Abstract
Persons with previous extrapulmonary tuberculosis have reduced peripheral blood mononuclear cell cytokine production and CD4+ lymphocytes compared to persons with previous pulmonary tuberculosis or latent tuberculosis infection, but specific defects related to Mycobacterium tuberculosis infection of macrophages have not been characterized. The objective of this study was to further characterize the in vitro immune responses to M. tuberculosis infection in HIV-seronegative persons with previous extrapulmonary tuberculosis. Peripheral blood mononuclear cells were isolated from HIV-seronegative persons with previous extrapulmonary tuberculosis (n = 11), previous pulmonary tuberculosis (n = 21), latent M. tuberculosis infection (n = 19), and uninfected tuberculosis contacts (n = 20). Experimental conditions included M. tuberculosis-infected macrophages cultured with and without monocyte-depleted peripheral blood mononuclear cells. Concentrations of interleukin 1β (IL-1β), IL-4, IL-6, CXCL8 (IL-8), IL-10, IL-12p70, IL-17, CCL2 (monocyte chemoattractant protein 1), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) were measured by multiplex cytokine array. When M. tuberculosis-infected macrophages were cocultured with monocyte-depleted peripheral blood mononuclear cells, IFN-γ (P = 0.01), TNF-α (P = 0.04), IL-10 (P < 0.001), and IL-6 (P = 0.03) exhibited similar continua of responses, with uninfected persons producing the lowest levels, followed by extrapulmonary tuberculosis cases, pulmonary tuberculosis controls, and persons with latent M. tuberculosis infection. A similar pattern was observed with CXCL8 (P = 0.04), IL-10 (P = 0.02), and CCL2 (P = 0.03) when monocyte-depleted peripheral blood mononuclear cells from the four groups were cultured alone. Persons with previous extrapulmonary tuberculosis had decreased production of several cytokines, both at rest and after stimulation with M. tuberculosis. Our results suggest that persons who develop extrapulmonary tuberculosis have a subtle global immune defect that affects their response to M. tuberculosis infection.
INTRODUCTION
Tuberculosis (TB) presents an interesting paradox: while one-third of the world's population is infected with Mycobacterium tuberculosis, only 5 to 10% of those infected will develop active disease (1, 23). This observation suggests that factors other than the mycobacterium play a role in the development of TB. Our understanding of protective immunity to M. tuberculosis infection is incomplete. The immune response to M. tuberculosis involves cells such as monocytes, macrophages, and T lymphocytes that produce cytokines such as CXCL8 (interleukin-8 [IL-8]), IL-12, gamma interferon (IFN-γ), and tumor necrosis alpha (TNF-α) (16). While hematogenous dissemination occurs in most persons soon after infection, subsequent extrapulmonary TB disease does not (25). Development of extrapulmonary disease could be a result of a breakdown in host immune surveillance, a change in the mycobacteria from a dormant to an active state, or a combination of the two. Discovery of factors that predispose to extrapulmonary disease will advance TB prevention efforts by identifying immune responses that could be boosted by TB vaccines. It will also help identify individuals at increased risk for progression from latent infection to clinical disease, so that more intensive observation and treatment of latent infection can be initiated.
Extrapulmonary TB is associated with underlying immune defects. For example, HIV-infected persons are at increased risk of extrapulmonary TB, and this risk increases as the CD4+ T lymphocyte count declines (30). Young children also have an increased incidence of extrapulmonary TB, specifically TB meningitis, presumably due to an immature immune system (31). Our group has previously noted reduced peripheral blood mononuclear cell (PBMC) cytokine production and CD4+ T lymphocytes in HIV-seronegative adults with previous extrapulmonary TB compared to persons with previous pulmonary TB or latent M. tuberculosis infection (2, 38). In a subset of patients from the present study, we also found that persons with previous extrapulmonary TB had increased regulatory T cell frequency and CD4+ lymphocyte activation, indicating possible immune dysregulation (8). However, an evaluation of the sequential activation of innate and adaptive immune responses to M. tuberculosis infection in such patients has not been performed. We therefore assessed in vitro immune responses in persons with previous extrapulmonary TB under several experimental conditions, including one in which autologous monocyte-depleted PBMCs were cocultured with M. tuberculosis-infected host macrophages.
(This work was presented at the annual American Thoracic Society meeting in New Orleans, LA, May 2010, in abstract form [abstract 3799] [12].)
MATERIALS AND METHODS
Subject recruitment.
Cases were defined as persons with previously treated extrapulmonary TB. There were three sets of controls: (i) persons with previously treated pulmonary TB, (ii) persons with latent M. tuberculosis infection, and (iii) persons who had been exposed to culture-positive pulmonary TB but were not infected (i.e., tuberculin skin test [TST] negative). Inclusion criteria consisted of the following: age >18 years at the time of diagnosis of TB disease or infection; HIV seronegative; culture-confirmed disease and either near completion (within 1 month) or completion of therapy (for extrapulmonary cases and pulmonary controls); and TST induration of >10 mm (for latent M. tuberculosis infection controls). We did not require persons to complete therapy for latent infection in order to be enrolled. Only contacts of culture-positive pulmonary TB cases or known TST converters were included as controls. Contacts of culture-positive pulmonary TB cases were tested for latent infection at the beginning of the contact investigation and after 8 to 12 weeks (5). Exclusion criteria consisted of the following: serum creatinine of >2 mg/dl; use of corticosteroids or other immunosuppressive agents at the time of diagnosis or study entry; malignancy; and diabetes mellitus. Pleural TB represents an exaggerated (not diminished) cell-mediated immune response (3). Because persons with pleural TB may exhibit a unique immunopathogenesis compared with the other forms of extrapulmonary TB, we excluded these individuals from our study.
All cases and controls were enrolled in Tennessee. Extrapulmonary TB cases and pulmonary TB controls were identified by review of the Tennessee Department of Health TB registry. Ongoing contact investigations at local and regional TB clinics were reviewed to identify patients in the remaining control groups. Demographic and clinical characteristics were collected from the patient or the Tennessee TB registry.
The institutional review boards of Vanderbilt University, Nashville Davidson Metro Public Health Department, and the Tennessee Department of Health approved the study. Study participants provided written informed consent.
Sample processing.
HIV serology and a complete blood count were performed for each subject. PBMCs were isolated within 24 h under sterile conditions by Ficoll-Paque (GE Healthcare Bio-Science) density centrifugation. Viability was estimated by trypan blue dye exclusion. PBMCs were immediately suspended at a concentration of 107/ml in complete RPMI (2).
CD14+ monocytes were positively selected from PBMCs according to the manufacturer's instructions (Stemcell Technologies). Monocytes and monocyte-depleted PBMCs (PBMC-M) were stained with trypan blue to estimate viability. After isolation, aliquots of PBMCs, monocytes, and PBMC-M were stained for viability with the Live Dead Fixable Aqua Dead Cells stain kit (Invitrogen), followed by surface staining with anti-CD14 antibody (PE-Cy7; eBioscience) to assess purity. Flow cytometry results were obtained by gating on viable cells.
Isolated monocytes were plated into a 48-well plate at a concentration of 2.5 × 105/ml in complete RPMI. PBMC-M were frozen at a concentration of 107/ml in freezing media (10% dimethyl sulfoxide [DMSO], 90% fetal bovine serum [FBS]). Monocytes were incubated for 7 days at 37°C and 5% CO2. Following incubation, wells were examined under magnification of ×40 for confirmation of morphological transformation of monocytes to confluent macrophages.
Infection of macrophages with M. tuberculosis H37Rv.
Mycobacterium tuberculosis H37Rv (ATCC 25618) was cultivated as previously described (10). All stocks were quantified by serial dilution, and aliquots were frozen. On the day of infection, one vial of H37Rv was thawed, washed once with media (complete RPMI without penicillin or streptomycin), and suspended in media to a final concentration of 1.25 × 106 CFU/ml (multiplicity of infection, 5:1). One milliliter of H37Rv suspension was added to selected macrophage wells. After 24 h of infection, the same wells were washed twice with Dulbecco's phosphate-buffered saline 1× (DPBS×1) to eliminate extracellular mycobacteria, and 1 ml of antibiotic-free medium was replaced.
Twenty-four hours after the wash, autologous PBMC-M were thawed and stained with trypan blue to assess viability. PBMC-M were suspended in R-10 antibiotic-free medium at a concentration of 3 × 106/ml. In one infected macrophage well and one uninfected macrophage well, medium was removed and replaced with 1 ml of the PBMC-M suspension. Separate wells with PBMC-M alone and PBMC-M plus staphylococcal enterotoxin B (SEB) (10 ng/ml) were also plated as negative and positive controls, respectively.
Cytokine bead array.
After 48 h of incubation following addition of PBMC-M, 150 μl of culture supernatant was withdrawn from each of six conditions and frozen at −80°C. Cell culture supernatant was thawed once and examined for IL-1β, IL-4, IL-6, CXCL8 (IL-8), IL-10, IL-12p70, IL-17, CCL2 (monocyte chemoattractant protein 1 [MCP-1]), TNF-α, and IFN-γ by multiplex cytokine array analysis using the Bio-Plex protein multiarray system (Bio-Rad, Hercules, CA). All cytokine determinations were performed with the same lot of reagents. Laboratory personnel performing the cytokine bead array were blind to the case-control status of the specimens.
Statistical analysis.
The sample size was calculated based on log-transformed means of unstimulated cytokines (2). Twenty subjects in each group were required to detect an effect size of 1.7 standard deviation (SD) of the log-transformed mean, with 80% power and 2-sided 5% significance level using analysis of variance. Clinical and demographic characteristics were compared among the 4 patient groups using the Kruskal-Wallis test for continuous variables and the chi-square and Fisher exact tests for categorical variables. A P value of <0.05 was considered significant.
In order to minimize the risk of increased type I error caused by multiple comparisons of cytokine responses, we conducted a generalized estimating equation (GEE) as a global test to simultaneously compare differences in the 10 cytokine measurements among the four patient groups, using proportional odds logistic regression. A robust sandwich estimator was used to address heavily skewed cytokine responses. The global test was performed separately within each experimental condition and was controlled for white blood cell (WBC) count. We also controlled for the effect of race (black versus nonblack) because our previous epidemiologic work suggested that blacks were more likely to present with extrapulmonary TB (13). Also, persons of the black race have low numbers of WBCs and differences in monocyte function compared to persons of other races (6, 35). When the global test was rejected, showing a significant effect of patient groups, we further analyzed the effect of group on each cytokine through a similar multivariable regression model, again controlling for WBC count and race. This global test is not able to predict where the difference lies, and post hoc pairwise comparisons among the four patient groups were not performed to minimize the risk of type I error. To determine if a correlation existed between cytokine values and the time between TB therapy completion and study enrollment, a Spearman's rank correlation was performed with each cytokine only under experimental conditions that were found to be globally significant. If a correlation was found, a proportional odds regression model adjusting for the time variable was performed to determine if this correlation differed by group.
RESULTS
Clinical characteristics of the study population.
Between 2008 and 2009, we enrolled 11 persons with previous extrapulmonary TB, 21 with previous pulmonary TB, 19 with latent M. tuberculosis infection, and 20 uninfected contacts of pulmonary TB cases. All of the persons with previous clinical TB had completed therapy. Two persons with latent infection declined preventive treatment, and three latently infected persons were enrolled during their final month of therapy. The clinical and demographic characteristics of the study population are listed in Table 1. Sites of disease among extrapulmonary cases included lymphatic sites (n = 4), areas near the spine (n = 1), vertebra (n = 1), miliary sites (n = 1), and the genitourinary tract (n = 1), peritoneum (n = 1), skin (n = 1), and breast (n = 1).
Table 1.
Demographic characteristics of the study populationa
| Characteristic | TST negative (n = 20) | Latent TB infection (n = 19) | Pulmonary TB (n = 21) | Extrapulmonary TB (n = 11) | P valueb |
|---|---|---|---|---|---|
| Age (yr) (IQR)c | 43 (33–52) | 45 (39–64) | 49 (40–59) | 41 (27–71) | 0.27 |
| Male | 7 (35) | 7 (37) | 13 (62) | 6 (55) | 0.27 |
| Black raced | 7 (25) | 2 (11) | 9 (43) | 6 (55) | 0.02 |
| Foreign borne | 0 (0) | 2 (11) | 5 (24) | 8 (73) | 0.007 |
| Homeless | 0 (0) | 1 (6) | 5 (24) | 0 (0) | 0.03 |
| Alcohol use | 1 (5) | 1 (5) | 7 (33) | 0 (0) | 0.03 |
| Tobacco use | 6 (30) | 4 (19) | 11 (52) | 1 (9) | 0.05 |
| Illicit drug use | 0 (0) | 0 (0) | 2 (9) | 0 (0) | 0.18 |
| No. of WBCs/mm3 (IQR)c | 8.2 (7.0–9.1) | 7.2 (6.3–7.8) | 5.9 (5.5–7.7) | 5.4 (4.3–7.5) | 0.01 |
| No. of CD4+ cells/mm3 (IQR)c | 1,078 (882–1,332) | 1,175 (797–1,495) | 1,058 (708–1,206) | 917 (673–1,058) | 0.15 |
| No. of mos. from treatment completion to study entryf | NAg | 18 (2–26) | 5 (0.25–11) | 21 (6–39) | 0.09 |
Data are presented as numbers (%) of individuals unless otherwise noted.
Kruskal-Wallis test and Pearson's chi-square test.
Values are median numbers, with IQRs (interquartile ranges) in parentheses.
Other races that were represented in the study population were Caucasian, Asian, and Hawaiian/Pacific Islander.
The classification of foreign-born individuals by race is as follows: Caucasians (n = 4), Black (n = 5), Asian (n = 5), Hawaiian/Pacific Islander (n = 1).
Two persons with latent TB infection did not complete therapy.
NA, not applicable.
Cytokine bead array.
All median cytokine results (pg/ml) measured at 48 h after the addition of PBMC-M are presented in Table 2, with the exception of IL-4, which was detectable only when PBMC-M were stimulated with SEB. Levels of IL-12p70 were low under each condition tested, perhaps due to the time point at which supernatants were collected (4). The two experimental conditions that were globally significant were (i) autologous PBMC-M cocultured with M. tuberculosis-infected macrophages and (ii) autologous PBMC-M cultured alone. Thus, differences between the patient groups for each cytokine could be evaluated statistically under these conditions.
Table 2.
Cytokine production of PBMCs 48 h after addition of PBMC-Ma
| Cytokine | Group | Median cytokine concn (IQR) or P value 48 h after addition of: |
|
|---|---|---|---|
| Macrophage + M. tuberculosis H37Rv + PBMC-M | PBMC-M | ||
| IL-1β | TST− | 1,959 (754–3,502) | 155 (69–252) |
| EPTB | 2,035 (906–3,813) | 126 (60–188) | |
| PTB | 2,253 (1,876–4,209) | 236 (120–314) | |
| LTBI | 3,722 (1,783–6,243) | 225 (136–606) | |
| P value | 0.15 | 0.11 | |
| IL-6 | TST− | 2,159 (710–4,206) | 3,514 (950–5,490) |
| EPTB | 3,867 (1,375–10,212) | 2,431 (1,311–5,880) | |
| PTB | 4,266 (2,047–6,116) | 4,835 (3,844–7,599) | |
| LTBI | 4,827 (2,492–10,475) | 5,387 (3,639–9,228) | |
| P value | 0.04 | 0.07 | |
| CXCL8 | TST− | 16,777 (8,700–28,034) | 27,790 (10,158–54387) |
| EPTB | 15,644 (8,465–64,617) | 14,461 (94,301–44,438) | |
| PTB | 21,495 (13,762–44,211) | 36,949 (27,993–72,137) | |
| LTBI | 27,499 (14,935–59,700) | 46,454 (21,853–85,471) | |
| P value | 0.12 | 0.04 | |
| IL-10 | TST− | 18 (11–37) | 27 (12–44) |
| EPTB | 33 (23–103) | 32 (16–49) | |
| PTB | 69 (35–139) | 44 (26–65) | |
| LTBI | 64 (37–142) | 51 (28–152) | |
| P value | <0.001 | 0.02 | |
| IL-12 | TST− | 1.3 (1.1–2.0) | 0.53 (0–1.3) |
| EPTB | 1.7 (0–5.9) | 0 (0–1.3) | |
| PTB | 1.8 (1.4–2.5) | 1.1 (0–1.6) | |
| LTBI | 1.6 (1.1–2.4) | 1.2 (1–1.7) | |
| P value | 0.49 | 0.27 | |
| IL-17 | TST− | 5.6 (2.4–16) | 2.0 (0.6–7.4) |
| EPTB | 22 (5.2–45) | 1.7 (0–3.2) | |
| PTB | 8.4 (5.6–53) | 2.2 (1.1–4.7) | |
| LTBI | 19 (12–79) | 4.7 (2.0–15) | |
| P value | 0.01 | 0.17 | |
| CCL2 | TST− | 146 (83–278) | 988 (226–5,567) |
| EPTB | 358 (116–1,434) | 623 (457–3,218) | |
| PTB | 436 (167–655) | 4,044 (1,565–6,484) | |
| LTBI | 213 (121–1,686) | 4,685 (1,678–6,381) | |
| P value | 0.05 | 0.03 | |
| TNF-α | TST− | 293 (141–694) | 26 (12–44) |
| EPTB | 684 (223–1860) | 20 (9.5–72) | |
| PTB | 708 (416–2,000) | 31 (17–45) | |
| LTBI | 989 (268–2,107) | 46 (21–79) | |
| P value | 0.04 | 0.17 | |
| IFN-γ | TST− | 1,301 (607–2,672) | 46 (10–157) |
| EPTB | 2,599 (1,770–13,064) | 19 (12–49) | |
| PTB | 3,533 (1,931–7,743) | 33 (9.7–91) | |
| LTBI | 3,126 (1,974–9,709) | 83 (17–177) | |
| P value | 0.01 | 0.07 | |
Values are in pg/ml; P values of <0.05 are in bold. Abbreviations: EPTB, extrapulmonary TB; PTB, pulmonary TB; LTBI, latent TB infection; IQR, interquartile range.
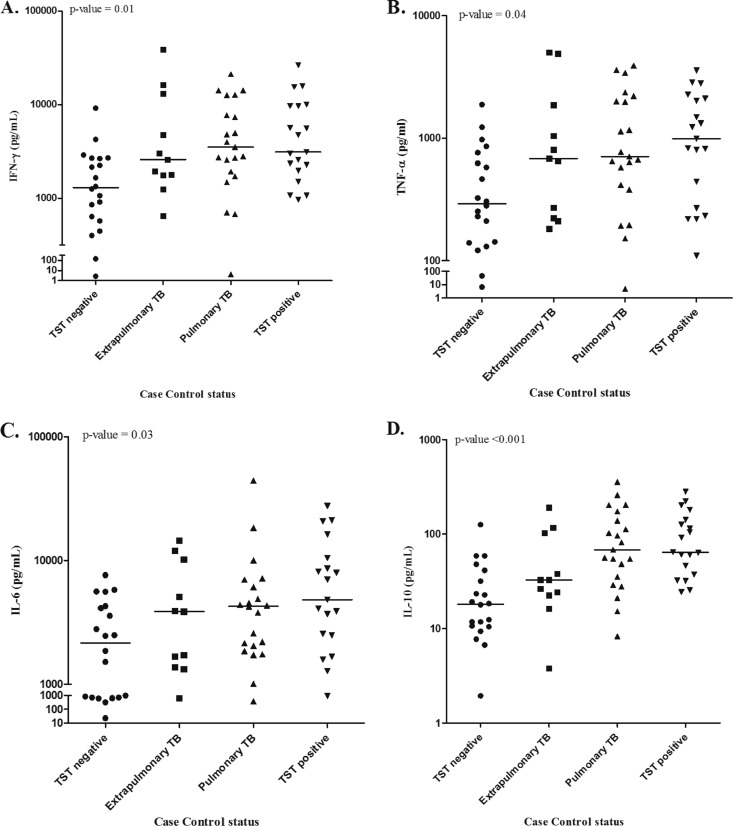
Compared to infected macrophages alone, all cytokines tested increased when autologous PBMC-M were added to infected macrophages, but a pattern emerged in the latter condition when evaluating by patient group. After controlling for race and WBCs, median production of IL-6 (P = 0.03), IL-10 (P < 0.001), TNF-α (P = 0.04), and IFN-γ (P = 0.01) was lowest in TST-negative contacts, followed by persons with previous extrapulmonary TB, latently infected persons, and persons with previous pulmonary TB (Fig. 1). A similar trend was seen for IL-1β and CXCL8, but it was not statistically significant. Production of IL-17 was highest in persons with previous extrapulmonary TB (P = 0.01). Persons with previous pulmonary TB had the highest level of CCL2 (P = 0.05). There was no significant correlation between cytokine production and time between TB treatment completion and study entry (data not shown).
Fig 1.
Levels of IFN-γ (A), TNF-α (B), IL-6 (C), and IL-10 (D) across study groups when autologous PBMCs are added to M. tuberculosis-infected macrophages. Macrophages from study participants were cultured for 7 days and then infected with M. tuberculosis H37Rv. Forty-eight hours later, autologous monocyte-depleted PBMCs were added to the macrophage culture. Supernatants were collected 48 h later and stored at −70°C until submission for cytokine bead array.
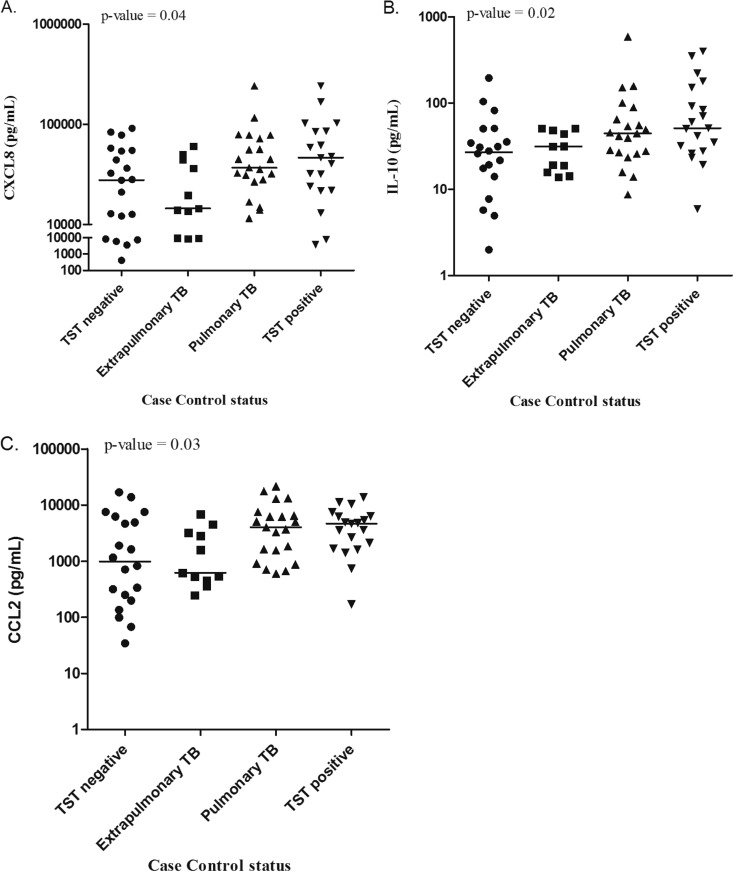
A similar pattern of cytokine production by patient group was noted when PBMC-M were cultured alone. TST-negative contacts produced the lowest median levels of CXCL8 (P = 0.04), IL-10 (P = 0.02), and CCL2 (P = 0.03), followed by persons with previous extrapulmonary TB, persons with previous pulmonary TB, and latently infected persons after controlling for race and WBCs (Fig. 2). A similar trend was seen for IL-1β, IL-6, TNF-α, and IFN-γ but was not statistically significant. A positive correlation between IL-1β and IL-10 production and time between TB treatment completion and study entry was noted (Spearman's coefficient, 0.21 [P = 0.03] and 0.23 [P = 0.03], respectively). A proportional odds logistic regression model adjusting for time as well as race and WBCs was performed, and the observed differences in these two cytokines between the four patient groups remained statistically significant (data not shown).
Fig 2.
Levels of CXCL8 (A), IL-10 (B), and CCL2 (C) across study groups when autologous monocyte-depleted PBMCs were cultured alone. Supernatants were collected from monocyte-depleted PBMCs after 48 h of culture and stored at −70°C until submission for cytokine bead array.
Similar to what was observed in our previous work, persons with previous extrapulmonary TB had lower levels of IL-6, IL-10, CCL2, TNF-α, and IFN-γ than did the other groups when uninfected macrophages were cocultured with autologous PBMC-M, but this trend was not statistically significant (see Table S1 in the supplemental material) (2). With the exception of CXCL8 and CCL2, cytokine levels increased in all study groups when autologous PBMC-M were cultured with infected macrophages compared to levels in PBMC-M cultured with uninfected macrophages. IFN-γ showed the greatest fold increase when all groups were compared under these two conditions, with increases ranging from 245 to 1,000-fold compared to the other cytokines.
Levels of IL-β, IL-6, CXCL8, IL-10, and CCL2 decreased when comparing uninfected to infected macrophages (see Table S1 in the supplemental material). This was most striking for CCL2, which decreased 200- to 600-fold when uninfected macrophages were compared to infected macrophages. The decrease in cytokine levels was not different between cases and controls.
DISCUSSION
Previous studies comparing the immune responses of persons with extrapulmonary TB to those with pulmonary TB and healthy controls have measured in vivo or in vitro cytokine production and have arrived at different conclusions. Some have found similar cytokine levels in sera from persons with various TB manifestations (40), while others such as Hasan et al. found that circulating levels of cytokines such as IFN-γ and CXCL9 differ based on the site of TB disease (19, 40). Other groups have stimulated whole blood or PBMCs from persons with various forms of TB and measured cytokine levels and T lymphocyte proliferation (20, 21, 36, 41, 42). These studies have focused more on the immune response to M. tuberculosis in persons with active TB, in some cases to identify diagnostic biomarkers. The present study differs from previous studies in the literature and builds on prior work in that we used a more precise and biologically relevant model of the sequential activation of innate and adaptive immunity following human infection with M. tuberculosis. We enrolled only persons who had completed TB therapy in order to evaluate for immune defects that may predispose to development of TB and to avoid possible confounding of our results with the tumultuous immune response that accompanies active TB (28). We observed a continuum of immune responses in which uninfected persons produced the lowest levels of several cytokines, followed by persons with previous extrapulmonary TB, persons with previous pulmonary TB, and persons with latent TB infection. Similar to our previous work (2, 38), our results suggest that people who develop extrapulmonary TB appear to have a subtle immune defect compared to persons with other TB manifestations.
Our findings could signify inadequate activation of memory T lymphocytes by antigen-presenting macrophages in persons with previous extrapulmonary TB compared to latently infected persons and persons with previous pulmonary TB. If so, it is not surprising that uninfected persons had the lowest cytokine responses to in vitro challenge with M. tuberculosis. A recent study found that IFN-γ production after stimulation with M. tuberculosis peptides in persons with pulmonary TB was mediated by effector memory T lymphocytes and this response was different based on severity of disease (17). Inadequate production or maintenance of memory T lymphocytes could potentially lead to the development of TB at sites distant from the site of primary infection after initial control of M. tuberculosis infection, and this may explain why persons with previous extrapulmonary TB produced low cytokine levels. There were differences between the groups in the time between completion of therapy and study enrollment, which could affect the memory immune response, but the differences in the immune response between study groups did not change when adjusting for this time variable.
We found that persons with previous extrapulmonary TB had the highest levels of IL-17. IL-17 is a proinflammatory cytokine that participates in the early defense against M. tuberculosis infection and has been shown in murine models to be essential for granuloma formation (33). In a separate study that included a subset of the current study's patient population, we observed that persons with previous extrapulmonary TB have increased frequencies of regulatory T cells, a category of T cells characterized by surface expression of CD4 and CD25, and intracellular expression of FoxP3 (8). In active TB, regulatory T cells suppress IFN-γ production but have less suppressive effects on IL-17 production (32). IFN-γ also regulates production of IL-17 (7). Our findings suggest a model whereby increased regulatory T cell frequency suppresses IFN-γ responses to M. tuberculosis infection, leading to elevated levels of IL-17. Future studies where production of IFN-γ and IL-17 is measured after regulatory T cell depletion could test this hypothesis.
We found that levels of CCL2 were elevated in persons with previous pulmonary TB, which is consistent with our previous work involving children (39). Flores-Villanueva et al. found that a polymorphism in the CCL2 promoter was associated with increased levels of CCL2 in Korean and Mexican adults with active pulmonary TB compared to healthy controls (15). The consistent finding of elevated CCL2 in various groups of patients in different settings suggests that it may have a direct effect on the pathogenesis of pulmonary TB.
Interestingly, we found that in addition to lower levels of inflammatory cytokines such as IL-6 and TNF-α, PBMCs from persons with previous extrapulmonary TB also had lower levels of IL-10, which is traditionally considered an anti-inflammatory cytokine that suppresses the immune response. We found a similar result in a previous study (2). These findings suggest a global defect in cytokine production rather than a defect in production of a specific cytokine or chemokine.
Several of the cytokines were noted to decrease significantly when infected macrophages were compared to uninfected macrophages. This was most striking with CCL2. All cytokines tested increased with the addition of autologous PBMC-M to infected macrophages. One possibility for this finding is that infected macrophages became “burned out” after several days of infection and required activating signals from PBMC-M to produce inflammatory cytokines. Also, certain cytokines, such as IL-6 and CXCL8, as well as IFN-γ and IL-17, were likely contributed by the added lymphocytes. Finally, it is possible that infected macrophages became nonviable after several days of incubation and that is why lower production of cytokines was observed. We did not assess for viability when obtaining supernatant from macrophage cultures.
Our study has several limitations. First, it is unclear whether cytokine production in response to M. tuberculosis after completion of anti-TB therapy is similar to cytokine production prior to developing TB disease. Previous studies have demonstrated that cytokine profiles differ between persons with active TB disease and uninfected controls (24) and before and after TB therapy (22, 29). Because the aim of our study was to identify predisposing immunologic factors leading to development of extrapulmonary TB, we chose to study persons who had completed TB therapy to avoid the fluctuations in cytokine production that have been observed in early-stage TB (28). Second, extrapulmonary TB is a complex disease, and different sites of disease manifestation may have different pathophysiology (9, 26, 34, 37). Previous studies have suggested that persons with different sites of TB disease have various immune responses (18). We included a wide spectrum of TB manifestations in the present study. The heterogeneous nature of the extrapulmonary group potentially makes our findings more broadly applicable to all persons with extrapulmonary TB, although it makes it impossible to draw conclusions about factors that may influence whether a person develops a limited or disseminated form of extrapulmonary TB. We are planning future studies to compare immune responses between persons with various forms of extrapulmonary TB. Also, immune activation in the periphery may differ from the immune response at the site of disease (i.e., in the lung or lymph nodes). Finally, persons with previous pulmonary TB were more likely to smoke than persons in the other study groups. Smoking is strongly associated with pulmonary disease (11, 27), likely due to its local effect in the lung rather than an effect on systemic immune function. Alcohol use is often associated with tobacco smoking and has also been associated with pulmonary TB (14).
Taken together, our data suggest that persons with previous extrapulmonary TB may have a subtle immune defect that leads to an inability to control M. tuberculosis at the site of primary infection. This defect may lie in signaling pathways upstream of cytokine production. Our results expand our knowledge of the immune response to M. tuberculosis infection and help us to further understand the factors that may influence progression from infection to active TB disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Vanderbilt Physician Scientist Development Program; a Vanderbilt CTSA grant (UL1 RR024975 NCRR/NIH); NIH K24 AI065298; and P60 AR056116. Flow cytometric cell acquisition and sorting were performed by the Vanderbilt-Meharry Center for AIDS Research (CFAR) Immunopathogenesis Core, an NIH-funded program (P30 AI 54999).
We gratefully acknowledge the assistance of the Tennessee Department of Health and Metro Nashville Public Health (Jon Warkentin, Celia Goodson, Diedra Freeman, Amy Kerrigan, Imelda Villarosa, and Diane Peterse) in recruitment of study subjects. We also acknowledge Steve Holland and Shanmugalakshmi Sadagopal for their insight and assistance in experimental design. We also thank Holly Algood and Luc van Kaer for their critical review of the manuscript.
We report no potential conflicts of interest.
Footnotes
Published ahead of print 6 June 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. American Thoracic Society/Centers for Disease Control and Prevention 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:S221–S247 [DOI] [PubMed] [Google Scholar]
- 2. Antas PRZ, et al. 2006. Decreased CD4(+) lymphocytes and innate immune responses in adults with previous extrapulmonary tuberculosis. J. Allergy Clin. Immunol. 117:916–923 [DOI] [PubMed] [Google Scholar]
- 3. Barnes PF, et al. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castano D, Barrera LF, Rojas M. 2011. Mycobacterium tuberculosis alters the differentiation of monocytes into macrophages in vitro. Cell. Immunol. 268:60–67 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2005. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recommend. Rep. 54:1–47 [PubMed] [Google Scholar]
- 6. Crowle AJ, Elkins N. 1990. Relative permissiveness of macrophages from black and white people for virulent tubercle bacilli. Infect. Immun. 58:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz A, et al. 2006. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416–1420 [DOI] [PubMed] [Google Scholar]
- 8. de Almeida AS, Fiske CT, Sterling TR, Kalams SA. 2012. Increased frequency of regulatory T cells and T lymphocyte activation in persons with previously treated extrapulmonary tuberculosis. Clin. Vaccine Immunol. 19:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djoba Siawaya JF, et al. 2009. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine 47:132–136 [DOI] [PubMed] [Google Scholar]
- 10. Edwards KM, et al. 2001. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 164:2213–2219 [DOI] [PubMed] [Google Scholar]
- 11. Feng Y, et al. 2011. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect. Immun. 79:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiske CT, de Almeida AS, Kalams SA, Sterling TR. 2010. Abnormal immune responses in persons with previous extrapulmonary tuberculosis in an in vitro macrophage model that simulates in vivo infection with M. tuberculosis. Am. J. Respir. Crit. Care Med. 181:A3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiske CT, et al. 2010. Black race, sex, and extrapulmonary tuberculosis risk: an observational study. BMC Infect. Dis. 10:16 doi:10.1186/1471-2334-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiske CT, Hamilton CD, Stout JE. 2009. Alcohol use and clinical manifestations of tuberculosis. J. Infect. 58:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flores-Villanueva PO, et al. 2005. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J. Exp. Med. 202:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129 [DOI] [PubMed] [Google Scholar]
- 17. Goletti D, et al. 2006. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J. Infect. Dis. 194:984–992 [DOI] [PubMed] [Google Scholar]
- 18. Hasan Z, et al. 2009. ESAT6-induced IFNgamma and CXCL9 can differentiate severity of tuberculosis. PLoS One 4:e5158 doi:10.1371/journal.pone.0005158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasan Z, et al. 2009. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand. J. Immunol. 69:259–267 [DOI] [PubMed] [Google Scholar]
- 20. Hasan Z, et al. 2011. M. tuberculosis sonicate induced IFNgamma, CXCL10 and IL10 can differentiate severity in tuberculosis. Scand. J. Immunol. 75:220–226 [DOI] [PubMed] [Google Scholar]
- 21. Hasan Z, et al. 2005. Elevated ex vivo monocyte chemotactic protein-1 (CCL2) in pulmonary as compared with extra-pulmonary tuberculosis. BMC Immunol. 6:14 doi:10.1186/1471-2172-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirsch CS, et al. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069–2073 [DOI] [PubMed] [Google Scholar]
- 23. Horsburgh CR. 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350:2060–2067 [DOI] [PubMed] [Google Scholar]
- 24. Hussain R, et al. 2002. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J. Immunol. Methods 264:95–108 [DOI] [PubMed] [Google Scholar]
- 25. Iseman MD. 2000. A clinician's guide to tuberculosis, p 63–96 Lippincott, Williams, and Wilkins, Philadelphia, PA [Google Scholar]
- 26. Jamil B, et al. 2007. Interferon gamma/IL10 ratio defines the disease severity in pulmonary and extra pulmonary tuberculosis. Tuberculosis (Edinb.) 87:279–287 [DOI] [PubMed] [Google Scholar]
- 27. Jee SH, et al. 2009. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am. J. Epidemiol. 170:1478–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jo EK, et al. 2000. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand. J. Immunol. 51:209–217 [DOI] [PubMed] [Google Scholar]
- 29. Jo EK, Park JK, Dockrell HM. 2003. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 16:205–210 [DOI] [PubMed] [Google Scholar]
- 30. Jones BE, et al. 1993. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. Rev. Respir. Dis. 148:1292–1297 [DOI] [PubMed] [Google Scholar]
- 31. Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. 2004. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int. J. Tuberc. Lung Dis. 8:658–674 [PubMed] [Google Scholar]
- 32. Marin ND, et al. 2010. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis (Edinb.) 90:252–261 [DOI] [PubMed] [Google Scholar]
- 33. Okamoto Yoshida Y, et al. 2010. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184:4414–4422 [DOI] [PubMed] [Google Scholar]
- 34. Porcel JM. 2009. Tuberculous pleural effusion. Lung 187:263–270 [DOI] [PubMed] [Google Scholar]
- 35. Reed WW, Diehl LF. 1991. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch. Intern. Med. 151:501–505 [PubMed] [Google Scholar]
- 36. Sai Priya VH, et al. 2009. In vitro levels of interleukin 10 (IL-10) and IL-12 in response to a recombinant 32-kilodalton antigen of Mycobacterium bovis BCG after treatment for tuberculosis. Clin. Vaccine Immunol. 16:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma SK, Mitra DK, Balamurugan A, Pandey RM, Mehra NK. 2002. Cytokine polarization in miliary and pleural tuberculosis. J. Clin. Immunol. 22:345–352 [DOI] [PubMed] [Google Scholar]
- 38. Sterling TR, et al. 2001. Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clin. Infect. Dis. 33:976–982 [DOI] [PubMed] [Google Scholar]
- 39. Sterling TR, et al. 2007. Immune function in young children with previous pulmonary or miliary/meningeal tuberculosis and impact of BCG vaccination. Pediatrics 120:e912–e921 [DOI] [PubMed] [Google Scholar]
- 40. Verbon A, et al. 1999. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin. Exp. Immunol. 115:110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkinson RJ, et al. 1998. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J. Infect. Dis. 178:760–768 [DOI] [PubMed] [Google Scholar]
- 42. Wilsher ML, Hagan C, Prestidge R, Wells AU, Murison G. 1999. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber. Lung Dis. 79:371–377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.