Abstract
Vaccination of neonatal calves with Mycobacterium bovis bacillus Calmette-Guérin (BCG) induces a significant degree of protection against bovine tuberculosis, caused by infection with virulent M. bovis. In two independent experiments, we assessed the duration of the protective immunity induced in calves by neonatal vaccination with BCG Danish. Protection from disease was assessed at 12 and 24 months postvaccination in cattle challenged via the endotracheal route with M. bovis. We also assessed antigen-specific immune responses to assess their utility as correlates of protection. At 12 months postvaccination, significant reductions in lung and lymph node pathologies were observed compared to nonvaccinated M. bovis-challenged control cattle. At 24 months post-BCG vaccination, there was a reduction in lung and lymph node pathology scores and in bacterial burden. However, when comparing vaccinated and control groups, this did not reach statistical significance. Vaccination induced long-lived antigen (purified protein derivative [PPD])-specific gamma interferon (IFN-γ) release in whole-blood cultures, which remained above baseline levels for more than 20 months (approximately 90 weeks). The number of antigen-specific IFN-γ-secreting central memory T cells present at the time of M. bovis challenge was significantly higher in vaccinated than in control animals at 12 months postvaccination, but not at 24 months. Vaccination of neonatal calves with BCG Danish induced protective immune responses against bovine TB which were maintained for at least 12 months postvaccination. These studies provide data on the immunity induced by BCG vaccination in calves; the results could inform vaccination strategies for the control of bovine TB in United Kingdom cattle herds.
INTRODUCTION
Bovine tuberculosis (bTB) affects a significant number of cattle in the United Kingdom, with associated economic and animal welfare concerns. The incidence of bovine TB continues to rise, with more than 10% of cattle herds in England currently under TB restrictions. Currently, the diagnosis of bovine TB is by tuberculin skin test combined with ancillary blood tests to detect antigen-specific gamma interferon (IFN-γ) secretion. Cattle which test positive are subsequently culled from affected herds. However, the control of bTB is complicated by the presence of M. bovis-infected wildlife species, including the Eurasian badger, which act as reservoirs of infection. It is clear that improved control strategies are required to reduce the incidence of bTB.
Vaccination could be an important element of bovine TB control in the future. Currently, the only licensed vaccine against human TB is bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis. Alternative approaches to BCG vaccination have been assessed for efficacy in humans and in cattle (reviewed in references 6 and 23). However, it has been shown that the most effective strategies that induce protective immunity against bTB are those that include BCG, either alone delivered to calves or as the priming agent in heterologous prime-boost strategies (24, 30). Vaccination of badgers with injectable BCG has been shown in experimental studies to reduce the severity of disease and excretion of M. bovis (8). In field trials, BCG vaccination of badgers significantly reduced the number of badgers which tested positive for TB with the Stat-Pak antibody test, suggesting that vaccination controlled the progression of disease (8, 20). Vaccination of badgers with BCG has been deployed, on a limited basis, as a control mechanism in England since 2010. Importantly, vaccination of calves with BCG has been shown in a number of studies to induce significant protection against M. bovis infection (5, 7, 16, 34). Following vaccination with BCG Danish (the strain of BCG licensed for human, and now badger, vaccination), significant reductions in the number of TB lesions and the bacterial burden were observed in the lungs and respiratory tract-associated lymph nodes (LN) of M. bovis-challenged calves (16, 34).
Although a major constraint to the use of BCG as a cattle vaccine is interference with detection of bTB by tuberculin-based skin test and IFN-γ detection methods, diagnostic tests utilizing M. bovis-specific antigens can be employed to distinguish vaccinated from infected animals in tests for differentiating infected from vaccinated animals (DIVA tests) (3, 4, 9, 29, 31). The implementation of such tests could facilitate the deployment of BCG as a cattle vaccine for bTB control, subject to amendments in EU legislation.
An important consideration is the duration of immunity induced by BCG vaccination. We performed studies to determine the duration of protective immunity: calves were vaccinated with BCG and challenged with M. bovis 12 or 24 months postvaccination. We report here that reductions in pathology and bacterial burden were evident at 12 and 24 months following vaccination, with significant protection from M. bovis challenge evident at 12 months.
MATERIALS AND METHODS
Animals and experimental plan.
Cattle were British Holstein-Friesian calves (Bos taurus) bred at the Institute for Animal Health. The IAH herd has been confirmed free from bovine TB for more than 10 years. Two separate experiments, each with groups of age-matched vaccinated and control calves, were performed. Animals were vaccinated with BCG when between 10 days and 29 days of age (experiment 1: median age at vaccination, 17 days; range, 10 to 27 days; experiment 2: median age at vaccination, 25 days; range, 19 to 29 days). Controls were age-matched, nonvaccinated calves. Calves were assigned to groups (9 animals per group) according to age and preexisting responses to mycobacterial antigens (determined as purified protein derivative [PPD]-specific IFN-γ [see below]). Approximately 12 (experiment 1) or 24 (experiment 2) months after vaccination, cattle were transferred into a high-security Advisory Committee on Dangerous Pathogens (ACDP) category 3 animal housing unit at the Animal Health and Veterinary Laboratories Agency (AHVLA) in Weybridge and infected with M. bovis via the endotracheal route. Twelve weeks after infection, tuberculin skin tests were performed. The animals were killed 1 week later, and postmortem examinations were performed as described below. The experiments were approved by the local ethics committee according to national United Kingdom guidelines.
Bacteria and calf inoculations.
BCG Danish (batch numbers 104051C and 106052A, used in experiments 1 and 2, respectively) was purchased from Statens Serum Institut (SSI), Denmark, and reconstituted immediately prior to use in 1 ml Sauton medium. Calves were inoculated subcutaneously in the left shoulder with 0.5 ml (5 times the recommended human dose) of BCG Danish (16). This corresponds to a dose of 1 × 106 to 4 × 106 CFU according to the manufacturer's specifications. For challenge, M. bovis (strain AF 2122/97 [13]) was diluted immediately prior to inoculation from frozen stock in 7H9 medium. Cattle were inoculated endotracheally as previously described (30). The dose of M. bovis was confirmed by titration on modified 7H11 agar (12) and determined to be approximately 2 × 103 CFU per calf.
Postmortem examination and bacteriology.
Lymph nodes of the head (lateral and medial retropharyngeal, submandibular, and parotid) and thorax (caudal and cranial mediastinal, bronchial left and right, and cranial tracheo-bronchial), tonsils, nasal and tracheal mucosa, and pulmonary lobes were examined for gross lesions following the cutting of 0.5- to 1-cm slices. Macroscopic lesions were scored using the semiquantitative systems described here and previously (28).
(i) Lungs.
Lung lobes (left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic, and right accessory) were examined individually. For each lobe, the following scoring system was applied: 0, no visible lesions; 1, no gross lesions but lesions apparent upon slicing; 2, <5 gross lesions with diameters of <10 mm; 3, >6 gross lesions with diameters of <10 mm or a single distinct gross lesion with a diameter of >10 mm; 4, >1 distinct gross lesion with diameter of >10 mm; 5, gross coalescing lesions. The scores of the individual lobes were added up to calculate the lung score for each animal.
(ii) Lymph nodes.
The severity of the observed gross pathology in individual lymph nodes was scored by use of the following scoring system: 0, no necrosis or visible lesions; 1, small focus (1 to 2 mm in diameter); 2, several small foci or a necrotic area of at least 5 by 5 mm; 3, multiple necrotic areas of at least 5 by 5 mm distributed throughout the node or one necrotic area affecting >5% of the node. Individual lymph node scores were added up to calculate the lymph node score. Both lymph node scores and lung pathology scores were added to determine the total pathology score per animal. All scoring was performed by the same operator for all animals to ensure scoring consistency. Tissues were fixed in 10% formal saline and processed for histological examination following staining with hematoxylin and eosin. Tissues with typical lesions of TB evident by microscopy, but no gross lesions, were given a score of 1 in the overall pathology comparison. Tissue samples were frozen at −70°C for subsequent bacteriological examination by titration of tissue homogenates on modified 7H11 agar (12). Colonies per individual lymph node sample were calculated, and CFU loads of individual lymph node samples were added up to estimate the bacillary content in a given animal.
Antigens for whole-blood assays.
Purified protein derivatives from Mycobacterium avium (PPD-A) and M. bovis (PPD-B) were obtained from the Tuberculin Production Unit at the AHVLA, Weybridge. For all in vitro assays, Weybridge PPDs were used (1 mg/ml; 25,000 IU/ml). Recombinant ESAT-6 and CFP-10 were as described previously (9). These antigens are expressed by M. bovis but not by BCG and can be used to discriminate between vaccinated and infected individuals.
Tuberculin skin tests.
The single comparative intradermal tuberculin test with avian and mammalian PPDs (PPD-A and PPD-B, respectively) was by intradermal inoculation of 0.1 ml of PPD-A and PPD-B, and reactions were read 72 h later. In experiment 1 (12 months postvaccination), Weybridge PPD was used (1 mg/ml; 25,000 IU/ml). For experiment 2 (24 months postvaccination), PPD was obtained from Prionics AG, Switzerland (potencies of 25,000 IU/ml and 30,000 IU/ml for PPD-A and PPD-B, respectively). Results were recorded as the increase in skin thickness at 72 h compared to thickness preinjection and interpreted according to the standard protocol (European Commission Directive 64/432/EEC, as amended [25]).
Immunological assays.
Blood was collected into tubes with heparin (10 U/ml). For cytokine assays, 4 ml of blood was incubated at 37°C for 24 h with PPD-A or PPD-B (final concentration of 20 μg per ml) diluted in RPMI with 50 μg/ml gentamicin. ESAT-6 and CFP-10 were used at a final concentration of 5 μg/ml. An equal volume of RPMI with gentamicin was used as a control. The supernatant was removed after centrifugation and stored at −20°C until assayed. IFN-γ levels were determined in an enzyme-linked immunosorbent assay (ELISA) as described elsewhere, using recombinant bovine IFN-γ as a standard (19). Each sample assayed was measured in duplicate by ELISA; the variability between samples was less than 5%.
Cultured ELISPOT.
Secretion of IFN-γ by cultured peripheral blood mononuclear cells (PBMC) was also assessed by cultured enzyme-linked immmunosorbent spot (ELISPOT) assay, as previously described (36, 37). Briefly, PBMC were stimulated in 24-well plates (2 × 106 PBMC/ml, 1-ml aliquots) with PPD-B at 10 μg/ml. Cells were fed on days 5 and 8 with recombinant bovine intereukin-2 (IL-2; 10 U/ml) and on days 10 and 12 with fresh tissue culture medium. On day 13, 104 cultured cells were added to wells of ELISPOT plates (17) and cultured together with antigen (PPD-A and PPD-B at 10 μg/ml). To each well, 1 × 103 autologous monocyte-derived dendritic cells (15) were added. Cells were cultured for 24 h, and spots were developed (17, 37). Results are expressed as the number of spot-forming cells (SFC) per 106 PBMC.
Statistical analyses.
Analyses were performed using Minitab version 15. The degree of pathology between BCG-vaccinated and control calves was compared using the Mann-Whitney test, and the degree of bacterial colonization between BCG-vaccinated and control calves was compared using the t test on logarithmically transformed counts. For analysis of central memory antigen-specific T cell responses, the data for the control groups were pooled and compared to the vaccinated groups in nonparametric analyses of variance. P values of <0.05 were considered significant.
RESULTS
Vaccination with BCG Danish in neonatal calves induces significant protection against M. bovis challenge at 12 months, but not 24 months, postvaccination.
In order to establish the duration of immunity induced by neonatal BCG vaccination, groups of neonatal calves were vaccinated with BCG Danish within 1 month of birth. Age-matched control calves were not vaccinated. Approximately 12 months (week 54) or 24 months (week 112) later, calves were challenged endotracheally with M. bovis. The protective efficacy of BCG vaccination against disease was assessed by postmortem examinations performed 12 weeks postchallenge for all animals (Tables 1 and 2). None of the animals showed clinical signs of disease. Although the extent of lesions varied between animals, as reflected by the lesion scores (Table 1), in each case lesions were primarily restricted to the respiratory tract-associated lymph nodes and lungs.
Table 1.
Pathology scores and bacteriological counts in animals infected with M. bovis 12 months postvaccination
| Test group and animal ID | Gross pathology score for: |
CFU/LN sample | Skin test (PPD-B − PPD-A)a | ||
|---|---|---|---|---|---|
| Total LN samples | Total lung samples | All samples | |||
| Control animals | |||||
| 402340 | 14 | 16 | 30 | 587,000 | 47 |
| 602342 | 20 | 6 | 26 | 1,142,000 | 53 |
| 502348 | 19 | 19 | 38 | 128,400 | 27 |
| 302353 | 9 | 7 | 16 | 483,480 | 25 |
| 502355 | 11 | 4 | 15 | 47,000 | 26 |
| 401361 | 15 | 13 | 28 | 990,400 | 37 |
| 102365 | 2 | 5 | 7 | 11,400 | 27 |
| 302367 | 8 | 5 | 13 | 92,200 | 36 |
| 602370 | 0 | 0 | 0 | 0 | 0 |
| Median or mean valueb | 11 | 6 | 16 | 386,875 | 30.9 |
| BCG-vaccinated group | |||||
| 502341 | 10 | 3 | 13 | 842,020 | 49 |
| 702343 | 8 | 0 | 8 | 29,740 | 22 |
| 702350 | 1 | 0 | 1 | 0 | 7 |
| 402354 | 12 | 0 | 12 | 46,060 | 18 |
| 302360 | 9 | 7 | 16 | 310,020 | 8 |
| 202366 | 7 | 4 | 11 | 77,400 | 30 |
| 502369 | 0 | 0 | 0 | 0 | 0 |
| 102372 | 0 | 7 | 7 | 7,260 | 32 |
| 402375 | 0 | 0 | 0 | 0 | 0 |
| Median or mean valueb | 7 | 0 | 8 | 145,833 | 18.4 |
Skin test thickness was recorded at day 0 and day 3 following inoculation with PPD-A and PPD-B. PPD-B-specific increases in skin thickness at 72 h are shown (i.e., response to PPD-B minus the response to PPD-A).
For pathology scores, medians are shown; for CFU and skin test data, means are shown.
Table 2.
Pathology scores and bacteriological counts in animals infected with M. bovis 24 months postvaccination
| Test group and animal ID | Gross pathology score for: |
CFU/LN sample | Skin test (PPD-B − PPD-A)a | ||
|---|---|---|---|---|---|
| Total LN samples | Total lung samples | All samples | |||
| Control animals | |||||
| C09-5134 | 4 | 7 | 11 | 2,400 | 40 |
| C09-5135 | 4 | 6 | 10 | 1,753 | 18 |
| C09-5136 | 5 | 4 | 9 | 62,700 | 63 |
| C09-5137 | 4 | 4 | 8 | 8,297 | 27 |
| C09-5138 | 3 | 5 | 8 | 467 | 18 |
| C09-5139 | 10 | 5 | 15 | 79,020 | 17 |
| C09-5140 | 7 | 10 | 17 | 53,500 | 57 |
| C09-5141 | 10 | 7 | 17 | 47,187 | 21 |
| C09-5142 | 5 | 5 | 10 | 2,167 | 28 |
| Median or mean valueb | 5 | 5 | 10 | 28,610 | 32.1 |
| BCG vaccination group | |||||
| C10-6123 | 4 | 4 | 8 | 1,813 | 27 |
| C10-6124 | 3 | 4 | 7 | 45,900 | 20 |
| C10-6125 | 5 | 4 | 9 | 47,027 | 10 |
| C10-6126 | 9 | 4 | 13 | 5,040 | 30 |
| C10-6129 | 3 | 4 | 7 | 2,373 | 15 |
| C10-6130 | 8 | 9 | 17 | 11,420 | 28 |
| C10-6127 | 7 | 7 | 14 | 16,260 | 38 |
| C10-6128 | 0 | 5 | 5 | 1,040 | 45 |
| C10-6131 | 3 | 3 | 6 | 4,147 | 16 |
| Median or mean valueb | 4 | 4 | 8 | 15,002 | 25.4 |
Skin test thickness was recorded at day 0 and day 3 following inoculation with PPD-A and PPD-B. PPD-B-specific increases in skin thickness at 72 h are shown (i.e., response to PPD-B minus the response to PPD-A).
For pathology scores, medians are shown; for CFU and skin test data, means are shown.
In animals infected with M. bovis 12 months postvaccination (Table 1), lesions typical of TB were observed in all but one of the nonvaccinated controls, in both lymph nodes and lung. Two animals in the BCG-vaccinated group had no evidence of lesions. BCG Danish conferred significant protection against M. bovis challenge, with reduced total gross pathology (P = 0.0373) and significantly reduced lung pathology scores (P = 0.0303). Lymph node pathology scores were also substantially reduced in vaccinated animals, although this reduction did not quite reach statistical significance (P = 0.0685).
Lesions typical of TB were observed in all of the animals challenged 24 months postvaccination, irrespective of their vaccination status (Table 2). Although there was a tendency toward lower lesion scores within the BCG-vaccinated group, no significant difference in overall pathology between BCG-vaccinated and control cattle was observed (P = 0.1428). No statistically significant difference was observed in lymph node (P = 0.3473) or lung (P = 0.1304) pathology scores between BCG-vaccinated and control animals. Overall, although an approximately 20% reduction in lesion scores was evident in the BCG-vaccinated animals (Table 2), this was not a statistically significant decrease.
BCG vaccination of neonatal calves reduces the level of bacterial colonization.
Neonatal BCG vaccination reduced the severity of disease following M. bovis infection. In order to assess whether this was associated with effects on bacterial burden, we measured numbers of bacteria present within the respiratory tract-associated lymph nodes. Bacteriological examination of tissues indicated that M. bovis was present in the majority of tissues with gross lesions in both experiments (Tables 1 and 2). In cattle challenged 12 months postvaccination (Table 1), 3/9 animals in the BCG-vaccinated group had no viable M. bovis cultured from tissues. However, overall there was no significant difference in the number of viable M. bovis (CFU/LN) between control and vaccinated cattle (P = 0.189). In the cattle challenged 24 months postvaccination (Table 2), viable bacteria were isolated from the tissues of all of the animals, and no significant differences were observed between the BCG-vaccinated and nonvaccinated control animals (P = 0.733).
Skin test reactivity in BCG-vaccinated and control calves following M. bovis challenge.
Reactions to PPD in the skin test were observed in the majority of animals 12 weeks after challenge with M. bovis (Tables 1 and 2). Visible local clinical signs and increases in comparative skin thickness (the skin test thickness response to PPD-B minus the response to PPD-A) ranging from 7 to 63 mm were recorded. Relatively low responses to PPD-A were noted in these animals.
In experiment 1 (animals challenged with M. bovis at 12 months postvaccination [Table 1]), one nonvaccinated animal and two BCG-vaccinated animals showed no skin test reactivity. These animals also had no visible lesions or viable M. bovis present in tissues. However, 7/9 BCG-vaccinated and 8/9 control animals would be classified as reactors according to the standard interpretation of the skin test. There were no significant differences between the groups (P = 0.112).
All of the animals challenged at 24 months postvaccination had significant skin test reactivity (Table 2). There were no significant differences between the groups (P = 0.266), and each of the animals would be classified as reactors according to the standard interpretation of the skin test.
BCG Danish induces long-lived antigen-specific IFN-γ responses in whole-blood cultures.
We measured antigen-specific IFN-γ secretion in BCG-vaccinated and control calves throughout the experiment to monitor the course of the immune response. Calves were vaccinated with BCG Danish and challenged with M. bovis at 12 months postvaccination (experiment 1) or 24 months postvaccination (experiment 2) (Fig. 1).
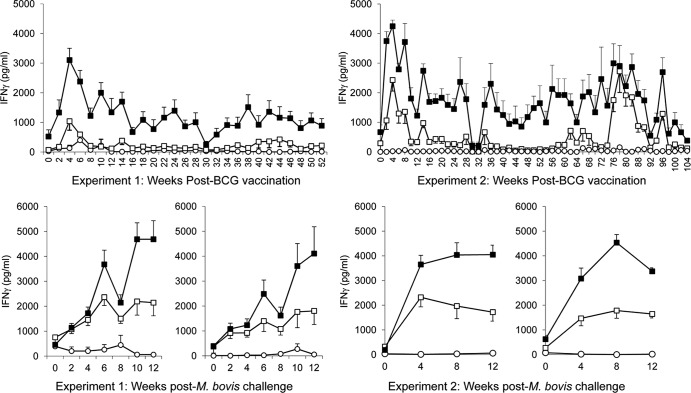
Fig 1.
Antigen-specific IFN-γ secretion in whole-blood cultures. IFN-γ release from bovine whole-blood cultures stimulated with PPD-A (open squares), PPD-B (closed squares), or medium (open circles) from animals vaccinated with BCG Danish (left panel, top graph), nonvaccinated control animals challenged with M. bovis at 12 months (week 54) (left panel, bottom left graph), or vaccinated with BCG Danish at week 0 and challenged with M. bovis at 12 months (week 54) (left panel, bottom right graph). The panel on the right shows graphs for animals vaccinated with BCG Danish in experiment 2 (top graph), nonvaccinated control animals challenged with M. bovis at 24 months (week 114) (bottom left graph), and animals vaccinated with BCG Danish at week 0 and challenged with M. bovis at 24 months (week 114) (bottom right graph). IFN-γ levels are expressed as group mean (± standard error) plasma cytokine concentrations (in pg/ml).
In experiment 1 vaccination, with BCG Danish induced PPD-B-specific immune responses that were evident at 2 weeks postvaccination (Fig. 1). Although the PPD-B-specific IFN-γ response was reduced after 6 weeks postvaccination, this was above the level of PPD-B-specific IFN-γ observed in the control group for the duration of the vaccination study (52 weeks). No PPD-B-specific IFN-γ (i.e., levels were <500 pg/ml) was observed in the control calves at any time point (data not shown).
Post-M. bovis challenge PPD-B-specific immune responses in whole-blood cultures were observed from 4 weeks in both control and vaccinated animals (Fig 1). No differences between vaccinated and control groups were observed for PPD-B-specific IFN-γ at any time point post-M. bovis challenge.
In experiment 2 similar early kinetics of PPD-B-specific IFN-γ induction were observed with the peak response evident between 2 to 8 weeks postvaccination. These responses remained generally above the prevaccination levels up to 90 weeks post vaccination (with the exception of weeks 32, 34, 64 and 66) (Fig. 1).
Post-M. bovis challenge, ESAT-6/CFP-10-specific IFN-γ secretion was observed in all animals irrespective of vaccination status. In the animals challenged at 12 months (Fig. 2), higher responses were observed in the control group (closed symbols) from 4 weeks to 8 weeks following infection. No differences in reactivity to ESAT-6/CFP-10 were observed between control and vaccinated animals challenged at 24 months.
Fig 2.
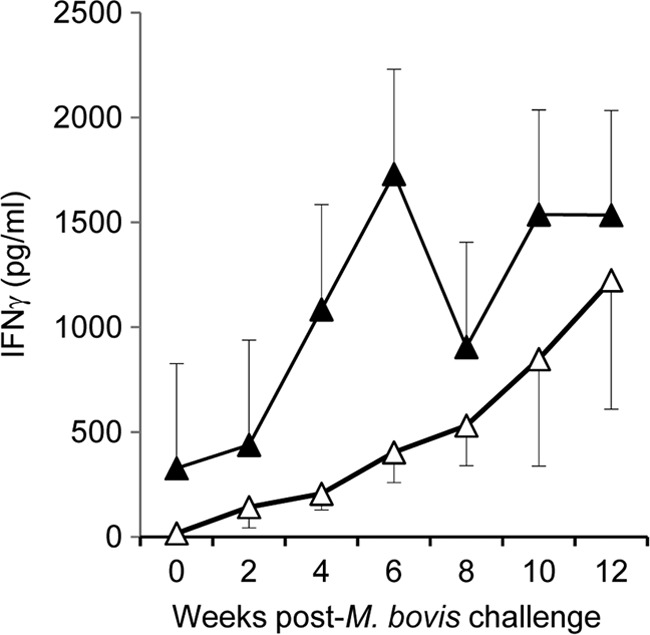
IFN-γ released following stimulation of whole blood with an ESAT-6/CFP-10 cocktail. IFN-γ secretion was assessed post-M. bovis challenge in BCG-vaccinated (open symbols) and nonvaccinated control (closed symbols) animals. Animals were challenged with M. bovis 12 months postvaccination, and blood was sampled and assessed biweekly. IFN-γ levels are expressed as the group mean (± standard error) plasma cytokine concentration (in pg/ml).
The frequency of antigen-specific IFN-γ-secreting memory cells present at the time of challenge correlates with protective immunity.
Detection of IFN-γ-secreting central memory T lymphocytes in cultured ELISPOT assays was previously shown to predict protection in BCG-vaccinated calves (16, 36) and was used here to assess whether the detection of memory T cells (Tcm) at 12 or 24 months postvaccination was also predictive of efficacy. At 12 months (Fig. 3) or 24 months postvaccination (data not shown), immediately prior to M. bovis challenge, PBMC were isolated from all calves and cultured in the presence of antigen and IL-2 to establish short-term T cell lines. Following 2 weeks of culture, the presence of central memory T cells secreting IFN-γ was assessed by ELISPOT. The frequency of PPD-B-specific SFC (response to PPD-B minus response to PPD-A) was calculated. The data for the control animals were pooled for analysis, as no significant differences were noted between the two control groups.
Fig 3.
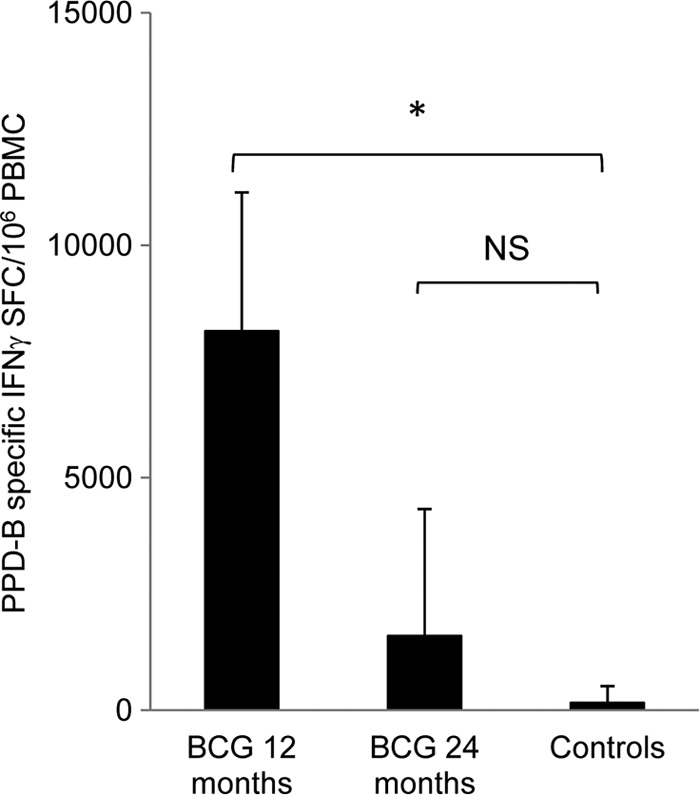
Frequency of antigen-specific IFN-γ-secreting T lymphocytes present at the time of M. bovis challenge. At 12 months (BCG 12) or 24 months (BCG 24) post-BCG vaccination, memory cell responses were assessed by cultured ELISPOT. The responses in control animals were also assessed: these were not significantly different at either time point, and the data were therefore pooled for analysis. The number of PPD-B-specific IFN-γ SFC present within PBMC was significantly higher in vaccinated animals at 12 months, but not at 24 months, postvaccination, compared with control, nonvaccinated animals. Mean ± standard deviations are shown (n = 9 for vaccinated groups; n = 18 for controls). *, P < 0.05.
Twelve months after vaccination of cattle with BCG Danish (Fig. 3, black bars), numbers of antigen-specific IFN-γ-expressing central memory T lymphocytes were significantly higher than in those in nonvaccinated control animals (P = 0.02) (Fig. 3). In contrast, the number of PPD-B-specific IFN-γ-expressing central memory T cells present in cattle 24 months postvaccination was not significantly different from that observed in control animals.
DISCUSSION
Bovine tuberculosis is an economically important disease of increasing incidence in United Kingdom cattle herds. Disease control is currently through the skin test and slaughter policy and ancillary blood tests detecting tuberculin-induced cell-mediated immune responses. The presence of the causative agent of bovine TB, Mycobacterium bovis, in wildlife reservoirs impacts significantly on the effectiveness of disease control. Hence, additional control tools are required to reduce the incidence of bovine TB in areas where bTB is endemic. An important mechanism for control is likely to be vaccination. As an attenuated form of M. bovis, BCG shares high homology with the virulent strain, and therefore vaccinated cattle also show skin test reactivity to tuberculin (PPD-B) and also secrete antigen-specific IFN-γ in blood-based diagnostic tests. However, despite numerous vaccine candidates having undergone trials in cattle for efficacy against M. bovis infection, the most successful strategies are those which include BCG either as a single vaccine or as the priming agent in heterologous prime-boost regimens (reviewed in references 6 and 33). Thus, differentially expressed antigens between M. bovis and BCG, such as ESAT-6, CFP-10, and other RD1 gene products, have been identified and used in diagnostic assessments to provide the capacity for DIVA determinations (reviewed in reference 32).
Vaccination of calves with BCG is associated with significant protective efficacy against virulent challenge with M. bovis (5, 7, 16, 17, 34). In these studies, vaccination within the first 4 weeks of life was shown to induce significant reductions in disease-associated parameters, including lesion (pathology) scores and bacterial burdens within the respiratory tract tissues. Protective efficacies of approximately 70% were reported, with a significant number of animals completely protected from disease.
In experimental studies of BCG vaccination of neonatal calves (5, 7, 16, 17, 34), there was a relatively short duration between vaccination and infectious challenge with M. bovis: on average, a vaccination period of 3 months was utilized. Given that dairy cows have an average life span on farm of ≥3 years, we sought to determine whether a single vaccination of calves would give long-lasting protection, or to define at what time point protective immunity wanes. This would enable determination of time scales for enhancing immunity through heterologous boosting or homologous revaccination.
In humans, neonatal vaccination with BCG has a significant impact on childhood TB; however, a decline in BCG efficacy has been reported over time/with age. These studies imply that a relatively short duration of protection induced by BCG vaccination may account, at least in part, for the variable efficacy of BCG vaccination that has been reported in human populations (10, 14). In mice, the duration of BCG-induced immunity was shown to be less than 40 weeks, with a significant reduction in protective efficacy observed between 20 and 40 weeks postvaccination (26).
Here, we have shown that vaccination of calves induces protective immunity against M. bovis challenge and that this is maintained for at least 12 months following vaccination. We observed a protective efficacy at 12 months similar to that previously reported at 3 months neonatal vaccination (16). However, the protective immunity we observed had waned by 24 months. Thus, although there was a reduction in each of the parameters assessed in cattle challenged 2 years after vaccination (lung and lymph node pathology scores and bacterial burdens), with the relatively small group sizes utilized in our studies these reductions did not reach statistical significance. However, this protective effect could potentially have a positive impact on disease control in larger cattle herds by reducing the overall level of disease and the numbers of bacilli present.
It should also be noted that experimental challenge whereby ∼103 CFU M. bovis are placed directly into the respiratory tract is a more aggressive challenge than natural exposure, whereby transmission occurs primarily through droplet inhalation of relatively small numbers of bacilli. Interestingly, in a recent field study in Ethiopia in which calves were vaccinated with BCG, Ameni et al. showed that immunity against naturally transmitted M. bovis infection was long-lived, with a duration of at least 23 months (1). In their studies, a significant proportion of completely protected cattle with no visible lesions at postmortem examination was observed. In similar studies performed in Mexico (22), the duration of BCG-induced immunity against bTB was reported to wane at around 12 months of age, in line with the results of our experimental studies.
However, consideration will need to be given to the likelihood that boosting of immunity will be required. In studies where short-interval (6-week) homologous boosting of BCG-induced immunity was assessed, no positive effect on the outcome of infectious challenge was observed (7). However, the effects of homologous BCG boosting or revaccination with longer prime-boost intervals (e.g., 1 year post-initial BCG vaccination) have not yet been assessed, but such boosting could stimulate longer-duration protection, as the initial immunity starts to wane. Heterologous prime-boost strategies, utilizing viral vectors such as human adenovirus or modified vaccinia virus Ankara which express immunodominant antigens such as Ag85A, have been shown to be superior to BCG alone in protecting cattle from M. bovis (30) and are currently in phase IIB clinical trials in humans for vaccination against Mycobacterium tuberculosis infection (23, 24). The capacity for these strategies to boost BCG-induced immunity for life-long protection for cattle should be assessed.
Identification of predictors of protective immunity is an essential part of vaccine research. The capability to determine whether vaccines bestow protective immune responses prior to infectious challenge will enable more high-throughput studies, allowing prioritization of candidates for efficacy studies. This not only will reduce the time scales for vaccine evaluation but also will significantly reduce the costs of vaccine studies and reduce the severity of animal procedures. A number of candidate immunological predictors have been identified in TB vaccine studies. In studies of neonatal BCG vaccination, we showed that protective efficacy was correlated with the number of PPD-B-specific central Tcm present at the time of M. bovis challenge (16), in line with previous studies of vaccine-induced protection from bTB (30, 36). Confirming this observation, we showed here that when measured 12 months postvaccination, the numbers of Tcm determined by cultured ELISPOT were higher in vaccinated than control animals, and this corresponded to protection from infection overall. However, by 24 months postvaccination we were unable to show a significant difference in the numbers of Tcm between vaccinated and control cattle. Significantly increased numbers of PPD-B-specific Tcm in vaccinated cattle are thus only present in groups of cattle where protective immunity is observed, suggesting that this may be a predictive correlate of BCG-induced protection.
The relationship of IFN-γ secretion to protective efficacy is complex: while it is clear that successful vaccination requires the induction of IFN-γ, detection of IFN-γ alone is not a sufficient biomarker of protection (18, 27, 33). We observed increased antigen-specific IFN-γ secretion for up to 90 weeks following vaccination, but we could not correlate this with the observed levels of protection from M. bovis challenge. In studies of the duration of BCG-induced immunity in mice, IFN-γ responses of CD4+ T cells to M. tuberculosis antigens were maintained despite a loss of protection between 20 and 40 weeks postvaccination (26). More complex measurements of polyfunctional T cells have revealed that multiparametric responses of lymphocytes are required for protective immunity against M. tuberculosis in humans and mice (2, 11, 21). Recent studies in cattle have established methods for the measurement of multifunctional T cells expressing IFN-γ, IL-2, and tumor necrosis factor alpha in M. bovis infection (35), and these may now be applied to vaccine studies to facilitate the definition of predictors of vaccine success. Application of such techniques to future vaccination studies of neonatal calves may be useful in defining predictors and correlates of protection.
In summary, we have shown that BCG vaccination of calves induces a significant degree of protective immunity that lasts for at least 12 months but that this wanes by 24 months postvaccination. These results will inform future studies of the use of BCG vaccination in conjunction with strategies for boosting immunity or revaccination for longer-term protection from M. bovis infection.
ACKNOWLEDGMENTS
This work was supported by grants from the Department for the Environment, Food and Rural Affairs (DEFRA) UK. J. C. Hope, H. M. Vordermeier, and R. G. Hewinson are Jenner Institute Investigators.
We gratefully acknowledge the staff of the IAH and VLA animal facilities for care of the cattle and members of the TB group at VLA for assistance with necropsy and bacteriological evaluations. Statistical advice was received from G. Gettinby at Strathclyde University.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Ameni G, Vordermeier M, Aseffa A, Young DB, Hewinson RG. 2010. Field evaluation of the efficacy of Mycobacterium bovis bacillus Calmette-Guerin against bovine tuberculosis in neonatal calves in Ethiopia. Clin. Vaccine Immunol. 17:1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beveridge NE, et al. 2007. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37:3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buddle BM, et al. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615–620 [DOI] [PubMed] [Google Scholar]
- 4. Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37–46 [DOI] [PubMed] [Google Scholar]
- 5. Buddle BM, Wards BJ, Aldwell FE, Collins DM, de Lisle GW. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126–1133 [DOI] [PubMed] [Google Scholar]
- 6. Buddle BM, Wedlock DN, Denis M, Vordermeier HM, Hewinson RG. 2011. Update on vaccination of cattle and wildlife populations against tuberculosis. Vet. Microbiol. 151:14–22 [DOI] [PubMed] [Google Scholar]
- 7. Buddle BM, et al. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71:6411–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers MA, et al. 2011. Bacillus Calmette-Guerin vaccination reduces the severity and progression of tuberculosis in badgers. Proc. Biol. Sci. 278:1913–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cockle PJ, et al. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comstock GW, Palmer CE. 1966. Long-term results of BCG vaccination in the southern United States. Am. Rev. Respir. Dis. 93:171–183 [DOI] [PubMed] [Google Scholar]
- 11. Forbes EK, et al. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallagher J, Horwill DM. 1977. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. J. Hyg. (Lond.) 79:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garnier T, et al. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100:7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart PD, Sutherland I. 1977. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br. Med. J. 2:293–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hope JC, Kwong LS, Sopp P, Collins RA, Howard CJ. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285–291 [DOI] [PubMed] [Google Scholar]
- 16. Hope JC, et al. 2011. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin. Vaccine Immunol. 18:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hope JC, et al. 2005. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 139:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hope JC, Vordermeier HM. 2005. Vaccines for bovine tuberculosis: current views and future prospects. Expert Rev. Vaccines 4:891–903 [DOI] [PubMed] [Google Scholar]
- 19. Kwong LS, et al. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85:213–223 [DOI] [PubMed] [Google Scholar]
- 20. Lesellier S, et al. 2009. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 27:402–409 [DOI] [PubMed] [Google Scholar]
- 21. Lindenstrom T, et al. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047–8055 [DOI] [PubMed] [Google Scholar]
- 22. Lopez-Valencia G, et al. 2010. Field evaluation of the protective efficacy of Mycobacterium bovis BCG vaccine against bovine tuberculosis. Res. Vet. Sci. 88:44–49 [DOI] [PubMed] [Google Scholar]
- 23. McShane H. 2011. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:2782–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McShane H, et al. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240–1244 [DOI] [PubMed] [Google Scholar]
- 25. Morrison WI, et al. 2000. Pathogenesis and diagnosis of infections with Mycobacterium bovis in cattle. Independent Scientific Group on Cattle TB. Vet. Rec. 146:236–242 [PubMed] [Google Scholar]
- 26. Ozeki Y, et al. 2011. Loss of anti-mycobacterial efficacy in mice over time following vaccination with Mycobacterium bovis bacillus Calmette-Guerin. Vaccine 29:6881–6887 [DOI] [PubMed] [Google Scholar]
- 27. Parida SK, Kaufmann SH. 2010. The quest for biomarkers in tuberculosis. Drug Discov. Today 15:148–157 [DOI] [PubMed] [Google Scholar]
- 28. Vordermeier HM, et al. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vordermeier HM, Cockle PJ, Whelan AO, Rhodes S, Hewinson RG. 2000. Toward the development of diagnostic assays to discriminate between Mycobacterium bovis infection and bacille Calmette-Guerin vaccination in cattle. Clin. Infect. Dis. 30(Suppl. 3):S291–S298 [DOI] [PubMed] [Google Scholar]
- 30. Vordermeier HM, et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vordermeier HM, et al. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vordermeier M, Jones GJ, Whelan AO. 2011. DIVA reagents for bovine tuberculosis vaccines in cattle. Expert Rev. Vaccines 10:1083–1091 [DOI] [PubMed] [Google Scholar]
- 33. Waters WR, Palmer MV, Buddle BM, Vordermeier HM. 2012. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine 30:2611–2622 [DOI] [PubMed] [Google Scholar]
- 34. Wedlock DN, Denis M, Vordermeier HM, Hewinson RG, Buddle BM. 2007. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induce different levels of IFNγ post-vaccination, but induce similar levels of protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 118:50–58 [DOI] [PubMed] [Google Scholar]
- 35. Whelan AO, Villarreal-Ramos B, Vordermeier HM, Hogarth PJ. 2011. Development of an antibody to bovine IL-2 reveals multifunctional CD4 T(EM) cells in cattle naturally infected with bovine tuberculosis. PLoS One 6:e29194 doi:10.1371/journal.pone.0029194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whelan AO, et al. 2008. Evidence for enhanced central memory priming by live Mycobacterium bovis BCG vaccine in comparison with killed BCG formulations. Vaccine 26:166–173 [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, et al. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307–3318 [DOI] [PubMed] [Google Scholar]