Abstract
The aim of this study was to estimate the rate of misclassification in treated HIV patients who initiated treatment at the chronic stage of HIV infection using an enzyme immunoassay (EIA) that discriminates between recent infection (RI; within 6 months) and established infection. The performance of EIA-RI was evaluated in 96 HIV-1 chronically infected patients on highly active antiretroviral therapy (HAART) with an undetectable viral load (VL) for at least 3 years. Demographic data, HIV-1 viral load, CD4+ T-cell count, viral subtype, and treatment duration were collected. The subset of misclassified patients was further analyzed using samples collected annually. The impact on incidence estimates was evaluated by simulation. The specificity in treated patients was significantly lower (70.8 to 77.1%) than that observed in untreated patients (93.3 to 99.3%, P < 0.001). Patients falsely classified as recently infected had been treated for a longer period and had longer-term viral suppression than those correctly classified. The loss of specificity of the test due to treatment may have a dramatic impact on the accuracy of the incidence estimates, with a major impact when HIV prevalence is high. The cross-sectional studies intended to derive HIV incidence must collect information on treatment or, alternatively, should include detection of antiretroviral drugs in blood specimens to rule out treated patients from the calculations.
INTRODUCTION
Monitoring the incidence of human immunodeficiency virus type 1 (HIV-1) infections is critical both for surveillance of the epidemic and evaluation of prevention programs. The concept of immunoassays for recent infections, named serological testing algorithm for recent HIV seroconversion (STARHS), was introduced more than 10 years ago by Janssen et al. (9) and, since then, has been considered a major tool allowing the estimation of HIV incidence in cross-sectional studies (for recent reviews, see references 4, 6, and 16). Although several technical approaches have been used, the shared rationale for recent infection testing algorithms (RITA) is to discriminate recent from long-standing infections based on maturation of HIV-specific antibody responses, predominantly using the measurement of antibody levels or antibody avidity toward major antigenic proteins or epitopes of HIV-1 (2, 9, 18, 20, 24, 28, 31). Several limitations of the RITA have been reported regularly, and there have been debates about their real validity and, hence, their value for incidence measurements (6). Among these limitations, the interfering effect of highly active antiretroviral treatment (HAART) has been clearly documented when HAART was initiated in patients with primary HIV-1 infection and, also, in patients with chronic infection (2, 8, 17, 25). By stopping the viral replication, the early virostatic treatment may prevent the development of the HIV-1-specific antibody response, either quantitatively (antibody level) or qualitatively (avidity), leading to an unacceptably high rate of false-recent results in samples collected more than 1 year after infection (2, 8, 25). In accordance with this, although not analyzed in the context of RITA, it was previously reported that even entire seroreversion could be reached when HAART is initiated during acute/early HIV infection, suggesting that ongoing antigenic stimulation may be required to maintain HIV-1-specific humoral responses (7, 10, 12). The effect of HAART on RITA results when treatment is initiated later during the course of HIV-1 infection, when the antibody level has already plateaued at a high level, has been much less well documented. However, this corresponds to the most frequent timing for treatment initiation, as considered by international guidelines, when CD4+ cells reach a threshold of 350 to 500/μl (27). Although seroreversion seems to be a very rare event when HAART is initiated at the chronic stage of infection (1), it would be useful to estimate the false-recent rate in such patients in order to document the level of impairment of incidence estimation based on RITA results. Such knowledge is essential when incidence estimations are provided through cross-sectional studies where the information on antiretroviral treatment is not routinely collected.
For several years, we have been using an enzyme immunoassay for recent infection (EIA-RI) to monitor the dynamics of the HIV epidemic at a national level in France (2, 13, 14, 21, 26). Until now, the EIA-RI has been used only for surveillance of new diagnoses, and thus, in untreated patients (13, 14, 21, 26). We undertook the present study to document the false-recent rate of the EIA-RI and the consequence for incidence estimation if it were used in typical cross-sectional samples of populations, including treated patients.
(Presented in part at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 27 February to 2 March 2011.)
MATERIALS AND METHODS
Study population.
We used cryopreserved plasma samples from 96 HIV-1 patients regularly followed up at the Infectious Department of the Tours University Hospital. All patients had been under HAART for at least 3 years and initiated treatment at the chronic phase of infection. Because our aim was to evaluate the false-recent rate of the EIA-RI, we selected patients who would be representative of a worst-case scenario, i.e., patients with a sustained undetectable viral load defined initially as <1.6 log10 copies/ml (i.e., <40 copies/ml) at two 3-year-distant time points (2006 and 2009). Seventy-three and 19 patients were infected by B viruses and non-B viruses, respectively (Table 1). The subtype of the infecting strain was not identified for the remaining 4 patients. The following complementary data were collected: age, gender, minimum duration of HIV infection, duration on HAART, CD4+ T-cell count, and viral load (VL) during HAART (Table 1). The route of infection and nature of antiretroviral combination were also collected. Although the 96 patients were initially considered to have an undetectable VL for at least 3 years, three subgroups were defined based on viral load during the entire period. Forty-two patients were defined as viral suppressors (VL of <40 copies/ml with no residual viremia at any time point; “undetectable”), and 45 were defined as viremic controllers (intermittent residual viremia of <40 copies/ml; “residual viremia”). Albeit considered eligible because they had viremia of <40 copies/ml at the two initial time points for selection (2006 and 2009), 9 patients presented occasional viral rebounds (<1,000 copies/ml; “viral blippers”) during the follow-up (Table 1). Ethics approval of the study was obtained from the Comité de Protection des Personnes of the Région Centre (CPP, Tours, France).
Table 1.
Characteristics of patients on HAART and recent-infection classification according to EIA-RI measuring either IDE-V3 or IDE alonea
| Characteristic | Overall (n = 96) | No. of patients with indicated RI classification according to EIA-RI measuring: |
|||||
|---|---|---|---|---|---|---|---|
| IDE and V3 |
IDE only |
||||||
| Misclassified (n = 28) | Correctly classified (n = 68) | P value | Misclassified (n = 22) | Correctly classified (n = 74) | P value | ||
| Age (yr) | 50 (47–52) | 56 (52–59) | 48 (45–51) | NS | 56 (52–59) | 48 (45–51) | NS |
| Sex ratio (male/female) | 2.3 (67/29) | 6.0 (24/4) | 1.7 (43/25) | 0.029 | 4.5 (18/4) | 2.0 (49/25) | NS |
| CD4+ T-cell count: | |||||||
| At inclusion (cells/μl) | 417 (360–473) | 431 (316–545) | 399 (359–549) | NS | 418 (364–563) | 412 (398–530) | NS |
| At last follow-up (cells/μl) | 518 (461–574) | 467 (278–519) | 531 (504–635) | NS | 458 (375–672) | 531 (478–605) | NS |
| HAART duration (mo) | 138 (128–147) | 155 (140–171) | 123 (111–135) | 0.02 | 156 (133–171) | 123 (110–135) | 0.02 |
| Minimum duration of infection (mo) | 183 (163–193) | 181 (160–207) | 186 (159–197) | NS | 189 (157–211) | 178 (158–193) | NS |
| Strict viral suppression (mo) | 61 (56–69) | 80 (70–94) | 56 (51–65) | 0.005 | 95 (83–112) | 57 (51–66) | 0.002 |
| Virological suppression [no. (%)] | |||||||
| Viral rebound | 9 | 1 (11.1) | 8 (88.9) | 1 (11.1) | 8 (88.9) | ||
| Residual viremia | 45 | 14 (31.1) | 31 (68.9) | NS | 9 (20.0) | 36 (80.0) | NS |
| Strict viral suppressors | 42 | 13 (31.0) | 29 (69.0) | 12 (28.6) | 30 (71.4) | ||
| Virus subtype [no. (%)] | |||||||
| B | 73 (76.0) | 22 (30.1) | 51 (69.9) | 17 (23.3) | 56 (76.7) | ||
| Non-B | 19 (19.8) | 5 (26.3) | 14 (73.7) | NS | 4 (21.1) | 15 (78.9) | NS |
| Nontypeable | 4 (4.2) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 3 (75.0) | ||
The specificity of the EIA-RI in this treated population was compared to that observed in 143 never-treated chronically infected patients at the clinical AIDS stage and 150 never-treated patients with chronically established infection not suffering from AIDS from the French ANRS SEROCO and HEMOCO cohorts (5). Ethics approval for use of the ANRS cohorts was obtained from the Commission Nationale de l'Informatique et des Libertés. Written informed consent was obtained from each participant enrolled in the ANRS cohorts.
EIA-RI testing.
The EIA-RI test was developed initially to detect recent HIV infection through an algorithm that combined standardized measures of antibody binding to both the immunodominant epitope (IDE) of gp41 and the V3 region of gp120 (2). At that time, although IDE was the most discriminatory antigen, we included antibody binding measures for both IDE and V3. The properties of the assay were modeled as a function of time using logistic regression. Recent infection was defined as being infected for less than 180 days, and the biomarker threshold was estimated for the specific purpose of classification according to time since infection. The result was expressed as an IDE-V3 formula (P value) ranging from 0 to 1, with a cutoff value of 0.5. Infection was defined as recent if the P value was under 0.5, and patients with P value of >0.5 were considered chronically infected (>180 days) (2). Secondarily, to perform an incidence estimation based on the test results, we calibrated the duration of the assay window period by modeling the growth of IDE measures only (13). Using IDE only, the result was expressed as a normalized ratio of the optical density (ODspecimen/ODnegative control). Samples with a ratio of less than 15.4 were classified as recent infections, and those with values of >15.4 were classified as long-term infections. For the present study, we analyzed the data using the two methods, P value based on both IDE and V3 values and IDE ratio.
The plasma samples from the 96 HIV-1 chronically infected patients on HAART collected at the last follow-up were tested first. The subset of patients who were misclassified as recently infected at the last sample (2009) were further tested by EIA-RI in the same run using samples collected 3 years earlier (2006) and 2 intermediate samples collected at approximately 12-month intervals (2007 and 2008) in order to analyze the kinetics of the EIA-RI results for each patient.
Statistical analysis and modeling.
Data were analyzed with R statistical software (22). Chi-square tests for comparison of specificity and sex ratio were conducted with P values from the standard chi-square distribution. We used the parametric Student's t test and nonparametric Pearson's chi-square test to compare parameters associated with false-recent results. We used 5% significance levels for all comparisons.
In order to assess the impact on incidence estimates of using a test that has decreased specificity in individuals on ART, we simulated a cross-sectional population fulfilling the steady-state assumptions: the total population of infected and uninfected subjects remains constant over time and incidence remains constant over time. We differentiated the HIV-positive population into four categories by combining two possible states: being diagnosed or undiagnosed and being recently or not recently infected. We assumed that only those diagnosed could be on treatment. The fractions of diagnosed cases among recently and not recently infected individuals, equal to 0.26 and 0.86, respectively, were those obtained from the Prevagay study, a cross-sectional serosurvey carried out in Paris in men having sex with men (MSM) attending gay venues (29). We set the sample size at 1,000 individuals, corresponding approximately to that of the Prevagay study, and we let either the “true” underlying incidence or the “true” underlying prevalence vary and fixed the other value to either a prevalence of 15% or an incidence of 4 per 100 person-years, similar to what has been observed in the Prevagay study. We also let the proportion of diagnosed HIV-infected individuals under effective antiretroviral treatment vary. We calculated the expected number of recent infections as a function of the true incidence rate by inverting the formula given by Welte et al. (30) as follows: I = [P − (ε/1 − ε)N]/[ωS], where I is the incidence rate, P is the number of recent infections, ε is the proportion of infected individuals that do not progress out of the recent state (false-recent rate), N is the number of nonrecent infections, ω is the mean duration spent in recent infection for individuals who progress (mean RITA duration), and S is the number of susceptible individuals (HIV negative). We used the calibration parameters estimated for the EIA-RI test for the modeling of population-based incidence in France (13) and accounted for the misclassification due to effective treatment by introducing the specific false-recent rate determined in the present study. As a measure of the impact of ignoring the “treatment effect” on incidence estimation, we estimated the bias as the relative difference between true and observed incidence.
RESULTS
False-recent rate in chronically infected HIV-1 patients under treatment.
Based on the IDE-V3 formula, 28 of the 96 patients on HAART with viral suppression for more than 3 years were falsely classified as recently infected (Table 1). Specificity in treated patients was therefore 70.8%, significantly lower than that observed in 150 untreated chronically infected patients without AIDS (99.3%; P < 0.001) or 143 patients suffering from AIDS (93.3%; P < 0.001). Based on the IDE ratio threshold, 22 patients were falsely classified as recently infected. Specificity in treated patients was therefore 77.1% (false-recent rate = 22.9%), compared to 99.3% in untreated patients without AIDS (P < 0.001) or suffering from AIDS (95.8%; P < 0.001). The 22 samples misclassified based on the IDE-only assay were all included in the 28 misclassified samples based on the IDE-V3 formula. The distributions of the EIA-RI values (P value for the IDE-V3 formula or IDE specimen/negative-control ratio) are shown in Fig. 1.
Fig 1.
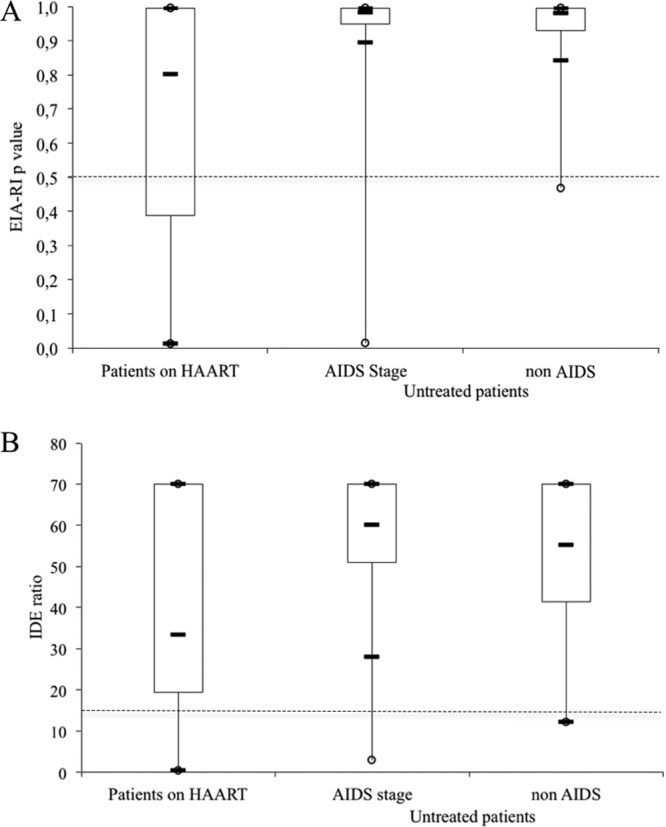
Distribution of the EIA-RI values determined with the IDE-V3 formula (A) or IDE ratio (B). Horizontal black bars denote the 10th, 50th (median), and 90th percentiles. Boxes represent the 25th to 75th percentiles. The threshold is shown by the dotted line.
The kinetics of the EIA-RI values were analyzed over a 3-year period for all the patients in whom a false-recent result was identified for the last sample. A regular decrease of the EIA-RI values was observed for all 28 misclassified patients (Fig. 2). Twelve of the 28 patients misclassified at the last follow-up were above the 0.5 threshold (IDE-V3 assay) when tested 3 years earlier (Fig. 2A). Albeit continuing to decrease, the P values (IDE-V3 assay) were already below the threshold 3 years earlier for the 16 other patients. A similar trend was observed when using the IDE-only ratio, 14 of the 22 falsely classified patients being correctly classified 3 years earlier (Fig. 2B). These results are consistent with a continuous decrease in the specificity of the EIA-RI when patients remain under efficient treatment, from 85.4% to 70.8% 3 years later when using the IDE-V3 formula and from 91.2% to 77.1% when using the IDE ratio alone.
Fig 2.
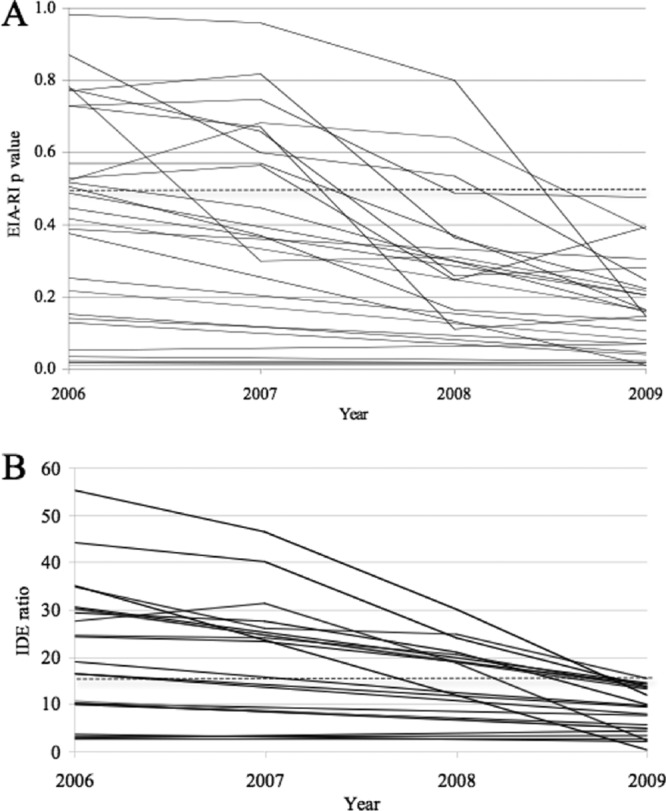
Kinetics of EIA-RI values over a 3-year period among patients classified as “false recent” at the last sample, determined using the IDE-V3 formula (A) or the IDE ratio (B). The threshold is shown by the dotted line.
Parameters associated with false-recent results in treated patients.
There was no statistically significant difference between the patients with a false-recent result and those correctly classified at the last follow-up visit according to age, the viral subtype, the CD4+ T-cell counts either at the last follow-up or 3 years earlier, or the duration of infection (Table 1). We observed a statistically significant difference according to the treatment duration and viral suppression duration: patients falsely classified as recently infected had been treated for a longer period than those correctly classified (median, 155 versus 123 months, respectively, P = 0.02) and had longer-term viral suppression (median, 80 versus 56 months, respectively, P = 0.005) when using the IDE-V3 formula. Similar significant differences were observed when using the IDE ratio alone (Table 1). We observed a statistically significant difference according to gender but only when considering the combined IDE-V3 assay (P = 0.029). The relative risk of being misclassified with this assay was 1.39 (95% confidence interval, 1.07 to 1.69). However, due to the nonsignificant difference in the IDE-only assay and the limited population size (only 4 women among the misclassified samples), this difference remains questionable.
Because sustained viral suppression could influence the EIA-RI results, we took into account all VLs measured during the last 3 years of follow-up in the 96 HIV-1-infected patients on HAART. Based on the IDE-V3 formula, the false-recent rate was similar for patients who were classified as viral suppressors (13/45 [31.1%]) and those classified as viremic controllers (14/42 [31.0%]). In contrast, among the 9 patients with viral rebounds (blips of <1,000 copies/ml), only 1 (11.1%) had a false-recent result. The same trend was observed when using the IDE ratio alone (Table 1).
Consequences for incidence estimates.
The bias in incidence estimates expected in a partly treated population where treatment information is not available is shown in Fig. 3. In these contour plots, the iso-line represents the change in bias as a function of the proportion of the diagnosed-positive population under effective treatment and of either the incidence (Fig. 3A) or prevalence (Fig. 3B) level. Incidence varied from 0 to 5 per 100 person-years while prevalence was kept at 15%, as shown by the results in Fig. 3A. Prevalence varied from 0 to 20% while incidence was kept constant at 4 per 100 person-years, as shown by the results in Fig. 3B. As an example, with a true incidence of 4 per 100 person-years and a true prevalence of 15% (figures observed in the MSM Prevagay study), if 30% of the individuals diagnosed as positive receive an effective treatment, both figures show that the incidence would be overestimated by 50%. The data in Fig. 3A indicate that with even a modest level of ART coverage (from a few percentage points and up), when the prevalence is high in the population, fixed at 15%, there is almost no way to estimate precisely the incidence (with a bias below 5 or 10%). In contrast, the results in Fig. 3B show that the incidence can be estimated with a bias below 5%, irrespective of the treatment coverage, if prevalence remains below 2 to 3%.
Fig 3.
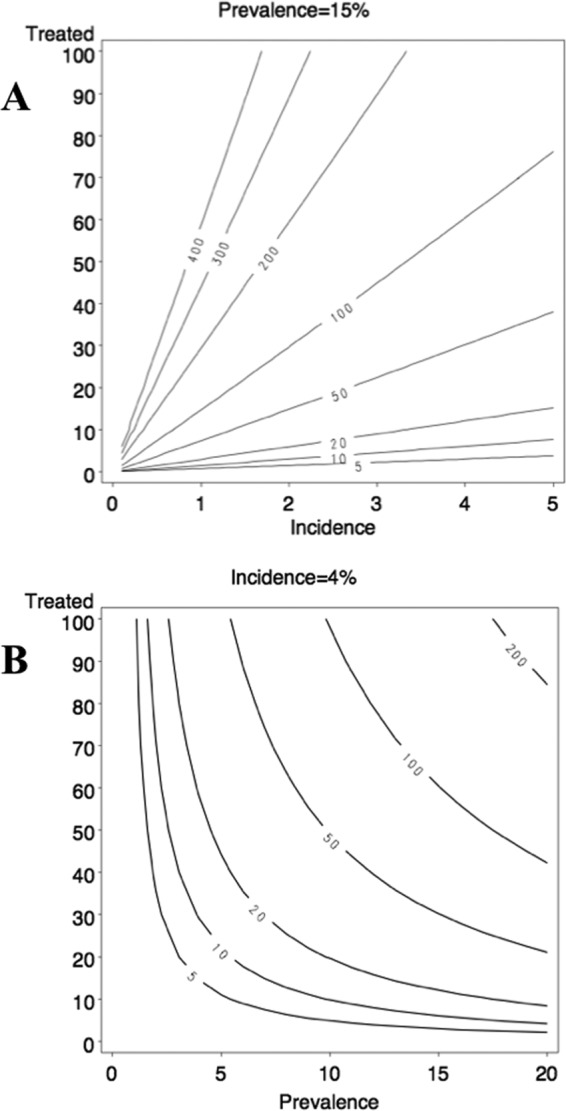
Simulations of the bias in incidence according to the fraction of the population that benefits from HAART. (A) Incidence was varied from 0 to 5 per 100 person-years, while prevalence was fixed at 15%. (B) Prevalence was varied from 0 to 20%, while incidence was fixed at 4 per 100 person-years. Values on the lines of the plots represent the bias as the relative difference between true and observed incidence. y axes: percentage of the population that benefits from HAART.
DISCUSSION
The assays for recent HIV-1 infection have generated considerable interest due to the possibility that they offer to provide estimates of HIV incidence through cross-sectional studies. However, in addition to the variability of the immune response at the individual level among recently HIV-1-infected persons, these assays continue to be challenged by several interfering factors, such as reduced specificity in immunosuppressed patients at late-stage AIDS (2, 4, 9) and differential performances in populations with different genetic backgrounds or infected by different subtypes (3, 11, 14, 19, 23). It is also clearly shown that antiretroviral treatment initiated early after infection downregulates the HIV-specific antibody response, leading to a high rate of false-recent results (2, 25). The effect of ART on the false-recent rate in assays for recent infection when patients initiated treatment at the chronic stage of infection has been less clearly documented. A significantly diminished long-term specificity of the BED enzyme immunoassay was reported in a recent study performed in South Africa (15). In that study, the patients had a CD4+ T-cell count of less than 200/ml or suffered from an AIDS-defining condition to qualify for ART at a late stage, when the risk of a false-recent result is already high. The aim of our study was to evaluate the specificity of the EIA-RI assay in a less immunosuppressed population of HIV-1-infected patients in whom ART was initiated at the chronic stage of infection following the recommendations applied in developed countries, usually when CD4+ T-cell counts were higher than 200/ml. Because we wanted to challenge the impact of ART on the long-term specificity of the EIA-RI as much as possible in the context of a developed country, our strategy was to deliberately include cases representative of a worst-case scenario for the assay. All the cases that were included were patients with prolonged efficient viral suppression with an undetectable viral load (<1.6 log copies/ml) at two 3-year-distant time points.
We confirm a decreased specificity of the EIA-RI among patients with continuous suppressed viral replication on ART. Depending on the mode of interpretation of the assay (IDE-V3 formula or IDE ratio only), the specificity in treated patients was 70.8 to 77.1%, significantly lower than that observed in untreated patients without AIDS (99.3%) or suffering from AIDS (93.3 to 95.8%). The study also clearly showed that the combined EIA-RI assay based on the detection of antibodies directed to IDE and V3 was not more informative than the simpler assay that measures absorbance values with IDE only. This observation confirms the data presented in a previous work in which we selected the combined assay based on a slightly higher specificity for patients without AIDS, although both the sensitivity and the specificity of the combined assay and the IDE-only assay were almost the same for patients suffering from AIDS (2). The longitudinal analysis of samples from all the misclassified patients clearly demonstrated a continuous regular decrease of the EIA-RI values. The association of the false-recent results with the viral suppression was illustrated by a statistically significant difference according to the treatment duration and minimum duration of viral suppression: patients falsely classified as recently infected had been treated for a longer period than those correctly classified and had a longer viral suppression duration. We also showed that the false-recent rate was reduced in patients with viral rebounds (blips of <1,000 copies/ml) compared to the rate in patients who were classified as strict viral suppressors or viremic controllers, again suggesting the link between sustained viral suppression and decrease of the antibody level.
By simulating a cross-sectional study in which an incidence estimation based on recent infection testing would be performed, we showed that the loss of specificity of the test due to treatment may have a dramatic impact on the accuracy of the estimation. Because the source of false-recent cases is the long-term-infected population that is under treatment, the major impact is observed when HIV prevalence is high. It is important to note that we arbitrarily set the background incidence and prevalence level, as well as the proportion of diagnosed infections, at the levels observed in a recent survey of French gay men (29).
The reduced specificity of assays for recent infection is now well demonstrated in two situations, end-stage AIDS and prolonged efficient antiretroviral treatment initiated during primary infection or at the chronic phase of HIV disease. This reduced specificity implies that information on both clinical status (AIDS stage or CD4+ T-cell count of <200/ml) and treatment regimen must be collected to avoid biased incidence estimates. In cases of population-based surveys among newly diagnosed patients, patients have not yet been treated and incidence estimates can be performed with good accuracy if information on the clinical stage is collected (13). In contrast, when no historical records nor data on clinical stage nor treatment status are available in cross-sectional studies, a high risk of overestimation of HIV-1 incidence must be considered. Our data show that cross-sectional studies intended to derive HIV incidence must collect information on treatment or, alternatively, should include the detection of antiretroviral drugs in blood specimens to rule out treated patients from the calculation.
ACKNOWLEDGMENTS
The EIA-RI was developed with support from the Agence Nationale de Recherche sur le Sida et les Hépatites (ANRS, Paris, France). The National Reference Centre is funded by a grant from the Institut de Veille Sanitaire. The Institut de Veille Sanitaire is funded by the French Ministry of Health.
There are no conflicts of interest for any author.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Amor A, et al. 2006. Seroreversion of HIV antibodies in patients with prolonged suppression of viraemia under HAART. AIDS 20:1460–1462 [DOI] [PubMed] [Google Scholar]
- 2. Barin F, et al. 2005. Development and validation of an immunoassay for identification of recent human immunodeficiency virus type 1 infections and its use on dried serum spots. J. Clin. Microbiol. 43:4441–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bärnighausen T, et al. 2008. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS One 3:e3640 doi:10.1371/journal.pone.0003640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busch MP, et al. 2010. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24:2763–2771 [DOI] [PubMed] [Google Scholar]
- 5. Carré N, et al. 1998. Predictive value of viral load and other markers for progression to clinical AIDS after CD4+ cell count falls below 200/microL. SEROCO & HEMOCO Study Group. Int. J. Epidemiol. 27:897–903 [DOI] [PubMed] [Google Scholar]
- 6. Guy R, et al. 2009. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect. Dis. 9:747–759 [DOI] [PubMed] [Google Scholar]
- 7. Hare CB, et al. 2006. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin. Infect. Dis. 42:700–708 [DOI] [PubMed] [Google Scholar]
- 8. Hayashida T, Gatanaga H, Tanuma J, Oka S. 2008. Effects of low HIV type 1 load and antiretroviral treatment on IgG-capture BED-enzyme immunoassay. AIDS Res. Hum. Retroviruses 24:495–498 [DOI] [PubMed] [Google Scholar]
- 9. Janssen RS, et al. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280:42–48 [DOI] [PubMed] [Google Scholar]
- 10. Jurriaans S, et al. 2004. HIV-1 seroreversion in an HIV-1-seropositive patient treated during acute infection with highly active antiretroviral therapy and mycophenolate mofetil. AIDS 18:1607–1608 [DOI] [PubMed] [Google Scholar]
- 11. Karita E, et al. 2007. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS 21:403–408 [DOI] [PubMed] [Google Scholar]
- 12. Kassutto S, Johnston MN, Rosenberg ES. 2005. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin. Infect. Dis. 40:868–873 [DOI] [PubMed] [Google Scholar]
- 13. Le Vu S, et al. 2010. Population-based HIV-1 incidence in France, 2003-08: a modelling analysis. Lancet Infect. Dis. 10:682–687 [DOI] [PubMed] [Google Scholar]
- 14. Le Vu S, et al. 2009. Performance of an immunoassay at detecting recent infection among reported HIV diagnoses. AIDS 23:1773–1779 [DOI] [PubMed] [Google Scholar]
- 15. Marinda ET, et al. 2010. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 53:496–499 [DOI] [PubMed] [Google Scholar]
- 16. Murphy G, Parry JV. 2008. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 13(36):pii=18966. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18966 [PubMed] [Google Scholar]
- 17. Novitsky V, et al. 2009. Better control of early viral replication is associated with slower rate of elicited antiviral antibodies in the detuned enzyme immunoassay during primary HIV-1C infection. J. Acquir. Immune Defic. Syndr. 52:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parekh BS, Pau CP, Kennedy MS, Dobbs TL, McDougal JS. 2001. Assessment of antibody assays for identifying and distinguishing recent from long-term HIV type 1 infection. AIDS Res. Hum. Retroviruses 17:137–146 [DOI] [PubMed] [Google Scholar]
- 19. Parekh BS, et al. 2011. Determination of mean recency period for estimation of HIV type 1 incidence with the BED-capture EIA in persons infected with diverse subtypes. AIDS Res. Hum. Retroviruses 27:265–273 [DOI] [PubMed] [Google Scholar]
- 20. Parekh BS, et al. 2002. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res. Hum. Retroviruses 18:295–307 [DOI] [PubMed] [Google Scholar]
- 21. Pillonel J, et al. 2008. Human immunodeficiency virus type 1 incidence among blood donors in France, 1992 through 2006: use of an immunoassay to identify recent infections. Transfusion 48:1567–1575 [DOI] [PubMed] [Google Scholar]
- 22. R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 23. Sakarovitch C, et al. 2007. Do tests devised to detect recent HIV-1 infection provide reliable estimates of incidence in Africa? J. Acquir. Immune Defic. Syndr. 45:115–122 [DOI] [PubMed] [Google Scholar]
- 24. Schüpbach J, et al. 2007. Assessment of recent HIV-1 infection by a line immunoassay for HIV-1/2 confirmation. PLoS Med. 4:e343 doi:10.1371/journal.pmed.0040343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selleri M, et al. 2007. Effective highly active antiretroviral therapy in patients with primary HIV-1 infection prevents the evolution of the avidity of HIV-1-specific antibodies. J. Acquir. Immune Defic. Syndr. 46:145–150 [DOI] [PubMed] [Google Scholar]
- 26. Semaille C, et al. 2007. Monitoring the dynamics of the HIV epidemic using assays for recent infection and serotyping among new HIV diagnoses: experience after 2 years in France. J. Infect. Dis. 196:377–383 [DOI] [PubMed] [Google Scholar]
- 27. Sterne JA, et al. 2009. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 373:1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suligoi B, et al. 2003. Identifying recent HIV infections using the avidity index and an automated enzyme immunoassay. J. Acquir. Immune Defic. Syndr. 32:424–428 [DOI] [PubMed] [Google Scholar]
- 29. Velter A, Barin F, Bouyssou A, Le Vu S, Guinard J. 2010. Prévalence du VIH et comportement de dépistage des hommes fréquentant les lieux de convivialité gay parisiens, Prevagay 2009. Bull. Epidemiol. Hebdom. 45–46:464–467 [Google Scholar]
- 30. Welte A, McWalter TA, Laeyendecker O, Hallett TB. 2010. Using tests for recent infection to estimate incidence: problems and prospects for HIV. Euro Surveill. 15(24):pii=19589. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19589 [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson KM, et al. 2004. Incidence immunoassay for distinguishing recent from established HIV-1 infection in therapy-naive populations. AIDS 18:2253–2259 [DOI] [PubMed] [Google Scholar]


