Abstract
We previously reported efficient transmission of the pathogenic R5 simian-human immunodeficiency virus SHIVSF162P3N isolate in Indian rhesus macaques by intravenous and intrarectal inoculations, with a switch to CXCR4 coreceptor usage in ∼50% of infected animals that progressed rapidly to disease. Since women continue to be disproportionately affected by HIV, we developed an animal model based on the intravaginal challenge of female rhesus monkeys with SHIVSF162P3N and sought to validate the utility of this model to study relevant aspects of HIV transmission and pathogenesis. The effect of viral dose on infection outcome was evaluated to determine the optimal conditions for the evaluation of HIV-1 preventive and therapeutic strategies. We found that the virus can successfully cross the vaginal mucosal surface to establish infection and induce disease with coreceptor switch, but with lower efficiencies compared to intravenous and rectal transmissions. In contrast to intrarectal infection, peak and cumulative viral load over a 1 year-infection period were significantly greater in macaques exposed intravaginally to lower rather than higher inoculum doses. Moreover, low and transient viremia was observed only in macaques that were challenged intravaginally twice within the same day with a high dose of virus, which can be seen as doubling the dose. Taken together, these results show that SHIVSF162P3N can successfully transmit across the genital mucosa, undergo coreceptor switch, and induce disease. However, the administered dose appears to impact SHIVSF162P3N vaginal infection outcome in an unexpected manner.
INTRODUCTION
Over 80% of all HIV transmissions occur across mucosal surfaces, with women accounting for 60% of adults infected with HIV in sub-Saharan Africa (18, 56, 60). Close to 80% of heterosexual transmissions with HIV-1 clades A, B, C, and D are established by a single viral variant from the donor, with higher numbers of transmitted/founder viruses detected in men who have sex with men and wider variations in variant numbers in intravenous drug users (2, 4, 15, 23, 24, 31, 48, 49). The majority of the transmitted/founder viruses are CCR5-tropic, with neutralization susceptibility profiles that are typical of primary viruses (24, 31, 49).
Nonhuman primate (NHP) models play a key role in providing a clearer understanding of the very early events in mucosal HIV transmission. Studies in the SIV/macaque model of vaginal transmission showed that within a few days of virus transmission, replication converges on the gut-associated lymphoid tissues (32, 63, 64), with loss of memory CD4+ T cells in this as well as in multiple lymphoid tissue compartments (40, 58). Furthermore, similar to the human situation, transmission of a very small number of variants was observed in rhesus macaques (RMs) exposed intrarectally (i.r.) or intravaginally (i.v.g.) to low-dose SIV and chimeric simian-human immunodeficiency virus (SHIV) (25, 36, 51, 57). Of note, macaques treated by depo-provera to thin the vaginal mucosa and exposed to a single supraphysiological dose of R5 SHIV also harbored few SHIV variants, indicating a particularly strong genetic bottleneck in vaginal transmission (7). NHP mucosal infection models, therefore, are relevant for the evaluation of intervention strategies such as vaccines, topical microbicides, and preexposure prophylaxis to prevent HIV-1 sexual transmission, providing information that allows clinical researchers to make informed decisions in the choice of intervention concepts or approaches with the greatest potential for success in humans.
Since HIV-1 strains transmitted between humans use primarily CCR5 as their coreceptor, prevention strategies must protect against R5 virus challenges. We recently documented successful intravenous (i.v.) and i.r. transmission of the late (time of overt immunodeficiency) R5 SHIVSF162P3N isolate in Indian RMs (20, 47), with pathogenic sequelae that consistently recapitulate key features of HIV-1 infection in humans. These include acute CD4+ T cell depletion in the gut, uncontrolled replication, and progression to AIDS with a switch in coreceptor preference toward CXCR4 in ∼50% of infected animals. Efficient transmission, consistency of high viral set points, and disease in RMs infected i.v. or i.r. with SHIVSF162P3N suggest that the virus will be useful not only for testing HIV-1 envelope-based prophylactic or early postinfection interventions but also for the discovery of intervention strategies that rely on reduction in steady-state viral loads and prevention or delay of disease as indicators of protection. However, because the risk of HIV-1 infection varies with different viral doses and exposure routes (19) and because vaginal transmission is responsible for a large number of new HIV-1 infections (18, 56, 60), it is necessary to demonstrate that R5 SHIVSF162P3N can successfully breach the vaginal mucosa and induce disease to establish the full utility of this model. An understanding of the biological consequences of infection with R5 SHIVSF162P3N via different routes and doses will also be important. We document here transmission, persistent infection, and coreceptor switching in macaques exposed intravaginally to R5 SHIVSF162P3N and examine the impact of viral dose on disease severity in the vaginally infected monkeys.
MATERIALS AND METHODS
Animal inoculation and clinical assessments.
All inoculations were carried out in adult rhesus monkeys (Macaca mulatta) of Indian origin housed at the Tulane National Primate Research Center in compliance with the Guide for the Care and use of Laboratory Animals. Animals were confirmed to be serologically negative for simian type D retrovirus, simian immunodeficiency virus (SIV), and simian T-cell lymphotropic virus prior to infection and screened for the presence of the Mamu-A*01, Mamu-B*17, and Mamu-B*08 class I alleles previously shown to be associated with the control of pathogenic SIVmac239 replication using standard PCR with allele-specific primers (13). They were also genotyped for TRIM5α expression, since this innate host defense mediator has been reported to contribute to SIV control as well (26, 35). Macaques received a single intrarectal (i.r.) or intravaginal (i.v.g.) inoculation with 104 or lower 50% tissue culture infectious dose (TCID50) of the cell-free challenge stock SHIVSF162P3N or two i.v.g. inoculations with 104 TCID50 virus, 4 h apart. The challenge virus was propagated and titered in RM peripheral blood mononuclear cells and was generated through successive rapid transfer in RMs of the CCR5 molecular clone SHIVSF162, recovered from passage 3 in animal T353 at end-stage disease (17, 20). Whole blood from the inoculated animals was collected weekly for the first 8 weeks, biweekly for another 16 weeks, and monthly thereafter. Surgery was performed at peak (2 to 3 weeks postinfection [wpi]) and postacute (∼12 wpi) infection for collection of tissues from one external and one internal lymph node and from internal organs such as the gut, bone marrow, thymus, and spleen. Animals were euthanized at the end of the study period by intramuscular administration of telazol and buprenorphine, followed by an overdose of sodium pentobarbital. Euthanasia was considered to be AIDS related if the animal exhibited peripheral blood CD4+ T cell depletion (<200/mm3), >25% loss of body weight, and combinations of the following conditions: diarrhea unresponsive to treatment, opportunistic infections, peripheral lymph node atrophy, and abnormal hematology (e.g., anemia, thrombocytopenia, or leukopenia). Tissues from multiple sites were collected. Plasma viremia was quantified by branched DNA analysis (Siemens Medical Solutions Diagnostic Clinical Lab, Emeryville, CA), and absolute CD4+ and CD8+ cell counts were monitored in TruCount tubes (BD Biosciences, Palo Alto, CA). The percentages of CD4+ T cells in the tissue cells were analyzed by flow cytometry (FACSCalibur) using CD3-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE), and CD8-peridinin chlorophyll protein (PerCP) antibodies. Except for CD3-FITC (BioSource, Camarillo, CA), all antibodies were obtained from BD Biosciences.
Viral RNA isolation, env amplification, and sequencing.
Viral RNA was prepared from 300 to 500 μl of plasma using a commercially available RNA extraction kit (Qiagen, Chatsworth, CA,) followed by reverse transcription (RT) with Superscript III RT (Invitrogen, Carlsbad, CA) and random hexamer primers (Amersham Pharmacia, Piscataway, NJ). The V1 to V5 region of gp120 was amplified from the RT products using Taq DNA polymerase (Qiagen) with the primers ED5 and ED12 or ES7 and ES8 as previously described (9), and full-length gp160 was amplified using the outer primers SH50 (5′-TAGAGCCCTGGAAGCATCCAGGAAGTCAGCCTA and SH51 (5′- TCCAGTCCCCCCTTTTCTTTTATAAAA) and the inner primers SH43 (5′-AAGACAGAATTCATGAGAGTGAAGGGGATCAGGAAG-3′) and SH44 (5′-AGAGAGGGATCCTTATAGCAAAGCCCTTTCAAAGCCCT-3′) (20). For single-genome amplification (SGA) of full-length env, limited endpoint dilution PCR was performed with primers SH50/51 and SH43/44 to identify the DNA dilution that gives <30% positive reactions in the total number of reactions (48). SGA amplicons were subjected to direct automated sequencing while standard PCR products were cloned with the TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions, followed by automated sequencing of cloned amplicons (Genewiz, South Plainfield, NJ). Nucleotide sequences were aligned with CLUSTAL X (29) and edited manually using BioEdit v7.0.9. A phylogenetic tree was constructed using a maximum-composite-like model in the MEGA, version 5.03 software, and bootstrap values were generated with 1,000 repetitions.
Plasmid constructs and pseudotyped virus production.
For the expression of envelope glycoproteins, full-length gp160 coding sequence amplified from RT products was subcloned into the pCAGGS vector (20). To generate luciferase reporter viruses capable of only a single round of replication, envelope trans-complementation assay was used. Briefly, Env expression plasmid and the NL4.3LucE-R+ vector were cotransfected by polyethyleneimine (Polyscience, Warrington, PA) into 2.5 × 106 293T cells plated in a 100-mm plate. Cell culture supernatants were harvested 72 h later, filtered through 0.45-μm-pore-size filters, and stored at −70°C in 1-ml aliquots. Pseudotyped viruses were quantified for p24 Gag content by enzyme-linked immunosorbent assay (Beckman Coulter, Fullerton, CA).
Determination of coreceptor usage.
Coreceptor usage of pseudotyped reporter viruses was determined by infection of U87.CD4 indicator cell lines. Briefly, 7 × 103 U87.CD4.CCR5 or U87.CD4.CXCR4 cells were seeded in 96-well plates 24 h before use and infected, in triplicates, with 5 ng of p24 Gag equivalent of the indicated pseudotyped viruses, followed by incubation for 72 h at 37°C. At the end of the incubation period, the cells were harvested, lysed, and processed for luciferase activity according to the manufacturer's instructions (luciferase assay system; Promega, Madison, WI). Entry, as quantified by luciferase activity, was measured with an MLX microtiter plate luminometer (Dynex Technologies, Inc., Chantilly, VA).
Statistical analysis.
Disease-free survival curves for the i.v.-, i.r.-, and i.v.g.-infected macaques were estimated using the Kaplan-Meier method. Differences in time to AIDS between groups were assessed using log-rank tests, while differences in peak and cumulative viral loads (log-transformed RNA copies/ml plasma) were examined using Mann-Whitney U tests. The cumulative viral load was computed as an integration of the area under the curve for a 1-year infection duration. A P value of <0.05 was considered statistically significant.
RESULTS
Persistent infection and disease induction in macaques exposed intravaginally to a single high dose R5 SHIVSF162P3N challenge.
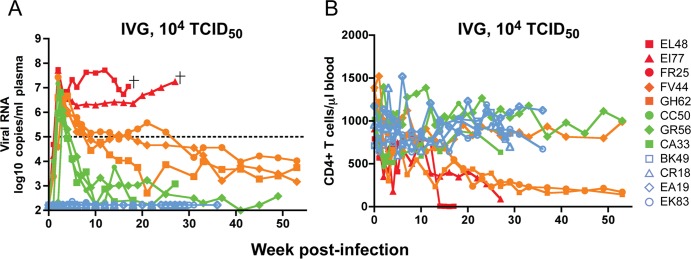
To determine whether R5 SHIVSF162P3N can transmit across the vaginal mucosa, we inoculated 12 female Indian RMs once with a high inoculum dose (104 TCID50 virus). To develop a more relevant model of vaginal transmission, the animals were not treated with Depo-provera, a procedure that thins the vaginal epithelium to achieve consistent rates of SIV/SHIV infection (39). Eight of the twelve depo-naive macaques were systemically infected, with an infection rate (66.7%) that is comparable to the rate we observed for single high dose i.v.g. transmission of the lineage-related early (20 wpi) R5 SHIVSF162P3 virus in depo-naive Indian rhesus monkeys (16). Peak viremia of 6 to 8 log10 RNA copies/ml of plasma was seen at 2 wpi (Fig. 1A). This was followed by three different patterns of postacute viremia. Two of the eight infected animals (EL48 and EI77) continued to replicate virus at high levels, with progression to disease and euthanasia within 30 wpi. Three (FR25, FV44, and GH62) had a viral set-point of 4 to 5 log10 RNA copies/ml of plasma, while the remaining three (CA33, CC50, and GR56) replicated only to levels of 3 log10 RNA copies/ml or lower. In contrast, macaques BK49, CR18, EA19, and EK83 remained aviremic over 30 weeks of observation, suggesting that they resisted high dose SHIV i.v.g. infection. However, substantial amount of mucus was noted in the vaginal vault of EK83 at the time of challenge, providing an impediment which could have limited virus transmission (27). Transient peripheral CD4+ T cell loss accompanied peak viremia in half of the infected macaques. The exceptions were the highly viremic macaques EL48, EI77, FR25, and GH62 (Fig. 1B), where blood CD4+ T cell count continued to decline, with precipitous drop in EL48 and EI77 toward end-stage disease. In contrast to the other infected monkeys, these two latter animals failed to sustain an anti-SHIV antibody response (data not shown) and were euthanized at 17 and 27 wpi, respectively, with clinical symptoms of AIDS.
Fig 1.
Virologic (A) and immunologic (B) measurements in RMs (n = 12) receiving a single i.v.g. inoculum of 104 TCID50 R5 SHIVSF162P3N. A “+” sign indicates euthanasia with clinical symptoms of AIDS, open symbols denote those animals that resisted challenge, and the dotted line in panel A marks a set point of 5 log10 RNA copies/ml of plasma.
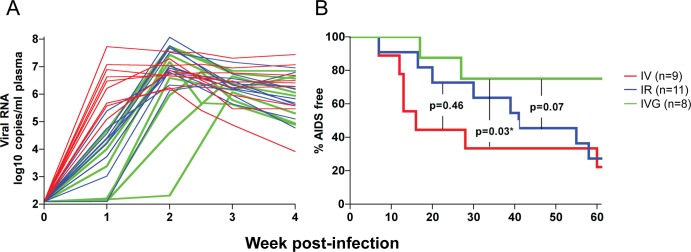
Compared to macaques challenged i.v. or i.r. with a similar high virus dose, the transmission efficiency of R5 SHIVSF162P3N i.v.g. exposure was lower (66.7% versus 100% for the i.v. and i.r. routes) (Table 1). Moreover, the kinetics of early virus dissemination (Fig. 2A) and the rate of disease progression over a 1-year infection period (Fig. 2B) were slower in high dose R5 SHIVSF162P3N i.v.g.-infected macaques than in the i.v.- or i.r.-infected macaques. The percentages of animals AIDS-free at 1 year were 22.2 and 27.3%, respectively, for the i.v.- and i.r.-infected macaques, compared to 75% for animals infected i.v.g. The difference in the rate of disease progression between the i.v.- and i.v.g.-infected macaques is statistically significant (P = 0.03), with the difference between the i.r.- and i.v.g.-infected monkeys approaching significance (P = 0.07).
TABLE 1.
Summary of single-dose i.v., i.r., and i.v.g. challenges with R5 SHIVSF162P3N
| Route | Dose (TCID50) | No. of animals | No. (%) of infected animals | No. (%) of infected animals with: |
Source or reference | |||
|---|---|---|---|---|---|---|---|---|
| AIDS | RP phenotype | Controlled infection | CoR switch | |||||
| i.v. | 3,000 | 9 | 9 (100) | 8 (89) | 6 (66.7) | 0 | 4 (44.4) | 20, 21 |
| i.r. | 10,000 | 11 | 11 (100) | 10 (91) | 4 (36.4) | 0 | 5 (45.4) | 47; this study |
| ≤1,000 | 6 | 6 (100) | 3 (50) | 1 (16.7) | 2 (33.3) | 0 | This study | |
| i.v.g. | 10,000 | 12 | 8 (66.7) | 2 (25) | 2 (25) | 2 (25) | 2 (25) | This study |
| 1,000 | 6 | 4 (66.7) | 2 (50) | 2 (50) | 2 (50) | 1 (25) | This study | |
Fig 2.
Kinetics of early virus dissemination (A) and disease-free survival curves (B) for macaques infected i.v. (n = 9), i.r. (n = 11), or i.v.g. (n = 8) with a single high dose of R5 SHIVSF162P3N. Plasma RNA levels within the first 4 weeks of infection (A) and AIDS development over a 1-year infection period (B) are shown. A P value of <0.05 is considered statistically significant.
Coreceptor switching in R5 SHIVSF162P3N vaginally infected monkeys that progressed rapidly to disease.
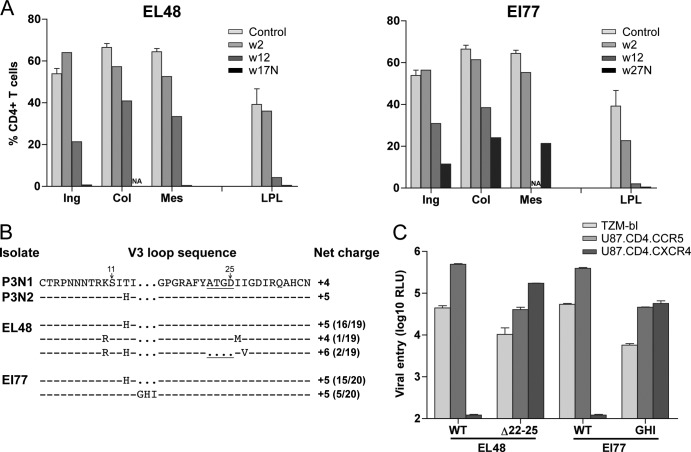
The precipitous decline in peripheral CD4+ T lymphocytes toward end-stage disease in the two i.v.g. rapid progressor (RP) macaques (EL48 and EI77) prompted us to investigate the emergence of X4 viruses. Analyses of the percentages of CD4+ T cells in tissue compartments during peak viremia (2 wpi) revealed minimal CD4+ T cell loss (<20%) in peripheral lymph nodes (LN), with a modest drop in CD4+ T lymphocytes at effector sites such as the lamina propria (LP) of the gut seen only in EI77 (∼40%; Fig. 3A). Depletion of CD4+ T lymphocytes increases in lymphoid tissues during chronic infection, with close to 90% loss in the gut of both animals at 12 wpi. At the time of death, >99% of CD4+ T lymphocytes in the gut and LN of EL48 were depleted. Massive depletion of CD4+ T cells was also seen in the gut of EI77 at the time of euthanasia, but 20 to 35% of this T cell subset was preserved in secondary lymphoid tissues. Sequence analysis showed that 2 of 19 envelope (env) clones amplified from plasma of EL48 at the time of necropsy had four amino acid deletions in the C terminus of the V3 loop (Δ22-25) that increased the net charge of this domain to +6 (Fig. 3B). This Δ22-25 V3 deletion sequence had previously been shown to confer CXCR4 usage to variants that retained and preferred CCR5 for entry in R5 SHIVSF162P3N i.v.- and i.r.-infected macaques (50). Indeed, V3 Δ22-25-bearing Envs from EL48 were found to be dualtropic, infecting U87.CD4 cells that expressed either the CCR5 or CXCR4 coreceptor (Fig. 3C). In comparison, 5 of 20 env amplicons from plasma of EI77 at the time of necropsy harbored three amino acid insertions (GHI) immediately upstream of the GPGR crown in the V3 loop. This V3 insertion was also found in a dualtropic variant recovered from a RP macaque infected i.v. with R5 SHIVSF162P3N, and GHI-bearing viruses in EI77 infected CCR5, as well as CXCR4-expressing cells (Fig. 3C). Furthermore, and consistent with the findings in i.v.- and i.r.-infected macaques (50, 53), the dualtropic variants in the i.v.g.-infected animals entered TZM-bl less efficiently than the coexisting R5 viruses. These results show coreceptor switching in the two i.v.g. R5 SHIVSF162P3N-infected RP monkeys, supporting the notion of similar pathogenic sequela of R5 SHIVSF162P3N i.v., i.r., and i.v.g. infections.
Fig 3.
Tissue CD4+ T cell frequency (A), V3 loop sequence (B), and coreceptor usage (C) of viral variants in macaques EL48 and EI77. (A) Percentages of CD4+ T cells in the inguinal (Ing), colonic (Col), and mesenteric (Mes) lymph nodes and lamina propria lymphocyte (LPL) from the jejunum during peak (w2) and chronic (w12) stage of infection and at time of necropsy (N) are reported. Baseline values generated from three uninfected macaques (control) are shown for reference. NA, not available. (B) V3 loop sequence comparison of representative SHIVSF162P3N clones (P3N1 and P3N2) and plasma viruses in macaques EL48 and EI77 at time of necropsy. Dots indicate gaps, and dashes stand for identity in sequences, with the net positive charge of the V3 region shown on the right. Positions 11 and 25 within the V3 loop are indicated by arrows, and the 4-amino-acid deletion is underlined. The numbers in parentheses represent the numbers of clones matching the indicated sequence per total number of clones sequenced. (C) Relative entry of pseudoviruses bearing EL48 and EI77 Envs into TZM-bl, U87.CD4.CCR5, and U87.CD4.CXCR4 indicator cells. RLU, relative light units. The data are means and standard deviations from triplicate wells and are representative of at least two independent experiments.
Effect of dose on virus replication in R5 SHIVSF162P3N i.v.g.-infected macaques.
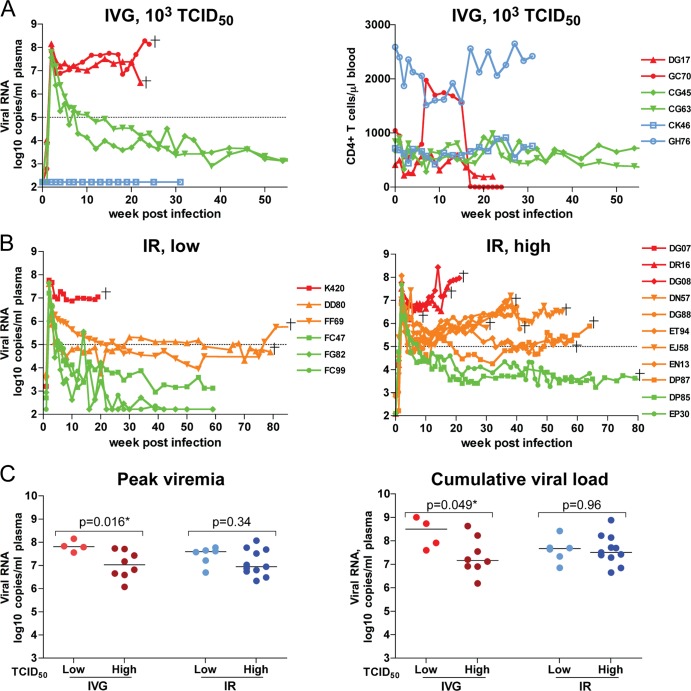
Because low-dose challenge models are believed to more closely reflect the viral concentrations found in semen (44), we investigated the effect of challenge dose on i.v.g. R5 SHIVSF162P3N transmission and used a 10-fold-lower inoculum than in the first series of animals studied. We found that four of six female RMs exposed once i.v.g. to 1,000 TCID50 of SHIVSF162P3N were systemically infected (66.7% transmission rate), with a high peak viremia of 7 to 8 log10 RNA copies/ml of plasma at 2 wpi (Fig. 4A). Viremia remained high (≥6 log10 RNA copies/ml of plasma) in two (DG17 and GC70) of the four infected macaques, with progression to disease at 22 to 24 wpi in the absence of sustained seroconversion. This contrasts with the other two infected animals, CG45 and CG63, where infection leveled off at 3 to 4 log10 RNA copies/ml of plasma, with seroconversion at 4 to 6 wpi. The peripheral CD4+ T cell count remained relatively stable throughout the course of infection in the two i.v.g.-infected animals that had low chronic viremia, but with protracted decline in DG17 and dramatic loss in GC70, the two highly viremic monkeys. At the time of euthanasia, the gut CD4+ T cells were severely depleted in both animals, with drastic LN CD4+ T cell loss only in GC70 (data not shown). Sequence analysis of GC70 plasma viruses showed the presence of variants harboring the Δ22-25 V3 deletion sequence (data not shown), suggesting that it represented another i.v.g.-infected RP with a coreceptor switch.
Fig 4.
Comparison of virologic and immunologic measurements in RMs inoculated i.v.g. or i.r. with various doses R5 SHIVSF162P3N. (A) Viral load and peripheral CD4+ T cell counts in macaques receiving a single low intravaginal inoculum of 103 TCID50 R5 SHIVSF162P3N. A “+” indicates death due to euthanasia, and open symbols designate those macaques that resisted challenge. (B) Viral load in macaques inoculated i.r. with a single low (102 to 103 TCID50) or high (104 TCID50) dose of R5 SHIVSF162P3N. A “+” indicates euthanasia with clinical symptoms of AIDS. Dotted line in panels A and B mark a set-point of 5 log10 RNA copies/ml of plasma. (C) Peak and cumulative viral load (area under the curve over a 1-year infection period) comparison of R5 SHIVSF162P3N i.v.g.- and i.r.-infected macaques. The line represents the median viral RNA copies for each group. An asterisk (*) indicates statistical significance (P < 0.05).
The apparent lack of a dose effect on R5 SHIVSF162P3N i.v.g. transmission efficiency and virus replication contrasts with prior observations using the lineage-related early R5 SHIVSF162P3 (55), the dualtropic SHIVDH12R (10), and SIVmac251 (43), where animals infected i.v.g. with low inoculum doses often developed transient viremia. Accordingly, we examined whether the lack of a dose effect on R5 SHIVSF162P3N i.v.g. transmission and replication was a feature unique for this route of mucosal challenge by comparing the infection outcome in 11 RMs challenged i.r. with high doses (104 TCID50) to six RMs that were exposed to 1- to 2-log-lower inoculum sizes (Fig. 4B). All i.r.-exposed macaques were infected, irrespective of the challenge dose. Nine of eleven macaques infected i.r. with a high inoculum dose sustained a viremia of >5 log10 RNA copies/ml of plasma and developed AIDS, with a rapid progressor phenotype in four monkeys and coreceptor switching in ∼50% of the infected animals (Table 1). Of the two animals that had a 1-log-lower steady-state viremia, one (DP85) developed clinical symptoms of AIDS and was euthanized at 80 wpi. Peak viremia was of the same order of magnitude (7 to 8 log10 RNA copies/ml) among macaques infected i.r. with lower inoculum doses, but only three of six macaques infected with a lower dose progressed to disease, with one displaying a rapid progressor phenotype (K420). Of the remaining three animals, two controlled their infection to undetectable levels at 40 to 50 wpi (<2 log10 RNA copies/ml of plasma). Comparison of the peak and cumulative viral load (area under the curve) over a 1-year infection period for macaques infected i.v.g. or i.r. with various R5 SHIVSF162P3N doses showed that the peak (P = 0.016) as well as the overall (P = 0.049) viral burden was significantly lower in macaques inoculated i.v.g. with high inoculum doses (104 TCID50) than in macaques inoculated i.v.g. with lower inoculum doses (103 TCID50), and this was not observed in the i.r.-infected animals (Fig. 4C). These findings suggest that an inverse association between challenge dose and R5 SHIVSF162P3N replication and pathogenesis exists but that it is route dependent, i.e., it is true for i.v.g. challenge but not for i.r. challenge.
Atypical pattern of virus replication in macaques exposed intravaginally twice within the same day to high dose R5 SHIVSF162P3N.
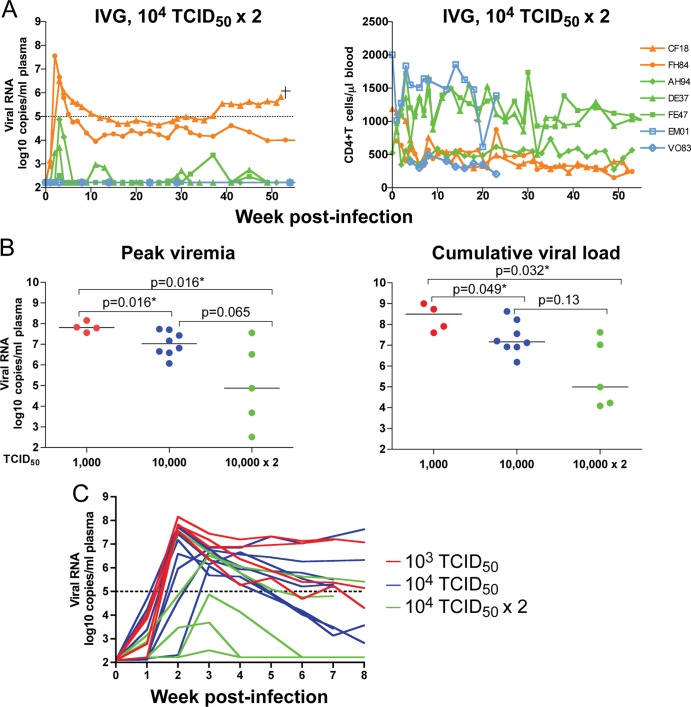
In several studies, two high doses of SIV administered i.v.g. 4 h apart were shown to infect a higher proportion of animals than single-dose exposures (37, 38, 41). We therefore investigated whether this exposure regimen would increase the vaginal transmission rate of R5 SHIVSF162P3N and abrogate the seemingly inverse effect of high inoculum dose on i.v.g. infection. A wide variation in peak viremia, ranging from 2 to 8 log10 RNA copies/ml of plasma, was detected at 2 to 3wpi in five of seven depo-naive macaques exposed twice to 104 TCID50 SHIVSF162P3N within a 4-h period (Fig. 5A). We observed a 71.4% transmission rate for R5 SHIVSF162P3N using two high doses administered within the same day (five of seven exposed monkeys), which was comparable to that observed using a single high dose (66.7%). However, viremia was transient in three of the five infected monkeys (AH94, DE37, and FE47), with intermittent viral blips of ≤3 log10 RNA copies/ml of plasma detected in DE37 and FE47 over a 1-year infection period. The two remaining infected macaques (CF18 and FH84) established a set-point of 4 to 5 log10 RNA copies/ml of plasma, with a 1-log increase in virus replication seen in CF18 at 40 wpi. This macaque developed clinical signs of AIDS and was euthanized at 52 wpi.
Fig 5.
(A) Virologic and immunologic measurements in RMs (n = 7) receiving two doses of 104 TCID50 R5 SHIVSF162P3N administered i.v.g. twice within the day. A “+” indicates euthanasia with clinical symptoms of AIDS, open symbols designate the macaques that resisted challenge, and dotted line marks a set-point of 5 log10 RNA copies/ml of plasma. (B) Peak viremia and cumulative viral load comparison of macaques infected i.v.g. with various doses. The line represents the median viral RNA copies for each group. An asterisk (*) indicates statistical significance (P < 0.05). (C) Virus dissemination in macaques infected i.v.g. with different doses of SHIVSF162P3N. Plasma RNA levels within the first 8 weeks of infection are shown.
Peripheral CD4+ T cell counts remained relatively stable in three of the five macaques infected i.v.g. following two virus exposures, with a gradual decline in the two animals (CF18 and FH84) with chronic viremia. CF18, FH84, and AH94 seroconverted at 4 to 6 wpi, but two of the animals with transient viremia, DE37 and FE47, remained seronegative at the end of a 1-year study period. This phenotype of infection without seroconversion, or occult infection, was seen only in the 2 × 104 TCID50 i.v.g. challenge group, suggesting very rapid control of viral spread (Table 2). A comparison of the peak and cumulative viral load in macaques infected i.v.g. with R5 SHIVSF162P3N using various doses showed an inverse trend between the i.v.g. R5 SHIVSF162P3N inoculum and virus replication (Fig. 5B). Peak and cumulative viral load were significantly lower in animals infected with 104 and 2 × 104 TCID50 of virus than in those receiving 10-fold-lower doses (P < 0.05), with a difference in peak viremia that approaches statistical significance (P = 0.065) between the 104 and 2 × 104 TCID50 challenge conditions. Furthermore, the kinetics of virus dissemination was slower in macaques exposed to 104 TCID50 R5 SHIVSF162P3N twice than in those challenged once, even with 10-fold-lower inoculum sizes (Fig. 5C). The time of peak viremia was week 2 in all four macaques infected with 103 TCID50 and in five of the eight animals inoculated with 104 TCID50 virus, whereas it took 3 weeks to reach peak viremia in the majority (four of five) of monkeys receiving 104 TCID50 virus twice within the same day.
TABLE 2.
Effects of dose on i.v.g. infection with R5 SHIVSF162P3
| Dose (TCID50) | No. of exposed animals | No. (%) infected animals | No. (%) of infected animals with transient viremia | No. (%) of infected animals with an RP phenotype | No. of strong seroconvertersa/no. of infected animals (%) |
|---|---|---|---|---|---|
| 10,000 × 2 | 7 | 5 (71.4) | 3 (60) | 0 | 3/5 (60) |
| 10,000 | 12 | 8 (66.7) | 0 | 2 (25) | 6/8 (75) |
| 1000 | 6 | 4 (66.7) | 0 | 2 (50) | 2/4 (50) |
RPs that mounted a transient anti-SHIV antibody response are not considered strong seroconverters.
The effect of challenge dose on i.v.g. R5 SHIVSF162P3N infection cannot be explained by differences in host genetics or in the nature of the transmitted viruses.
Since the presence of the Mamu-A*01, Mamu-B*17, and Mamu-B*08 class I haplotypes and that of certain TRIM5α alleles has previously been shown to be associated with control of pathogenic SIV replication (13, 26, 34), we examined whether these host restriction factors played a role in R5 SHIVSF162P3N i.v.g. infection. None of the animals that resisted i.v.g. challenge expressed the three restrictive Mamu alleles (Table 3), and there was no clear association between restrictive major histocompatibility complex (MHC) class I expression and SHIV control. For example, one of the three macaques that expressed Mamu-B*08 showed an RP phenotype (GC70), with the other two sustaining (FV44) or controlling (CC50) viremia (Fig. 1). The only monkey that tested positive for Mamu-B*17 (CG45; Fig. 4A) also remained viremic over a 1-year study period. As for TRIM5, four of the eight resistant macaques expressed the restrictive homozygous TFP/TFP allele, while the remaining four had the permissive heterozygous TFP/Q genotype (Table 3), with similar representation of these two alleles among the animals in the three dosage groups. There was also no apparent association between TRIM5 allelic polymorphisms and SHIV virus replication levels (see Fig. S1 in the supplemental material). The one macaque with TRIMcyp had low and transient viremia (FE47). However, low and transient viremia were also seen in macaque AH94 with the permissive heterozygous TFP/Q genotype. The small cohort of animals studied may not have revealed subtle TRIM effects. However, because SHIVSF162P3N was derived from a SIVmac239 backbone into which an HIVSF162 env gene was inserted, our findings are consistent with reports of a much lower effect of TRIM5α on SIVmac239 and SIVmac251 infection, compared to its effect on SIVsmE660 (11, 26, 35).
TABLE 3.
Effect of host factors on R5 SHIVSF162P3N i.v.g. challenge
| Dose (TCID50) | Animal | Age (yrs) | Wt (kg) | No. of births | Infection outcomea | MHC (Mamu) allele |
TRIM5 allele | ||
|---|---|---|---|---|---|---|---|---|---|
| A*01 | B*08 | B*17 | |||||||
| 1,000 | DG17 | 8.98 | 5.83 | 3 | RP | – | – | – | TFP/TFP |
| GC70 | 5.76 | 5.6 | 0 | RP | – | + | – | TFP/Q | |
| CG45 | 11.47 | 9.4 | 4 | CP | – | – | + | TFP/Q | |
| CG63 | 11.55 | 8.0 | 4 | CP | – | – | – | TFP/TFP | |
| CK46 | 12.03 | 6.5 | 5 | Resistant | – | – | – | TFP/TFP | |
| GH76 | 6.05 | 6.75 | 0 | Resistant | – | – | – | TFP/Q | |
| 10,000 | EL48 | 7.3 | 6.05 | 1 | RP | – | – | – | TFP/Q |
| EI77 | 7.57 | 4.3 | 0 | RP | – | – | – | TFP/Q | |
| FR25 | 6.84 | 8.85 | 0 | CP | – | – | – | TFP/TFP | |
| FV44 | 6.53 | 8.3 | 0 | CP | – | + | – | TFP/Q | |
| GH62 | 5.66 | 6.5 | 0 | CP | – | – | – | Q/Q | |
| CC50 | 7.16 | 9.3 | 1 | CP | – | + | – | TFP/Q | |
| GR56 | 5.48 | 7.45 | 0 | CP | – | – | – | TFP/TFP | |
| CA33 | 7.25 | 7.8 | 1 | CP | – | – | – | TFP/TFP | |
| BK49 | 8.56 | 7.05 | 1 | Resistant | – | – | – | TFP/TFP | |
| CR18 | 6.59 | 6.8 | 0 | Resistant | – | – | – | TFP/TFP | |
| EA19 | 8.65 | 8.55 | 0 | Resistant | – | – | – | TFP/Q | |
| EK83 | 7.69 | 9.0 | 0 | Resistant | – | – | – | TFP/Q | |
| 10,000 × 2 | CF18 | 9.98 | 5.2 | 5 | CP | – | – | – | Q/Q |
| FH84 | 7.44 | 4.7 | 0 | CP | – | – | – | TFP/Q | |
| AH94 | 12.27 | 11.1 | 6 | Transient | – | – | – | TFP/Q | |
| DE37 | 9.73 | 5.6 | 3 | Transient | – | – | – | TFP/TFP | |
| FE47 | 6.7 | 10.14 | 1 | Transient | + | – | – | TFP/Cyp | |
| EM01 | 7.68 | 8.9 | 1 | Resistant | – | – | – | TFP/Q | |
| V083 | 14.84 | 14.6 | 8 | Resistant | – | – | – | TFP/TFP | |
RP, rapid progressor; CP, chronic progressor.
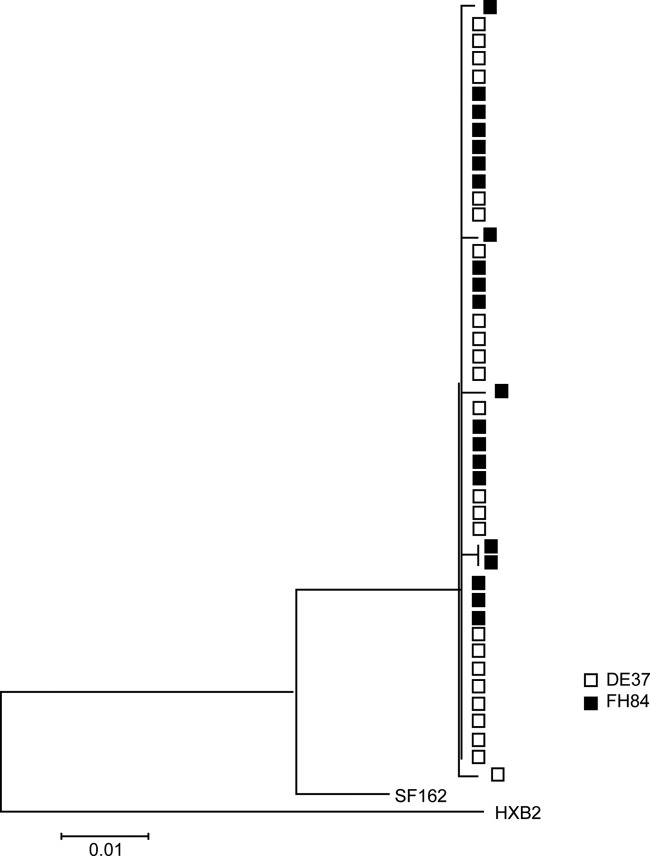
To investigate whether the differences in virus replication in the macaques infected intravaginally following two high-dose exposures were related to differences in the transmitted/founder virus, we performed a pilot study to analyze env sequences in the first viral RNA positive plasma samples from a macaque with high (FH84) or transient (DE37) viremia (Fig. 5A). Because of the low levels of virus replication in DE37, the envelope sequences were obtained by standard nested PCR/cloning and not by SGA. Nonetheless, increasing evidence suggests that with an adequate number of PCR templates analyzed, bulk sequencing captures a measure of population diversity similar to that determined by SGA (22, 30). Phylogenetic tree analysis of the early replicating viruses in the two monkeys showed clustering of DE37 (transient/low VL) env sequences with those of FH84 (high VL) (Fig. 6), suggesting that differences in the transmitted/founder virus were not the underlying reason for the variable infection outcome in the group of animals exposed twice within the same day to high doses of R5 SHIVSF162P3N.
Fig 6.
Early replicating viruses in viremic (FH84) and aviremic (DE37) R5 SHIVSF162P3N-infected macaques. A phylogenetic tree shows the relationship between Env variant sequences (V3 to V5) in plasma of macaques FH84 and DE37 at the first viral RNA-positive time point (2 wpi). A neighbor-joining tree rooted on the sequences of HxB2 and SF162 was generated. The scale bar indicates the genetic distance along the branches in nucleotides.
DISCUSSION
In this report, we document that R5 SHIVSF162P3N can breach the vaginal mucosa of RMs to establish persistent infection. Consistent with previous findings in macaques infected i.v. or i.r. with this virus, coreceptor switching was seen primarily in i.v.g.-infected animals that progressed rapidly to disease in the absence of a strong antiviral antibody response. Moreover, the V3 genetic sequence requirement for coreceptor switch in the i.v.g.-infected macaques overlapped with those found in i.v.- and i.r.-infected monkeys. Compared to the lineage-related early isolate R5 SHIVSF162P3, (16, 17, 52, 62), infection with R5 SHIVSF162P3N resulted in less variability in chronic viremia and disease outcome. The increase in replicative capacity and pathogenicity of R5 SHIVSF162P3N may be related to the fact that it is a late-stage AIDS-associated isolate that has a longer period of adaptation in rhesus monkeys and is more diverse and divergent than SHIVSF162P3 (20). R5 SHIVSF162P3N infection of RMs is therefore a useful model to study all modes of HIV-1 transmission and aspects of pathogenesis and for the testing of the efficacy of antiviral strategies during established infection.
Early studies with nonphysiological high doses of SIVs and SHIVs showed that there was no significant difference in the infection rates between macaques inoculated i.v. or i.r., but that this rate was lower after inoculation by the i.v.g. route (6, 8, 14, 38, 42, 54). Moreover, the kinetics of virus dissemination was slower, and the RNA levels were more variable in monkeys infected by the i.v.g. route than in monkeys infected by the i.v. or i.r. routes (3, 14, 43, 45). Our findings of reduced risk of infection (Table 1), slower dissemination (Fig. 2A), and greater variability in set-point viremia (Fig. 1A) with single high-dose i.v.g. R5 SHIVSF162P3N challenge in comparison to i.v. or i.r. infection with the same inoculum are in agreement with these earlier studies, further documenting the restrictions on virus transmission and spread imposed by the vaginal mucosa. Despite this barrier, we showed sufficient pathogenic sequela in female Indian RMs exposed i.v.g. Moreover, while the lower vaginal transmission efficiency could be a potential limitation in studies by this route, infection was achieved without the need for depo-provera treatment, which could perturb specific susceptibility factors such as mucosal antibodies and the levels of mucus and mucins, as well as innate antimicrobial mediators. Since the virus is CCR5-tropic, SHIVSF162P3N RM challenge provides a good model for studying the early stages of HIV-1 vaginal transmission and host response.
In this regard, although the number of animals studied was limited and the viral concentrations used could still be considered high compared to inoculum doses likely to be encountered in humans, an unexpected inverse association was seen between virus replication and the i.v.g.-administered dose of SHIVSF162P3N that produced a systemic infection. Virus replication was significantly lower in macaques inoculated with a single high inoculum dose (104 TCID50) than in those receiving a 1-log-lower dose challenge (Fig. 4C), with a higher proportion of the infected animals in the lower dose challenge group developing a RP phenotype (Table 2). Moreover, transient and low viremia was observed only in macaques exposed i.v.g. twice within the same day to high-dose inoculum, which can be seen as doubling the dose (Fig. 5A). These differences in SHIVSF162P3N i.v.g. infection could not be explained by MHC class I and TRIM5α genetic polymorphisms (Table 3 and see Fig. S1 in the supplemental material) or a selection for particular transmitted/founder viruses (Fig. 6), raising the possibility of modulation as a result of dose-dependent and exposure-induced immune factors. Because the virus stocks were propagated in activated macaque lymphocytes, the supernatants are bound to contain a range of secreted immune factors, which could have induced a protective effect. However, the inverse association was only seen with i.v.g. and not with i.r. challenge (Table 1), suggesting a differential host response to virus/supernatant dose at these two mucosal sites with structurally distinguishable epithelium barriers. A very-high-dose vaginal SHIVSF162P3N challenge could have tipped the balance between infection-promoting and inhibitory responses in favor of antiviral defense mechanisms that dampen SHIV expression or susceptibility (28, 46). Indeed, very early involvement of innate defenses, such as initiation of the interferon cascade to produce virus restriction factors and release of virus-inhibiting chemokines by pDCs that could blunt the initial wave of viral replication had been reported after i.v.g. SIV inoculation (1, 33). Nonetheless, other host factors such as age, preexisting genital inflammation and menstrual cycle status are also known to effect vaginal HIV/SHIV transmission efficiency (5, 12, 59, 61). SHIVSF162P3N i.v.g. challenge in additional animals will be required to draw firm conclusion on the effect of high inoculum dose. These studies might help to elucidate the nature of the factors that confer protection against vaginal transmission and systemic infection to guide microbicide and mucosal vaccine design.
In conclusion, we show that R5 SHIVSF162P3N can transmit across the female genital tract, with unexpected biological consequences at a very high inoculum dose. Whether decreased virus replication and disease modulation are the results of de novo innate immune factor synthesis triggered by the SHIV virion, the result of immune factors secreted into the cell culture supernatants along with virus or incorporated into the virion membrane and other host factors remains to be determined. Regardless, because mucosal infection with R5 SHIVSF162P3N recapitulates key effects of HIV-1 infection in humans and shows much more consistent pathogenicity than the lineage-related early SHIVSF162P3 isolate, this virus holds promise in studies to understand the impact of R5 virus infection on biological responses in the mucosa and for the development of HIV biomedical prevention methods.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lisa Chakrabarti for helpful discussion and critique of this work and Hiroshi Mohri for help with statistical analysis.
The following were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: reagents TAK779 (catalog no. 4983 from Takeda Chemical Industries, Ltd.), AMD3100 (8128 from AnorMed, Inc.), TZM-bl (catalog no. 8129 from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc.), and U87.CD4 indicator cell lines (catalog no. 4035 and 4036 from HongKui Deng and Dan Littman). This study was supported by the National Institutes of Health grants RO1AI046980 and AI084765 and by Primate Center Base grant P51-OD011104-51.
Footnotes
Published ahead of print 27 June 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 79:12164–12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrahams MR, et al. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambrose Z, et al. 2001. Evidence for early local viral replication and local production of antiviral immunity upon mucosal simian-human immunodeficiency virus SHIV(89.6) infection in Macaca nemestrina. J. Virol. 75:8589–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar KJ, et al. 2010. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J. Virol. 84:6241–6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benki S, McClelland RS, Overbaugh J. 2005. Risk factors for human immunodeficiency virus type-1 acquisition in women in Africa. J. Neurovirol. 11(Suppl 1):58–65 [PubMed] [Google Scholar]
- 6. Benson J, et al. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J. Virol. 72:4170–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton DR, et al. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chenine AL, et al. 2010. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J. Infect. Dis. 201:1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delwart EL, Gordon C. 1997. Tracking changes in HIV-1 envelope quasispecies using DNA heteroduplex analysis. Methods 12:348–354 [DOI] [PubMed] [Google Scholar]
- 10. Endo Y, et al. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fenizia C, et al. 2011. TRIM5alpha does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J. Virol. 85:12399–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2:33–42 [DOI] [PubMed] [Google Scholar]
- 13. Goulder PJ, Watkins DI. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenier JL, et al. 2001. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J. Virol. 75:3753–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haaland RE, et al. 2009. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 5:e1000274 doi:10.1371/journal.ppat.1000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harouse JM, et al. 2003. CD8+ T cell-mediated CXC chemokine receptor 4-simian/human immunodeficiency virus suppression in dually infected rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 100:10977–10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harouse JM, et al. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J. Virol. 75:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hladik F, Hope TJ. 2009. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 6:20–28 [DOI] [PubMed] [Google Scholar]
- 19. Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho SH, et al. 2007. Coreceptor switch in R5-tropic simian/human immunodeficiency virus-infected macaques. J. Virol. 81:8621–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho SH, Trunova N, Gettie A, Blanchard J, Cheng-Mayer C. 2008. Different mutational pathways to CXCR4 coreceptor switch of CCR5-using simian-human immunodeficiency virus. J. Virol. 82:5653–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan MR, et al. 2010. Comparison of standard PCR/cloning to single genome sequencing for analysis of HIV-1 populations. J. Virol. Methods 168:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kearney M, et al. 2009. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J. Virol. 83:2715–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keele BF, et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keele BF, et al. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirmaier A, et al. 2010. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8:e1000462 doi:10.1371/journal.pbio.1000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai SK, et al. 2009. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 83:11196–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lama J, Planelles V. 2007. Host factors influencing susceptibility to HIV infection and AIDS progression. Retrovirology 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larkin MA, et al. 2007. CLUSTAL W and CLUSTAL X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 30. Lerner P, et al. 2011. The gut mucosal viral reservoir in HIV-infected patients is not the major source of rebound plasma viremia following interruption of highly active antiretroviral therapy. J. Virol. 85:4772–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li H, et al. 2010. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 6:e1000890 doi:10.1371/journal.ppat.1000890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Q, et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 33. Li Q, et al. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim SY, et al. 2010. Contributions of Mamu-A*01 status and TRIM5 allele expression, but not CCL3L copy number variation, to the control of SIVmac251 replication in Indian-origin rhesus monkeys. PLoS Genet. 6:e1000997 doi:10.1371/journal.pgen.1000997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim SY, et al. 2010. TRIM5α modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6:e1000738 doi:10.1371/journal.ppat.1000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, et al. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84:10406–10412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma ZM, Abel K, Rourke T, Wang Y, Miller CJ. 2004. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J. Virol. 78:14048–14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ. 2001. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retrovir. 17:1455–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marx PA, et al. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- 40. Mattapallil JJ, et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 41. Miller CJ, et al. 1990. Effect of virus dose and nonoxynol-9 on the genital transmission of SIV in rhesus macaques. J. Med. Primatol. 19:401–409 [PubMed] [Google Scholar]
- 42. Miller CJ, et al. 1998. In vivo replication rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J. Virol. 72:3248–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller CJ, et al. 1994. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68:6391–6400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pilcher CD, et al. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189:1785–1792 [DOI] [PubMed] [Google Scholar]
- 45. Polacino P, et al. 2008. Differential pathogenicity of SHIV infection in pig-tailed and rhesus macaques. J. Med. Primatol 37(Suppl 2):13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Promadej-Lanier N, et al. 2010. Resistance to Simian HIV infection is associated with high plasma interleukin-8, RANTES, and Eotaxin in a macaque model of repeated virus challenges. J. Acquir. Immune Defic. Syndr. 53:574–581 [DOI] [PubMed] [Google Scholar]
- 47. Ren W, et al. 2010. Different tempo and anatomic location of dualtropic and X4 virus emergence in a model of R5 simian-human immunodeficiency virus infection. J. Virol. 84:340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salazar-Gonzalez JF, et al. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salazar-Gonzalez JF, et al. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shakirzyanova M, Ren W, Zhuang K, Tasca S, Cheng-Mayer C. 2010. Fitness disadvantage of transitional intermediates contributes to dynamic change in the infecting-virus population during coreceptor switch in R5 simian/human immunodeficiency virus-infected macaques. J. Virol. 84:12862–12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stone M, et al. 2010. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J. Virol. 84:7083–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Subbarao S, et al. 2006. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J. Infect. Dis. 194:904–911 [DOI] [PubMed] [Google Scholar]
- 53. Tasca S, Ho SH, Cheng-Mayer C. 2008. R5X4 viruses are evolutionary, functional, and antigenic intermediates in the pathway of a simian-human immunodeficiency virus coreceptor switch. J. Virol. 82:7089–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. ten Haaft P, et al. 2001. Comparison of early plasma RNA loads in different macaque species and the impact of different routes of exposure on SIV/SHIV infection. J. Med. Primatol. 30:207–214 [DOI] [PubMed] [Google Scholar]
- 55. Tsai L, et al. 2007. Efficient repeated low-dose intravaginal infection with X4 and R5 SHIVs in rhesus macaque: implications for HIV-1 transmission in humans. Virology 362:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. UNAIDS 2010. UNAIDS report on the global AIDS epidemic 2010. UNAIDS, New York, NY [Google Scholar]
- 57. Varela M, et al. 2011. Molecular evolution analysis of the human immunodeficiency virus type 1 envelope in simian/human immunodeficiency virus-infected macaques: implications for challenge dose selection. J. Virol. 85:10332–10345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Veazey RS, et al. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 59. Vishwanathan SA, et al. 2011. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 57:261–264 [DOI] [PubMed] [Google Scholar]
- 60. Voelker R. 2005. Women shoulder growing HIV/AIDS burden. JAMA 293:281–282 [DOI] [PubMed] [Google Scholar]
- 61. Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu H, Wang X, Morici LA, Pahar B, Veazey RS. 2011. Early divergent host responses in SHIVsf162P3 and SIVmac251-infected macaques correlate with control of viremia. PLoS One 6:e17965 doi:10.1371/journal.pone.0017965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Z, et al. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353–1357 [DOI] [PubMed] [Google Scholar]
- 64. Zhang ZQ, et al. 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. U. S. A. 101:5640–5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.