Abstract
The performance of gamma interferon (IFN-γ) release assays (IGRA) in the detection of latent tuberculosis (TB) infection is limited by the higher rates of indeterminate results in HIV-infected persons, who bear the brunt of TB disease in some high-burden settings. The objective of the study was to evaluate predictors of indeterminate IGRA results in the overall study population and in HIV-infected persons. The study setting is Khayelitsha, an informal township in the Western Cape of South Africa, with a high burden of TB and HIV infection. A total of 561 asymptomatic persons were recruited from the day hospital and youth centers. A questionnaire was used to collect demographic information, and blood tests, including CD4 counting and a 7-day in-house IGRA, were performed. The overall prevalence of indeterminate IGRA results was 8.6% (48/561), and this was higher in HIV-infected than in HIV-uninfected persons (11.5% [38/330] versus 4.3% [10/231], respectively; P = 0.003). In the overall study population, predictors of indeterminate IGRA results were the presence of HIV infection (odds ratio [OR], 2.36; 95% confidence interval [CI], 1.10 to 5.08) and the presence of a Mycobacterium bovis BCG scar (OR, 2.48; 95% CI, 1.23 to 5.01). Long-term township residents were significantly less likely to have indeterminate results than recent migrants (OR, 0.30; 95% CI, 0.11 to 0.80). Among HIV-infected persons, participants with CD4 counts of >200 cells/mm3 and long-term residents were significantly less likely to have indeterminate IGRA results (OR of 0.21 with a 95% CI of 0.09 to 0.48 and OR of 0.22 with a 95% CI of 0.07 to 0.68, respectively). We evaluated risk factors for indeterminate IGRA results and report a higher rate of indeterminate results among HIV-infected persons, particularly those with lower CD4 counts. Of note, a recent move to the township was associated with a higher risk of indeterminate IGRA results.
INTRODUCTION
The diagnosis of HIV-associated tuberculosis (TB) is challenging. Identification and treatment of latent TB infection (LTBI) has been shown to decrease the incidence of TB in HIV-infected persons, highlighting the importance of accurate detection of LTBI (20). In the diagnosis of LTBI, the sensitivity of the tuberculin skin test (TST) is impaired by the presence of HIV coinfection, and the test also lacks specificity due to cross-reactivity with nontuberculous mycobacteria (NTM) and the Mycobacterium bovis BCG vaccine.
Gamma interferon (IFN-γ) release assays (IGRA) represent a significant advance in latent TB diagnosis, and several studies have evaluated the ability of IGRA to detect LTBI in the presence of HIV infection in settings where HIV/TB are endemic. Chapman et al. enumerated TB-specific T cells and reported the response rates of the region of difference 1 (RD1) gene product-based enzyme-linked immunosorbent spot (ELISpot) assay, the purified protein derivative (PPD)-based ELISpot assay, and the TST; all methods showed that responses were reduced in healthy HIV-infected persons compared with those in HIV-uninfected Zambian individuals (4). In a study by Leidl et al. in Uganda, HIV-infected persons with CD4 counts of >250 cells/μl had the highest frequency of positive TST and QuantiFERON Gold In-Tube (QFT-GIT) results, indicating dependency on the degree of immunosuppression. In contrast, the T-SPOT.TB results were independent of the degree of immunodeficiency, with results for HIV-infected persons comparable to those of HIV-uninfected persons (13). These studies highlight some limitations of IGRA in HIV-infected persons.
Studies comparing HIV-infected to -uninfected persons have also shown a higher rate of indeterminate results in immunocompromised persons (19). Indeterminate IGRA results are due to either a high background level with the negative control or low response to the positive control. However, other predictors of an indeterminate gamma interferon response, particularly in HIV-infected persons, are less understood. A better understanding of these predictors could aid optimization of LTBI screening and interpretation of results in persons with identified risk factors.
The aim of this study was to evaluate predictors of indeterminate gamma interferon (IFN-γ) responses, particularly in HIV-infected persons.
MATERIALS AND METHODS
Study setting and participants.
Khayelitsha, the setting for this study, has a population of over 400,000 predominantly black Africans and an exceptionally high burden of TB and HIV, with a TB case notification rate of >1,600 cases/100,000 persons in 2008 and an HIV antenatal prevalence of 29% (15). Two-thirds of TB cases in Khayelitsha occur in HIV-coinfected persons (15).
Adults aged over 18 years were potentially eligible for inclusion as part of an observational TB diagnostics study recruiting asymptomatic persons with latent TB infections using the IGRA described. Written informed consent was obtained from all participants, and the study was approved by the University of Cape Town Faculty of Health Sciences Research Ethics Committee. From February 2008 to November 2010, asymptomatic HIV-infected and -uninfected patients were recruited from the Khayelitsha day hospital and youth clinic. Diagnosis of HIV infection was performed by the health facility using the Abbott Determine HIV 1/2 test and a confirmatory enzyme-linked immunosorbent assay (ELISA) processed by the National Health Laboratory Service, a reference laboratory.
A TB symptom screen was performed to identify and exclude suspected cases of TB. TB symptoms were defined by the presence of any of the following symptoms: cough, night sweats, fever, weight loss, or loss of appetite. The absence of all these symptoms has been reported to identify persons with a low probability of having TB disease (10). In addition, HIV-infected persons had TB excluded by induced sputum sent to the National Health Laboratory Service reference laboratory for TB microscopy and culture. Demographic details recorded included age, sex, body mass index (BMI), presence of a BCG scar, smoking status, history of previous TB, highest school grade achieved, employment status, duration of residency in Khayelitsha, HIV status, and CD4 count.
Gamma interferon release assay.
A 7-day in-house IGRA was performed at the laboratory at the Institute of Infectious Disease and Molecular Medicine, University of Cape Town. Samples of heparinized blood were transported at room temperature, and assays were set up on the same day within 4 h. Briefly, blood was diluted 1:10 in RPMI 1640 medium containing 1% l-glutamine and added to a 24-well flat-bottom plate at 1 ml/well. Antigens (ESAT-6, CFP-10, and an ESAT-6/CFP-10 fusion protein) (9) were added at a final concentration of 5 μg/ml. Phytohemagglutinin (PHA) at a concentration of 5 μg/ml acted as a positive control, and RPMI 1640 medium with 1% l-glutamine was the negative control. After gentle mixing, the plate was incubated at 37°C with 5% CO2 for 7 days. After incubation, the supernatants were harvested and stored at −80°C until assayed (up to a maximum of 2 weeks later). The IFN-γ ELISA was performed on the supernatants as described previously (5).
An indeterminate result was defined as having a high background level with the negative control or an inadequate positive-control response (defined as a PHA response of <1,000 pg/ml; 40 pg/ml is equivalent to 1 IU/ml [7]). This PHA cutoff point was selected based on the assumption that all participants should respond to the positive control and that the distribution of the real data should be normal. The distribution of the raw data when plotted was not normal. The log values of the IFN-γ response to PHA were then plotted, and low outliers were recursively excluded. At a cutoff point of 1,000 pg/ml, all tests of normality were passed, and this was therefore chosen as the cutoff point for a positive PHA response.
Statistical analysis.
Baseline characteristics were compared using simple proportions stratified according to whether IFN-γ responses were determinate or indeterminate. Risk factors associated with indeterminate IFN-γ results were analyzed using logistic regression built manually using two methods. First, negative and positive results were combined into one “determinate” variable, and logistic regression was performed. Second, multinomial regression was performed to compare the risk factors for indeterminate IFN-γ results to negative and positive IFN-γ results in turn. Nested models were compared using the likelihood ratio test. The Akaike's information criterion (AIC) was used to compare nonnested models, with a significantly lower AIC (>10%) indicating an improved model. Effect modification between confounding and exposure variables was also examined using interaction variables. The fit of the model was assessed using Pearson's goodness-of-fit test, with a P value of >0.05 indicating a good fit of the model to the data. Outlying and influential observations were identified using standardized residuals greater than +2 or less than −2 and the Hosmer and Lemeshow test of influence, respectively.
All data were analyzed using STATA, version 10.0 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics.
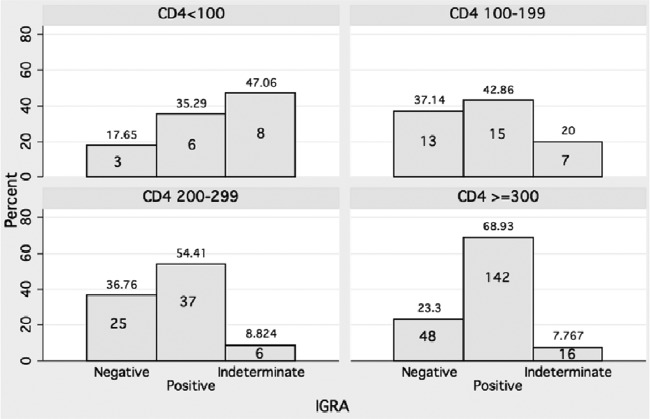
The overall prevalence of indeterminate IGRA results was 8.6% (48/561) and was higher in HIV-infected than in HIV-uninfected persons: 11.5% (38/330) versus 4.3% (10/231), respectively (P = 0.003). All indeterminate results were due to an inadequate positive-control response. Among persons with indeterminate IFN-γ responses, 72.9% were HIV infected, and 12.9% had lived in Khayelitsha for less than 1 year (compared to 4.4% in participants with determinate IGRA results) (Table 1). The median number of days since HIV diagnosis was 255 (interquartile range [IQR], 43 to 981 days) compared to 325 (IQR, 15 to 1,354 days) in those with determinate results. The median CD4 count was lower, at 241 (IQR, 101 to 420) versus 320 (IQR, 227 to 433) and 375 (IQR, 283 to 528) cells/mm3 in IFN-γ-negative and -positive persons, respectively. Figure 1 highlights the increasing prevalence of indeterminate results with decreasing CD4 counts. A higher proportion of HIV-infected persons than uninfected persons were recent migrants (6.8% versus 2.4%; P = 0.024).
Table 1.
Baseline characteristics of determinate and indeterminate IGRA resultsa
| Patient characteristic | Determinate IFN-γ response | Indeterminate IFN-γ response | Overall total |
|---|---|---|---|
| Baseline characteristics (% [no. of positive subjects/total no. of subjects]) | |||
| Female | 66.6 (342/513) | 72.9 (35/48) | 67.2 (377/561) |
| HIV positive | 56.9 (292/513) | 79.2 (38/48) | 58.8 (330/561) |
| TST positive | 61.1 (301/493) | 59.6 (28/47) | 60.9 (329/540) |
| Smoker | 20.7 (106/511) | 8.3 (4/48) | 19.7 (110/559) |
| TB contact | 15.6 (79/507) | 14.6 (7/48) | 15.5 (86/555) |
| Previous episode of TB | 11.5 (59/512) | 14.6 (7/48) | 11.8 (66/560) |
| Presence of BCG scar | 48.1 (246/511) | 66.7 (32/48) | 49.7 (278/559) |
| On ARTb | 1.4 (4/290) | 2.6 (1/38) | 1.5 (5/328) |
| Employed | 30.6 (156/510) | 33.3 (16/48) | 30.8 (172/558) |
| Shack accommodation | 54.6 (261/478) | 61.4 (27/44) | 55.2 (288/522) |
| Resident <1 yr in Khayelitsha | 4.4 (21/476) | 12.9 (6/43) | 5.2 (27/519) |
| Other factors (median [IQR]) | |||
| Age (yr) | 28 (22.7–34.7) | 29.6 (25–35.5) | 28.1 (22.9–34.7) |
| BMI | 25.1 (22.0–30.0)) | 25.8 (23.4–29.3) | 25.2 (22.0–29.8) |
| Education (highest grade) | 11 (10–11) | 11 (10–12) | 11 (10–12) |
| No. of persons/bedroom | 2 (1.67–3) | 2 (1.9–3) | 2 (1.67–3) |
| CD4 count (cells/mm3) | 353 (260–507) | 241 (101–420) | 346.5 (241–502) |
| No. of days since HIV diagnosis | 325 (15–1354) | 255 (43–981) | 322.5 (17–1337) |
| Mantoux skin test result (mm) | 12 (1–20) | 10 (0–17) | 10 (0.5–20) |
Characteristics in bold represent significant differences between determinate and indeterminate IFN-γ responses.
ART, antiretroviral therapy.
Fig 1.
Histograms showing decreasing proportions of indeterminate results with increasing CD4 counts in HIV-infected persons. Numbers within the bars indicate sample sizes; percentages are given above the bars.
Comparison between in-house IGRA and QFT-GIT assay.
In the absence of a gold standard, the antigen-specific cutoff values were chosen using the QuantiFERON Gold In-Tube (QFT-GIT) test as an arbitrary gold standard. A comparison was conducted using 31 healthy donors (independent of the study), and statistical analysis was conducted by receiver operating characteristic curve analysis. The proportion of donors scoring any positive response to any peptide in the in-house assay was similar to the proportion scored positive by QFT-GIT (14/31 [45%] to any of the peptide mixtures). Significant values for the area under the curve (AUC) were obtained for ESAT-6 and CFP-10 and for the summed response (see the supplemental material). Overall, the most useful combination of specificity and sensitivity was provided by the response to CFP-10 at a cutoff of 300 pg/ml (sensitivity, 93% [95% confidence interval {CI}, 66% to 100%]; specificity 82% [95% CI, 57% to 96%]). The lowest cutoff values that provided 100% sensitivity at the highest likelihood ratio were therefore chosen. A positive IFN-γ response was defined as a response to ESAT-6 of >471 pg/ml, to CFP-10 of >188 pg/ml, or to the fusion protein of >857 pg/ml.
Factors associated with indeterminate IFN-γ results.
To explore factors that associate with indeterminate results, two methods were used. We compared determinate to indeterminate results as well as indeterminate to positive and negative results in a multinomial multivariable regression.
In the overall study population, factors found to be associated with indeterminate versus determinate results included HIV infection, presence of a BCG scar, and living in Khayelitsha for <1 year (Table 2). We examined the quantitative IFN-γ responses and found that all indeterminate results were due to low positive-control responses. Of these, 67% had a BCG scar. The quantitative antigen responses were examined in further detail, stratified by the presence or absence of a BCG scar. Table 3 demonstrates that the median antigen responses for indeterminate results were low and similar to IFN-γ-negative results. In the overall study population, 50% had a BCG scar; but this proportion was higher at 61% and 67% in persons with IFN-γ-negative and indeterminate results, respectively, than in IFN-γ-positive persons (43%).
Table 2.
Final model of predictors of indeterminate compared to determinate IGRA results
| Patient factor | Indeterminate vs determinate IFN-γ response |
||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| HIV infected | 2.36 | 1.10–5.08 | 0.028 |
| Presence of a BCG scar | 2.48 | 1.23–5.01 | 0.011 |
| Lived in Khayelitsha for >1 year | 0.30 | 0.11–0.80 | 0.016 |
Table 3.
Summary of quantitative IGRA antigen responses stratified by BCG scar status
| IFN-γ response | Median quantitative response (pg/ml [IQR]) |
|||||
|---|---|---|---|---|---|---|
| No BCG scar |
With BCG scar |
|||||
| ESAT-6 | CFP-10 | FPa | ESAT-6 | CFP-10 | FP | |
| Indeterminate | 0 (0–330) | 0 (0–115) | 0 (0–0) | 0 (0–165) | 0 (0–9) | 0 (0–579) |
| Negative | 72 (0–189) | 0 (0–56) | 62 (0–133) | 0 | 0 | 0 |
| Positive | 260 (863–5,085) | 1,189 (304–3,579) | 1,968 (576–4,819) | 1,106 (0–3,005) | 722 (148–2,536) | 1,808 (642–4,049) |
FP, fusion protein.
The multinomial model showed that the significant effects observed in the combined model were attributable to a comparison between indeterminate and positive IFN-γ results. Table 4 shows a comparison between indeterminate and positive IFN-γ results. A comparison of indeterminate and negative IFN-γ results showed no significant variables (data not shown).
Table 4.
Final model of predictors of indeterminate compared to positive IGRA results using multinomial regression
| Patient factor | Indeterminate vs positive IFN-γ response |
||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| HIV infected | 2.55 | 1.17–5.53 | 0.018 |
| Presence of a BCG scar | 3.02 | 1.48–6.16 | 0.002 |
| Lived in Khayelitsha for >1 yr | 0.29 | 0.10–0.82 | 0.020 |
In HIV-infected persons, factors that associated with indeterminate versus determinate results were similar to the overall model, with the presence of a BCG scar being associated with a 3-fold increase in odds of indeterminate results and persons living in Khayelitsha longer than 1 year having 74% lower odds of an indeterminate result (Table 5). Recent migrants were more likely to be HIV infected than longer-term residents (82% versus 60%, respectively). Stratifying HIV-infected persons by time in Khayelitsha, the median CD4 counts were similar in recent and long-term residents (422 [IQR, 248 to 513] and 345 [IQR, 230 to 498], respectively; P = 0.542), suggesting that higher rates in indeterminate results in recent migrants cannot be attributed to more advanced immunosuppression. In addition, we stratified all other variables by length of stay in Khayelitsha (less than 1 year/at least 1 year) and found that recent migrants had a significantly shorter median period since HIV diagnosis (38 [IQR, 15 to 178] days versus 348 [IQR, 18 to 1,411] days). They were also more likely to be HIV infected than longer-term residents. However, the proportions of participants living in informal accommodation and employment rates were similar in both groups.
Table 5.
Final model of predictors of indeterminate IGRA results in HIV-infected persons
| Patient factor | Indeterminate vs determinate IFN-γ response |
||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| CD4 count of >200 | 0.21 | 0.09–0.48 | <0.001 |
| Presence of a BCG scar | 3.05 | 1.30–7.17 | 0.002 |
| Lived in Khayelitsha for >1 yr | 0.22 | 0.07–0.68 | 0.009 |
In addition, among HIV-infected persons, a lower CD4 count was associated with higher risk of an indeterminate result, with an 80% lower risk of having an indeterminate result in those with CD4 counts of >200 (Table 5).
The multinomial model resulted in the same significant variables as the model that combined negative and positive results, with significant effects observed also being attributable to a comparison between indeterminate and positive IFN-γ results (Table 6).
Table 6.
Final model of predictors of indeterminate compared to positive IGRA results using multinomial regression in HIV-infected persons
| Patient factor | Indeterminate vs positive IFN-γ response |
||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| CD4 count of >200 | 0.16 | 0.07–0.40 | <0.001 |
| Presence of a BCG scar | 3.64 | 1.52–8.74 | 0.004 |
| Lived in Khayelitsha for >1 yr | 0.20 | 0.06–0.68 | 0.010 |
DISCUSSION
We describe, in this study, factors that associate with indeterminate IFN-γ responses. HIV is known to increase the risk of indeterminate results either through a high nil background or low positive-control responses. Our data are consistent with existing literature with regard to an association between low CD4 counts in HIV-coinfected persons and higher odds of indeterminate IFN-γ results.
A study in Cape Town, South Africa, examined healthy adults and found a decline in TST reactivity in HIV-infected persons (median CD4 count, 392 cells/mm3) compared with HIV-uninfected persons from the same community but no similar decline in response in the HIV-infected subjects tested by both commercial IGRA (19). The frequencies of positive T-SPOT.TB results were comparable across different degrees of immunosuppression, while the proportion of positive results of the QFT-GIT test were lower in those with more advanced HIV infection.
In a setting characterized by a high HIV burden, the lower sensitivity of QFT-GIT in HIV-infected persons has been noted to be due to a higher rate of indeterminate results, and no difference in sensitivity was observed when these results were excluded from analysis (1). A meta-analysis by Diel and colleagues reported the rate of indeterminate results to vary from 2.1% for the QFT-GIT to 3.8% for the T-SPOT.TB test, increasing to 4.4% and 6.1%, respectively, in immunocompromised persons (8). Other studies conducted in high-burden settings have shown similar results, with a decrease in sensitivity in HIV-infected persons (3, 18), while some studies have shown no association between degree of immunosuppression and rates of indeterminacy (11).
Among HIV-infected persons, previous studies have found an association between indeterminate IFN-γ results and advanced age, lower CD4 counts, and hypoalbuminemia (2, 12). Unexpectedly in this study, the presence of a BCG scar was associated with a 3-fold increase in odds of an indeterminate versus positive IFN-γ response. This could represent a limitation on the use of IGRA in persons with a BCG scar. Interestingly, the majority of indeterminate responses had low enough ESAT-6, CFP-10, and fusion protein responses to be classified negative, as shown in Table 3.
Another unexpected finding was that recent migrants to Khayelitsha (<1 year) had a higher risk of indeterminate than positive results. Although a higher proportion of recent migrants were HIV infected, this factor remained significant in the HIV-infected subgroup. The reason for this finding is unclear. The population of Khayelitsha is fairly mobile, with a constant (in and out) flux of new residents from the rural parts of South Africa as well as other parts of the continent. One explanation is that more recent migrants represent a more marginalized and vulnerable population living in more informal settings. Although our questionnaire ascertained whether participants lived in a permanent building or a shack, we did not collect data on the degree of informality of the shacks, as squatter camps exist in Khayelitsha with little or no access to basic hygiene or sanitation. This, along with increased chances of being malnourished, could increase the risk of indeterminate results. A study has reported an association between malnutrition and increased risk of indeterminate IGRA results in infants (6). Another study in adult patients with inflammatory bowel disease reported associations between indeterminate IGRA results and low serum albumin levels, a marker of malnutrition (17). In this study, there was no association between BMI and indeterminacy (median BMI, 25.3, 25.0, and 25.8 for negative, positive, and indeterminate IGRA results, respectively). South Africa is rapidly urbanizing, with changes in lifestyle behavior resulting in reduced physical activity and increasing prevalence of obesity across socioeconomic groups. The BMI is therefore likely to be a poor measure of malnutrition in adults in this setting.
Limitations.
The use of an in-house IGRA limits the ability to generalize from these findings. However, a comparison was carried out against the commercial QFT-GIT assay on healthy donors. The proportion of donors scoring positive to any antigen was similar to the proportion scoring positive by the QFT-GIT, suggesting comparability between these tests.
Another potential limitation in this study is the accuracy of recording the presence of a BCG scar. Previous studies conducted in this and other high-burden settings have found variability in the measurement of scars in both left and right deltoid regions and reported no association between TST positivity and the presence of a BCG scar (14, 16, 19).
Conclusion.
The finding of a positive association between HIV and indeterminate IFN-γ results is expected and congruent with existing literature. The presence of a BCG scar was also positively associated with indeterminate IFN-γ results; further analysis revealed that TB-specific antigen responses in subjects with indeterminate results and BCG scars were significantly lower than in those without BCG scars, suggesting that these were more likely to reflect a negative IGRA and a protective effect of BCG from LTBI. A recent move to Khayelitsha was associated with a higher risk of indeterminate IFN-γ results. These findings highlight the limitation in the performance of IGRA in these subpopulations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kees Franken for producing the fusion protein.
R.J.W. is funded by the Wellcome Trust (grants 084323 and 088316) and MRC (United Kingdom). This study was supported by the ILULU Consortium, which is funded by a grant from the European Union (Sante/2006/105-061).
The funders did not play a role in the design of the study or preparation of the manuscript.
T.O. was responsible for data collection, data analysis, and writing of the manuscript. H.P.G., N.B., R.S., and K.W. were responsible for laboratory processing of specimens. R.T. contributed to data collection. R.T.G. contributed to data collection and study coordination. T.H.M.O. supervised preparation of the fusion protein and contributed to the writing of the manuscript. K.A.W. supervised laboratory processing and contributed to the writing of the manuscript. R.J.W. contributed to writing of the manuscript.
Footnotes
Published ahead of print 20 June 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Aabye MG, et al. 2009. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS One 4:e4220 doi:10.1371/journal.pone.0004220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aichelburg MC, et al. 2009. Detection and prediction of active tuberculosis disease by a whole-blood interferon-γ release assay in HIV-1-infected individuals. Clin. Infect. Dis. 48:954–962 [DOI] [PubMed] [Google Scholar]
- 3. Aichelburg MC, et al. 16 May 2012. Prognostic value of indeterminate interferon-γ release assay results in HIV-1-infection. J. Clin. Microbiol. doi:10.1128/JCM.01054-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman ALN, et al. 2002. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 16:2285–2293 [DOI] [PubMed] [Google Scholar]
- 5. Connell TG, et al. 2007. Enhanced ex vivo stimulation of Mycobacterium tuberculosis-specific T cells in human immunodeficiency virus-infected persons via antigen delivery by the Bordetella pertussis adenylate cyclase vector. Clin. Vaccine Immunol. 14:847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connell TG, et al. 2010. Indeterminate interferon-γ release assay results in children. Pediatr. Infect. Dis. J. 29:285–286 [DOI] [PubMed] [Google Scholar]
- 7. Desem N, Jones SL. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diel R, Loddenkemper R, Nienhaus A. 2010. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a meta-analysis. Chest 137:952–968 [DOI] [PubMed] [Google Scholar]
- 9. Franken KLMC, et al. 2000. Purification of his-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr. Purif. 18:95–99 [DOI] [PubMed] [Google Scholar]
- 10. Getahun H, et al. 2011. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 8:e1000391 doi:10.1371/journal.pmed.1000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hang NTL, et al. 2011. Analysis of factors lowering sensitivity of interferon-γ release assay for tuberculosis. PLoS One 6:e23806 doi:10.1371/journal.pone.0023806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobashi Y, et al. 2009. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur. Respir. J. 33:812–815 [DOI] [PubMed] [Google Scholar]
- 13. Leidl L, et al. 2010. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur. Respir. J. 35:619–626 [DOI] [PubMed] [Google Scholar]
- 14. Mahomed H, et al. 2006. Comparison of Mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int. J. Tuberc. Lung Dis. 10:310–316 [PubMed] [Google Scholar]
- 15. Médecins Sans Frontierès 2011. Summary: Khayelitsha 2001–2011 activity report—10 years of HIV/TB care at primary health care level. Médecins Sans Frontierès, Sea Point, South Africa: http://www.msf.org.za/publication/summary-khayelitsha-activity-report-2001-2011 [Google Scholar]
- 16. Pai M, et al. 2005. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 293:2746–2755 [DOI] [PubMed] [Google Scholar]
- 17. Papay P, et al. 2011. Predictors of indeterminate IFN-γ release assay in screening for latent TB in inflammatory bowel diseases. Eur. J. Clin. Invest. 41:1071–1076 [DOI] [PubMed] [Google Scholar]
- 18. Raby E, et al. 2008. The effects of HIV on the sensitivity of a whole blood IFN-γ release assay in Zambian adults with active tuberculosis. PLoS One 3:e2489 doi:10.1371/journal.pone.0002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rangaka MX, et al. 2007. Effect of HIV-1 infection on T-cell-based and skin test detection of tuberculosis infection. Am. J. Respir. Crit. Care Med. 175:514–520 [DOI] [PubMed] [Google Scholar]
- 20. Samandari T, et al. 2011. 6-Month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 377:1588–1598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.