Abstract
Three serologic methods for antibody detection in elephant tuberculosis (TB), the multiantigen print immunoassay (MAPIA), ElephantTB STAT-PAK kit, and DPP VetTB test, were evaluated using serial serum samples from 14 captive elephants infected with Mycobacterium tuberculosis in 5 countries. In all cases, serological testing was performed prior to the diagnosis of TB by mycobacterial culture of trunk wash or tissue samples collected at necropsy. All elephants produced antibody responses to M. tuberculosis antigens, with 13/14 recognizing ESAT-6 and/or CFP10 proteins. The findings supported the high serodiagnostic test accuracy in detecting infections months to years before M. tuberculosis could be isolated from elephants. The MAPIA and/or DPP VetTB assay demonstrated the potential for monitoring antimycobacterial therapy and predicting TB relapse in treated elephants when continuously used in the posttreatment period. History of exposure to TB and past treatment information should be taken into consideration for proper interpretation of the antibody test results. Data suggest that the more frequent trunk wash culture testing of seropositive elephants may enhance the efficiency of the TB diagnostic algorithm, leading to earlier treatment with improved outcomes.
INTRODUCTION
Tuberculosis (TB) has been recognized as a reemerging disease of captive elephants worldwide (22, 23, 25) with serious zoonotic concerns (19, 26, 27). In the past 2 decades, increasing numbers of elephant TB cases have been reported from different countries (1, 13, 20). In the United States, after two Asian elephants were diagnosed with Mycobacterium tuberculosis infection in 1996, a TB Advisory Panel was formed to investigate the situation (21). In order to address growing concern, the National Tuberculosis Working Group for Zoo and Wildlife Species, in coordination with the United States Department of Agriculture (USDA), developed the Guidelines for the Control of Tuberculosis in Elephants. The document was published in 1997 and updated in 2000, 2003, 2008, and 2010, as new information became available. The guidelines determined criteria for risk groups, specified protocols for surveillance and diagnostic testing, and provided recommendations for treatment of elephants diagnosed with TB. Under the current guidelines (2010 version), all elephants in the United States should be tested annually by both trunk wash culture and serology using the USDA-licensed ElephantTB STAT-PAK kit (6). Elephants that are ElephantTB STAT-PAK reactive are subjects for confirmatory testing by multiantigen print immunoassay (MAPIA) (15).
Recently, a new generation test for rapid point-of-care TB serodiagnosis in elephants has been developed using dual path platform (DPP) technology (7). This assay format has been evaluated in different host species infected with M. tuberculosis complex organisms to demonstrate the promising potential for multispecies applications (2, 3, 4, 18, 28, 29). The goal of the present work was to evaluate the predictive diagnostic value of three serologic methods, the ElephantTB STAT-PAK kit, MAPIA, and DPP VetTB assay, in a group of selected elephants which were identified as antibody positive when routinely tested by serology and eventually diagnosed with TB by culture.
MATERIALS AND METHODS
Animals.
The study group (Table 1) consisted of 11 Asian (Elephas maximus) and 3 African (Loxodonta africana) elephants, all females 14 to 64 years of age, from 11 facilities in the United States (n = 9), Nepal (n = 2), Australia (n = 1), France (n = 1), and Sweden (n = 1) that agreed to participate. The following inclusion criteria were adopted for the longitudinal study design: (i) at the time of serological testing of live elephants, the true infection status was unknown (with consistently negative trunk wash culture results), (ii) specific antibody was detected, and (iii) M. tuberculosis was isolated at a later time from trunk wash specimens or from tissues at necropsy. Of the 7 elephants with postmortem TB diagnosis, 3 died and 4 were humanely euthanized. All 7 showed granulomatous lesions in the lungs, lymph nodes, and other organs. For 6/7 elephants diagnosed antemortem, treatment with first-line anti-TB drugs was initiated, in accordance with the Guidelines for the Control of Tuberculosis in Elephants (6). Previous exposure to TB was known for 9 elephants, 4 of which had reportedly received prophylactic treatment in the past. Signs suggestive of TB (chronic weight loss, dyspnea, trunk discharge) were noticed in 7 elephants prior to culture-based diagnosis. Serum samples were serially collected before and after confirmation of M. tuberculosis infection for use in the antibody assays.
Table 1.
Clinical, epidemiological, and diagnostic data obtained for the M. tuberculosis-infected elephantsa
| Elephant | TB sign(s) | Known exposure | TB diagnosis, yr | Yr of serum specimen collection | No. of mo from seroconversion to positive culture |
|---|---|---|---|---|---|
| 1 | CWL | Yes | PM, 2004 | 2002–2004 | >24 |
| 2 | None | Yes | PM, 2005 | 2002–2004 | >31 |
| 3 | CWL | Yes | PM, 2006 | 1998–2006 | 20 |
| 4 | CWL | No | PM, 2009 | 2006–2009 | 31 |
| 5 | None | No | PM, 2010 | 2009 | >9 |
| 6 | CWL | No | PM, 2010 | 2010 | >3 |
| 7 | None | Yes | PM, 2011 | 2002–2011 | >6 |
| 8 | TMD | Yes | AM, 2008 | 2006–2011 | −4 |
| 9 | Dyspnea, TMD | No | AM, 2010 | 2008–2010 | 24 |
| 10 | None | No | AM, 2010 | 2004–2011 | >72 |
| 11 | None | Yes | AM, 2010 | 2008–2011 | 16 |
| 12 | None | Yes | AM, 2011 | 2011 | >3 |
| 13 | TMD | Yes | AM, 2011 | 1996–2011 | >168 |
| 14 | None | Yes | AM, 2011 | 1996–2011 | >180 |
CWL, chronic weight loss; TMD, trunk mucous discharge; PM, postmortem; AM, antemortem.
Mycobacterial culture.
The procedure for collecting triple trunk wash samples for culture was performed as previously described (22). Feces, urine, vaginal discharge, and various tissues obtained at necropsy were also submitted for culture testing. Isolation and identification of M. tuberculosis and other mycobacteria were performed at the National Veterinary Services Laboratories (Ames, IA) and other certified laboratories, in accordance with the Guidelines for the Control of Tuberculosis in Elephants (6). Briefly, Middlebrook 7H10 with glycerol, Middlebrook 7H11 with glycerol, Stonebrinks, and BBL Mycobactosel L-J and, also, Bactec 12B vials were inoculated with 0.5 ml of sample supplemented with polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) and erythromycin (32 μg/ml). Processed specimens were inoculated on media and incubated at 37°C with 10% CO2 for up to 8 weeks. All suspicious colonies and Bactec bottles with a growth indicator value > 25 were subjected to acid-fast staining and, if results were positive, confirmed with an AccuProbe Mycobacterium tuberculosis complex culture identification test (Gen-Probe, San Diego, CA). If positive on the DNA probe, spoligotyping was performed to confirm M. tuberculosis. Atypical mycobacteria were identified by partial 16S, rpoB, or internal transcribed spacer (ITS) sequencing as previously described (8, 9).
ElephantTB STAT-PAK kit.
The ElephantTB STAT-PAK (Chembio Diagnostic Systems, Inc., Medford, NY) test, a one-step lateral-flow screening test, detects antibodies to ESAT-6, CFP10, and MPB83 antigens (15). The assay was performed according to the manufacturer's instructions by veterinarians at elephant facilities using 30 μl of freshly collected serum or plasma and 3 drops of sample buffer (included in the kit) that were added to the device sequentially. Results were read visually 20 min later. Any visible band in the test area, in addition to the control line, was considered an antibody-positive result, whereas the absence of a test band was considered an antibody-negative result. ElephantTB STAT-PAK reactivity was scored visually as follows: −, none; +, weak; ++, moderate; and +++, strong.
Dual path platform (DPP) VetTB assay.
The DPP format is a two-step point-of-care test recently developed for rapid detection of TB in elephants and other host species (5, 28). The assay has two test antigen bands on the membrane strip, T1 (MPB83 protein) and T2 (CFP10/ESAT-6 fusion), for differential IgG antibody detection by colloidal gold particles coupled with hybrid protein A/G. The DPP VetTB assay was performed at Chembio (for 11 elephants) or on site at elephant facilities (for 3 elephants in Australia and Nepal) as previously described (7) using 5 μl of serum, 2 drops of buffer added to the sample well, and 4 drops of buffer added to the conjugate well. Results were evaluated at 15 min visually and by a DPP optical reader device to measure reflectance in relative light units (RLU). Reactivity of T1 and/or T2 above the cutoff value of 30 RLU was considered an antibody-positive result. Absence of reactivity with either of the two test antigens was interpreted as an antibody-negative result.
MAPIA.
The testing was performed at Chembio as previously described (15). The antigen panel consisted of 12 recombinant proteins of M. tuberculosis and 2 native antigen preparations of M. bovis as follows: ESAT-6 and CFP10 proteins as well as hybrids CFP10/ESAT-6 and Acr1/MPB83 (from Statens Serum Institut, Copenhagen, Denmark); MPB59, MPB64, MPB70, and MPB83 proteins as well as bovine protein purified derivative (B-PPD) tuberculin and M. bovis culture filtrate (MBCF) from the Veterinary Sciences Division of Stormont (United Kingdom); Mtb8 and polyepitope fusion TBF10 developed by Corixa Corp. (Seattle, WA); and alpha-crystallin (Acr1) and the 38-kDa protein from Standard Diagnostics (Seoul, South Korea). Elephant IgG antibody bound to the immobilized antigens was detected by peroxidase-conjugated protein G (Sigma, St. Louis, MO) diluted at 1:1,000 and visualized with 3,3′,5,5′-tetramethyl benzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD). MAPIA results were evaluated visually, with a band of any intensity being read as an antibody-positive reaction.
RESULTS
Antibody responses to M. tuberculosis.
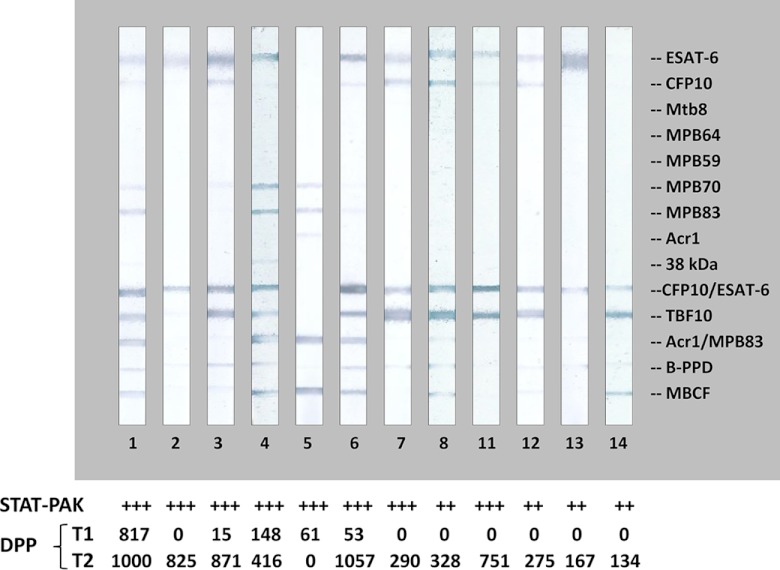
Clinical, epidemiological, and diagnostic data obtained for the 14 elephants studied are summarized in Table 1. Testing of serially collected serum specimens with the ElephantTB STAT-PAK kit, MAPIA, and DPP VetTB assay demonstrated agreement between the results with respect to relative intensities of test bands reflecting the antibody levels in individual elephants and the kinetics of the immune responses (Fig. 1, 2, and 3). Two elephants (9 and 10) were tested in the field by the ElephantTB STAT-PAK kit and DPP VetTB assay, but not by MAPIA, as the serum samples were not available for testing in the United States. Time of seroconversion was determined for 9 of the 14 elephants, while the other 5 were already antibody positive at the time the first serum sample was available for testing.
Fig 1.
Differential antibody reactivity patterns obtained by the MAPIA with serum samples from M. tuberculosis-positive elephants. Images show MAPIA results for individual elephants, with identification numbers shown below the strips. Preprinted antigens are indicated in the right margin. ElephantTB STAT-PAK test results for the respective elephant serum samples are presented as +++ (strong reaction) or ++ (moderate reaction). Semiquantitative DPP VetTB assay results measured by a reflectance reader device are shown for the T1 band (IgG reactivity to MPB83 protein) and for the T2 band (IgG reactivity to CFP10/ESAT-6 fusion protein).
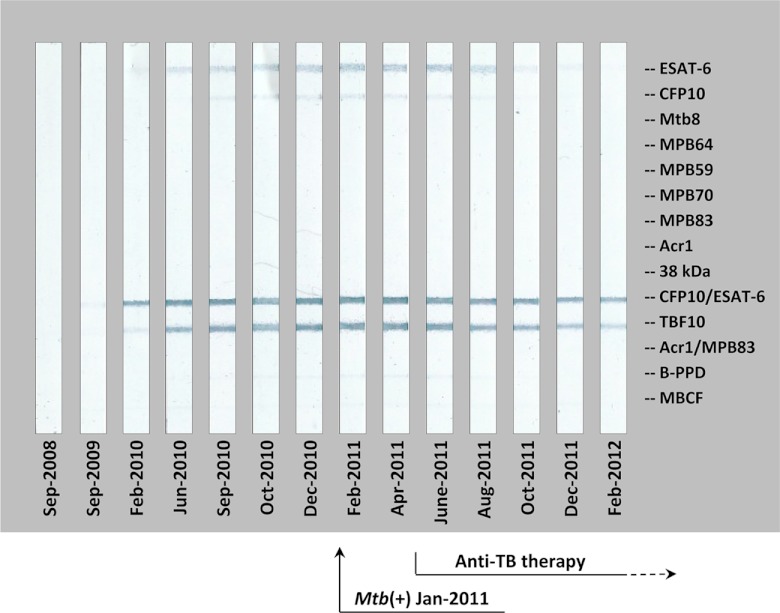
Fig 2.
Antibody response detected by MAPIA in elephant 11 and the effect of antimycobacterial therapy: an example of early seroconversion and successful treatment. Strips show MAPIA results obtained with serum samples collected on the indicated dates. Printed antigens are listed in the right margin. The time of M. tuberculosis isolation from trunk wash specimens (January 2011) is indicated by a solid arrow. The time frame of anti-TB therapy (May 2011 to May 2012) is shown on the bottom (dashed arrow).
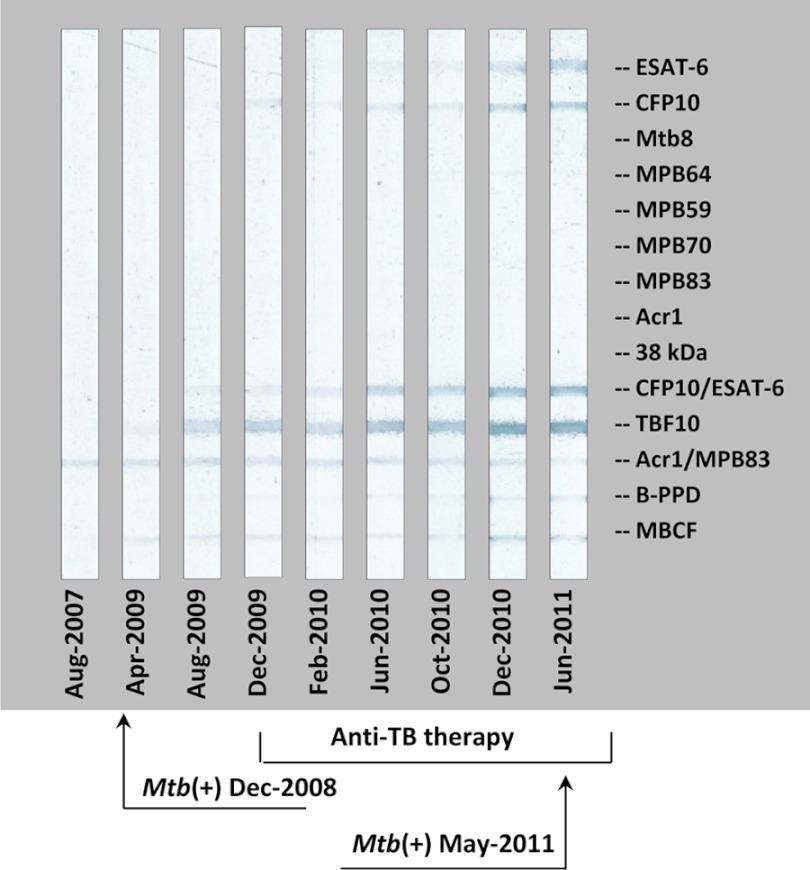
Fig 3.
Antibody response detected by MAPIA in elephant 8 and the effect of antimycobacterial therapy: an example of delayed seroconversion and unsuccessful treatment. Strips show MAPIA results obtained with serum samples collected on the indicated dates. Printed antigens are listed in the right margin. Arrows show the dates of M. tuberculosis isolation from trunk washes: December 2008 (left) and May 2011 (right). The time frame of anti-TB therapy (December 2009 to July 2011) is indicated on the bottom.
Of the 9 elephants with a history of known exposure to TB-infected elephants, 4 had received prophylactic treatment prior to serologic testing. One of these elephants (elephant 8) had M. tuberculosis isolated from trunk wash specimens 4 months before an initial seropositive result (Fig. 4). In contrast, the other 13 elephants in the study group produced serum antibodies prior to (ranging from 3 months to 15 years) culture-based TB diagnosis (Table 1). Importantly, elephant 8 had received antimycobacterial therapy twice during previous years, which might have resulted in delayed seroconversion.
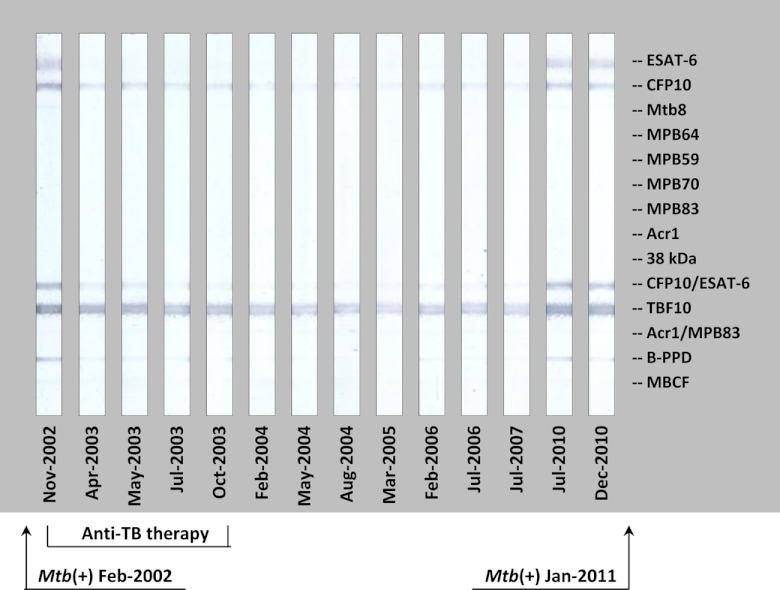
Fig 4.
Antibody response detected by MAPIA in elephant 7 before and after antimycobacterial therapy: an example of successful treatment followed by a relapse. Strips show MAPIA results obtained with serum samples collected on the indicated dates. Printed antigens are listed in the right margin. Arrows show the dates of M. tuberculosis isolation: February 2002, from trunk washes (left), and January 2011, at postmortem examination (right). The time frame of anti-TB therapy (December 2002 to December 2003) is indicated on the bottom.
The ElephantTB STAT-PAK scores and DPP VetTB assay reading values (for the T2 band) did not appear to be associated with the presence of clinical signs indicative of TB. However, while 4/7 elephants with antemortem diagnosis showed relatively low antibody levels, all 7 elephants diagnosed postmortem (2 of them died of chronic TB) had strong serological reactions (Table 1 and Fig. 1; serology data not shown for elephants 9 and 10).
Variable seroreactivity patterns.
Use of multiple antigens enabled further analysis of antibody profiles during elephant TB infection. Serum IgG antibodies to ESAT-6 and/or CFP10 proteins were detected by the DPP VetTB assay and/or MAPIA in 13/14 elephants (Fig. 1; data not shown for elephants 9 and 10). Five elephants (including elephant 9; data not shown) responded to MPB83 protein, with one (elephant 5) recognizing this antigen in the absence of measurable IgG reactivity to ESAT-6 or CFP10 proteins, in contrast to the other elephants in this subgroup. Interestingly, the antibody responses to MPB83 protein, with or without ESAT-6 and/or CFP10 involved, were found in 4/7 elephants diagnosed with TB postmortem and in only 1/7 elephants with antemortem diagnosis.
The more frequent trunk wash testing of seropositive elephants facilitates earlier culture-based diagnosis.
The current Guidelines for the Control of Tuberculosis in Elephants recommend annual testing by trunk wash culture and ElephantTB STAT-PAK kit (6). However, increased frequency of trunk wash testing is required for elephants with antibody-positive results confirmed by the MAPIA and/or DPP VetTB assay, as these elephants are considered to be at higher risk of having TB, especially if seropositivity is combined with known history of exposure to a culture-positive TB case. The present study provided important examples (three are described below) to support the diagnostic value of this approach.
Asymptomatic elephant 10 with no history of TB exposure had been trunk wash culture negative in annual testing performed since 2004. When the ElephantTB STAT-PAK kit was first used to test this elephant in April 2009, it yielded a reactive result which was subsequently confirmed by the DPP VetTB assay. Furthermore, these serodiagnostics detected antibody in archived serum samples collected in 2006 (at the time the facility acquired this elephant) and in 2004 (prior to acquisition). Once the facility implemented quarterly trunk wash cultures for this antibody-positive elephant (April 2009), M. tuberculosis was isolated within 19 months (November 2010), followed by initiation of antimycobacterial treatment (January 2011).
Annual trunk wash culture results had been consistently negative in asymptomatic elephant 11 with long-term direct exposure to an M. tuberculosis-positive herd mate which was diagnosed by culture in 2000 and treated during 2000 to 2001. In February 2010, routine serological screening revealed an antibody response to ESAT-6 and CFP10 proteins that gradually increased in the subsequent months (Fig. 2). Based on the finding of seroconversion in the TB-exposed elephant, in October 2010 the facility introduced daily trunk wash collections every other week for submission of pooled (up to 5) specimens for culture. Using this intensified testing schedule, a positive culture was obtained from elephant 11 within 3 months (January 2011) or 16 months after the onset of antibody response. The strain of M. tuberculosis was indistinguishable, based on spoligotyping and variable-number tandem-repeat (VNTR) testing, from that isolated from the treated elephant (likely source of infection) which had remained trunk wash culture negative since 2000. Antibiotic treatment was initiated for both elephants in May 2011. Importantly, of 13 trunk wash pooled specimens submitted for culture testing between September 2009 (seroconversion) and May 2011 (treatment start), only one yielded a positive result (January 2011), while 8 preceding and 4 following samples were culture negative. In contrast, the serologic assays yielded consistently antibody-positive results during the same time period.
Elephants 13 and 14 were housed together, and both had been in close contact with an M. tuberculosis-positive elephant diagnosed in 1996. They also had a very long history of being antibody positive (since 1997 for elephant 13 and since 1996 for elephant 14; first tested in 2004 retrospectively), while both remained consistently culture negative at annual or semiannual trunk wash testing. In late 2011, however, when the facility began daily trunk wash sampling from these two elephants for culture testing, M. tuberculosis was isolated from pooled specimens collected within 3.5 weeks from elephant 13 and from samples collected within 2 weeks from elephant 14.
Serological monitoring of treatment.
In elephants 10, 11, and 12, antibody levels measured by the MAPIA and/or DPP VetTB assay gradually declined within several months of anti-TB therapy (Fig. 2 shows a representative example). Considering previous observations (13), these results implied that the treatment of these elephants was effective. In sharp contrast, this trend was not found for elephant 8, in which the antibody levels continued increasing gradually over the course of anti-TB therapy (Fig. 3) and treatment failed, as, 17 months after its initiation, M. tuberculosis was isolated from a trunk discharge.
In elephant 7, M. tuberculosis was isolated on two different occasions, first in 2002 from trunk wash specimens and the second time in 2011 at necropsy. This elephant was treated during 2003 and believed to be cured at the time as evidenced by the absence of clinical signs, consistently negative trunk wash culture results during annual testing, and significantly decreased antibody reactivity in the following years (Fig. 4). However, a sharp increase of levels of antibody to ESAT-6 and CFP10 was detected by the MAPIA and DPP VetTB assay about 6 months prior to euthanasia. The strain of M. tuberculosis isolated at necropsy proved to be indistinguishable, based on spoligotyping and VNTR testing, from the 2002 isolate, suggesting a reactivation of previously treated TB rather than reinfection. Thus, regular serological monitoring in the posttreatment period predicted TB relapse in this elephant.
DISCUSSION
Mycobacterial culture remains the gold standard method for elephant TB diagnosis, despite its poor accuracy, particularly for antemortem diagnosis (7, 22, 23). The reported estimates of the trunk wash culture sensitivity obtained from serial testing of multiple Asian elephants infected with M. tuberculosis ranged from 3% (1) to 4% (24), with some TB cases described that had never produced culture-positive results until postmortem examination, even when elephants were tested monthly after detection of antibody responses. In the present study, one elephant (elephant 11) that was subjected to a more frequent trunk wash testing protocol after seroconversion yielded only 1/13 culture-positive result, with 8 negative samples preceding and 4 following the positive trunk wash culture. This finding further demonstrates the intermittent nature of shedding previously suggested for elephants (22) and other animal hosts (5) as a likely contributing factor to the poor sensitivity and consistency of the trunk wash culture method.
Previous work has shown high accuracy of serology in detecting elephants with mycobacterial infection months to years before positive culture, even in the absence of clinical signs in most cases (7). The antibody responses to M. tuberculosis in Asian and African elephants were characterized in retrospective studies using available collections of archived serum samples obtained from animals with an already-established infection status (11, 15). With the ESAT-6 and CFP10 proteins identified as the key antigens, the ElephantTB STAT-PAK, MAPIA, and DPP VetTB assay showed a sensitivity of 100% and specificity of 95% to 100% (7). A recent case report described four Asian elephants diagnosed with TB in Thailand (1). Three of these elephants had ElephantTB STAT-PAK reactive results long before (10, 19, and 32 months) M. tuberculosis was isolated (Victor Rutten, personal communication).
In the present study, a longitudinal design was used to validate the predictive diagnostic value of the antibody immunoassays in animals of unknown infection status at the time of serological testing. On annual field testing, 14 elephants from 11 facilities in 5 countries were primarily identified as ElephantTB STAT-PAK reactive, and the results were confirmed by the MAPIA and/or DPP VetTB assay for the presence of M. tuberculosis-specific antibodies while having trunk wash-negative results before being diagnosed with TB by culture at a later date, either antemortem or postmortem. The results supported the high serodiagnostic sensitivity reported previously (7) and provided several new insights.
The magnitude of antibody responses and the number of antigens recognized in elephant TB appeared to be associated with advanced stages of disease. A close correlation between antibody titers and development of pathology has been shown for different host species infected with M. tuberculosis or M. bovis (14, 16, 17). Thus, semiquantitative measurements of antibody responses in elephant TB offer a potentially useful correlate of disease progression, providing a convenient tool for the treatment and care of affected elephants as well as risk assessment for exposed animals and humans.
As reported previously (7), the serological signature of elephant TB is closely associated with ESAT-6/CFP10 reactivity. However, the present findings suggest that elephant antibody responses to M. tuberculosis may not necessarily be limited to recognition of these two immunodominant targets. Elephant 5 responded to MPB83 protein but not to ESAT-6 or CFP10. In our experience based on 42 elephants with confirmed TB tested to date (reference 7, the present report, and our unpublished observations), this is the first case with such an atypical pattern of seroreactivity. It may be of particular interest that elephant 5 had a mixed infection, with M. tuberculosis and M. szulgai found postmortem. Interestingly, the presence of antibody only to MPB83 protein has been reported in two African elephants with fatal pulmonary disease caused by M. szulgai (10). Thus, one may speculate that the unusual antibody profile found for elephant 5 in the present study might have been influenced by coinfection with M. szulgai.
Past antimycobacterial therapy may pose a confounding factor for interpretation of existing elephant TB diagnostics. The antibody response can be affected by treatment in different ways, including a decline in magnitude, modification of antigen recognition patterns, and/or delay in onset of the antibody response. In the present study, the latter was exemplified by finding seroconversion after trunk wash culture became positive in elephant 8, which had received anti-TB prophylactic treatment in the past. A similar phenomenon was reported for M. bovis BCG-vaccinated Eurasian badgers whose antibody responses to experimental M. bovis challenge were delayed for various numbers of weeks (12).
Prophylactic administration of antimycobacterial antibiotics can also reduce M. tuberculosis shedding to undetectable levels, thereby limiting the value of antemortem culture testing (7, 22). The present study suggests that more-frequent trunk wash sampling in high-risk elephants (i.e., clinical signs of TB, history of exposure, recent seroconversion, or continuing antibody reactivity in the MAPIA and/or DPP VetTB assay) may accelerate TB diagnosis. Exposed elephants 13 and 14 had specific antibody responses but remained negative upon annual trunk wash testing for over a decade thereafter, presumably due to the preventive antimycobacterial treatment they had received. These two elephants were finally diagnosed with TB by isolation of M. tuberculosis within only a few weeks of implementation of daily trunk wash collection.
In conclusion, the serologic assays for elephant TB evaluated longitudinally on a group of 14 captive elephants with culture-diagnosed disease in 5 countries were confirmed to have a high predictive value in rapidly detecting infection months or years before M. tuberculosis could be isolated from trunk wash samples or at necropsy. Background information and medical history, including past exposure and treatments, should be taken into consideration for proper interpretation of the antibody test results. The more frequent antemortem culture testing of seropositive elephants may significantly improve efficiency of the TB diagnostic algorithm, leading to earlier treatment with potentially improved outcomes.
ACKNOWLEDGMENTS
We are grateful to the Albuquerque Biological Park elephant veterinary team for their assistance with the project; to Roberto Aguilar for consultation and support; to Claudia Quinn for skillful technical assistance with the serological assays; and to the numerous veterinarians and elephant caretakers for supplying serum samples, medical records, and historical information from elephants included in the study.
Gratitude is extended to the Department of National Parks and Wildlife Conservation, the National Trust for Nature Conservation, the World Wildlife Fund—Nepal, and Sarad Paudel, Jeewan Thapa, and Barbara Vincent for TB work in Nepal as well as to the U.S. Fish and Wildlife Service, Asian Elephant Conservation Fund, for financial support.
Footnotes
Published ahead of print 13 June 2012
REFERENCES
- 1. Angkawanish T, et al. 2010. Mycobacterium tuberculosis infection of domesticated Asian elephants, Thailand. Emerg. Infect. Dis. 16:1949–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boadella M, et al. 2012. Performance of immunochromatographic and ELISA tests for detecting fallow deer infected with Mycobacterium bovis. Prev. Vet. Med. 104:160–164 [DOI] [PubMed] [Google Scholar]
- 3. Boadella M, et al. 2011. Serologic tests for detecting antibodies against Mycobacterium bovis and Mycobacterium avium subspecies paratuberculosis in Eurasian wild boar (Sus scrofa scrofa). J. Vet. Diagn. Invest. 23:77–83 [DOI] [PubMed] [Google Scholar]
- 4. Buddle BM, et al. 2010. Sensitivity, specificity, and confounding factors of novel serological tests used for the rapid diagnosis of bovine tuberculosis in farmed red deer (Cervus elaphus). Clin. Vaccine Immunol. 17:626–630 doi:10.1128/CVI.00010-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chambers MA, et al. 2008. Validation of the BrockTB STAT-PAK assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 46:1498–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Committee on Tuberculosis 2010. Guidelines for the control of tuberculosis in elephants, p 578–639 Proceedings of 114th Annual Meeting. U.S. Animal Health Association, St. Joseph, MO [Google Scholar]
- 7. Greenwald R, et al. 2009. Highly accurate antibody assays for early and rapid detection of tuberculosis in African and Asian elephants. Clin. Vaccine Immunol. 16:605–612 doi:10.1128/CVI.00038-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harmsen D, et al. 2003. Ridom: comprehensive and public sequence database for identification of mycobacterium species. BMC Infect. Dis. 3:26 doi:10.1186/1471-2334-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins J, et al. 2011. Identification of Mycobacterium spp. of veterinary importance using rpoB gene sequencing. BMC Vet. Res. 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacasse C, et al. 2007. Two cases of atypical mycobacteriosis caused by Mycobacterium szulgai associated with mortality in captive African elephants (Loxodonta africana). J. Zoo Wildl. Med. 38:101–107 [DOI] [PubMed] [Google Scholar]
- 11. Larsen RS, et al. 2000. Evaluation of a multiple-antigen enzyme-linked immunosorbent assay for detection of Mycobacterium tuberculosis infection in captive elephants. J. Zoo Wildl. Med. 31:291–302 [DOI] [PubMed] [Google Scholar]
- 12. Lesellier S, et al. 2009. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 27:402–409 doi:10.1016/j.vaccine.2008.10.068 [DOI] [PubMed] [Google Scholar]
- 13. Lewerin SS, et al. 2005. Outbreak of Mycobacterium tuberculosis infection among captive Asian elephants in a Swedish zoo. Vet. Rec. 156:171–175 [DOI] [PubMed] [Google Scholar]
- 14. Lyashchenko K, et al. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyashchenko KP, et al. 2006. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin. Vaccine Immunol. 13:722–732 doi:10.1128/CVI.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyashchenko KP, et al. 2007. PrimaTB STAT-PAK assay, a novel rapid lateral-flow test for tuberculosis in nonhuman primates. Clin. Vaccine Immunol. 14:1158–1164 doi:10.1128/CVI.00230-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lyashchenko KP, et al. 2008. Animal-side assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132:283–292 [DOI] [PubMed] [Google Scholar]
- 18. Lyashchenko KP, et al. 2011. Diagnostic value of animal-side antibody assays for rapid detection of Mycobacterium bovis or Mycobacterium microti infection in South American camelids. Clin. Vaccine Immunol. 18:2143–2147 doi:10.1128/CVI.05386-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michalak K, et al. 1998. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg. Infect. Dis. 4:283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikota SK. 2008. Tuberculosis in elephants, p 355–364 In Fowler ME, Miller RE. (ed), Zoo and wild animal medicine, current therapy, 6th ed Saunders Elsevier, St. Louis, MO [Google Scholar]
- 21. Mikota SK, Larsen RS, Montali RJ. 2000. Tuberculosis in elephants in North America. Zoo Biol. 19:393–403 doi:10.1002/1098-2361(2000)19:5<393::AID-ZOO9>3.0.CO;2-T [Google Scholar]
- 22. Mikota SK, et al. 2001. Epidemiology and diagnosis of Mycobacterium tuberculosis in captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 32:1–16 [DOI] [PubMed] [Google Scholar]
- 23. Mikota SK, Maslow JN. 2011. Tuberculosis at the human-animal interface: an emerging disease of elephants. Tuberculosis 91:208–211 doi:10.1016/j.tube.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 24. Moller T, Roken B, Petersson L, Vitaud C, Lyashchenko K. 2005. Preliminary results of a new serological test for detection of TB infection (Mycobacterium tuberculosis) in elephants (Elephas maximus and Loxodonta africanum)—Swedish case studies. Verh. ber. Erkrg. Zootiere 42:173–181 [Google Scholar]
- 25. Montali RJ, Mikota SK, Cheng LI. 2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Sci. Tech. 20:291–303 [DOI] [PubMed] [Google Scholar]
- 26. Murphree R, Warkentin JV, Dunn JR, Schaffner W, Jones TF. 2011. Elephant-to-human transmission of tuberculosis, 2009. Emerg. Infect. Dis. 17:366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oh P, et al. 2002. Human exposure following Mycobacterium tuberculosis infection of multiple animal species in a metropolitan zoo. Emerg. Infect. Dis. 8:1290–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rhodes SG, et al. 2011. Comparative study of IFNγ and antibody tests for feline tuberculosis. Vet. Immunol. Immunopathol. 144:129–134 doi:10.1016/j.vetimm.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 29. Waters WR, et al. 2011. Bovine tuberculosis in a Nebraska herd of farmed elk and fallow deer: a failure of the tuberculin skin test and opportunities for serodiagnosis. Vet. Med. Int. 2011:953985. [DOI] [PMC free article] [PubMed] [Google Scholar]