Abstract
Recently, it has been demonstrated that disease progression during HIV infection is not determined merely by the number of HIV-specific T cells but also by their quality (J. R. Almeida, et al., J. Exp. Med. 204:2473–2485, 2007; C. T. Berger, et al., J. Virol. 85:9334–9345, 2011; M. R. Betts, et al., Blood 107:4781–4789, 2006; V. V. Ganusov, et al., J. Virol. 85:10518–10528, 2011; P. Kiepiela, et al., Nat. Med. 13:46–53, 2007; and F. Pereyra, et al., J. Infect. Dis. 197:563–571, 2008). Therefore, strategies to specifically enhance or induce high-quality, HIV-specific T-cell responses are necessary to develop effective immune therapies. Thalidomide, lenalidomide, and pomalidomide have a strong capacity to boost immune responses and are therefore referred to as immunomodulatory drugs (IMiDs). We evaluated the effects of lenalidomide and pomalidomide on HIV-specific T cells. We found that the presence of IMiDs during in vitro T-cell stimulation with dendritic cells electroporated with Gag- or Nef-encoding mRNA resulted in higher numbers of cytokine-secreting HIV-specific CD8+ T cells, particularly inducing polyfunctional HIV-specific CD8+ T cells with an enhanced lytic capacity. Furthermore, CD8+ T-cell responses were detected upon stimulation with lower antigenic peptide concentrations, and a higher number of Gag epitopes was recognized upon addition of IMiDs. Finally, IMiDs reduced the proliferation of the HIV-specific CD4+ T cells while increasing the number of polyfunctional CD4+ T cells. These results provide new information about the effects of IMiDs on antigen-specific T cells and suggest that these drugs increase the efficacy of immune therapies for infectious diseases and cancer.
INTRODUCTION
The host immune responses greatly influence the clinical course of an HIV infection. In this regard, the presence and, more importantly, the quality of HIV-specific T-cell responses may determine the degree of disease progression (3, 9, 43). Since long-term administration of combination antiretroviral therapy (cART) is associated with several limitations, attempts have been made to manipulate the immune system in such a way that HIV infection could be prevented or controlled in the absence of cART (12). Although several studies have shown that HIV-specific immune responses can be enhanced by therapeutic vaccinations, such as dendritic cell (DC)-based vaccines (reviewed in reference 25), these interventions lacked sustained clinical responses. Therefore, new therapeutic strategies that specifically enhance or induce high-quality HIV-specific CD8+ T-cell responses are needed to design more effective immune therapies.
Thalidomide (α-N-phthalimido-glutarimide) was developed in 1954 as a sedative agent during pregnancy but was removed from the market in the 1960s due to its teratogenic characteristics. Afterwards, the drug was revived as an effective therapy for a number of disorders, since it was shown to inhibit tumor necrosis factor alpha (TNF-α) production by human monocytes (46) and was found to have antiangiogenic effects (17). More recently, it has been demonstrated that thalidomide has immunomodulatory properties, resulting in T-cell costimulation (35) and natural killer (NK) cell and NK T-cell activation (13, 19). Accordingly, thalidomide has been successfully used to treat immune-mediated diseases, such as erythema nodosum leprosum (36) and inflammatory bowel diseases (5). Currently, its most frequent clinical application is in the treatment of multiple myeloma, generally in combination with dexamethasone (reviewed in reference 44).
Given the potency of thalidomide in the treatment of several diseases and the fact that important side effects are associated with its use, a quest for more potent thalidomide analogues with less toxicity was initiated and resulted in the development of the immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide. Interestingly, these IMiDs not only are up to 50,000-fold more potent in inhibiting TNF-α production in vitro (16) but are also characterized by a stronger T-cell costimulatory capacity (15), which is an interesting feature in the search for more effective HIV immunotherapies. Indeed, Haslett et al. previously showed that lenalidomide is a potent costimulator of virus-specific CD8+ T cells (27).
DCs are powerful stimulators of HIV-specific T cells in vitro (2, 50) and are currently being tested as a therapeutic HIV vaccine in several clinical trials (25). Although HIV-specific immune responses are clearly enhanced after vaccination with DCs (1, 23, 24, 37, 45), the clinical responses induced by DC-based vaccines are generally disappointing (1, 23). We investigated the effects of low doses of lenalidomide and pomalidomide on HIV-specific T cells stimulated by DCs in vitro. We evaluated the effects of these IMiDs on CD4+ and CD8+ T-cell proliferation, cytokine production, and degree of (poly)functionality. Furthermore, we analyzed the influence of IMiDs on the breadth of the CD8+ T-cell responses.
MATERIALS AND METHODS
Study subjects.
HIV-1-infected patients were recruited from the Infectious Diseases Department of the Universitair Ziekenhuis Brussel (UZ Brussel, Brussels, Belgium). Approval for this study was obtained from the institutional review board, and informed consent was provided according to the Declaration of Helsinki. Patient selection criteria were the following: HIV-1 seropositivity as determined by Western blotting, stable cART regimen with a CD4+ T-cell count of ≥500/mm3 for at least 3 months, and plasma viral load (pVL) of <50 RNA copies/ml for at least 6 months. Eleven patients were included in this study. These patients were on cART for a median of 48 months (range, 12 to 168 months).
IMiDs.
Lenalidomide (IMiD3; CC-5013) and pomalidomide (IMiD1; CC-4047) were obtained from Selleck Chemicals LLC (Houston, TX), dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO) at a concentration of 80 mM, and stored in aliquots at −20°C.
mRNA constructs.
The mRNA constructs carrying pGEM-sig-Nef-DC-LAMP and pST1-sig-Gag-DC-LAMP were described previously (1). The pGEM-sig-MelanA-DC-LAMP mRNA construct was used as the control mRNA and was described earlier (49).
Synthetic peptides.
HIV-1 subtype B consensus Gag and Nef 15-mer peptides overlapping by 11 amino acids were obtained from the NIH AIDS Research & Reference Reagent Program (Rockville, MD). The peptides were dissolved in DMSO, further diluted in 10 mM acetic acid, and stored at a concentration of 2 mg/ml at −20°C. Gag peptides were pooled (12 pools of 10 to 13 peptides) and used at a concentration of 2 μg/ml.
T-cell stimulations.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll-Hypaque gradient centrifugation (Axis-Shield, Oslo, Norway), after which they were frozen in heat-inactivated human AB serum (PAA Laboratories, Linz, Austria) supplemented with 10% DMSO and stored in liquid nitrogen. PBMCs were thawed and rested overnight in Iscove's modified Dulbecco's medium (IMDM; Cambrex, Verviers, Belgium) supplemented with 10% heat-inactivated human AB serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 0.24 mM l-asparagine, and 0.55 mM l-arginine (all from Cambrex), here referred to as the lymphocyte medium, in the presence of 25 U/ml interleukin-2 (IL-2) (Chiron, Emeryville, CA) and 10 U/ml DNase I (Sigma-Aldrich). The next day, purified CD4+ and CD8+ T cells were obtained using CD4- and CD8-coated MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively. T-cell stimulations were performed in lymphocyte medium at a concentration of 2 million T cells/ml. One hour before they were cocultured with DCs, the T cells were pretreated with the IMiDs or an equal volume of lymphocyte medium (no-IMiD cultures). Afterwards, DCs electroporated with mRNA encoding either Gag or Nef were added at a DC-to-T-cell ratio of 1:10. Every 48 h, IMiDs or an equal volume of lymphocyte medium (no-IMiD cultures) were added to the T-cell stimulations.
DC generation, maturation, and antigen loading.
To generate DCs, PBMCs were seeded in T-175 (175-cm2) tissue culture flasks (Falcon, Becton, Dickinson [BD], San Jose, CA) in X-vivo 15 medium (Cambrex) supplemented with 1% heat-inactivated human AB serum, here referred to as DC medium, and incubated for 2 h at 37°C to allow plastic adherence. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS; Cambrex) and cryopreserved until further use as a T-cell source. Adherent cells were cultured in DC medium, which was supplemented with 1,000 IU/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and 500 IU/ml IL-4 (both produced in-house) on days 0, 2, and 4 of DC generation.
On day 5, the DCs were matured by adding a mixture of inflammatory cytokines containing 100 IU/ml IL-1β, 1,000 U/ml IL-6 (both produced in house), 100 U/ml TNF-α (Bachem, Bubendorf, Switzerland), 1 μg/ml PGE2 (Pfizer, Vienna, Austria), 1,000 IU/ml GM-CSF, and 500 IU/ml IL-4.
On day 6 the mature DCs were harvested, after which they were electroporated with mRNA encoding Gag-DC-LAMP or Nef-DC-LAMP, as described earlier (40). Alternatively, mature DCs were loaded with Gag or Nef peptide (pools) for 2 h in serum-free medium.
Flow cytometry.
All flow cytometry analyses were performed on an LSR Fortessa flow cytometer (BD Biosciences). Automatic compensation was performed using CompBeads (BD Biosciences).
Proliferation assays.
For proliferation assays, T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) as described previously (20). Following a 6-day stimulation with DCs electroporated with antigen-encoding mRNA, the T cells were harvested and stained with CD3 V450 (BD Biosciences), CD4 peridinin chlorophyll protein (PerCP)-Cy5.5 (eBioscience, San Diego, CA), and CD8 allophycocyanin (APC)-Cy7 (BD Biosciences) before being analyzed for antigen-specific T-cell proliferation. Aspecific T-cell proliferation, measured after coculture with mock-electroporated DCs, was considered background. To test cytokine production by proliferating T cells, the CellTrace Violet cell proliferation kit (Invitrogen, Paisley, United Kingdom) was used. T cells were resuspended at 1 million cells/ml in PBS and were incubated with 0.5 μM CellTrace Violet for 20 min at 37°C. Afterwards, the cells were washed thoroughly with lymphocyte medium and stimulated with DCs electroporated with antigen-encoding mRNA. On day 7, the T cells were harvested and cocultured with DCs that had been electroporated, in the absence of IMiDs, with mRNA encoding either the relevant antigen (Gag or Nef) or the control antigen MelanA. After 3 h, GolgiPlug (BD Biosciences) was added to the cocultures. The following day, the T cells were harvested and stained with CD4 PerCP-Cy5.5 and CD8 APC-Cy7. Intracellular cytokine and chemokine staining was performed as described below. Aspecific T-cell cytokine production, measured upon screening with DCs electroporated with mRNA encoding MelanA-DC-LAMP, was considered background.
Intracellular staining.
After 10 days of stimulation with DCs, T cells were harvested and cocultured with DCs electroporated, in the absence of IMiDs, with mRNA encoding either the relevant antigen (Gag or Nef) or the control antigen MelanA. For some assays, a CD107a antibody was added to the cocultures (Brilliant Violet 421 anti-human CD107a; clone H4A3; BioLegend, San Diego, CA) in the presence of monensin (GolgiStop; BD Biosciences). After 3 h, brefeldin (GolgiPlug; BD Biosciences) was added to the cocultures. The following day, the T cells were harvested and stained using the following antibodies: CD3 V450 or CD3 eFluor 605NC (eBioscience), CD4 PerCP-Cy5.5, and/or CD8 APC-Cy7. Fixation and permeabilization of the cells was performed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Intracellular stainings were performed using the following antibodies: gamma interferon (IFN-γ) phycoerythrin (PE; clone 45.B3; eBioscience), TNF-α fluorescein isothiocyanate (FITC; clone MAb11; eBioscience), IL-2 APC (clone MQ1-17H12; eBioscience), macrophage inflammatory protein 1β (MIP-1β) PE-Cy7 (clone D21-1351; BD Pharmingen), and/or perforin FITC (clone B-D48; Abcam, Cambridge, United Kingdom). Aspecific T-cell responses, measured upon screening with DCs electroporated with mRNA encoding MelanA-DC-LAMP, were considered background.
IFN-γ ELISA.
After 10 days of stimulation with DCs, T cells were harvested. Ten thousand CD8+ T cells were cocultured with 20,000 peptide-pulsed DCs in round-bottom, 96-well plates in the absence of IMiDs. Each condition was performed in triplicate. After 16 h, culture supernatants were collected and analyzed for IFN-γ production by enzyme-linked immunosorbent assay (ELISA; Endogen; PerBio Science, Helsingborg, Sweden) according to the manufacturer's instructions. T-cell responses were considered positive when the IFN-γ production exceeded 50 pg/ml. Aspecific IFN-γ production by the CD8+ T cells, measured upon coculture with DCs that were not pulsed with peptide, was considered background.
Data analysis.
Flow cytometry data were analyzed using FACS DIVA (BD Biosciences) and FlowJo (Tree Star Inc.) software. In addition, SPICE version 5.2 software (provided by Mario Roederer, National Institute of Allergy and Infectious Diseases, Bethesda, MD) was used to analyze polyfunctional T-cell responses. Unless the background is shown, data were corrected for the background by subtraction of the responses measured under the negative-control conditions (screening with mock-electroporated DCs in the case of proliferation assays, screening with MelanA-electroporated DCs in the case of intracellular cytokine stainings, and screening with DCs that were not pulsed with peptide in the case of ELISA). Statistical analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA). The results shown in Fig. 1A were analyzed by a two-way analysis of variance (ANOVA). The results shown in Fig. 1B, C, and F, 2B, 5A, B, and D, and 6C and D were analyzed by repeated-measure ANOVA. Post hoc Bonferroni tests were performed to compare individual groups. A chi-square test was used to compare the number of recognized pools between different conditions shown in Fig. 3.
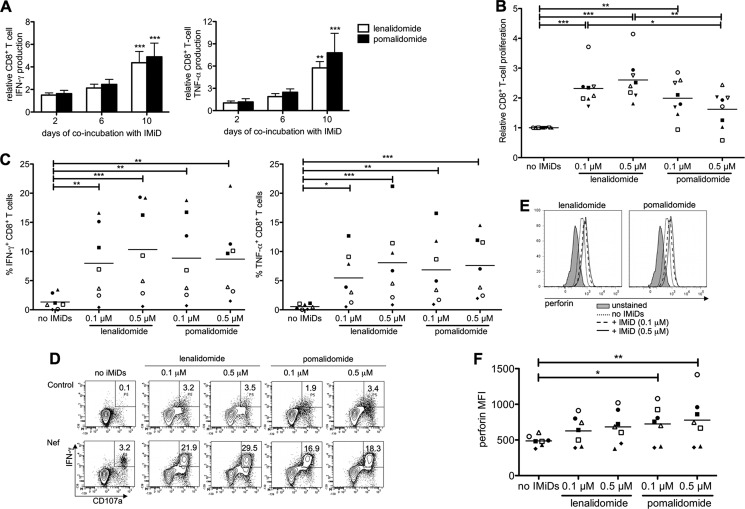
Fig 1.
IMiDs enhance the magnitude of HIV-specific CD8+ T-cell responses. Purified CD8+ T cells were stimulated with DCs electroporated with mRNA encoding Gag or Nef. (A) T cells were stimulated in the presence of IMiDs (0.5 μM) for 2, 6, or 10 days before the IMiDs were removed by washing the cells. On day 10, the CD8+ T cells were cocultured overnight with DCs and cytokine secretion was determined by intracellular staining. The bar graphs show the percentages of IFN-γ+ (left) or TNF-α+ (right) CD8+ T cells relative to the no-IMiD condition (means from 5 experiments). The error bars show the standard errors of the means (SEM). T-cell responses that differed significantly from those under the no-IMiD condition are indicated. (B) T cells were stimulated in the presence of IMiDs (0.1 to 0.5 μM) for 6 days, after which CD8+ T-cell proliferation was measured by CFSE dilution assays. CD8+ T-cell proliferation, relative to that under the no-IMiD condition, is depicted. (C to F) After 10 days of stimulation, CD8+ T cells were cocultured overnight with DCs electroporated with Gag or Nef, after which antigen-specific T-cell responses were analyzed. (C) The overview graphs show the percentages of IFN-γ (left)- and TNF-α (right)-producing CD8+ T cells. (D) Flow cytometry plots showing the coexpression of IFN-γ and CD107a on HIV-specific CD8+ T cells. One representative out of 7 experiments is shown. (E) Histogram overlays showing the perforin expression in CD107a+ CD8+ T cells. One representative out of 7 experiments is shown. (F) Overview graphs showing the perforin MFI in CD107a+ CD8+ T cells. On the overview graphs shown in panels B, C, and F, each symbol represents one experiment. Equal shapes indicate that the Gag-specific (filled symbols) or the Nef-specific (open symbols) CD8+ T-cell responses of the same patient were analyzed. Horizontal lines indicate the means from all experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
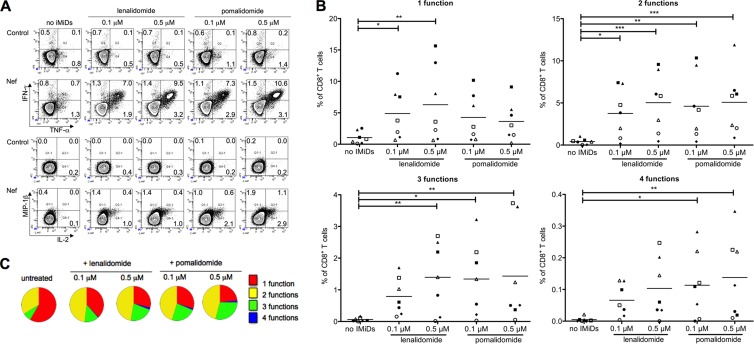
Fig 2.
IMiDs induce polyfunctional HIV-specific CD8+ T cells. Purified CD8+ T cells were stimulated with DCs electroporated with mRNA encoding Gag or Nef. After 10 days of stimulation, the cells were cocultured with DCs electroporated with Gag or Nef to measure antigen-specific cytokine and chemokine production by intracellular stainings. (A) Flow cytometry plots showing the (co)expression of IFN-γ, TNF-α, IL-2, and MIP-1β by CD8+ T cells. (B) Overview graphs showing the percentages of CD8+ T cells producing 1, 2, 3, or 4 cytokines/chemokines. Each symbol represents one experiment. Equal shapes indicate that the Gag-specific (filled symbols) or the Nef-specific (open symbols) CD8+ T-cell responses of the same patient were analyzed. Horizontal lines indicate the means from all experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Pie charts showing the proportion of CD8+ T cells displaying 1 to 4 functions. In panels A and C, one representative example out of 7 experiments is shown.
Fig 5.
IMiDs abrogate the proliferation but not the cytokine production of HIV-specific CD4+ T cells. Purified CD4+ T cells were stimulated with DCs electroporated with mRNA encoding Gag or Nef. (A) After 6 days of stimulation, CD4+ T-cell proliferation was measured by CFSE dilution assays. The CD4+ T-cell proliferation, relative to the no-IMiD condition, is depicted. (B to E) After 10 days of stimulation, the cells were cocultured overnight with DCs electroporated with Gag- or Nef-encoding mRNA to measure antigen-specific cytokine production. (B) The overview graphs show the percentages of IFN-γ (left)- and TNF-α (right)-producing, antigen-specific CD4+ T cells. (C) Flow cytometry plots showing the (co)expression of IFN-γ, TNF-α, IL-2, and MIP-1β by CD4+ T cells. (D) Overview graphs showing the percentages of CD4+ T cells producing 1, 2, 3, or 4 cytokines/chemokines. (E) Pie charts showing the proportion of the total CD4+ T cells displaying 1 to 4 functions. On the overview graphs shown in panels A, B, and D, each symbol represents one experiment. Identical shapes indicate that the Gag-specific (filled symbols) or the Nef-specific (open symbols) CD4+ T-cell responses of the same patient were analyzed. Horizontal lines indicate the means from all experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. In panels C and E, one representative out of 6 experiments is shown.
Fig 6.
Relationship between the effects of IMiDs on HIV-specific T-cell proliferation and cytokine production. Purified CD8+ (A) and CD4+ (B) T cells were labeled with CellTrace Violet and stimulated with DCs electroporated with antigen-encoding mRNA. On day 7, the T cells were cocultured overnight with DCs electroporated with Gag- or Nef-encoding mRNA before the cells were stained intracellularly to measure cytokine production. The percentages on the flow cytometry plots indicate the percentages of cytokine-producing (IFN-γ+, TNF-α+, or IL-2+) cells within the proliferating (CellTrace Violetlow) CD4+ or CD8+ T-cell population. In panel A, one representative out of 5 experiments is shown, whereas one representative out of 6 experiments is shown in panel B. (C and D) IFN-γ (left), TNF-α (middle), and IL-2 (right) MFI of cytokine+ CellTrace Violetlow CD8+ (C) and CD4+ (D) T cells. Each symbol represents one experiment. Identical shapes indicate that the Gag-specific (filled symbols) or the Nef-specific (open symbols) T-cell responses of the same patient were analyzed. Horizontal lines indicate the means from all experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
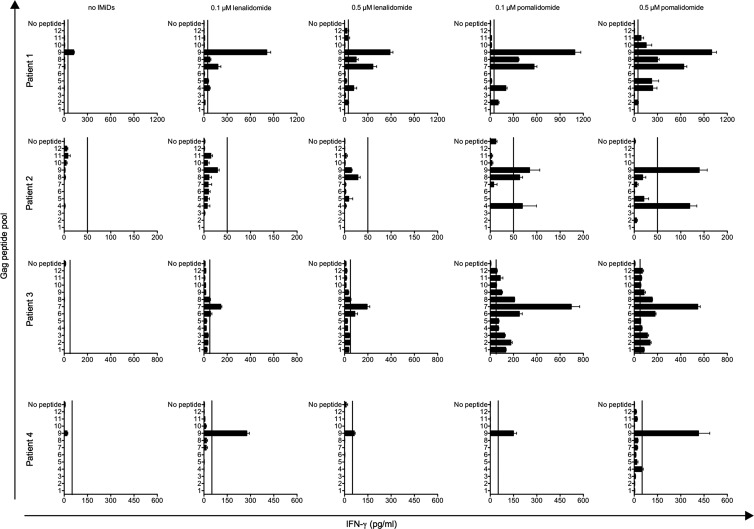
Fig 3.
Breadth of HIV-specific CD8+ T-cell responses is increased upon stimulation in the presence of IMiDs. Purified CD8+ T cells were stimulated with Gag-electroporated DCs. After 10 days of stimulation, the cells were cocultured with DCs pulsed with Gag peptide pools. IFN-γ secretion in the culture supernatants was detected by ELISA. Error bars indicate the SEM of 3 replicate wells. Vertical lines on the graphs indicate IFN-γ production of 50 pg/ml (the cutoff for positivity).
RESULTS
Immunomodulatory drugs enhance the magnitude of HIV-specific CD8+ T-cell responses.
We first tested the effects of IMiDs on HIV-specific T-cell responses during overnight assays. Our preliminary results showed that IMiDs did not affect T-cell cytokine production when they were present only during an overnight ex vivo stimulation with DCs (data not shown). Therefore, we tested the effect of a longer coincubation of CD8+ T cells with IMiDs during a 10-day stimulation with DCs. The presence of IMiDs at a concentration of 0.5 μM during the first 2 days of CD8+ T-cell stimulation had already resulted in enhanced IFN-γ production but did not increase TNF-α production. The presence of IMiDs during the first 6 days of stimulation resulted in higher percentages of IFN-γ+ and TNF-α+ CD8+ T cells. Cytokine production was even more enhanced when the IMiDs were present during the 10 days of stimulation (Fig. 1A). Based on these results, we decided to stimulate T cells with DCs in the presence of IMiDs for at least 6 days in all further experiments.
We tested the effect of the IMiDs on CD8+ T-cell proliferation by performing a CFSE dilution assay (Fig. 1B). The presence of lenalidomide or, to a lesser extent, pomalidomide during the stimulation of CD8+ T cells with DCs presenting either Gag- or Nef-derived epitopes resulted in enhanced CD8+ T-cell proliferation. Remarkably, low concentrations of IMiDs (0.1 μM) had already resulted in significantly increased HIV-specific CD8+ T-cell proliferation (P < 0.01) (Fig. 1B).
The functionality of the CD8+ T cells was analyzed by performing intracellular cytokine staining. Stimulation of CD8+ T cells by DCs in the presence of 0.1 μM lenalidomide or pomalidomide had already resulted in highly increased percentages of IFN-γ- and TNF-α-producing HIV-specific CD8+ T cells (Fig. 1C). Finally, we analyzed the cytotoxic potential of the HIV-specific CD8+ T cells by measuring the expression of CD107a and perforin. A higher percentage of CD107a+ IFN-γ+ CD8+ T cells was found under conditions stimulated in the presence of IMiDs (Fig. 1D). The high expression of perforin within the CD107a+ CD8+ T cells confirmed their cytotoxic potential (Fig. 1E). In addition, in the majority of the experiments, perforin expression within the CD107a+ CD8+ T cells was enhanced when the cells were stimulated in the presence of IMiDs (Fig. 1E and F).
Immunomodulatory drugs induce polyfunctional HIV-specific CD8+ T cells.
We further investigated the effect of IMiDs by determining the number of polyfunctional CD8+ T cells after antigen-specific T-cell stimulation. The CD8+ T cells were stained intracellularly to simultaneously detect IFN-γ, TNF-α, IL-2, and MIP-1β production (Fig. 2A). CD8+ T cells stimulated in the absence of IMiDs were characterized by a mono- or bifunctional phenotype, whereas the presence of lenalidomide or pomalidomide induced CD8+ T cells with a polyfunctional cytokine expression profile (simultaneous production of 3 to 4 cytokines/chemokines) (Fig. 2B and C; also see Fig. S1 in the supplemental material). Upon stimulation in the presence of IMiDs, the percentages of CD8+ T cells producing 1, 2, 3, or 4 cytokines/chemokines increased up to 6-, 12-, 24-, and 29-fold, respectively. Whereas the HIV-specific CD8+ T-cell response was generally dominated by IFN-γ-producing cells under the conditions stimulated without IMiDs, the presence of lenalidomide or pomalidomide during the stimulation resulted in increased numbers of TNF-α-, IL-2-, and MIP-1β-producing CD8+ T cells as well (see Fig. S1).
The breadth of HIV-specific CD8+ T-cell responses is increased upon stimulation in the presence of IMiDs.
To test the influence of IMiDs on the breadth of epitope recognition, we stimulated CD8+ T cells for 10 days with DCs electroporated with Gag-encoding mRNA in the presence or in the absence of lenalidomide or pomalidomide. Afterwards, the IFN-γ production by the CD8+ T cells in response to DCs pulsed with Gag peptide pools was analyzed by ELISA. The number of peptide pools recognized by the CD8+ T cells increased when the stimulation was performed in the presence of lenalidomide. Upon treatment with pomalidomide, this number was even higher: more than 6 out of 12 Gag peptide pools were recognized by the CD8+ T cells present in 2 out of 4 patients (Fig. 3).
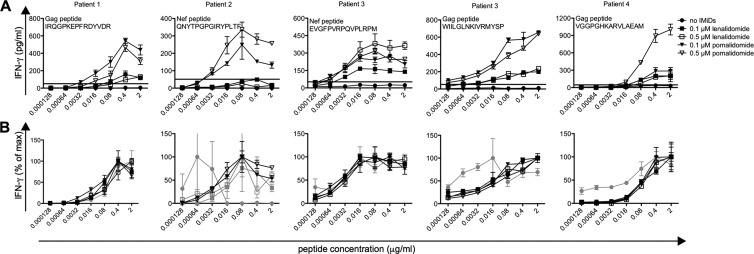
Effects of IMiDs on the antigen sensitivity of HIV-specific CD8+ T-cell responses.
We next evaluated the antigen sensitivity of the HIV-specific CD8+ T cells. Purified CD8+ T cells were stimulated for 10 days with Gag- or Nef-electroporated DCs. Afterwards, the CD8+ T cells were cocultured with DCs pulsed with increasing concentrations (ranging from 0.128 ng/ml to 2 μg/ml) of a Gag or a Nef peptide for which an antigen-specific T cell response was previously detected with an ex vivo enzyme-linked immunospot (ELISpot) assay. The IFN-γ concentration in the culture supernatants was measured by ELISA. CD8+ T cells stimulated in the presence of IMiDs responded to lower peptide concentrations. This effect was more pronounced for pomalidomide than lenalidomide (Fig. 4A). A chi-square test showed that for patients 1 and 3, a significantly increased breadth of the Gag-specific CD8+ T-cell response was observed after in vitro stimulation in the presence of pomalidomide (P = 0.0108 and <0.0001, respectively). However, the 50% effective peptide concentration was not altered in the presence of IMiDs, indicating that IMiDs do not affect the antigen avidity of HIV-specific CD8+ T cells (Fig. 4B).
Fig 4.
Effects of IMiDs on the antigen sensitivity of HIV-specific CD8+ T cells. Purified CD8+ T cells were stimulated with DCs electroporated with Gag-encoding mRNA. After 10 days of stimulation, the cells were cocultured with DCs pulsed with a 5-fold serial dilution of a Gag or a Nef peptide for which a T-cell response was previously detected by an ex vivo ELISpot. IFN-γ secretion in the culture supernatants was detected by ELISA. Error bars indicate the SEM for 3 replicate wells. The IFN-γ concentration in response to peptide-pulsed DCs is indicated on the graphs shown in panel A. Horizontal lines on the graphs indicate an IFN-γ production of 50 pg/ml (the cutoff for positivity). The percentage of the maximal IFN-γ concentration reached upon coculture with peptide-pulsed DCs is shown on the graphs in panel B. Gray curves indicate that the IFN-γ concentration remained below 50 pg/ml for all peptide concentrations. Patient numbers correspond to the patient numbers shown in Fig. 3.
IMiDs abrogate the proliferation but not the cytokine production of HIV-specific CD4+ T cells.
Based on the stimulatory effects of IMiDs on HIV-specific CD8+ T cells, we further investigated whether IMiDs had an influence on HIV-specific CD4+ T cells. Purified CD4+ T cells were stimulated with Gag- or Nef-electroporated DCs in the absence or in the presence of IMiDs, and CD4+ T-cell proliferation was determined by performing CFSE dilution assays. In contrast to the increased CD8+ T-cell proliferation observed upon in vitro treatment with either lenalidomide or pomalidomide (Fig. 1B), IMiDs significantly dampened the proliferation of HIV-specific CD4+ T cells in a dose-dependent manner (P < 0.05). This effect was more pronounced for pomalidomide than for lenalidomide (Fig. 5A). However, we observed that the cytokine secretion by HIV-specific CD4+ T cells was not reduced upon stimulation in the presence of IMiDs (Fig. 5B and C). In contrast, upon treatment with lenalidomide, there was a trend toward higher numbers of cytokine-producing HIV-specific CD4+ T cells which did not reach statistical significance (Fig. 5B). In addition, we investigated the degree of polyfunctionality of HIV-specific CD4+ T cells, which was, in analogy to the CD8+ T cells, increased upon treatment with IMiDs: the percentages of CD4+ T cells producing 1, 2, 3, or 4 cytokines/chemokines increased up to 1.3-, 1.5-, 2.4-, and 3.7-fold, respectively (Fig. 5C to E; also see Fig. S2 in the supplemental material). Interestingly, while pomalidomide did not result in an increased percentage of HIV-specific CD4+ T cells characterized by a mono- or bifunctional phenotype, treatment with pomalidomide did result in significantly higher percentages of CD4+ T cells exhibiting 3 or 4 functions (P < 0.05) (Fig. 5D). However, the effect of the IMiDs on the polyfunctionality of CD4+ T cells was less pronounced than the effects observed for the CD8+ T cells (Fig. 2; also see Fig. S1).
The relationship between the effects of IMiDs on HIV-specific T-cell proliferation and cytokine production.
To gain more insight into the different effects of IMiDs on CD4+ versus CD8+ T-cell proliferation (decreased and increased, respectively) and on the CD4+ T-cell proliferation versus cytokine secretion (decreased and slightly increased, respectively), we simultaneously measured HIV-specific T-cell cytokine production and proliferation (Fig. 6). Interestingly, when looking at individual divisions, it was clear that the CD8+ T-cell fraction that underwent the highest number of divisions (the lowest CellTrace Violet intensity) was strongly enhanced upon stimulation in the presence of IMiDs (Fig. 6A). Moreover, it was within this population that the highest level of cytokine production was observed. Nevertheless, the enhanced CD8+ T-cell cytokine production upon stimulation in the presence of IMiDs could not be attributed only to the reinforced CD8+ T-cell proliferation, since the percentages of IFN-γ-, TNF-α-, and IL-2-producing cells within the proliferating CD8+ T-cell population were all higher after stimulation with DCs in the presence of IMiDs, even within the CD8+ T-cell population that underwent the highest number of divisions (Fig. 6A). In addition, IMiDs dampened HIV-specific CD4+ T-cell proliferation, while the CD4+ T-cell cytokine production, which could mainly be assigned to the proliferating CD4+ T cells, was not affected by their coincubation with IMiDs. The percentage of cytokine-producing cells within the proliferating CD4+ T-cell population was higher after stimulation with DCs in the presence of IMiDs (Fig. 6B). Interestingly, the HIV-specific CD8+ and CD4+ T cells also produced higher concentrations of cytokines per cell when they were stimulated in the presence of IMiDs (Fig. 6C and D).
DISCUSSION
In this study, we show that the immunomodulatory drugs lenalidomide and pomalidomide costimulate HIV-specific T cells, resulting in an expansion of HIV-specific T cells endowed with several features that were previously shown to be associated with HIV control. In vitro T-cell stimulation with DCs electroporated with mRNA encoding HIV antigens in the presence of IMiDs results in higher numbers of proliferating and cytokine-producing CD8+ T cells, showing high CD107a and perforin expression. Furthermore, to our knowledge, we show for the first time that the in vitro addition of IMiDs results in the induction of polyfunctional HIV-specific CD8+ T cells, characterized by an increased breadth of epitope recognition. Although the addition of IMiDs reduced the proliferation of HIV-specific CD4+ T cells, it resulted in higher percentages of polyfunctional CD4+ T cells.
Thalidomide and its IMiD analogues were previously shown to be strong costimulators of naïve and memory T-cell responses, including virus-specific CD8+ T-cell responses (27, 35, 41). Although the mechanism whereby these agents trigger T cells is not completely understood, it has been shown that lenalidomide induces tyrosine phosphorylation of CD28 on T cells, resulting in the activation of nuclear factor κB (NF-κB) (35). Since important defects in CD28 expression and/or signaling in T cells have been described in both cancer patients and HIV-infected individuals (11, 48, 51), and given the need for new approaches to improve the potency of currently available tools in the search for an effective HIV vaccine, we decided to analyze the effects of IMiDs on HIV-specific T-cell responses. In our study, we used lenalidomide and pomalidomide concentrations of 0.1 to 0.5 μM. The maximum plasma lenalidomide concentration following a standard daily dose of 25 mg is 2.2 μM (14). Thus, the IMiD concentrations we used are relatively low, even compared to physiologically attainable concentrations, and likely are relevant for the in vivo effects of IMiDs.
In the presence of IMiDs, we observed enhanced proliferation and cytokine production by HIV-specific CD8+ T cells stimulated with dendritic cells, which is in accordance with previously published studies showing a costimulatory effect of IMiDs on antigen-specific T-cell responses (27, 35, 41). Furthermore, we found increased numbers of CD8+ T cells degranulating (CD107a+), expressing perforin, and showing a high degree of functionality (i.e., expressing ≥3 of the following factors: the cytokines IFN-γ, TNF-α, and IL-2 and the chemokine MIP-1β) upon in vitro addition of IMiDs. It was previously shown that the capacity to produce several cytokines/chemokines per antigen-specific T cell is a T-cell characteristic that is associated with HIV nonprogression (9). Therefore, the induction of so-called polyfunctional CD8+ T-cell responses is increasingly regarded as an important outcome for an effective therapeutic vaccine (4). As far as we know, a significant improvement in the number of HIV-specific CD8+ T cells that exert 3 or more functions upon in vitro T-cell stimulation, as we show in Fig. 2, has never been reported before. IL-2 production was one of the CD8+ T-cell functions that was strongly increased upon in vitro stimulation in the presence of IMiDs, which could explain the enhanced CD8+ T-cell proliferation. We also observed that T cells stimulated in the presence of IMiDs produced higher concentrations of each of the individual cytokines (IFN-γ, TNF-α, or IL-2) on a per-cell basis (Fig. 6C and D). This confirms their high degree of polyfunctionality, since it has been shown that cells making multiple cytokines simultaneously produce higher levels of each of these cytokines (18).
The stimulatory effects of IMiDs in combination with Gag- or Nef-electroporated DCs on the magnitude and the quality of HIV-specific CD8+ T-cell responses indicate that these drugs are promising candidates to enhance the efficacy of DC-based HIV vaccines. Besides inducing polyfunctional HIV-specific CD8+ T-cell responses, IMiDs resulted in a broadening of antigenic epitope recognition and detectable IFN-γ production by CD8+ T cells upon stimulation with low antigen doses (Fig. 3 and 4). The breadth of the HIV-specific T-cell response and the CD8+ T-cell antigen sensitivity are also important T-cell characteristics in the context of HIV immunotherapy (8, 31). First, the number of epitopes recognized by CD8+ T cells may play an important role in the immune control of HIV replication. Indeed, HIV often evades cytotoxic T-lymphocyte (CTL) responses by generating viral escape variants, which are then no longer recognized by the CD8+ T cells. An increased number of epitope-specific CTL responses can reduce the rate of viral escape (22). Moreover, Kiepiela et al. showed that an increased breadth of Gag-specific CD8+ T-cell responses is associated with reduced viremia (33). Second, CD8+ T-cell responses triggered by low antigenic concentrations are able to recognize cognate peptide-major histocompatibility complex class I complexes present at low densities on the surface of an infected cell in vivo. Consequently, effector functions can be triggered more readily, corresponding to a rapid and effective clearance of virus-infected cells (7, 47). Interestingly, it has been shown that T cells with high levels of antigen avidity exert more T-cell functions at a given antigen density (4). However, IMiDs did not affect the antigen avidity of CD8+ T cells. Thus, the effects we observed in Fig. 4A mainly result from the enhanced cytokine production upon stimulation in the presence of IMiDs.
It has been described that IMiDs can increase Th1-type immunity (21). Indeed, we found slightly increased numbers of IFN-γ-producing, antigen-specific CD4+ T cells upon in vitro stimulation in the presence of IMiDs. Moreover, CD4+ T cells exhibited a high degree of polyfunctionality when IMiDs were present during the antigen-specific stimulation. In contrast, proliferation of HIV-specific CD4+ T cells was inhibited by lenalidomide and pomalidomide (Fig. 5 and 6B). These results are in accordance with a previous study by Hsu et al., who demonstrated that CD4+ T cells stimulated polyclonally in the presence of lenalidomide show decreased proliferative capacity while producing significantly larger amounts of IL-2 (29). Although decreased proliferation of HIV-specific CD4+ T cells is a characteristic of HIV-infected patients with a high viral load (39), the increased functionality of the CD4+ T cells, observed upon in vitro addition of IMiDs, might compensate for their lower proliferative capacity. Moreover, a decreased CD4+ T-cell proliferation might reduce the number of potential target cells for HIV replication in vivo and therefore could slow down disease progression. Thus, the decreased proliferation but increased functionality of HIV-specific CD4+ T cells, the major target cells for HIV infection, upon addition of IMiDs might be beneficial in the context of HIV immunotherapy.
Interestingly, while both lenalidomide and pomalidomide improved HIV-specific T-cell responses to some degree, the effects of both IMiDs differed depending on the T-cell function investigated. While the effect of lenalidomide on CD8+ T-cell proliferation was higher, pomalidomide increased the number of T-cell epitopes recognized by the CD8+ T cells, the CD8+ T-cell perforin production, and the CD8+ T-cell antigen sensitivity to a higher extent and resulted in a more pronounced decrease of CD4+ T-cell proliferation. It was previously reported that, compared to thalidomide, lenalidomide is 100 to 1,000 times more potent in stimulating T-cell proliferation and IFN-γ and IL-2 production. In addition, pomalidomide enhances the expression of the transcription factor T-bet, resulting in more pronounced Th1-like effector cells in vitro (34). Further research is necessary to determine which of these T-cell characteristics, and thus which type of IMiD, would be most beneficial in the context of HIV immunotherapy.
Since we tested the effects of IMiDs on T cells stimulated with DCs, the T-cell costimulatory effects of the drugs observed in our experiments could have been influenced by their direct effects on DCs. Therefore, we evaluated the expression of several maturation markers (CD40, CD80, CD83, CD86, CD137L, CD70, PD-L1, and PD-L2) and cytokines (IL-10 and IL-12) by DCs cultured in the presence of IMiDs. We did not observe any major effect of IMiDs on the phenotype and cytokine production of cytokine cocktail-matured DCs (data not shown), which is in accordance with previously published results (27, 42). These results suggest that the enhanced HIV-specific T-cell responses upon stimulation with antigen-presenting cells in the presence of IMiDs mainly result from the direct effects of IMiDs on the T cells.
Apart from its T-cell costimulatory effects, thalidomide improves the outcome of wasting syndrome and aphthous ulceration in HIV-infected individuals (30, 32). In addition, lenalidomide was recently tested as a treatment for AIDS-related Kaposi's sarcoma and HIV-associated plasmablastic lymphoma (10, 38). Surprisingly, there are only a limited number of reports investigating the effects of thalidomide treatment on immune responses in HIV-infected patients. Thalidomide stimulates antigen-specific T-cell responses and IL-12 production in HIV-infected patients (6, 28). Furthermore, Hanekom et al. showed that the expression of CD28 and CD45RO increases upon treatment with thalidomide, and that the treatment results in higher numbers of IFN-γ-producing Gag-specific CD8+ T cells in 75% of the patients (26). A case report investigating a cART-treated HIV patient with 5q minus syndrome showed that lenalidomide treatment resulted in a modest rise of CD4+ T-cell counts concomitant with an increase in CD8+ T-cell counts. Since thalidomide treatment needs to be discontinued regularly due to adverse effects, lenalidomide and pomalidomide could be alternative treatment options, because these IMiDs are associated with a lower incidence of adverse effects. In addition, lenalidomide is not teratogenic in rabbit models (34). We show here that in addition to its application as a treatment for HIV-related pathologies, low doses of IMiDs could be a valuable addition to HIV immunotherapies currently being investigated, such as DC-based therapeutic vaccines. In this study, we used cellular material derived from patients that had an undetectable plasma viral load under stable cART. In the future, it would be very interesting to evaluate whether IMiDs restore HIV-specific CD8+ T-cell responses using exhausted T cells derived from viremic HIV-infected patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Carolien Wylock for her help with patient recruitment and Lukasz Bialkowski for technical assistance. We acknowledge Carlo Heirman, Elsy Vaeremans, and Xavier Debaere for mRNA preparation. We thank Rob Gruters (Department of Virology, Erasmus Medical Center, Rotterdam, The Netherlands) and Michel Moutschen (Department of Infectious Diseases and General Internal Medicine, University of Liège, Belgium) for their critical comments and helpful suggestions. We thank the patients who agreed to participate in this study.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 consensus B Gag and Nef (15-mer) peptides, complete sets.
This work was supported by the Institute for Science and Technology (IWT; IWT-TBM 60511), by the University Research fund (VUB; OZR1801), and by the Stichting Tegen Kanker.
S.D.A. is a Ph.D. student and J.L.A. is a postdoctoral fellow of the Fund for Scientific Research, Flanders, Belgium (FWO).
We have no competing financial interests.
Footnotes
Published ahead of print 20 June 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Allard SD, et al. 2012. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev, and Nef expressing dendritic cells followed by treatment interruption. Clin. Immunol. 142:252–268 [DOI] [PubMed] [Google Scholar]
- 2. Allard SD, et al. 2008. Functional T-cell responses generated by dendritic cells expressing the early HIV-1 proteins Tat, Rev, and Nef. Vaccine 26:3735–3741 [DOI] [PubMed] [Google Scholar]
- 3. Almeida JR, et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appay V, Douek DC, Price DA. 2008. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14:623–628 [DOI] [PubMed] [Google Scholar]
- 5. Bariol C, et al. 2002. Early studies on the safety and efficacy of thalidomide for symptomatic inflammatory bowel disease. J. Gastroenterol. Hepatol. 17:135–139 [DOI] [PubMed] [Google Scholar]
- 6. Bekker LG, Haslett P, Maartens G, Steyn L, Kaplan G. 2000. Thalidomide-induced antigen-specific immune stimulation in patients with human immunodeficiency virus type 1 and tuberculosis. J. Infect. Dis. 181:954–965 [DOI] [PubMed] [Google Scholar]
- 7. Bennett MS, Ng HL, Dagarag M, Ali A, Yang OO. 2007. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J. Virol. 81:4973–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger CT, et al. 2011. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted Gag-derived epitopes associated with relative HIV control. J. Virol. 85:9334–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bibas M, et al. 2010. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J. Clin. Oncol. 28:e704–e708doi:10.1200/JCO.2010.30.0038 [DOI] [PubMed] [Google Scholar]
- 11. Brinchmann JE, et al. 1994. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J. Infect. Dis. 169:730–738 [DOI] [PubMed] [Google Scholar]
- 12. Cafaro A, Macchia I, Maggiorella MT, Titti F, Ensoli B. 2009. Innovative approaches to develop prophylactic and therapeutic vaccines against HIV/AIDS. Adv. Exp. Med. Biol. 655:189–242 [DOI] [PubMed] [Google Scholar]
- 13. Chang DH, et al. 2006. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood 108:618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen N, et al. 2007. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J. Clin. Pharmacol. 47:1466–1475 [DOI] [PubMed] [Google Scholar]
- 15. Corral LG, et al. 1999. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 163:380–386 [PubMed] [Google Scholar]
- 16. Corral LG, et al. 1996. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol. Med. 2:506–515 [PMC free article] [PubMed] [Google Scholar]
- 17. D'Amato RJ, Loughnan MS, Flynn E, Folkman J. 1994. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 91:4082–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darrah PA, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 19. Davies FE, et al. 2001. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98:210–216 [DOI] [PubMed] [Google Scholar]
- 20. De Keersmaecker B, et al. 2011. The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J. Leukoc. Biol. 89:989–999 [DOI] [PubMed] [Google Scholar]
- 21. Dredge K, et al. 2002. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. J. Immunol. 168:4914–4919 [DOI] [PubMed] [Google Scholar]
- 22. Ganusov VV, et al. 2011. Fitness costs and diversity of the cytotoxic T lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. J. Virol. 85:10518–10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García F, et al. 2011. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 203:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García F, et al. 2005. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J. Infect. Dis. 191:1680–1685 [DOI] [PubMed] [Google Scholar]
- 25. García F, Routy JP. 2011. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection. Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine 29:6454–6463 [DOI] [PubMed] [Google Scholar]
- 26. Hanekom WA, et al. 2001. The immunomodulatory effects of thalidomide on human immunodeficiency virus-infected children. J. Infect. Dis. 184:1192–1196 [DOI] [PubMed] [Google Scholar]
- 27. Haslett PA, Hanekom WA, Muller G, Kaplan G. 2003. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J. Infect. Dis. 187:946–955 [DOI] [PubMed] [Google Scholar]
- 28. Haslett PA, et al. 1999. Thalidomide stimulates T cell responses and interleukin 12 production in HIV-infected patients. AIDS Res. Hum. Retrovir. 15:1169–1179 [DOI] [PubMed] [Google Scholar]
- 29. Hsu AK, et al. 2011. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood 117:1605–1613 [DOI] [PubMed] [Google Scholar]
- 30. Jacobson JM, et al. 1997. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N. Engl. J. Med. 336:1487–1493 [DOI] [PubMed] [Google Scholar]
- 31. Julg B, et al. 2010. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84:5540–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaplan G, et al. 2000. Thalidomide for the treatment of AIDS-associated wasting. AIDS Res. Hum. Retrovir. 16:1345–1355 [DOI] [PubMed] [Google Scholar]
- 33. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 34. Kotla V, et al. 2009. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LeBlanc R, et al. 2004. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 103:1787–1790 [DOI] [PubMed] [Google Scholar]
- 36. Levy L, Fasal P, Levan NE, Freedman RI. 1973. Treatment of erythema nodosum leprosum with thalidomide. Lancet ii:324–325 [DOI] [PubMed] [Google Scholar]
- 37. Lu W, Arraes LC, Ferreira WT, Andrieu JM. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359–1365 [DOI] [PubMed] [Google Scholar]
- 38. Martinez V, et al. 2011. Lenalidomide in treating AIDS-related Kaposi's sarcoma. AIDS 25:878–880 [DOI] [PubMed] [Google Scholar]
- 39. McNeil AC, et al. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 98:13878–13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michiels A, et al. 2005. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 12:772–782 [DOI] [PubMed] [Google Scholar]
- 41. Neuber B, et al. 2011. Lenalidomide enhances antigen-specific activity and decreases CD45RA expression of T cells from patients with multiple myeloma. J. Immunol. 187:1047–1056 [DOI] [PubMed] [Google Scholar]
- 42. Noonan K, et al. 2012. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin. Cancer Res. 18:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pereyra F, et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571 [DOI] [PubMed] [Google Scholar]
- 44. Quach H, et al. 2010. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 24:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Routy JP, et al. 2010. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin. Immunol. 134:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. 1991. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 173:699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Speiser DE, Kyburz D, Stübi U, Hengartner H, Zinkernagel RM. 1992. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J. Immunol. 149:972–980 [PubMed] [Google Scholar]
- 48. Speiser DE, et al. 1999. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur. J. Immunol. 29:1990–1999 [DOI] [PubMed] [Google Scholar]
- 49. Tuyaerts S, et al. 2003. Induction of Influenza Matrix Protein 1 and MelanA-specific T lymphocytes in vitro using mRNA-electroporated dendritic cells. Cancer Gene Ther. 10:696–706 [DOI] [PubMed] [Google Scholar]
- 50. Van Gulck ER, et al. 2006. Efficient stimulation of HIV-1-specific T cells using dendritic cells electroporated with mRNA encoding autologous HIV-1 Gag and Env proteins. Blood 107:1818–1827 [DOI] [PubMed] [Google Scholar]
- 51. Yadav A, et al. 2006. HIV-1 transgenic rat CD4+ T cells develop decreased CD28 responsiveness and suboptimal Lck tyrosine dephosphorylation following activation. Virology 353:357–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.