Abstract
This retrospective study proposes a new reading of immunoblotting (IB) in the diagnosis of congenital toxoplasmosis. Our findings demonstrate that a three-IgM-band association at 75, 90, and 100 kDa called the IgM triplet increases the sensitivity to 95.8% when combined with prenatal and serological neonatal tests.
TEXT
Primary infection with Toxoplasma gondii during pregnancy can have severe consequences for the fetus such as miscarriage or severe neurological or ocular lesions (5, 10, 11, 14).
Detection of Toxoplasma gondii infection in pregnancy has been implemented at a national or regional level in several European countries (1) and is also performed worldwide at the initiative of clinicians. When seroconversion occurs, tests are performed to detect and treat congenital toxoplasmosis (CT) as early as possible in an attempt to reduce the severity of the disease (2, 3, 4, 10, 17, 18). In this way, CT diagnosis is confirmed if one of the following tests is positive: prenatal diagnosis based on the detection of Toxoplasma gondii in the amniotic fluid or neonatal diagnosis primarily based on the detection of neosynthesis of IgM and/or IgA specific antibodies in the infant serum (Table 1).
Table 1.
Contribution of each biological test for congenital toxoplasmosis diagnosis
| Biological test | No. of infected children with positive biological test (n = 118) |
|---|---|
| Prenatal diagnosis (amniotic fluid) (PCR targeting the 529-bp repeat element and/or gene B1) | 49 |
| Neonatal IgM serology (<1 mo of life) (Toxo-ISAGA [bioMérieux]; IgM Enzygnost Toxoplasmosis [Dade Behring]) | 54 |
| Neonatal IgA serology (<1 mo of life) (Toxo-ISAGA [bioMérieux]; ToxoIgA [SFRI]) | 56 |
| Persistence of IgG within 12 mo (VIDIA Toxo IgGII [bioMérieux]; VIDAS Toxo IgG [bioMérieux]; IgG Enzygnost Toxoplasmosis [Dade Behring]) | 18 |
| Immunoblot mother-infant profile comparison (Toxoplasma WB IgG IgM [LD-Bio]) | 93 |
This diagnosis can be done through comparison of IgG and IgM mother and infant serological profiles using immunoblotting (IB) (7, 13, 14): each additional band smaller than 120 kDa found in the infant serum but not in the maternal serum demonstrates a specific antibody synthesis by the neonate. However, the bands between 75 and 120 kDa are often disregarded (8, 12) due to a lower resolution reported by the manufacturer, leading to difficulties in interpreting immunoblot profiles that differ only by bands in this molecular mass range. This study aimed to assess the potential of 75- to 120-kDa-molecular-mass bands for the neonatal diagnosis of CT.
The study was performed on 236 mother-child serum pairs collected from 1999 to 2010 at the parasitology laboratories of the university hospitals of Lyon and Marseille. All mothers (n = 236) had acquired maternal Toxoplasma infection during pregnancy, and their children had therefore undergone postnatal follow-up to confirm (n = 118) or exclude (n = 118) congenital infection (5). The samples of the 118 infected children were consecutively collected from 1999 to 2010 whereas the samples of the 118 noninfected children were drawn randomly from all cases with CT excluded in the same period. In 145 cases, amniotic fluid had been sampled after 16 weeks of gestation and at least 4 weeks after the estimated date of maternal infection for PCR targeting the 529-bp repeat element (6, 21) and/or mouse inoculation. Comparison of mother and child profiles using cord blood (Marseille) or peripheral blood sampled at 3 days of life (Lyon) had been performed prospectively with the Toxoplasma WB IgG IgM assay (LD-Bio, Lyon, France) according to the manufacturer's recommendations. However, in our retrospective study immunoblot results were not taken into account and congenital infection was defined solely as the detection of Toxoplasma gondii in amniotic fluid or the presence of specific IgM and/or IgA antibodies after 10 days of life detected by immunocapture assay or the persistence of specific IgG at 1 year of age. Retrospective reading of all IgM and IgG mother-infant immunoblot pair profiles was performed by two independent operators who were unaware of the status of the child.
Characteristics of the congenital toxoplasmosis (CT) and no-congenital-toxoplasmosis (NCT) groups are detailed in Table 2.
Table 2.
Characteristics of congenital toxoplasmosis and no-congenital-toxoplasmosis groups
| Characteristic | No. of patients |
|
|---|---|---|
| Congenital toxoplasmosis | No congenital toxoplasmosis | |
| Total no. of patients | 118 | 118 |
| Gestational age at contamination (wk of gestation) | ||
| 0–13 | 8 | 62 |
| 14–26 | 53 | 47 |
| 27–39 | 57 | 9 |
| Prenatal diagnosis (amniotic fluid) | 61 | 84 |
| Immunoblot mother-infant profile pairs (cord blood or day 3 of life) | 118 | 118 |
| Neonatal serology (<1 mo of life) | 98 | 96 |
In the NCT group, no additional band was detected by IB in infants compared to their mothers, neither with IgG nor with IgM.
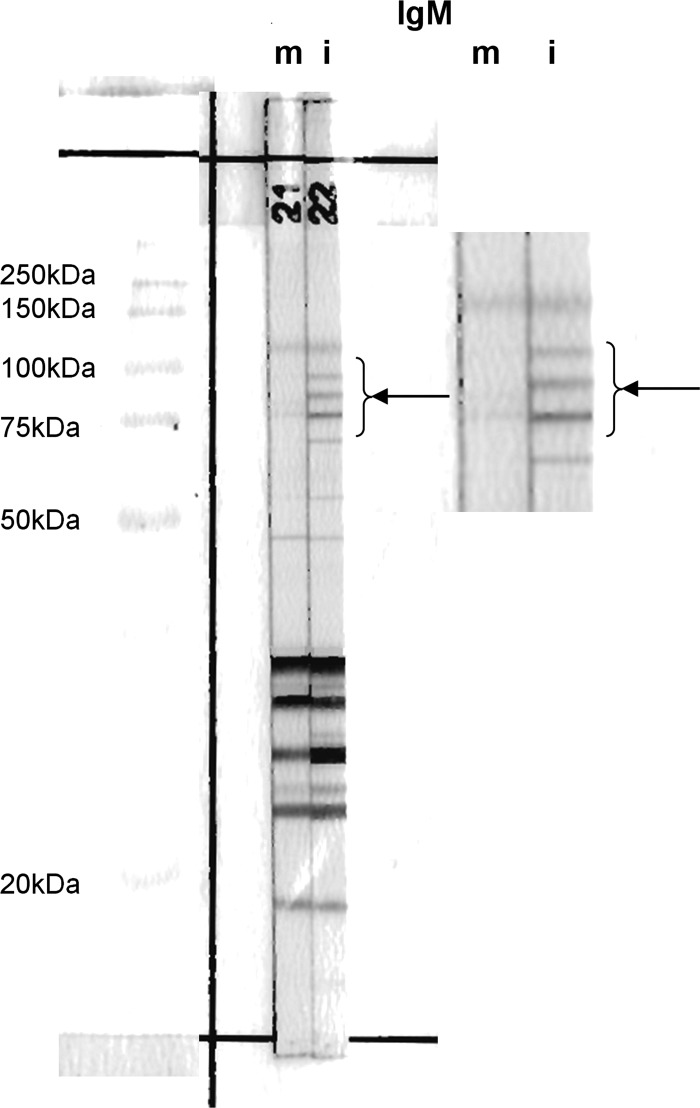
In the CT group, additional bands smaller than 75 kDa were observed for IgM in 51 newborns (43.2%), for IgG in 39 newborns (33.0%), and for both IgM and IgG in 67 newborns (56.8%). Regarding all immunoblot bands, including those of 75 to 120 kDa, additional bands were detected in 84 (71.2%) newborns by IgM and 43 (36.4%) newborns by IgG. Taken together, IgM and IgG immunoblot assays led to detection of neosynthesis of antibodies in 93 (78.8%) newborns. Among the latter, 57 (61.3%) had an IgM profile that included a specific set of 3 equidistant bands: 75, 90, and 100 kDa (Fig. 1). The presence of this IgM triplet enabled diagnosis in 25 infected cases (21.2%) for whom no differences could be detected based either on IgM molecular mass bands of less than 75 kDa or on IgG immunoblotting.
Fig 1.
IgM immunoblot analysis with paired mother and newborn sera from an infant congenitally infected with Toxoplasma gondii. Arrows show the IgM triplet defined by the association of 3 equidistant bands at 75, 90, and 100 kDa. (Left panel) The 90- and 100-kDa bands in the blot of the infant's serum were not present in the corresponding blot of the mother's serum. These supplementary bands were associated with the 75-kDa band in the IgM triplet. Other supplementary bands are present in low- and medium-molecular-mass bands in the blot of this infant's serum. (Right panel) Higher-magnification image from blot on the left highlighting the IgM triplet. m, mother; i, infant.
When considering the IgM triplet, the sensitivity of the immunoblot increased from 56.8% to 78.8%. Moreover, the sensitivity of CT diagnosis increased to 92.4% when prenatal diagnosis was combined with immunoblotting and to 95.8% with the addition of a conventional serological test (specific IgM/IgA) performed during the first month of life. Notably, consideration of the IgM triplet reduced the number of cases for whom CT could not be diagnosed within 1 month of life from 14 to 5 newborns. These five cases could be diagnosed, through the detection of IgM and IgG synthesis, at 6 (n = 1), 9 (n = 2), and 12 (n = 2) months of life.
Most of the infected newborns identified through prenatal screening have no clinical sign of congenital toxoplasmosis at birth (5, 9, 16, 20) but are at risk of developing retinal diseases later in life. Biological tests play a central role in identifying those who have congenital infection and need to be treated to reduce the risk of long-term sequelae (15) and those who do not warrant treatment but need to be followed up to exclude infection. Increasing the sensitivity of tests is an important challenge. It is recognized that the comparison of mother and infant IgG and IgM immunoblot profiles allows early neonatal diagnosis (19). However, due to the weak resolution of the immunoblot between 75 and 120 kDa, additional bands in the high-molecular-mass range (12) are often disregarded when comparing mother and child profiles. Our findings demonstrate that taking high-molecular-mass bands into account greatly improves the sensitivity of the test without yielding false-positive results. The biologists who interpret immunoblot profiles should be particularly aware of the great diagnostic value of this three-IgM-band association at 75, 90, and 100 kDa, called the IgM triplet. In our series, this specific association markedly improved the performance of this test with a resulting sensitivity of 95.8% when combined with prenatal and conventional serological neonatal tests without loss of specificity. Despite the significant progress brought about by the analysis of these additional immunoblot bands, postnatal follow-up remains, however, necessary in the first year of life to fully identify infected children.
Footnotes
Published ahead of print 13 June 2012
REFERENCES
- 1. Bénard A, et al. 2008. European Toxo Prevention Study Group (EUROTOXO). Survey of European programmes for the epidemiological surveillance of congenital toxoplasmosis. Euro Surveill. 13(15):18834 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bessières MH, et al. 2009. Diagnosis of congenital toxoplasmosis: prenatal and neonatal evaluation used in Toulouse University Hospital and incidence of congenital toxoplasmosis. Mem. Inst. Oswaldo Cruz 104:389–392 [DOI] [PubMed] [Google Scholar]
- 3. Buffolano W, et al. 2005. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 43:5916–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Carlo P, Romano A, Schimmenti MG, Mazzola A, Titone L. 2008. Materno-foetal Toxoplasma gondii: critical review of available diagnostic methods. Infez. Med. 16:28–32 [PubMed] [Google Scholar]
- 5. Faucher B, et al. 2012. Long-term ocular outcome in congenital toxoplasmosis: a prospective cohort of treated children. J. Infect. 64:104 doi:10.1016/j.jinf.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 6. Faucher B, Miermont F, Ranque S, Franck J, Piarroux R. 2012. Optimization of Toxoplasma gondii NA extraction from amniotic fluid using NucliSENS easyMAG and comparison with QIAamp DNA minikit. Eur. J. Clin. Microbiol. Infect. Dis. 31:1035–1039 [DOI] [PubMed] [Google Scholar]
- 7. Franck J, Mary C, Laugier M, Dumon H, Quilici M. 1990. Interest of Western blot technique in the diagnosis of congenital toxoplasmosis. Bull. Soc. Fr. Parasitol. 10:3–11 (In French.) [Google Scholar]
- 8. Franck J, Lanza-Silhol F, Vuillemot L, Pelissier V, Dumon H. 2000. Congenital toxoplasmosis diagnosis by immunoblot; a prospective study using a new commercial immunoblotting kit, abstract. In Niewiadomska K. (ed), VIII European Multicolloquium of Parasitology. Witold Stefanski Institute of Parasitology, Warsaw, Poland [Google Scholar]
- 9. Gilbert RE, et al. 2007. Screening for congenital toxoplasmosis: accuracy of immunoglobulin M and immunoglobulin A tests after birth. J. Med. Screen. 14:8–13 [DOI] [PubMed] [Google Scholar]
- 10. Gras L, et al. 2005. European Multicenter Study on Congenital Toxoplasmosis. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatr. 94:1721–1731 [DOI] [PubMed] [Google Scholar]
- 11. Kieffer F, et al. 2008. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr. Infect. Dis. J. 27:27–32 [DOI] [PubMed] [Google Scholar]
- 12. Machado AS, et al. 2010. IgG and IgM western blot assay for diagnosis of congenital toxoplasmosis. Mem. Inst. Oswaldo Cruz 105:757–761 [DOI] [PubMed] [Google Scholar]
- 13. Magi B, Migliorini L. 2011. Western blotting for the diagnosis of congenital toxoplasmosis. New Microbiol. 34:93–95 [PubMed] [Google Scholar]
- 14. Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554–566 [DOI] [PubMed] [Google Scholar]
- 15. Peyron F, et al. 2011. Long-term impact of treated congenital toxoplasmosis on quality of life and visual performance. Pediatr. Infect. Dis. J. 30:597–600 [DOI] [PubMed] [Google Scholar]
- 16. Pinon JM, et al. 2001. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M and A antibodies. J. Clin. Microbiol. 39:2267–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Remington JS, Thulliez P, Montoya JG. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert-Gangneux F, et al. 2010. Clinical relevance of placenta examination for the diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 29:33–38 [DOI] [PubMed] [Google Scholar]
- 19. Tissot Dupont D, et al. 2003. Usefulness of Western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:122–125 [DOI] [PubMed] [Google Scholar]
- 20. Villena I, et al. 1998. Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Reims Toxoplasmosis Group. Scand. J. Infect. Dis. 30:295–300 [DOI] [PubMed] [Google Scholar]
- 21. Wallon M, et al. 2010. Accuracy of real-time polymerase chain reaction for Toxoplasma gondii in amniotic fluid. Obstet. Gynecol. 115:727–733 [DOI] [PubMed] [Google Scholar]