Abstract
Immunization with attenuated lentiviruses is the only reliable method of protecting rhesus macaques (RM) from vaginal challenge with pathogenic simian immunodeficiency virus (SIV). CD8+ lymphocyte depletion prior to SIVmac239 vaginal challenge demonstrated that a modest, Gag-specific CD8+ T cell response induced by immunization with simian-human immunodeficiency virus 89.6 (SHIV89.6) protects RM. Although CD8+ T cells are required for protection, there is no anamnestic expansion of SIV-specific CD8+ T cells in any tissues except the vagina after challenge. Further, SHIV immunization increased the number of viral target cells in the vagina and cervix, suggesting that the ratio of target cells to antiviral CD8+ T cells was not a determinant of protection. We hypothesized that persistent replication of the attenuated vaccine virus modulates inflammatory responses and limits T cell activation and expansion by inducing immunoregulatory T cell populations. We found that attenuated SHIV infection decreased the number of circulating plasmacytoid dendritic cells, suppressed T cell activation, decreased mRNA levels of proinflammatory mediators, and increased mRNA levels of immunoregulatory molecules. Three days after SIV vaginal challenge, SHIV-immunized RM had significantly more T regulatory cells in the vagina than the unimmunized RM. By day 14 postchallenge, immune activation and inflammation were characteristic of unimmunized RM but were minimal in SHIV-immunized RM. Thus, a modest vaccine-induced CD8+ T cell response in the context of immunoregulatory suppression of T cell activation may protect against vaginal HIV transmission.
INTRODUCTION
Although an attenuated human immunodeficiency virus (HIV) vaccine is unlikely to be used in humans due to safety concerns (63), the immune mechanisms that confer protection against pathogenic lentiviral infection can be tested in monkeys vaccinated with attenuated virus (38). In fact, live attenuated lentivirus infection is the only immunization strategy that elicits consistent protection after vaginal challenge with pathogenic simian immunodeficiency virus (SIV); 60% of rhesus macaques (RM) immunized with attenuated simian-human immunodeficiency virus 89.6 (SHIV89.6) avoid infection or control viral replication after intravaginal challenge with SIVmac239 (3, 52). SHIV immunization induces modest but persistent polyfunctional SIV Gag-specific CD4+ and CD8+ T cell responses in the vagina (26, 27), and CD8+ lymphocyte depletion in vivo suggests that these SIV-specific CD8+ T cells are responsible for protection from vaginal SIV challenge in immunized RM (26, 27). However, the strength of the CD8+ T cell responses in SHIV-immunized RM is less than has been elicited by vaccines that do not protect humans from HIV infection. With a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay, 56% (5/9) of SHIV-immunized RM had SIV-specific T cells (range of 30 to 165/106 peripheral blood mononuclear cells [PBMC]) in blood at the time of vaginal SIV challenge (26). For comparison, 77% (258/354) of people that participated in the phase IIB step trial of the MRKAd5 HIV-1 gag/pol/nef vaccine responded with IFN-γ-secreting HIV-specific T cells (range of 163 to 686/106 PBMC) by ELISPOT; and HIV-specific CD8+ T cells were found in 73% (117/160) of participants in a flow cytometry-based intracellular cytokine assay (49). In the phase III efficacy trial (RV144) of the prime-boost vaccine containing ALVAC-HIV, vaccination induced an HIV Env- and/or Gag-specific T cell response, as measured by IFN-γ ELISPOT assay, in 19.7% of volunteers 6 months after the final dose of vaccine. Further, HIV-specific CD8+ T cells were found in 11% (16/144) of participants in a flow cytometry-based intracellular cytokine assay (62). The STEP trial vaccine elicited moderate T cell responses but did not protect from HIV infection. While SHIV immunization elicited weak CD8+ T cell responses in blood, it does protect monkeys from vaginal SIV challenge. The lack of a direct relationship between the strength of virus-specific CD8+ T cell immunity and protection from infection in immunized people (49, 62) and SHIV-immunized RM (26, 27) suggests that the strength of vaccine-elicited antiviral CD8+ T cell responses is not a reliable predictor of vaccine efficacy.
In SHIV-immunized monkeys, a number of features of the antiviral T cell response were unusual. After vaginal SIV challenge, SIV-specific CD8+ T cells in the vaginal mucosa of immunized RM proliferated, but SIV-specific CD8+ T cells in the blood or other tissues did not (27). Furthermore, the levels of systemic T cell activation, proliferation, and apoptosis in SHIV-immunized RM were low and stable after SIV challenge (27), in contrast to the aberrant T cell activation that occurs in unimmunized RM infected with SIV (21). Thus, systemic anamnestic expansion of memory T cells to produce large numbers of activated CD8+ T cells, a process that has been invoked to explain the effectiveness of memory antiviral T cell responses, was not required for protection in SHIV-immunized RM.
The nature of infection with attenuated SHIV89.6 may explain the effectiveness of the elicited SIV Gag-specific CD8+ T cell responses in protecting RM from vaginal SIV challenge. SHIV immunization results in a persistent infection. SHIV RNA is readily detected in plasma for 8 to 12 weeks after immunization, and low-level SHIV89.6 replication persists up to the time of SIV challenge, 6 to 8 months after SHIV infection (26). CD8+ T cell depletion of SHIV-immunized RM prior to SIV challenge results in a rapid rebound of plasma SHIV vRNA in most RM (C. J. Miller, unpublished data; 70). Thus, an antiviral CD8+ T cell response effectively controls SHIV replication in the first few weeks after immunization, and this is followed by an extended period of low-level antigenic stimulation from residual SHIV replication. Chronic antigen production is characteristic of attenuated lentiviral immunization, and this temporal pattern of antigen exposure differs from the limited period of antigen exposure typical of other vaccines. The presence of effector stage antiviral CD8+ T cells in the vaginal mucosa, at the time of SIV challenge, due to this chronic low-level antigenic stimulation may explain the effectiveness of this SIV vaccine model (26). However, SHIV immunization also induced SIV-specific CD4+ T cells in the vagina (26, 27) and increased the total number of CD4+ T cells and activated CD4+ T cells (see below). Thus, SHIV immunization increases viral target cells in the site of viral challenge, and this should limit the effectiveness of SIV-specific CD8+ T cells at this site. In fact, the CD8+ lymphocyte depletion experiment demonstrated that the SHIV-induced CD4+ T cells in the vaginal mucosa have the capacity to enhance viral replication, as SIV RNA levels in the plasma at day 7 and in the vaginal mucosa at day 14 postchallenge (PC) were significantly higher in the CD8+ lymphocyte-depleted, SHIV-immunized RM than in the unimmunized control RM (70).
To explain the protective effect of the modest SIV-specific effector CD8+ T cell response in the vagina (27), we hypothesized that persistent SHIV89.6 infection induces changes in the immunoregulatory and inflammatory environment that limit immune activation in the SHIV-infected host even after vaginal SIV challenge. In support of this hypothesis, it was recently reported that people who have been repeatedly exposed to HIV but resist infection have reduced frequencies of activated T cells and elevated frequencies of regulatory CD4+ T cells (Tregs) compared with those of HIV-unexposed individuals (15). Tregs suppress T cell proliferation, limiting the size of the HIV target cell pool, and low levels of immune activation may contribute to the ability of some individuals to resist HIV infection (15). Indeed, after infection with attenuated SHIV89.6, we found that there were significant changes in plasmacytoid dendritic cell (pDC) number and function, mRNA levels for proinflammatory and regulatory molecules, and the phenotype of activated T cells. These changes persisted up to the day of challenge. After vaginal SIV challenge, CD4+ Tregs rapidly expanded in the vaginal mucosa of SHIV-immunized RM, and the mRNA levels of innate immune genes and proinflammatory mediators increased. However, by 14 days after SIV challenge, all innate and inflammatory responses were suppressed in SHIV-immunized RM compared to those in unimmunized control RM. Thus, SHIV immunization of RM has specific effects on innate immunity and immunoregulation that collectively increase the threshold for T cell activation in RM exposed to SIV, and this may be critical for the effectiveness of a modest SIV-specific effector CD8+ T cell response at the site of mucosal challenge.
MATERIALS AND METHODS
Experimental design.
The SHIV vaccination and SIV challenge are described in detail in previous publications that characterized the SIV-specific CD8+ T cell responses and the extent of viral replication of the SHIV89.6 vaccine virus and the SIVmac239 challenge virus (26, 27, 70). A total of 30 female monkeys were intravenously infected with live, virulence attenuated SHIV89.6 for 6 to 8 months, as previously described (3). After the 6- to 8-month immunization period, at the day zero time point, nine of these RM were necropsied, and a detailed study of the SIV-specific T cell responses has been published (26). The remaining immunized RM (n = 21) were challenged with pathogenic SIVmac239 by intravaginal inoculation, as described previously (27), and necropsied at 3 (n = 3), 7 (n = 6), or 14 (n = 12) days postchallenge (PC). Twenty-one unimmunized female RM were vaginally challenged with pathogenic SIVmac239 and necropsied at 3 (n = 3), 7 (n = 9), and 14 (n = 9) days PC as unimmunized, SIV control RM. In addition, to establish baseline values for parameters measured, 6 normal control female RM were necropsied for sample collection. The identifying animal number, age at necropsy, and number of copies of SIV GAG per ml of plasma at necropsy are listed in Table S1 in the supplemental material.
Flow cytometric analysis of cell populations.
The percentage of CD3+ CD4+ and CD3+ CD8+ T cells in blood samples was determined using a FACSCalibur (Becton, Dickinson) and RM-reactive antibodies (Abs) anti-CD3-peridinin chlorophyll protein (PerCP) clone no. SP34, anti-CD4-PE clone no. L-200, and anti-CD8-FITC clone no. SK1 (NIAID AIDS Reagent Repository) (where PE is phycoerythrin and FITC is fluorescein isothiocyanate). Additional combinations of surface markers were examined on the same blood samples for CD3 and CD8: CD45RA-FITC/CD28-PE (clone no. RA3-6B2 and L293, respectively) and HLA-DR-PE/CD38-FITC (clone no. G46-6 and OKT10, respectively).
Flow cytometric analysis of Tregs.
Freshly isolated PBMC and lymph node (LN) cell suspensions were labeled with anti-CD3-Pacific Blue, anti-CD4-AmCyan, anti-CD25-Cy7PE, anti-CTLA-4-Cy5PE (clone no.BN13), and anti-HLA-DR-Cy5.5PerCP antibodies. Samples were permeabilized with Foxp3 Fix/perm buffer (Biolegend) for intracellular staining with anti-Foxp3-allophycocyanin antibody (clone no.206D; Biolegend). Samples were incubated for 15 min at room temperature. After being washed with Foxp3 Fix/perm buffer, cells were fixed with 1% paraformaldehyde. Data were acquired using a FACSAria flow cytometer (Becton, Dickinson) and analyzed using FlowJo software (Treestar, Inc.) and Macintosh G5 computers (Apple Inc.). A minimum of 150,000 small lymphocyte events were collected per sample. All Abs were purchased from Pharmingen unless another source is noted.
Relative quantification of mRNA expression levels in tissues.
mRNA levels were determined by real-time PCR, as described previously (3, 76), for CD4, interleukin 1 (IL-1), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, IL-23, IL-29, tumor necrosis factor (TNF), CCL3/MIP1-α, CCL4/MIP1-β, CCL20/LARC, CXCR3, CCR5, tripartite motif 5 α (TRIM5α), IFN-α, IFN-β, IFN-γ, monokine-induced by IFN-γ (MIG), 2′-5′-oligoadenylate synthetase (OAS), IFN-inducible protein 10 (IP-10), MxA, interferon regulatory factor 7 (IRF7), melanoma differentiation-associated gene 5 (MDA5), retinoic-acid-inducible protein 1 (RIG-1), virus-induced signaling adapter (VISA), Siglecs 3 and 5, transforming growth factor β (TGF-β), FoxP3, perforin, granzyme, and GAPDH. Samples were tested in duplicate, and the GAPDH housekeeping gene and the target gene were tested in the same plate. The reaction was carried out in a 96-well optical plate (Applied Biosystems) in a 25-μl reaction volume containing 5 μl cDNA plus 20 μl Mastermix (Applied Biosystems). All sequences were amplified using the 7900 default amplification program: 2 min at 50°C, 10 min at 95°C, followed by 40 to 45 cycles of 15 s at 95°C and 1 min at 60°C. The results were analyzed with the SDS 7900 system software, version 2.3 (Applied Biosystems).
The comparative threshold cycle (ΔCT) method was used for quantification of mRNA levels (User Bulletin no. 2; ABI PRISM 7700 Sequence Detection System, Applied Biosystems) (2, 77). GAPDH was used as the reference gene for all gene targets except FoxP3, which was normalized to CD4 mRNA levels, and all samples were tested in duplicate. CT values of both GAPDH and target genes were recorded. A ΔCT value was generated by subtracting the CT value of GAPDH from the CT value of the target mRNA. Thus, the smaller the resulting ΔCT value, the higher the target mRNA level in the sample. The analysis of a sample was repeated if the standard deviation (SD) of the ΔCT of the duplicate wells was above 0.5. Tissues from 6 sex- and age-matched uninfected normal control RM were used to determine the reference range of all target mRNA levels. We estimated the number of FoxP3 mRNA copies/CD4+ T cell by dividing the ΔCT of FoxP3 mRNA by the ΔCT of CD4 mRNA in a tissue sample as an estimate of the number of FoxP3 transcripts/CD4+ T cell in a tissue. This approach accounts for the relative number of CD4+ T cells in the sample and allows FoxP3 mRNA levels to be meaningfully compared in samples with widely disparate numbers of CD4+ T cells, i.e., LNs and the vagina.
Treg suppression assay.
Cryopreserved mononuclear cells from spleen were thawed and rested overnight at 37°C in 5% CO2 in AIM-V medium supplemented with 10% fetal bovine serum (FBS). An aliquot of the cells was depleted of CD25+ cells using a previously titrated anti-CD25-PE monoclonal antibody (MAb) (Miltenyi Biotec, Auburn, CA) for 15 min at 4° and positive selection using the CD25 selection kit as described by the manufacturer (StemCell Technologies, Inc.). Both the undepleted cell fraction and the CD25-depleted fraction were counted and resuspended at 2 × 106/ml and plated in triplicate in a 96-well plate at 2 × 105 cells/well and either left unstimulated or stimulated with ConA at 1 or 10 μg/ml. Cells were then pulsed overnight with 3H-thymidine and harvested at 72 h, and radioactivity was counted in a beta counter (Wallac Microbeta Trilux; Perkin Elmer, Sheldon, CT). For each cell fraction, a stimulation index was calculated from the mean of triplicate data as the cpm of stimulated/unstimulated cultures. The results of the assay are reported as a “suppression index” that was calculated by dividing the stimulation index of the CD25+ T cell-depleted fraction by the stimulation index of the unfractionated cells from a sample.
Plasmacytoid DC enumeration and assessment of IFN-α production.
As previously described (5), pDC were phenotyped by flow cytometry using an FITC lineage marker Ab cocktail (lin 1; CD3, CD14, CD16, CD19, CD20, CD56; BD Biosciences), anti-HLA-DR-PerCP antibody, and anti-CD123-PE antibody (clone no. 7G3; BD Pharmingen). To assess IFN-α production, PBMC were cultured in 24-well plates at 1 × 106 cells/ml in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin-streptomycin, and l-glutamine. PBMC were cultured with herpes simplex virus (HSV; multiplicity of infection [MOI] = 1) and harvested at 6 h poststimulation. During the last 2 h of stimulation, brefeldin A (10 mg/ml) was added to all cultures. Intracellular staining with an MAb for IFN-α (clone no. MMHA-2; PBL Biomedical Laboratories) was combined with surface marker staining described above to determine the phenotype of the IFN-α-producing cells. After cell surface marker staining, cells were fixed and permeabilized using phosphate-buffered saline (PBS) supplemented with 2% FBS and 0.5% saponin (Sigma). The IFN-α Ab was conjugated with allophycocyanin using the Molecular Probes' Zenon labeling kit (Invitrogen, Inc., Carlsbad, CA) according to the manufacturer's instructions. A total of 150,000 cells were collected on a FACSCalibur (Becton, Dickinson) and analyzed using FlowJo software (Treestar, Inc.) and Macintosh G5 computers (Apple Inc.). Since the PBMC concentration in blood varies among different time points, it is not reasonable to compare the pDC frequency or percentage in our experiments. Thus, we determined the concentration (absolute number per ml of blood) of both pDC and IFN-α-producing pDC by multiplying the proportion of mononuclear cells that are pDC (pDC number/total mononuclear cell number) with the number of mononuclear cells/ml of blood.
Immunohistochemistry and quantitative image analysis.
Tissue was fixed in 4% paraformaldehyde (Electron Microscopy Sciences) and embedded in paraffin. The following primary Abs were used: monoclonal anti-CD123 antibody (clone 7G3; BD Pharmingen Inc.), anti-CD4 mouse serum (clone IF6; Vector Inc.), polyclonal anti-Ki67 rabbit serum (Lab Vision), polyclonal anti-Foxp3 rabbit serum (Abcam Inc.), monoclonal anti-IDO mouse serum (clone 10.1; Millipore Inc.), and polyclonal anti-IDO sheep serum (AbD Serotec Inc.). Primary Abs were replaced by normal mouse (Dako Inc.) and rabbit IgG (Zymed Inc.) or sheep (R&D Systems Inc.) IgG and included with each staining series as the negative control. Binding of the CD4 and FoxP3 Abs was detected simultaneously using Alexa Fluor 568-labeled polyclonal goat anti-rabbit IgG and Alexa Fluor 488-labeled polyclonal goat anti-mouse IgG (Molecular Probes Inc.). For CD123 and IDO staining, Envision anti-mouse Ig polymer was used, while biotinylated rabbit anti-sheep IgG (Vector Lab Inc.) and streptavidin-horseradish peroxidase (HRP) (Invitrogen Inc.) were detected by diaminobenzidine (DAB; Dako Inc.). All the control experiments gave appropriate results with minimal nonspecific staining (data not shown).
Slides were visualized with light microscopy or epifluorescent illumination using a Zeiss Axioimager-1 microscope (Carl Zeiss Inc.) and appropriate filters. Digital images were captured and analyzed by using a Zeiss Axiocam System and Openlab software (Inprovision Inc.) as previously described (16, 21). Five high-power (40×) microscope fields of the T-cell-rich zone (paracortex) per LN section and five high-power fields of lamina propria from rectal and vaginal sections were randomly chosen and captured digitally with the system described above. Each captured field includes an area of approximately 0.09 mm2. Only clearly positive cells with distinctly labeled nuclei (DAPI [4′,6-diamidino-2-phenylindole]) and bright staining were considered positive. Individual positive cells in five high-power microscope fields of immunohistochemically labeled tissue sections were counted manually by a single observer. The number of positive cells is presented as cells per square millimeter in the paracortex of the LNs, the lamina propria of the colon, and vaginal lamina propria/submucosa.
Statistical analysis.
Statistical analyses were performed using Prism 4.0 software (GraphPad Software) and R software version 13.1 (http://cran.r-project.org/). In general, a two-sample t test was performed for comparison between two independent samples. For comparison between multiple independent samples, such as the RM necropsied at different time points, one-way analysis of variance (ANOVA) with Tukey's multiple comparison test was performed. Pearson's correlation was calculated between age and CD38 expression in T cells. In the gene expression analysis, which involves comparison of 35 different genes in 9 different tissues, the target genes' ΔCT values were compared using a Wilcoxon rank sum test. Multiple testing performed was adjusted by controlling the false discovery rate (FDR) at the 0.05 level. The following sets of comparisons were analyzed separately: SHIV versus control at baseline; control versus SIV-SHIV at days 3, 7, and 14; control versus SIV at days 3, 7, and 14; and SHIV versus SHIV-SIV at days 3, 7, and 14. All P values presented are two sided. Statistical significance was declared when the estimated FDR is less than 0.05 for gene expression analyses and when the P value is less than 0.05 otherwise.
RESULTS
Infection with SHIV89.6 increases the number of activated CD4+ T cells in the female genital tract.
We previously reported that SHIV immunization elicited SIV Gag peptide-specific CD4+ T cells in the vagina of 7 of 7 immunized RM and in the cervix of 5 of 7 immunized RM (26). To further characterize SIV target cell populations elicited by SHIV, we used quantitative immunohistochemistry (43) to enumerate total and activated CD4+ T cells in the genital tract. We found that compared to normal RM, the total number of CD4+ T cells in the endocervix and ectocervix of SHIV-immunized RM was not significantly different (Fig. 1A and C). However, in the vaginal mucosa of SHIV-immunized RM, the total number of CD4+ T cells was increased about 3-fold compared to that in normal RM (P = 0.0006, two-sample t test; Fig. 1E). Further, SHIV immunization increased the number of activated Ki67+/CD4+ T cells in both the vagina and ectocervix about 3-fold compared to that in normal RM (P = 0.025 and P = 0.003, respectively; Fig. 1D and F). A 3-fold increase in activated CD4+ target cells at the site of SIV challenge suggests that vaccine-induced CD8+ T cells are necessary (27, 70) but insufficient to explain the resistance of 60% of vaccinated RM. Thus, to determine the extent to which factors that limit target cell availability and dampen T cell activation could contribute to the protection from vaginal SIV challenge, we characterized the effect of SHIV immunization on proinflammatory and immunoregulatory responses.
Fig 1.
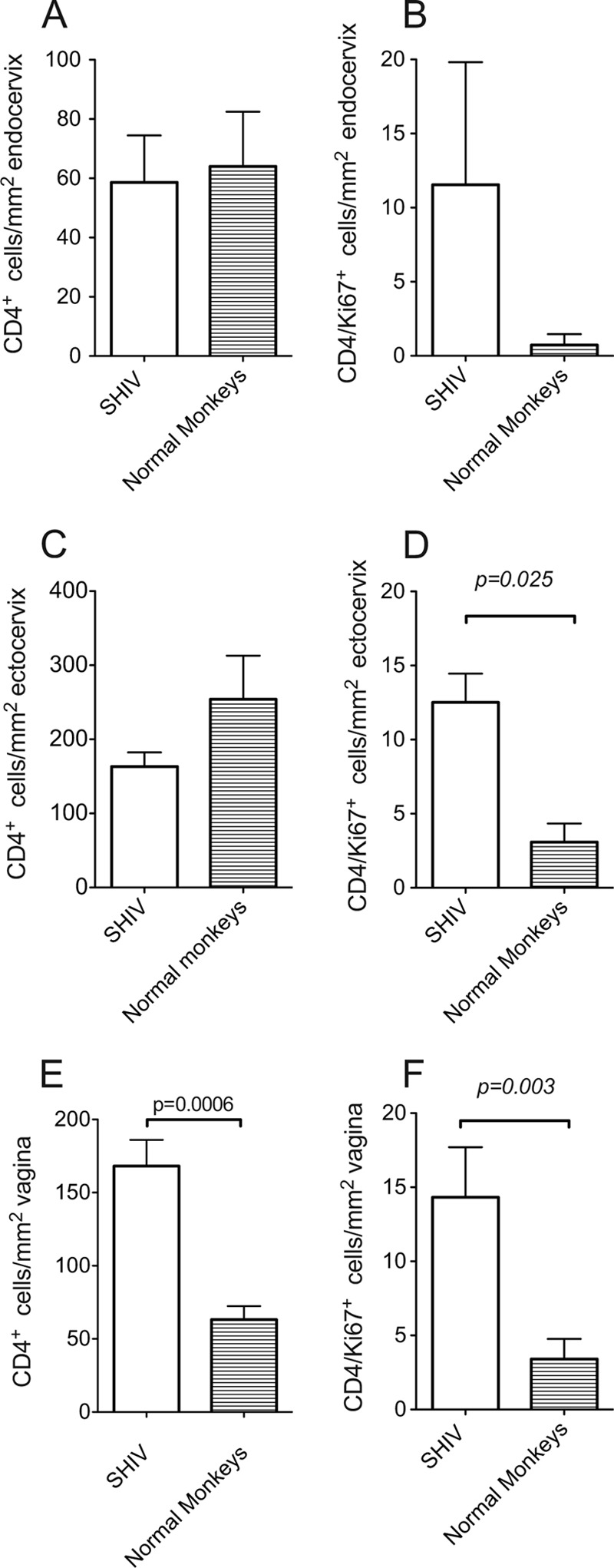
The total numbers of CD4+ T cells and activated CD4+ T cells are increased in the genital tract of SHIV-immunized RM compared to those of normal RM. The mean number of CD4+ T cells and Ki67+/CD4+ T cells per mm2 of tissue was assessed by immunohistochemistry 6 to 8 months after SHIV immunization (SHIV-immunized RM, n = 9 monkeys; normal controls, n = 6). (A) CD4+ T cells, endocervix; (B) Ki67+/CD4+ T cells, endocervix; (C) CD4+ T cells, ectocervix; (D) Ki67+/CD4+ T cells, ectocervix; (E) CD4+ T cells, vagina; (F) Ki67+/CD4+ T cells, vagina. Five randomly chosen fields were counted for each individual animal. P values were generated using a two-sample t test.
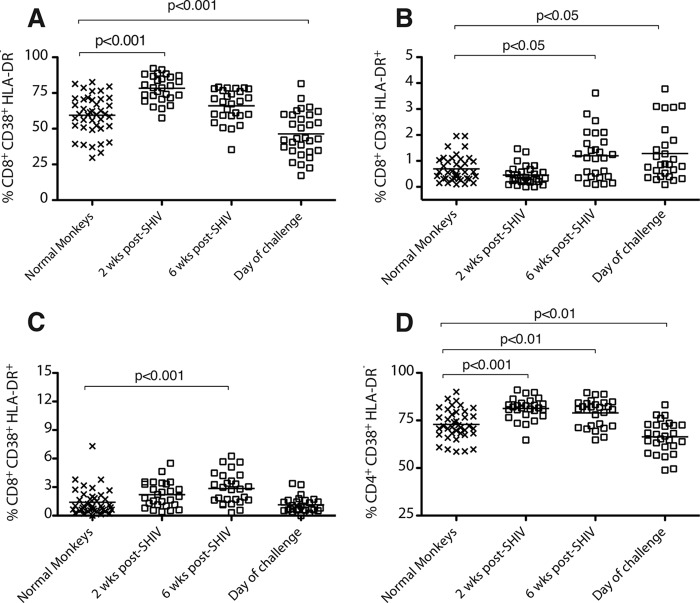
SHIV infection increases the frequency of circulating CD8+ HLA-DR+ CD38− T cells.
To determine the extent of immune activation caused by SHIV immunization, we determined the pattern of CD38 and HLA-DR expression on CD8+ T cells of immunized RM. Two weeks after SHIV89.6 immunization, the frequency of CD8+ CD38+ HLA-DR− T cells in blood was significantly elevated compared to preimmunization levels (Fig. 2A). However, 6 to 8 months after SHIV immunization, at the time of vaginal SIV challenge, the frequency of CD8+ CD38+ HLA-DR− T cells was significantly lower than preimmunization levels (Fig. 2A), and the frequency of CD8+ CD38− HLA-DR+ T cells was significantly increased in SHIV-immunized RM compared to that in normal, uninfected monkeys (Fig. 2B). Further, SHIV immunization also decreased the frequency of CD4+ CD38+ HLA-DR− T cells on the day of SIV challenge compared to that in normal monkeys (Fig. 2D). Finally, if analyzed without regard to the level of HLA-DR expression, the frequency of CD8+ CD38+ T cells, but not of CD4+ CD38+ T cells, was lower in SHIV-immunized RM than in normal monkeys (see Fig. S1A and D in the supplemental material).
Fig 2.
Infection with attenuated SHIV89.6 induces transient T cell activation and a sustained increase in CD38−/HLA-DR+ CD8+ T cells. Flow cytometry was used to determine the percentage of activated T cell subsets in the blood of SHIV-immunized RM from the day of SHIV immunization to the day of SIV challenge. (A) Percent of CD3+ CD8+ T cells expressing CD38 but not HLA-DR; (B) percentage of CD3+ CD8+ T cells expressing HLA-DR but not CD38; (C) percentage of CD3+ CD8+ T cells expressing both CD38 and HLA-DR; (D) percentage of CD3+ CD4+ T cells expressing CD38 but not HLA-DR. P values were generated using Tukey's multiple comparison post hoc test after a one-way ANOVA. Each animal is represented by a symbol.
Because decreased CD38 expression on T cells is associated with increasing age in humans (48), we determined if differences in age among the RM could account for the differences in CD38 expression among the animal groups. There was a significant correlation between age and the frequency of CD8+ CD38+ T cells (Pearson r = −0.54) and CD4+ CD38+ T cells (Pearson r = −0.33) (see Fig. S1B and E in the supplemental material). However, by the end of the immunization period, this correlation was no longer apparent in SHIV-immunized RM (see Fig. S1C and F). Thus, 6 to 8 months of live attenuated SHIV89.6 infection decreased the frequency of CD8+ CD38+ T cells, regardless of the age of the animal, and increased the frequency of CD8+ HLA-DR+ CD38− T cells in blood.
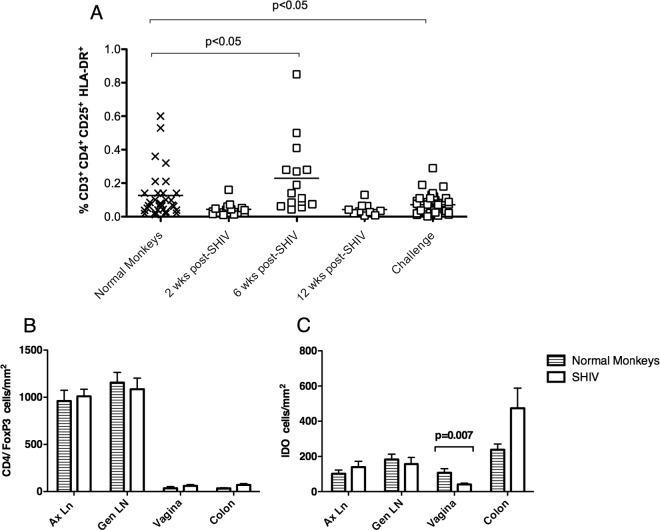
SHIV infection transiently increases the frequency of circulating Tregs.
As Tregs control immune activation and T cell proliferation (15), we sought to determine if changes in Tregs were associated with the limited immune activation and restricted anamnestic T cell response in SHIV-immunized monkeys before and after SIV challenge. We analyzed Treg frequency in SHIV and normal control RM by flow cytometry using a combination of FoxP3, CD25, HLA-DR, and cytotoxic T-lymphocyte antigen (CTLA-4, CD152) on CD4+ T cells. CD4+ CD25high HLA-DR+ T cells have been identified as a distinct subset of mature Tregs involved in contact-dependent in vitro suppression in humans (8), and 6 weeks after SHIV immunization, there was a significant, transient increase in the frequency of CD4+ CD25high HLA-DR+ T cells in blood (Fig. 3A). Approximately 6 months later, on the day of SIV challenge, the frequencies of CD4+ CD25high HLA-DR+, CD4+ Foxp3+ HLA-DR+, and CD4+ CD25+ Foxp3+ CTLA-4+ T cells were significantly lower in blood of the SHIV-immunized monkeys than in that of normal monkeys (P = 0.025, P = 0.019, and P = 0.003, respectively; two-sample t test; Fig. 3A and data not shown). Despite the decrease in peripheral Treg frequency, the numbers of CD4+ Foxp3+ cells in colon, vagina, axillary lymph node (Ax LN), and genital (Gen) LN of the SHIV-immunized RM at 6 to 8 months after immunization were similar to those in normal, age- and gender-matched RM (Fig. 3B).
Fig 3.
Infection with attenuated SHIV89.6 induces a transient increase in Tregs, while IDO+ cells in the vaginal mucosa are decreased. (A) Flow cytometry was used to determine the percentage of CD3+ CD4+ CD25+ HLA-DR+ T cells in blood of SHIV-immunized RM from the day of SHIV immunization to the day of SIV challenge. P values were generated using Tukey's multiple comparison post hoc test after a one-way ANOVA. In the tissues, the mean number of CD4+/Foxp3+ (B) and IDO+ (C) cells per mm2 of tissue was analyzed using immunohistochemistry and quantified in normal monkeys (n = 6) and SHIV-immunized monkeys (n = 9) on the day of challenge. P values were generated using a two-sample t test.
SHIV infection decreases the number of cells expressing IDO in the vaginal mucosa.
Indoleamine 2,3-dioxygenase (IDO) expression in antigen-presenting cells (APC) is induced by IFN-α (14, 51), and HIV-infected pDC expressing IDO (46) induce the differentiation of Tregs from naive T cells (18) and inhibit CD4+ T cell proliferation (10). Thus, we sought to determine if IDO expression was altered by SHIV immunization. Using quantitative morphometric analysis, we found that 6 to 8 months after SHIV infection, the immunized monkeys had almost 3 times fewer IDO+ cells in the vagina than normal RM did (Fig. 3C), indicating that SHIV immunization either depleted IDO+ APC or decreased the expression of this enzyme in vaginal APC.
SHIV infection reduces the frequency of circulating pDC.
To assess the role of pDC in limiting inflammation and immune activation in the SHIV-immunized RM, we analyzed pDC frequency and one function of pDC, IFN-α secretion. IFN-α is rapidly produced by pDC in response to viral infection, and increased IFN-α mRNA expression and protein secretion is detectable with the onset of viremia after SIV infection (1, 5, 28, 37, 45). Compared to preimmunization levels, SHIV immunization decreased the number of circulating pDC (Fig. 4A). Further, the number of pDC capable of producing IFN-α in response to in vitro stimulation with HSV type 2 was decreased at all time points after SHIV immunization (Fig. 4B).
Fig 4.
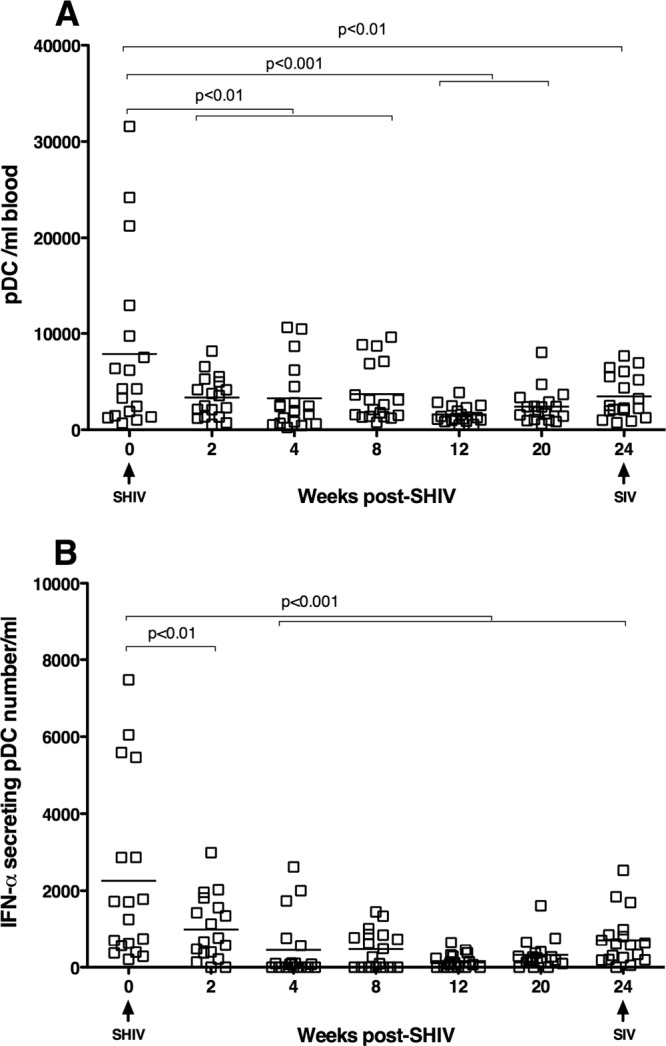
SHIV infection decreases the concentration of pDC and their ability to produce IFN-α in response to HSV-1 stimulation. (A) pDC concentration in peripheral blood from day of SHIV immunization to the day of SIV challenge. (B) The concentration of blood pDC that produce IFN-α in response to in vitro HSV-2 stimulation, from day of SHIV immunization to the day of SIV challenge. P values generated using Tukey's multiple comparison post hoc tests after a one-way ANOVA. Each animal is represented by a symbol.
SHIV infection alters gene expression for proinflammatory cytokine and regulatory molecules.
To better understand the immune environment in which the relatively modest SHIV-induced, SIV-specific CD8+ T cell response confers protection from uncontrolled virus replication after SIV challenge, we assessed the relative mRNA levels of proinflammatory and regulatory molecules in tissues of SHIV-infected monkeys by TaqMan reverse transcription (RT)-PCR. In the 9 SHIV-immunized RM that were necropsied on the day of SIV challenge, the mean mRNA levels of a number of genes were significantly different compared to those of normal RM (Table 1). Thus, IL-8, IL-4, and IL-2 mRNA levels were lower in LN but not in vagina or cervix, while levels of CCL3/MIP1-a were depressed in LN and vagina. In contrast, IFN-γ and RIG-1 mRNA levels were elevated in the genital tract (Table 1). TNF mRNA levels were elevated in mesenteric LN, and the mRNA of sialic acid-grabbing IgG-like lectin-5 (Siglec-5) was higher in the iliac LN of SHIV-immunized RM than of normal monkeys (Table 1). Siglecs are surface receptors on T cells that are thought to promote cell-cell interactions and to regulate T cell activation through glycan recognition. Thus, the increased expression of Siglec-5 and decreased expression of CCL3/MIP1-α could contribute to the altered pattern of T cell activation in SHIV-immunized RM. There was no significant difference in the mRNA levels of VISA, MDA5, Siglec-3, CCR5, CXCR3, IFN-α, IFN-β, MxA, OAS, IP-10, IRF7, CxCL9 (MIG), CCL4 (MIP1-β), CCL20, IL-1, IL-6, IL-10, IL-17, IL-23, TGF-β, FoxP3, TRIM5α, and perforin in any tissues tested (data not shown).
Table 1.
Modulation of chemokine, cytokine, and regulatory mRNA levels by SHIV infection compared to their expression in normal, age- and sex-matched RM
| Gene product | Tissue | Median ΔCT |
Modulation by SHIV relative to normal | P valuea | |
|---|---|---|---|---|---|
| Normal | SHIV | ||||
| IFN-γ | Vagina | 16.74 | 14.89 | ↑ | 0.001 |
| Cervix | 16.88 | 13.44 | ↑ | 0.0004 | |
| IL-2 | Obturator LN | 9.72 | 11.58 | ↓ | 0.0016 |
| IL-4 | Axillary LN | 12.47 | 14.1 | ↓ | 0.0018 |
| IL-8 | Iliac LN | 13.92 | 17.01 | ↓ | 0.0016 |
| Obturator LN | 13.89 | 17.6 | ↓ | 0.002 | |
| MIP1-α | Axillary LN | 8.02 | 11.03 | ↓ | 0.0008 |
| Iliac LN | 6.5 | 10.91 | ↓ | 0.0004 | |
| Obturator LN | 7.82 | 10.09 | ↓ | 0.0018 | |
| Vagina | 13 | 16.91 | ↓ | 0.001 | |
| RIG-1 | Vagina | 8.51 | 7.65 | ↑ | 0.0016 |
| Siglec-5 | Iliac LN | 9.28 | 7.01 | ↑ | 0.0004 |
| TNF-α | Mesenteric LN | 7.34 | 5.88 | ↑ | 0.0016 |
Significant difference based on a Wilcoxon rank sum test with an estimated false discovery rate for multiple comparisons of <0.05.
After vaginal SIV challenge, SHIV-immunized RM have an increased frequency of circulating CD8+ CD38− HLA-DR+ T cells.
At day 3 postchallenge (PC), a time before there are any detectable differences in SIV replication between SHIV-immunized and unimmunized SIV-infected control RM (referred to here as “SIV RM”) (70), CD8+ CD38− HLA-DR+ T cells were significantly more frequent in the Ax LN (P = 0.043, two-sample t test; data not shown) and mesenteric (Mes) LN (P = 0.06, two-sample t test; data not shown) of the SHIV-immunized RM than in the SIV RM. These relatively increased frequencies of CD8+ CD38− HLA-DR+ T cells were maintained through day 14 PC, when CD8+ CD38− HLA-DR+ T cells were significantly more frequent in the Gen (P = 0.032, two-sample t test; data not shown) and Mes (P = 0.034, two-sample t test; data not shown) LNs. While CD8+ CD38− HLA-DR+ T cells were relatively rare in the SIV RM, the frequency of CD8+ CD38+ HLA-DR− T cells steadily increased in these RM after SIV challenge. By day 14 PC, the frequencies of CD8+ CD38+ HLA-DR− T cells in the LNs and blood of SIV RM were significantly higher than in SHIV-immunized RM (90% versus 60%; P < 0.017 for each tissue in a two-sample t test; data not shown).
After SIV challenge, there are increased Tregs in the vagina of SHIV-immunized RM.
At day 3 PC, the number of Tregs in the vagina of the SHIV-immunized RM was significantly higher than in the SIV RM, but by day 7 PC, Treg levels in the SHIV-immunized RM had declined to the level of SIV RM (Fig. 5A and B). In the LNs and colon, Treg levels in the SHIV-immunized RM and the SIV RM were similar at days 3 and 7 PC (Fig. 5A and B). However, by day 14 PC, the frequencies of Tregs in the colon, Ax, and Gen LN of the unimmunized RM were significantly lower than in matched tissues of the SHIV-immunized RM (Fig. 5C). Based on flow cytometry, the frequency of CD4+ CD25high HLA-DR+ T cells significantly increased from days 0 to 7 PC in the Ax LN and Gen LN (P = 0.012 and P = 0.013, respectively, two-sample t test; data not shown) and spleen (P = 0.011, two-sample t test; data not shown) of SHIV-immunized RM. Further, at day 14 PC, the frequency of CD4+ Foxp3+ T cells in all LNs, but not in the blood, of SHIV-immunized RM were significantly higher than in the SIV RM (data not shown). Thus, after SIV challenge, there was a rapid and sustained expansion of CD4+ Tregs in the mucosal and lymphoid tissues of the SHIV-immunized RM compared to the marked depletion of CD4+ Tregs from tissues of the unimmunized SIV RM by day 14 PC.
Fig 5.
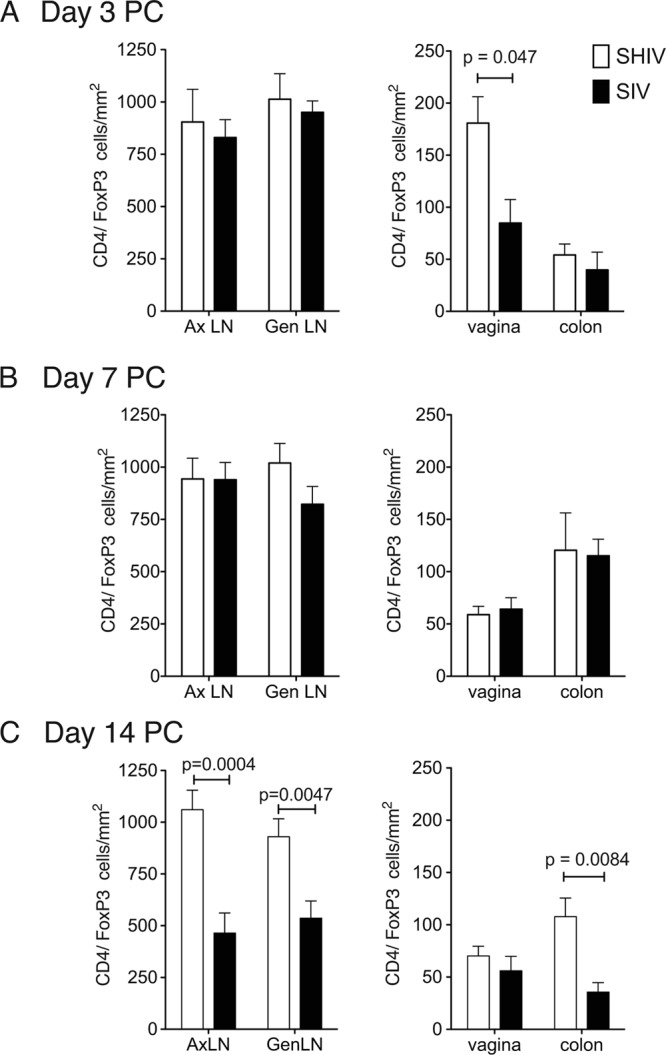
CD4+ FoxP3+ T cells rapidly increase in the vaginal mucosa of the immunized RM after SIV vaginal challenge. The mean number of CD4+/Foxp3+ T cells per mm2 of tissue was assessed by immunohistochemistry on day 3 PC (SHIV-immunized RM, n = 3; SIV controls, n = 3) (A), on day 7 PC (SHIV, n = 6; SIV, n = 9) (B), and on day 14 PC (SHIV, n = 12; SIV, n = 9) (C). Five randomly chosen fields were counted for each individual animal. P values were generated using a two-sample t test.
Because phenotypic markers do not determine Treg function, we assessed the in vitro suppressive capacity of CD4+ CD25+ T cells (33, 59) by comparing the proliferative capacity of mitogen-stimulated cells with or without CD25+ T cell depletion. Only the spleen samples provided a sufficient number of cells to perform this assay. At day 14 PC, there was significantly less suppressive activity in the CD25+ T cells isolated from the spleens of SIV RM than from the spleens of SHIV-immunized RM (Fig. 6). Thus, in addition to the marked loss of Tregs at day 14 PC, the Tregs that remain in the unimmunized SIV RM were functionally impaired.
Fig 6.
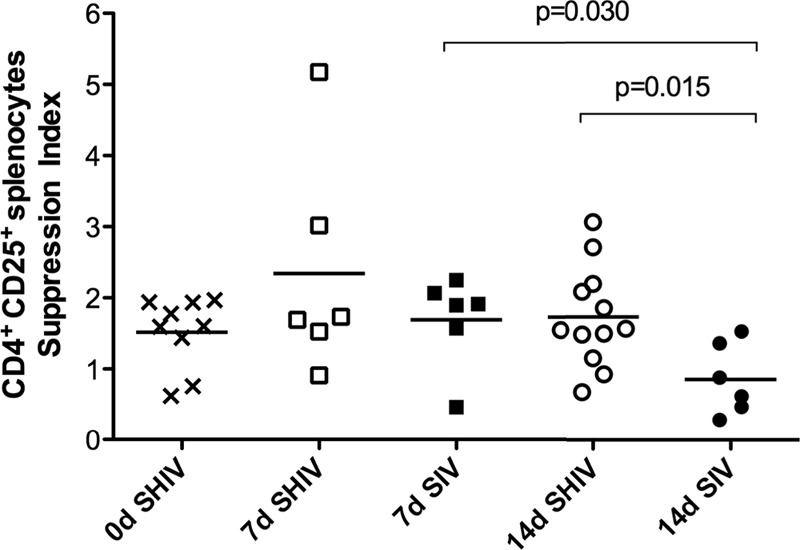
In vitro suppressive activity of CD4+ CD25+ T cells in spleen 14 days after vaginal SIV challenge. On the day of SIV challenge or on days 7 and 14 postchallenge, the suppressive capacity of CD25+ CD4+ splenic T cells was assessed by comparing the proliferative capacity of mitogen-stimulated cells with or without CD25+ T cell depletion. A suppression index was calculated as described in Materials and Methods. P values were generated using Tukey's multiple comparison post hoc tests after a one-way ANOVA.
After SIV challenge, there are reduced numbers of IDO+ cells in tissues of SHIV-immunized RM.
After SIV challenge, the number of IDO+ cells rapidly increased in the SIV RM, and by day 14 PC, IDO+ cells in the Ax LN (P = 0.03) and Gen LN (P = 0.049) of the SIV RM were significantly higher than in the SHIV-immunized RM (not shown). These findings are in agreement with a previous report documenting increased IDO activity during HIV/SIV infection (11). In both SHIV and SIV RM, IDO+ cells were highest in the colon at day 3 PC, but by day 7 PC in the SHIV-immunized RM, the number of IDO+ cells in the colon had dropped to the level seen prior to SIV challenge (not shown). Further, while the number of IDO+ cells in the vagina was much lower than that in any other tissue, at day 14 PC, the number of IDO+ cells in the vagina was significantly higher in the SIV RM than in the SHIV-immunized RM (P = 0.025; data not shown). Thus, SHIV immunization suppressed IDO expression in both mucosal and lymphoid tissues after SIV challenge.
After SIV challenge, there is an anti-inflammatory environment in the tissues of SHIV-immunized RM.
To understand the effects of inflammation and regulation of T cell activation on the outcome of vaginal SIV challenge, TaqMan RT-PCR was used to assess mRNA levels of specific target genes in tissues of SHIV-immunized and unimmunized RM at days 3, 7 and 14 PC. We first compared the gene expression levels in tissues of normal RM to SIV-infected RM at days 3, 7, and 17 PC (see Table S2 in the supplemental material). Three days after SIV challenge, SIV-infected RM had elevated granzyme mRNA levels in the colon and MxA mRNA was elevated in axillary LN. Further, TRIM5α and Siglec-5 mRNA levels were elevated in the vagina. In contrast, MIP1-β, IL-8, IL-4, IL-2, and IL-17 mRNA levels were low in LNs, while IRF7 and MDA5 mRNA levels were low in the genital tract 3 days after SIV challenge (see Table S2).
Seven days after SIV challenge, SIV-infected RM had elevated granzyme, MxA, IFN-γ, IL-29, MDA5, OAS, RIG-1, and TRIM5α mRNA levels in the colon. The mRNA levels of MxA, IFN-γ, IL-29, IRF7, MDA5, OAS, RIG-1, Siglec-5, and TGF-β were elevated in LNs. Further, IFN-γ, IL-6, IL-29, MxA, MDA5, RIG-1, Siglec-5, and TRIM5α mRNA levels were high in the genital tract. In contrast, CCL4/MIP1-β, IL-8, IL-4, IL-2, and IL-17 mRNA levels were low in LNs 7 days after SIV challenge (see Table S2 in the supplemental material).
Fourteen days after SIV challenge, SIV-infected RM had elevated granzyme, IFN-α, MxA, IP-10, IFN-γ, IL-29, MDA5, OAS, RIG-1, Siglec-5, perforin, and TRIM5α mRNA levels in the colon. The mRNA levels of perforin, granzyme, IFN-γ, IL-10, IL-29, IRF7, MDA5, IP-10, OAS, MIG/CXCL9, RIG-1, Siglec-3, Siglec-5, TGF-β, and TRIM5α were elevated in LNs. Further, perforin, IFN-α, IFN-γ, IL-10, IL-29, IL-6, IRF7, MDA5, IP-10, OAS, MIG/CXCL9, RIG-1, Siglec-3, Siglec-5, TGF-β, VISA, and TRIM5α mRNA levels were high in the genital tract. In contrast, IL-23, IL-17, IL-12, IL-8, IL-4, IL-2, and CCL4/MIP1-β mRNA levels were low in LNs 14 days after SIV challenge (see Table S2 in the supplemental material). These data clearly illustrate that the expression of innate immune genes is largely unaltered in all tissues until day 7 PC, when moderate changes associated with an innate immune response can be detected in some tissues. By day 14 PC, there is a marked increase in the expression of a wide variety of genes associated with innate and adaptive immunity in all tissues.
To understand the effect of SHIV immunization on gene expression, we compared gene expression levels in tissues of normal RM to SHIV-immunized RM at days 3, 7, and 14 PC (Table 2). After eliminating all comparisons with an estimated false discovery rate of >0.05, significant differences in gene expression between these 2 RM groups were not apparent until day 14 PC. While SHIV-immunized RM had few changes in gene expression in most tissues after vaginal SIV challenge, there was increased expression of 2 genes important for antiviral immunity. The mRNA level of CXCR3 was elevated in axillary LN, and IFN-γ mRNA levels were high in the vagina. In contrast, CCL3/MIP1-α, IL-8, IL-4, and IL-1 mRNA levels were low in LNs 14 days after SIV challenge of SHIV-immunized RM (Table 2). Thus, while SHIV-immunized RM had few changes in gene expression in most tissues after vaginal SIV challenge, there was increased expression of 2 genes (the IFN-γ and CXCR3 genes) important for antiviral immunity in the vagina and axillary LN.
Table 2.
mRNA levels for chemokine, cytokine, and regulatory genes from SHIV-immunized RM on day 14 after SIV challenge compared to their expression in normal, age- and sex-matched RM
| Gene product | Tissue | Median ΔCT |
Modulation by SHIV relative to normal | P value | |
|---|---|---|---|---|---|
| Normal | SHIV | ||||
| CXCR3 | Axillary LN | 9.85 | 6.23 | ↑ | 0.00032 |
| IFN-γ | Vagina | 16.88 | 13.62 | ↑ | 0.00043 |
| IL-1 | Cervix | 13.34 | 14.71 | ↓ | 0.00011 |
| IL-4 | Axillary LN | 12.47 | 15.58 | ↓ | 0.00088 |
| IL-8 | Iliac LN | 13.92 | 17.12 | ↓ | 0.00046 |
| MIP1-α | Iliac LN | 6.5 | 12.29 | ↓ | 0.00022 |
| Obturator LN | 7.82 | 10.38 | ↓ | 0.00043 | |
We also compared gene expression levels in tissues of SIV-infected RM to SHIV-immunized RM at days 3, 7, and 14 PC (Table 3), and no significant differences in gene expression between these 2 RM groups were apparent until day 14 PC, after eliminating all comparisons with an estimated false discovery rate of >0.05. Fourteen days after SIV challenge, mRNA levels of IL-29, IP-10, IRF7, and CCL3/MIP1-α were significantly lower in LNs of SHIV-immunized RM than in SIV RM, and levels of CXCR3, granzyme, perforin, IL-10, IL-29, IP-10, OAS, MxA, IRF7, MDA5, MIG/CXCL9, RIG-1, Siglec-5, and TRIM5α were significantly lower in the genital tract of SHIV-immunized RM than in SIV RM (Table 3).
Table 3.
mRNA levels for chemokine, cytokine, and regulatory genes from SHIV-immunized RM (SHIV) compared to their expression in unimmunized RM (SIV) on day 14 after SIV challenge
| Gene product | Tissue | Median ΔCT |
Modulation by SHIV relative to SIV | P valuea | |
|---|---|---|---|---|---|
| SIV | SHIV | ||||
| CXCR3 | Vagina | 8.49 | 9.34 | ↓ | 0.00095 |
| Granzyme | Vagina | 4.12 | 6.32 | ↓ | 0.000048 |
| IL-10 | Cervix | 11.41 | 13.87 | ↓ | 0.00066 |
| IL-29 | Axillary LN | 15.15 | 21.27 | ↓ | 0.00017 |
| Cervix | 17.77 | 25 | ↓ | 0.00011 | |
| Iliac LN | 16.06 | 22.12 | ↓ | 0.00017 | |
| Obturator LN | 14.63 | 21.2 | ↓ | 0.00042 | |
| Vagina | 16.72 | 25 | ↓ | 0.000075 | |
| IP-10 | Axillary LN | 13.77 | 16.52 | ↓ | 0.00013 |
| Cervix | 16.12 | 20.62 | ↓ | 0.00033 | |
| Iliac LN | 12.55 | 17.7 | ↓ | 0.00094 | |
| Vagina | 15.35 | 19.5 | ↓ | 0.00046 | |
| IRF7 | Cervix | 6.2 | 9.59 | ↓ | 0.0000068 |
| Vagina | 6.14 | 9.88 | ↓ | 0.00095 | |
| MDA5 | Colon | 5.36 | 8.16 | ↓ | 0.00066 |
| Vagina | 5.46 | 8.12 | ↓ | 0.0000068 | |
| MIG | Vagina | 7.19 | 11.06 | ↓ | 0.00094 |
| MIP1-α | Axillary LN | 8.48 | 11.61 | ↓ | 0.00083 |
| MX | Cervix | 0.87 | 5.38 | ↓ | 0.0000068 |
| Vagina | 0.64 | 5.29 | ↓ | 0.000082 | |
| OAS | Cervix | 6.2 | 9.37 | ↓ | 0.000048 |
| Vagina | 5.89 | 8.86 | ↓ | 0.000014 | |
| Perforin | Vagina | 5.93 | 7.56 | ↓ | 0.00095 |
| RIG-1 | Vagina | 5.11 | 7.23 | ↓ | 0.00014 |
| Siglec-5 | Vagina | 9.37 | 10.66 | ↓ | 0.00066 |
| TRIM5α | Vagina | 6.61 | 8.77 | ↓ | 0.00073 |
Significant difference based on a Wilcoxon rank sum test with an estimated false discovery rate for multiple comparisons of <0.05.
After SIV challenge, there are similar numbers of pDC in the genital tract of unimmunized and SHIV-immunized RM.
Based on a cross-sectional study of a limited number of control and SIV-inoculated rhesus macaques, it was reported that the density of CD4+ T cells increases in the cervix in the first few days after SIV inoculation (41). The authors also noted pDC strongly positive for type 1 interferons, and the CCR5+ cell-attracting chemokines MIP1-α and MIP1-β aligned just beneath the endocervical epithelium in a rhesus macaque 1 day after vaginal SIV inoculation (41) but not in the few control macaques examined. These findings led to the conclusion that the viral inoculum initiates an outside-in signaling pathway that recruits pDC and T cells to the cervix, and this putative influx of virus target cells supports the expansion of the nascent viral infection, allowing the infection to become established in the new host (41). To determine if the altered inflammatory environment in the tissues of SHIV-immunized animals limits the earliest stages of viral replication by affecting this process, we characterized the density of CD123+ pDC in sections of vagina and cervix of the SHIV-immunized and SIV RM necropsied at day 3 postchallenge (Fig. 7). We found that the density of pDC was quite variable among individual RM and was related to the levels of neutrophilic inflammation in the tissue. Thus, in the endocervix of one SIV animal with moderate neutrophilic cervicitis, CD123+ cells were relatively abundant below and within the columnar epithelium (Fig. 7A). In the other 2 SIV RM, CD123+ pDC were rare (Fig. 7B). The density of pDC in the SHIV-immunized RM also varied. In the endocervix of one SHIV-immunized animal with mild to moderate neutrophilic cervicitis, CD123+ cells were found below and within the columnar epithelium (Fig. 7C), but CD123+ pDC were rare in the cervix of the other 2 SHIV-immunized RM (Fig. 7D). pDC were also rare in the vaginal mucosa of all SHIV-immunized and SIV RM (not shown). In age-matched, normal RM, the number of pDC in the endocervix was also highly variable. In one normal animal, CD123+ cells were common just below and within the columnar epithelium (Fig. 7E), while in the endocervix of another normal animal, CD123+ pDC were rare (Fig. 7F).
Fig 7.
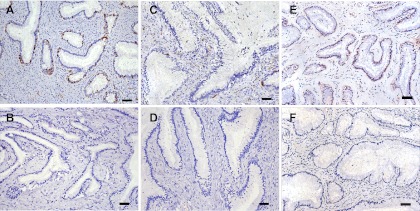
The number of plasmacytoid dendritic cells in the endocervix of RM is highly variable and unrelated to SIV exposure/infection status. (A) CD123+ pDC in the endocervix of unimmunized monkey 28617 3 days after vaginal SIV challenge; (B) CD123+ pDC in the endocervix of SHIV-immunized monkey 32644 3 days after vaginal SIV challenge; (C) CD123+ pDC in the endocervix of unimmunized monkey 28257 3 days after vaginal SIV challenge; (D) CD123+ pDC in the endocervix of SHIV-immunized monkey 31298 3 days after vaginal SIV challenge; (E) CD123+ pDC in the endocervix of an age-matched, SIV-naïve, normal RM; (F) CD123+ pDC in the endocervix of a second age-matched, SIV-naïve, normal RM. All tissue sections were stained immunohistochemically using a horseradish peroxidase-DAB chromogen system so that CD123+ cells appear dark brown. Scale bars indicate 50 μm.
Thus, large numbers of pDC in the endocervix are not a consistent finding in either unimmunized control RM or SHIV-immunized RM after vaginal SIV inoculation, and large numbers of pDC were seen in the endocervix of some SIV naïve normal RM. A pDC infiltrate similar to that previously reported (41) was found in 1 SIV-exposed/infected control animal (Fig. 7A) and in 1 SHIV-immunized SIV-exposed/infected animal (Fig. 7B) at day 3 after SIV inoculation, but the same pDC infiltrate was seen in some normal unexposed RM as well.
DISCUSSION
A critical role for CD8+ T cells in SHIV-induced protection can be inferred from the fact that immunized monkeys depleted of CD8α+ lymphocytes on the day of challenge were unable to control SIV replication after challenge. Thus, SIV-specific CD8+ T cells induced by SHIV immunization are necessary for protection from vaginal SIV challenge (3, 25, 27). Because natural killer cells also express CD8α, they might contribute to controlling viral replication after SHIV immunization, but the role of NK cells in effective anti-SIV immunity seems minor, as depletion of CD8+ CD16+ natural killer cells has no effect on SIV replication during acute infection (12). Our studies to date have not determined if an antiviral CD8+ T cell response alone is sufficient to protect immunized animals. Several features of the SHIV-induced antiviral T cell response are unusual. First, the strength of the SIV Gag-specific CD8+ T cell response in the SHIV-immunized RM is not impressive (26). In fact, the T cell responses in blood of SHIV-immunized RM were weaker than those elicited by an Ad5-HIV vaccine that provided no protection from HIV infection in a human clinical trial (49). Second, while the SIV Gag-specific CD8+ T cells in the vaginal mucosa expanded after SIV challenge, antiviral T cells in the blood and other tissues of SHIV-immunized RM remained sporadic and did not expand after SIV challenge (27), perhaps because the challenge virus did not disseminate to secondary lymphoid tissues (70). Third, SHIV immunization induced SIV-specific CD4+ T cells (26) and increased the frequency of total and activated CD4+ T cells in the vagina and cervix at the time of vaginal SIV challenge (Fig. 1). Thus, although SHIV immunization increases the number of target cells in the genital tract at the time of challenge, T cell activation is reduced after challenge, and that may allow the moderate antiviral CD8+ T cell response to control SIV replication before the infection can disseminate. After SIV challenge, there is much less activation and death of CD8+ T cells in SHIV-immunized RM compared to in unimmunized RM (27). We now show that even prior to SIV challenge, the nature and extent of generalized CD8+ T cell activation was significantly altered by SHIV immunization, as evidenced by the pattern of CD38 and HLA-DR expression. This CD8+ T cell activation phenotype in SHIV-immunized monkeys (CD8+ CD38− HLA-DR+) may define a highly effective killer cell that efficiently eliminates virus-infected cells (64).
We also found that SHIV infection dramatically altered many biomarkers of inflammation and immune activation in the immunized RM. The mRNA levels of some proinflammatory cytokines were increased, while others were decreased by SHIV immunization (Table 1). Importantly, expression of CCL3/MIP1-α, a chemokine that recruits T cells to tissues, was suppressed by SHIV infection, and expression of a cell surface molecule that dampens T cell activation, Siglec-5, was enhanced. Taken together, these results demonstrate that SHIV infection altered the gene expression of several molecules that control and shape immune activation in the host. After SIV challenge, altered expression of innate immune response genes was not detectable in tissues of unimmunized RM until 7 days after vaginal SIV inoculation, when increased expression of proinflammatory genes and genes involved in the innate antiviral response are detected in the genital tract and some lymph nodes. However, by day 14 PC, increased expression of these genes occurs in all mucosal and lymphoid tissues of the unimmunized RM. This temporal and anatomic pattern of altered gene expression is consistent with a previous report describing changes in gene expression after vaginal SIVmac251 inoculation (4). Further, this pattern of gene expression suggests that while SIV induces immune activation, it also blocks the elaboration of the full range of adaptive antiviral responses by decreasing IL-2 and IL-4 mRNA levels in lymph nodes. In contrast to unimmunized RM, expression of immune activation genes did not increase in SHIV-immunized RM after vaginal SIV challenge. Further, as Siglecs contribute to the control of T cell activation (7, 74, 75), the increased expression of Siglec-5 in lymph nodes may provide an explanation for the altered T cell activation patterns in SHIV-immunized RM. Taken together, these changes are consistent with the conclusion that SHIV immunization decreased immune activation and inflammation in the immunized RM after SIV challenge.
SHIV-immunized RM also had decreased numbers of pDC in blood. Although it is not clear if these cells were depleted, as in chronic SIV and HIV infection (13, 31, 42, 55, 61, 67), or had redistributed to tissues, there was no evidence of increased numbers of pDC in the genital tissues of the SHIV-immunized RM (Fig. 7). The pDC that remained in blood after SHIV immunization were hypofunctional, as they had a reduced ability to secrete IFN-α in response to in vitro HSV stimulation. These changes in pDC may contribute to the altered immune activation in SHIV-immunized RM, as these cells are key mediators of the innate antiviral immune response and secrete large amounts of IFN-α (17, 22, 66). The mechanisms behind the altered pDC biology have not been elucidated, but it seems unlikely that any loss of pDC is a direct effect of viral replication, because in RM infected with attenuated SHIV, pDC activity declines despite low viral loads and transient immune activation.
A critical role for pDC in explaining the reduced immune activation and lack of disease progression in African primates naturally infected with SIV (30, 56–58, 78) was proposed by Mandl et al. after finding that, compared to the pDC of RM, the pDC of sooty mangabeys (SM) produce markedly less IFN-α in response to SIV and other Toll-like receptor 7 and 9 ligands in vitro (47). More recently it was reported that the pDC of African green monkeys (AGM) rapidly produce IFN-α in vitro (20, 35), and thus an inherent inability of pDC to produce IFN-α does not explain the lack of immune activation in African primates. In fact, acute SIV infection in either natural or aberrant macaque hosts of SIV is associated with robust innate immune responses and increased expression of IFN-α and interferon-stimulated genes and other proinflammatory mediators (1, 5, 12, 35). However, SM and AGM rapidly suppress these innate immune responses while RM do not, leading to chronic immune activation (1, 5, 12, 35). Thus, active immunoregulatory mechanisms efficiently downregulate the immune activation that occurs soon after SIV infection of the natural host.
Although SHIV immunization had no effect on the number of pDC in the genital tract (Fig. 7), suppressed pDC activity may represent an active immunoregulatory response induced by SHIV immunization that contributes to the altered pattern of T cell activation and innate immune responses after SIV challenge, thus contributing to the ability of a modest antiviral CD8+ T cell response to control infection at the portal of entry.
SHIV immunization decreased the number of IDO+ cells in the vagina of immunized RM, providing additional evidence for altered immune activation. IDO-mediated tryptophan degradation by DC results in inhibition of T-cell proliferation, increased T-cell apoptosis, and de novo formation of Tregs (reviewed in reference 19). Macrophages and DC can up- and downregulate IDO expression in response to external stimuli (32, 53, 54), with proinflammatory signals inducing IDO expression in DC (reviewed in reference 19). Thus, the lower number of IDO+ cells in the vagina of SHIV-immunized RM than in normal RM is consistent with reduced levels of immune activation in the SHIV-immunized RM. On the other hand, the increased number of IDO+ cells in tissues of unimmunized RM after SIV challenge likely reflects generalized immune activation.
In addition to the changes noted above in SHIV-immunized RM prior to SIV challenge, after SIV challenge, CD4+ FoxP3+ Tregs in the vaginal mucosa expanded by >3-fold at day 3 PC (Fig. 5A). By day 14 PC, the levels of proinflammatory cytokines and IDO+ cells were much lower in SHIV-immunized RM than in unimmunized RM, indicating that the immunized RM had avoided or resolved their innate responses to challenge. The rapid expansion, conversion, or recruitment of Tregs into the vaginal mucosa after SIV challenge (Fig. 5A) is similar to the rapid expansion of Tregs after SIV infection of AGM (39), Friend leukemia virus infection of mice (34, 79), and West Nile virus infection of mice and humans (40). In all these viral infections, high levels of peripheral Tregs develop soon after infection, and these Tregs protect the immunocompetent host against severe immune-mediated disease. Further, people who have been exposed to HIV but resist infection have relatively low levels of immune activation, reduced frequencies of CD69+ T cells, and elevated frequencies of Tregs compared with those of HIV-unexposed controls (15), suggesting that Tregs may contribute to HIV resistance by controlling levels of T cell activation and minimizing the pool of CD4+ T cells susceptible to infection (15). The expanded vaginal Treg population in SHIV-immunized RM after SIV challenge suggests that there was priming of Treg responses and local expansion of Tregs after SIV challenge, but these cells did not eliminate the antiviral immune responses in the vagina of SHIV-immunized RM (27). Thus, the rapid expansion of Treg in the vagina may have contributed to the lack of inflammation and limited T cell activation after vaginal SIV challenge. By suppressing immune activation and preventing the generation of more activated target cells to support SIV replication, this rapid, local Treg response may contribute to a relatively quiescent tissue environment in immunized RM postchallenge.
In HIV-infected people, both the level of general immune activation and the specific nature of T cell activation clearly affect the progression to AIDS. HIV-infected individuals with large numbers of CD8+ HLA-DR+ CD38− T cells have a relatively good prognosis, while those with large numbers of CD8+ HLA-DR+ CD38+ T cells have more rapid CD4+ T cell declines and disease progression (29). In circulating T cells, CD38 is associated with activation, proliferation, and cytokine secretion in mature lymphocytes (44). It has been proposed that increased CD38 expression on T cells in HIV/SIV infection reflects aberrant, cytokine-driven immune activation in response to chronic high viral loads (60). We found that SHIV-immunized RM had a significantly lower level of this type of T cell activation than naive control RM. Thus, 6 months after SHIV immunization and before SIV challenge, the frequency of CD8+ CD38+T cells was lower and the frequency of CD8+ CD38− HLA-DR+ T cells was higher than prior to immunization (Fig. 2). The reduced expression of CD38 on CD8+ T cells in SHIV-infected RM may be a consequence of the reduced ability of pDC from SHIV-immunized RM to produce IFN-α in response to viral infection, because CD38 expression on T cells is enhanced by IFN-α secreted by pDC (9, 23, 47, 50).
Although it was previously reported that a key feature of vaginal SIV transmission is an influx of pDC and CD4+ T cells into the endocervix after vaginal inoculation (41), we could not confirm that finding in the present study. We did not find any differences in the frequency of pDC lining the endocervical epithelia among the naïve control animals or SHIV-immunized animals necropsied 3 days after SIV challenge (Fig. 7). In fact, among normal SIV-naïve animals, SHIV-immunized animals, and SIV control animals, the number and location of pDC in these tissues was highly variable and independent of virus exposure status (Fig. 7). Neutrophils were also often present in these tissues, a set of features that is consistent with ongoing inflammation in the cervix of these animals. Because the mixed immune cell infiltrate was found in the cervix of SIV-naive animals as well as SIV-exposed animals, pDC are likely to be a component of an inflammatory process that was present at the time of inoculation.
Although we did not attempt to determine the cause of the cervicitis found in some animals, the vaginal flora of captive rhesus and pigtail macaques is unique to each animal, and all animals are colonized with a large variety of bacterial vaginosis-type organisms (68, 69). In women, bacterial vaginosis is a source of genital inflammation that is associated with HIV acquisition (6, 65, 71–73). We recently found that in normal female RM at the CNPRC, there is a polymicrobial flora with TNF and IL-1 mRNA levels in cervicovaginal secretions that vary by up to 100-fold between animals (C. J. Miller and G. T. Spear, unpublished data). It seems likely that the unique polymicrobial vaginal flora in each animal contributes to the levels of inflammatory cytokine mRNAs in cervicovaginal secretions and the variability in the number of endocervical pDC in RM.
The generally accepted goal of conventional vaccines is to elicit the strongest possible B and T cell responses so that, upon exposure to the pathogen, strong antiviral effector mechanisms are in place and a rapid expansion of pathogen-specific memory lymphocytes occurs to amplify these antiviral responses. However, we have shown that a live-attenuated lentivirus vaccine that elicits a modest effector CD8+ T cell response protects rhesus monkeys from infection or uncontrolled viral replication after vaginal SIV challenge (24, 27). It is probably critical that this mucosal antiviral CD8+ T cell response occurs in a setting of minimal immune activation that is actively maintained by a population of local Tregs, as recently reported (36). By limiting the expansion of activated CD4+ viral target cells and antiviral CD8+ T cells immediately after vaginal SIV challenge, the mucosal immunoregulatory T cell population and the anti-inflammatory environment in the tissues of immunized RM likely contribute to the ability of the antiviral CD8+ T cell response to maintain control of SIV replication in SHIV-immunized RM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Murray Gardner for thoughtful review of the manuscript, the Primate Services Unit at the CNPRC, and Katie Lantz, Ding Lu, Lili Guo, Tracy Rourke, and Veronique de Silva for excellent technical assistance.
This work was supported by NIH grants RR00169, AI066314, and AI44480 and a gift from the James B. Pendleton Charitable Trust.
Footnotes
Published ahead of print 13 June 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abel K, et al. 2001. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 16:191–204 [DOI] [PubMed] [Google Scholar]
- 3. Abel K, et al. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 79:12164–12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abel K, et al. 2005. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clin. Diagn. Lab. Immunol. 12:606–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. 2005. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 280:19843–19851 [DOI] [PubMed] [Google Scholar]
- 8. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253 [DOI] [PubMed] [Google Scholar]
- 9. Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. 2008. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One 3:e2961 doi:10.1371/journal.pone.0002961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boasso A, et al. 2007. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 109:3351–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boasso A, et al. 2009. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J. Immunol. 182:4313–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosinger SE, et al. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119:3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown KN, Trichel A, Barratt-Boyes SM. 2007. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J. Immunol. 178:6958–6967 [DOI] [PubMed] [Google Scholar]
- 14. Capuron L, et al. 2003. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol. Psychiatry 54:906–914 [DOI] [PubMed] [Google Scholar]
- 15. Card CM, et al. 2009. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J. Infect. Dis. 199:1318–1322 [DOI] [PubMed] [Google Scholar]
- 16. Cecchinato V, et al. 2008. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J. Immunol. 180:5439–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cella M, et al. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type 1 interferon. Nat. Med. 5:919–923 [DOI] [PubMed] [Google Scholar]
- 18. Chung DJ, et al. 2009. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. 2009. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood 113:2394–2401 [DOI] [PubMed] [Google Scholar]
- 20. Diop OM, et al. 2008. Plasmacytoid dendritic cell dynamics and alpha interferon production during simian immunodeficiency virus infection with a nonpathogenic outcome. J. Virol. 82:5145–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Favre D, et al. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5:e1000295 doi:10.1371/journal.ppat.1000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feldman S, et al. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201–210 [DOI] [PubMed] [Google Scholar]
- 23. Funderburg N, et al. 2008. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One 3:e1915 doi:10.1371/journal.pone.0001915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Genesca M, McChesney MB, Miller CJ. 2009. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J. Intern. Med. 265:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genescà M, et al. 2007. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J. Immunol. 179:4732–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Genescà M, et al. 2008. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genesca M, et al. 2008. With minimal systemic T-cell expansion, CD8+ T cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus. J. Virol. 82:11181–11196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giavedoni LD, Velasquillo MC, Parodi LM, Hubbard GB, Hodara VL. 2000. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J. Virol. 74:1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giorgi JV, et al. 1994. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J. Infect. Dis. 170:775–781 [DOI] [PubMed] [Google Scholar]
- 30. Gordon SN, et al. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 179:3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grassi F, et al. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13:759–766 [DOI] [PubMed] [Google Scholar]
- 32. Grohmann U, et al. 2001. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J. Immunol. 167:708–714 [DOI] [PubMed] [Google Scholar]
- 33. Hartigan-O'Connor DJ, Abel K, McCune JM. 2007. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J. Exp. Med. 204:2679–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwashiro M, et al. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. U. S. A. 98:9226–9230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacquelin B, et al. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119:3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kastenmuller W, et al. 2011. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol. 187:3186–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khatissian E, et al. 1996. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res. Hum. Retroviruses 12:1273–1278 [DOI] [PubMed] [Google Scholar]
- 38. Koff WC, et al. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 7:19–23 [DOI] [PubMed] [Google Scholar]
- 39. Kornfeld C, et al. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 115:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lanteri MC, et al. 2009. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Invest. 119:3266–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Q, et al. 2009. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323:1726–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez C, Fitzgerald PA, Siegal FP. 1983. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J. Infect. Dis. 148:962–966 [DOI] [PubMed] [Google Scholar]
- 43. Ma Z, Lu FX, Torten M, Miller CJ. 2001. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin. Immunol. 100:240–249 [DOI] [PubMed] [Google Scholar]
- 44. Malavasi F, et al. 2006. CD38 and CD157 as receptors of the immune system: a bridge between innate and adaptive immunity. Mol. Med. 12:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malleret B, et al. 2008. Effect of SIVmac infection on plasmacytoid and CD1c+ myeloid dendritic cells in cynomolgus macaques. Immunology 124:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manches O, et al. 2008. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118:3431–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandl JN, et al. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077–1087 [DOI] [PubMed] [Google Scholar]
- 48. McCloskey TW, et al. 1997. Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children, and adults. Clin. Immunol. Immunopathol. 84:46–55 [DOI] [PubMed] [Google Scholar]
- 49. McElrath MJ, et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meier A, et al. 2007. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 81:8180–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mellor AL, Munn DH. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762–774 [DOI] [PubMed] [Google Scholar]
- 52. Miller CJ, et al. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Munn DH, et al. 2004. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 114:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orabona C, et al. 2006. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood 107:2846–2854 [DOI] [PubMed] [Google Scholar]
- 55. Pacanowski J, et al. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016–3021 [DOI] [PubMed] [Google Scholar]
- 56. Pandrea I, et al. 2006. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 80:4858–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pandrea I, et al. 2008. Cutting edge: experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 181:6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pandrea IV, et al. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pereira LE, et al. 2007. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 81:4445–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prince HE, Kleinman S, Czaplicki C, John J, Williams AE. 1990. Interrelationships between serologic markers of immune activation and T lymphocyte subsets in HIV infection. J. Acquir. Immune Defic. Syndr. 3:525–530 [PubMed] [Google Scholar]
- 61. Reeves RK, Fultz PN. 2007. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology 365:356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rerks-Ngarm S, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 63. Ruprecht RM. 1999. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol. Rev. 170:135–149 [DOI] [PubMed] [Google Scholar]
- 64. Saez-Cirion A, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sewankambo N, et al. 1997. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546–550 [DOI] [PubMed] [Google Scholar]
- 66. Siegal FP, et al. 1999. The nature of the principal type-1 interferon-producing cells in human blood. Science 284:1835–1837 [DOI] [PubMed] [Google Scholar]
- 67. Soumelis V, et al. 2001. Depletion of circulating natural type I interferon-producing cells in HIV-1 infected patients. Blood 98:906–912 [DOI] [PubMed] [Google Scholar]
- 68. Spear GT, et al. 2010. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retroviruses 26:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spear GT, et al. 3 May 2012. Longitudinal assessment of pigtailed macaque lower genital tract microbiota by pyrosequencing reveals dissimilarity to the genital microbiota of healthy humans. AIDS Res. Hum. Retroviruses. doi:10.1089/aid.2011.0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stone M, et al. 2009. Limited dissemination of pathogenic SIV after vaginal challenge of rhesus monkeys immunized with a live, attenuated lentivirus. Virology 392:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ. 2000. High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J. Infect. Dis. 182:467–473 [DOI] [PubMed] [Google Scholar]
- 72. Taha TE, et al. 1998. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699–1706 [DOI] [PubMed] [Google Scholar]
- 73. Thurman AR, Doncel GF. 2010. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am. J. Reprod. Immunol. 65:89–98 [DOI] [PubMed] [Google Scholar]
- 74. Toscano MA, et al. 2007. Dissecting the pathophysiologic role of endogenous lectins: glycan-binding proteins with cytokine-like activity? Cytokine Growth Factor Rev. 18:57–71 [DOI] [PubMed] [Google Scholar]
- 75. Varki A, Angata T. 2006. Siglecs—the major subfamily of I-type lectins. Glycobiology 16:1R–27R [DOI] [PubMed] [Google Scholar]
- 76. Wang Y, et al. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79:14355–14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yuan JS, Reed A, Chen F, Stewart CN., Jr 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zahn RC, et al. 2008. Simian immunodeficiency virus (SIV)-specific CD8+ T-cell responses in vervet African green monkeys chronically infected with SIVagm. J. Virol. 82:11577–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zelinskyy G, et al. 2009. The regulatory T cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T cell response. Blood 114:3199–3207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.