2.5,7,10,14,15,17,20,21 In our study, 10 patients lacked BSEP expression on immunohistochemical analysis. However, patients with clinical PFIC2 and mutations in ABCB11 may still have immunohistochemically detectable BSEP20; in such patients the protein expressed may be quantitatively insufficient or not fully functional. Patients 3 and 7 expressed BSEP on immunohistochemical analysis. Both had missense mutations (p.G116E and p.V1212F) predicted to affect the highly conserved nucleotide-binding folds of BSEP. These results are in keeping with the findings of Strautnieks et al,20 who detected BSEP expression in 28% of patients with PFIC2, most of whom had missense mutations within the nucleotide-binding folds.
An interesting question is whether the presence of intact BSEP expression correlates with clinical and/or histologic findings. The 2 BSEP-expressing patients in this study have followed a relatively slow progressive clinical course. Patient 7 was treated with ileal exclusion at 9 years of age and has not required additional surgical intervention. Patient 3 has not yet required surgical intervention till age 30 months. All biopsy specimens from these 2 patients showed only minimal or mild portal fibrosis (stages 1 or 2). These findings suggest that in PFIC2, patients with intact BSEP expression tend to develop less portal fibrosis than patients without BSEP expression, and follow a correspondingly more indolent clinical course. However, sinusoidal fibrosis, inflammatory features, and cholestatic changes in these BSEP-expressing patients varied, and did not substantially differ from those in patients without BSEP expression.
A related issue is whether specific genetic mutations are correlated with clinical and/or histologic findings. In this study, we found 6 ABCB11 mutations associated with clinical PFIC2 that have not been previously published, and identified 10 mutations that were previously published (Table 4).5,7,10,13,16,19,20 The diversity of ABCB11 mutations and the wide spectrum of findings on light microscopy, particularly the variations in samples from individual patients over time, indicate that severity of inflammatory and/or cholestatic features cannot be correlated with specific genetic mutations. Perhaps some of the variation reflects the realities of tissue sampling in a heterogeneous setting or reflects intercurrent, non-PFIC2-related hepatic injury. In a large study of patients with PFIC2, Strautnieks et al20 found that patients with 2 predicted protein-truncating mutations were at increased risk of developing hepatobiliary malignancy compared with other patients with PFIC2. That study raised the possibility that certain types of genetic mutations in PFIC2 may be correlated with clinical outcomes. A study of 180 families with PFIC1 or benign recurrent intra-hepatic cholestasis, type 1 (BRIC1), both caused by mutation in ATP8B1, correlated mutation type/location with clinical severity.8 An analogous study for ABCB11, correlating clinical presentations of PFIC2 and BRIC2 with mutation class, has not yet been conducted. Descriptive analysis in our small sample suggests that mutations predicted to abolish ABCB11 transcription are linked through lost BSEP expression, by more necrotic hepatocytes and more fibrosis, to worse outcome. Assessment in larger cohorts may validate this impression.
In summary, key light microscopy findings in PFIC2 include cholestasis, lobular fibrosis, lobular inflammation, portal inflammation, portal fibrosis, giant cell transformation, hepatocellular necrosis, and hepatocellular swelling. EM shows canalicular dilatation, partial-to-complete loss of microvilli, abnormal mitochondrial internal structure, and varying accumulation of finely granular bile. Our study underscores the variation in histologic findings that may be seen in PFIC2, both among patients and within the same patient over time. Light microscopic features are not clearly associated with clinical course in patients with PFIC2. Thus, although morphologic features can provide some support for the diagnosis of PFIC2, immunohistochemical or genetic studies, sometimes in combination, are required to establish the diagnosis.
Acknowledgments
Supported by the Cholestatic Liver Disease Consortium NIDDK grant U54 DK 078377 and by NIH grants U01 DK062500, U01 DK062470, and U01 DK062497.
Five study patients were enrolled in the longitudinal study of the Childhood Liver Disease Research and Education Network (ChiLDREN), which played an important role in fostering and supporting our collaboration.
References
- 1.Alissa FT, Jaffe R, Shneider BL. Update on progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2008;46:241–252. doi: 10.1097/MPG.0b013e3181596060. [DOI] [PubMed] [Google Scholar]
- 2.Alonso EM, Snover DC, Montag A, et al. Histologic pathology of the liver in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:128–133. doi: 10.1097/00005176-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bull LN, Carlton VE, Stricker NL, et al. Genetic and morphological findings in progressive familial intrahepatic cholestasis [Byler disease (PFIC-1) and Byler syndrome]: evidence for heterogeneity. Hepatology. 1997;26:155–164. doi: 10.1002/hep.510260121. [DOI] [PubMed] [Google Scholar]
- 4.Chen ST, Chen HL, Su YN, et al. Prenatal diagnosis of progressive familial intrahepatic cholestasis type 2. J Gastroenterol Hepatol. 2008;23:1390–1393. doi: 10.1111/j.1440-1746.2008.05432.x. [DOI] [PubMed] [Google Scholar]
- 5.Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- 6.Goto K, Sugiyama K, Sugiura T, et al. Bile salt export pump gene mutations in two Japanese patients with progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2003;36:647–650. doi: 10.1097/00005176-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jansen PL, Strautnieks SS, Jacquemin E, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 8.Klomp LW, Vargas JC, van Mil SW, et al. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27–38. doi: 10.1002/hep.20285. [DOI] [PubMed] [Google Scholar]
- 9.Knisely AS. Progressive familial intrahepatic cholestasis: an update. Pediatr Dev Pathol. 2004;7:309–314. doi: 10.1007/s10024-003-0625-0. [DOI] [PubMed] [Google Scholar]
- 10.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Aronow BJ, Jegga AG, et al. Novel resequencing chip customized to diagnose mutations in patients with inherited syndromes of intrahepatic cholestasis. Gastroenterology. 2007;132:119–126. doi: 10.1053/j.gastro.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LY, Wang ZL, Wang XH, et al. ABCB11 gene mutations in Chinese children with progressive intrahepatic cholestasis and low gamma glutamyltransferase. Liver Int. 2010;30:809–815. doi: 10.1111/j.1478-3231.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 13.Matte U, Mourya R, Miethke A, et al. Analysis of gene mutations in children with cholestasis of undefined etiology. J Pediatr Gastroenterol Nutr. 2010;51:488–493. doi: 10.1097/MPG.0b013e3181dffe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobili V, Di Giandomenico S, Francalanci P, et al. A new ABCB11 mutation in two Italian children with familial intrahepatic cholestasis. J Gastroenterol. 2006;41:598–603. doi: 10.1007/s00535-006-1816-z. [DOI] [PubMed] [Google Scholar]
- 15.Noe J, Kullak-Ublick GA, Jochum W, et al. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43:536–543. doi: 10.1016/j.jhep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Pawlikowska L, Strautnieks S, Jankowska I, et al. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. doi: 10.1016/j.jhep.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheimann AO, Strautnieks SS, Knisely AS, et al. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr. 2007;150:556–559. doi: 10.1016/j.jpeds.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 19.Strautnieks SS, Bull LN, Knisely AS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 20.Strautnieks SS, Byrne JA, Pawlikowska L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Treepongkaruna S, Gaensan A, Pienvichit P, et al. Novel ABCB11 mutations in a Thai infant with progressive familial intrahepatic cholestasis. World J Gastroenterol. 2009;15:4339–4342. doi: 10.3748/wjg.15.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]