Abstract
Induction of hepatitis B virus (HBV)-specific cytotoxic T cells by therapeutic immunization may be a strategy to treat chronic hepatitis B. In the HBV animal model, woodchucks, the application of DNA vaccine expressing woodchuck hepatitis virus (WHV) core antigen (WHcAg) in combination with antivirals led to the prolonged control of viral replication. However, it became clear that the use of more potent vaccines is required to overcome WHV persistence. Therefore, we asked whether stronger and more functional T-cell responses could be achieved using the modified vaccines and an optimized prime-boost vaccination regimen. We developed a new DNA plasmid (pCGWHc) and recombinant adenoviruses (AdVs) showing high expression levels of WHcAg. Mice vaccinated with the improved plasmid pCGWHc elicited a stronger WHcAg-specific CD8+ T-cell response than with the previously used vaccines. Using multicolor flow cytometry and an in vivo cytotoxicity assay, we showed that immunization in a DNA prime-AdV boost regimen resulted in an even more vigorous and functional T-cell response than immunization with the new plasmid alone. Immunization of naïve woodchucks with pCGWHc plasmid or AdVs induced a significant WHcAg-specific degranulation response prior to the challenge, this response had not been previously detected. Consistently, this response led to a rapid control of infection after the challenge. Our results demonstrate that high antigen expression levels and the DNA prime-AdV boost immunization improved the T-cell response in mice and induced significant T-cell responses in woodchucks. Therefore, this new vaccination strategy may be a candidate for a therapeutic vaccine against chronic HBV infection.
INTRODUCTION
Since the introduction of prophylactic vaccination programs against hepatitis B in over 170 countries, the number of new infections with hepatitis B virus (HBV) has been continuously decreasing. Despite the success of the prophylactic vaccines, chronic HBV infection is still a global health problem. The WHO estimates that over 360 million people are persistently infected with HBV, of whom 1 million die each year from HBV-associated liver cirrhosis or hepatocellular carcinoma. Currently, two types of antiviral therapies of chronic hepatitis B are approved: treatment with pegylated alpha interferon 2a (PEG-IFN-α) or nucleot(s)ide analogues, such as entecavir and tenofovir. Nevertheless, the efficacy of these therapies is still limited. Therapy with IFN-α results in a sustained antiviral response in only one-third of the patients, and treatment with nucleot(s)ide analogues needs a lifelong therapy (30, 39, 54, 55, 61).
It is well documented that an appropriate adaptive immune response is required to efficiently control HBV infection. Specific humoral immune responses to HBV, especially neutralizing anti-envelope antibodies, play a key role in preventing HBV spread to noninfected hepatocytes (12, 62). An early, vigorous, polyclonal, and multispecific T-cell immune response directed against HBV antigens is crucial for the resolution of acute HBV infection (22, 29, 45, 50, 52, 74). In contrast, chronic HBV carriers demonstrate weak, transient, or often undetectable CD8+ T-cell responses (38, 51, 79). Therefore, therapeutic vaccination approaches able to boost a functional antiviral T-cell response may be a promising strategy to overcome viral persistence.
Numerous clinical trials of therapeutic immunizations in chronically HBV-infected patients exploited the conventional HBV surface antigen (HBsAg)-based protein vaccines. However, the antiviral effect of these approaches was only transient in the best case, and none of them led to an effective control of HBV infection in patients (15, 20, 37, 56, 57, 63, 67, 78). The strategies designed to specifically stimulate an HBV-specific T-cell response by a DNA vaccine encoding small and medium HBsAgs were also not successful (46). The combination of the HBsAg-based vaccines with antiviral treatment using lamivudine did not lead to a satisfactory improvement of the therapies either (16, 36, 75). These findings clearly imply that new concepts of therapeutic vaccination are needed.
The woodchuck (Marmota monax) is a useful preclinical model for HBV research. The natural occurrence of chronic woodchuck hepatitis virus (WHV) infection allows the evaluation of potentially new therapeutic strategies in this model. Even though several innovative approaches combining antiviral treatment with nucleot(s)ide analogues, protein vaccines, and DNA vaccines were tested in chronically infected woodchucks, these strategies were not sufficient for resolution of the chronic infection (33, 34, 43, 44, 47, 65). The application of a DNA vaccine expressing WHV core antigen (WHcAg) in combination with antivirals in chronic WHV carriers led to the prolonged control of viral replication (44). This result was encouraging but also showed the weakness of the present technical approaches to induce efficient T-cell responses by DNA vaccination, especially in large animals.
Recent studies using recombinant viral vectors as vaccines demonstrated their potential to elicit vigorous and sustained T-cell responses. The recombinant adenoviruses (AdVs) have been one of the most intensively investigated vaccine carriers in many viral infections, such as Ebola virus, human immunodeficiency virus (HIV), and severe acute respiratory syndrome (SARS) virus (24, 64, 70, 72, 81). In the present study, we attempted to improve vaccines for immunotherapeutic approaches and tested new prime-boost strategies. We constructed a new DNA plasmid (pCGWHc) and an adenoviral serotype 5 vector (Ad5WHc) and a chimeric Ad5 displaying Ad35 fiber (Ad5F35WHc [Ad35WHc]) showing high expression levels of WHcAg. We investigated whether these new vaccines could prime quantitatively and qualitatively better T-cell responses in the animal models, C57BL/6 mice and naïve woodchucks, than previously used vaccines. In recent years, additional criteria were developed to assess the quality of the responses to the new vaccines. In addition to the determination of antibody titers, T-cell responses can be quantitatively and qualitatively assessed by the frequencies of antigen-specific T cells and their functionality, including the secretion of the cytokines and the cytolytic activity in vivo. Using these criteria developed for the assessment of the vaccines against HIV (7, 25, 28, 31), we could demonstrate an excellent multifunctionality of the elicited WHcAg-specific CD8+ T cells in the mouse model. For the first time, we could detect WHcAg-specific proliferative and cytotoxic T-cell responses in naïve woodchucks immunized with the optimized vaccines by using a [2-3H]adenine proliferation assay and a recently established CD107a degranulation assay (26).
MATERIALS AND METHODS
Laboratory animals.
Ten-week-old C57BL/6 female mice (genotype H-2b/b) were purchased from Harlan Winkelmann Laboratories (Borchen, Germany). Naïve woodchucks were purchased from Northeastern Wildlife (Harrison, ID). Animals were maintained according to the guidelines of the animal facility at the University Hospital Essen. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care and Use Committee (Animal Care Center, University of Duisburg-Essen, Essen, Germany, and the district government of Düsseldorf, Germany).
Cells and cell culture.
Baby hamster kidney cells 21 (BHK-21; ATCC, CCL-10) were grown in monolayers in Eagle's minimum essential medium (Invitrogen/Gibco, Karlsruhe, Germany). The human embryonic kidney cell line 293 (HEK-293A; Microbix Biosystems, Toronto, Ontario, Canada) was propagated in Dulbecco's modified Eagle medium with high glucose (Invitrogen/Gibco). Murine splenocytes and woodchuck peripheral blood mononuclear cells (PBMCs) were cultured in RPMI medium and AIM-V medium (Invitrogen/Gibco), respectively. Cell culture media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Biochrom AG, Berlin, Germany) and 10 U/ml penicillin-streptomycin (PAA Laboratories, Pasching, Austria). Cell lines were maintained in a humidified 5% CO2 atmosphere at 37°C.
Construction of pCGWHc plasmid for DNA vaccination.
The WHV strain 8 WHcAg gene was obtained from the WHcAg-encoding pWHcIm plasmid (41). The insert was cut out of the pWHcIm plasmid by using BamHI and XbaI restriction enzymes (New England BioLabs, Ipswich, MA) and then introduced between a β-globin intron sequence and polyadenylation signal into the BamHI/XbaI site of pCG vector (69).
Construction of recombinant adenoviral vectors expressing WHcAg.
The adenoviral vectors Ad5WHc and Ad5F35WHc (Ad35WHc) expressing WHcAg were constructed using the AdEasy system and vectors pShuttle, pAdEasy-1, and pAdEasy-1/F35 (Qbiogene, Carlsbad, CA).
For the construction of a pShuttle plasmid expressing WHcAg (pShuttleWHc.in), a cassette originating from the pCG plasmid containing the cytomegalovirus immediate-early (CMV-IE) promoter, β-globin intron, and polyadenylation signal was employed. The cassette insert was amplified by PCR using specific primers introducing KpnI and BglII restriction sites (pCG.KpnI, nucleotides [nt] 4391 to 4414, 5′-CATGGTACCTAATCGACTCACTATAGGGAGACC-3′; pCG.BglII, nt 1781 to 1804, 5′-CATAGATCTAGCTCCTCGAGTTCATAAGAGAAG-3′). The amplified fragment was cloned into the multicloning site (MCS) of pShuttle. In the second step, the WHcAg sequence was amplified by PCR using the pWHcIm plasmid as a template and specific primers introducing XbaI and SbfI restriction sites (WHc.XbaI, nt 954 to 976, 5′-AGCTTCTAGACCATGGACATAGATCCCTATAAA-3′; WHc.SbfI, nt 1514 to 1541, 5′-AGCTCCTGCAGGAATTCGGCTTCATTGAAGATCACAGTT-3′). The insert was introduced into the XbaI/SbfI site located between the β-globin intron and polyadenylation signal of the expression cassette. Recombinant Ad5WHc and Ad5F35WHc (Ad35WHc) were generated by homologous recombination of pShuttleWHc.in with pAdEasy-1 and pAdEasy-1/F35, respectively, and transfected into HEK-293A cells as described elsewhere (4). The vectors were purified with a Vivaspin AdenoPACK 100 kit (Vivascience/Sartorius, Göttingen, Germany) and titrated using the 50% tissue culture infectious dose (TCID50).
Transient expression of WHcAg in BHK-21 and HEK-293 cell lines and detection of WHcAg by IF staining and Western blotting.
Up to 1 × 106 BHK-21 cells per well of a six-well plate or up to 1 × 103 BHK-21 cells per well of an eight-well chamber slide (Nunc, Roskilde, Denmark) were incubated until the cells reached a confluence of 80%. Cells were transfected with 1 μg of plasmid using Effectene reagent (Qiagen, Hiden, Germany) or Lipofectamine reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's protocol. Up to 5 × 106 HEK-293A cells per well of a six-well plate were incubated until the cells reached a confluence of 80 to 90%. Cells were infected with medium containing 5 × 107 PFU of Ad5WHc or Ad35WHc (multiplicity of infection [MOI] of 10). For immunofluorescence (IF) staining, the cells were fixed with 50% methanol (20 min at 4°C). The expressed WHcAg was detected by indirect staining with rabbit antiserum and fluorescein isothiocyanate (FITC)-conjugated antibody as described previously (41). For Western blotting, cultured BHK-21 or HEK-293A cells were lysed in 1× SDS sample buffer supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany). Protein samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Schwalbach, Germany) using a semidry transfer method. The immunodetection of WHcAg was performed using primary cross-reactive anti-HBcAg 10E11 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary peroxidase-conjugated anti-mouse IgG antibody (Sigma-Aldrich). Blots were stripped and reprobed with anti-beta-actin antibody (Sigma-Aldrich) as a loading control. Protein bands were visualized using an ECL Western Blotting Detection Kit (GE Healthcare, Buckinghamshire, United Kingdom). The optical density of the protein bands was estimated by ImageJ software (National Institutes of Health, Bethesda, MD [http://imagej.nih.gov/ij/]). The relative densitometric values obtained for WHcAg bands were normalized using the values obtained for the respective beta-actin controls.
Immunization of mice by intramuscular injection of pCGWHc and pWHcIm plasmids and by a heterologous DNA prime-AdV boost regimen.
Mice were pretreated by intramuscular injection of 50 μl of cardiotoxin (10 μM in phosphate-buffered saline [PBS]; Latoxan, Valence, France) into tibialis anterior muscle 1 week before the plasmid immunization. Animals were then intramuscularly vaccinated three times with 100 μg of pWHcIm or pCGWHc (50 μg per muscle) at 2-week intervals, according to a previously described protocol (41). Mice of the control group received intramuscularly 100 μl of sterile PBS (50 μl per muscle). Mice were sacrificed 2 weeks after the last immunization.
For the heterologous DNA prime-AdV boost regimen, mice received cardiotoxin pretreatment and two DNA immunizations. Four weeks after the second DNA immunization, groups of mice were immunized with 2 × 109 PFU of Ad5WHc or 2 × 109 PFU of Ad35WHc or with 100 μg of pCGWHc as a reference. Mice of the control group were immunized twice with 100 μg of empty pCG vector and boosted with 2 × 109 PFU of Ad5 expressing green fluorescent protein ([Ad5GFP] kindly provided by W. Bayer, Institute of Virology, University Hospital of Essen). Mice were sacrificed 2 weeks after the last immunization.
Immunization of woodchucks with plasmid DNA or recombinant adenoviral vectors and WHV challenge experiment.
Two naïve woodchucks (animals 58063 and 70096) were pretreated by intramuscular injection of 250 μl of cardiotoxin (10 μM in PBS; Latoxan) into tibialis anterior muscle. One week later, animals were subsequently immunized three times with 1 mg of pCGWHc plasmid at 2-week intervals, according to a previously described protocol (41). Two naïve woodchucks (animals 46949 and 46957) were intramuscularly immunized with 5 × 109 PFU Ad5WHc and 4 weeks later boosted with 1 × 1010 PFU of Ad35WHc. Two weeks after the last immunization, the woodchucks were challenged with 107 WHV genome equivalents (GE). Two untreated woodchucks (animals 58055 and 58056) were infected only with WHV and served as controls.
Preparation and in vitro stimulation of murine splenocytes.
Preparation of single-cell suspensions of murine splenocytes was performed according to a previously described protocol (26). Up to 1 × 106 isolated splenocytes per well were plated in 96-well plates in 200 μl of cell culture medium. Splenic lymphocytes were stimulated for 6 h or 7 days (in the presence of 10 U/ml of recombinant murine interleukin-2 [IL-2]) (Roche) with a panel of 36 synthetic overlapping 15-mer or 15 overlapping 9-mer WHcAg-derived peptides (EMC Microcollections, Tübingen, Germany) (data not shown) added to a final concentration of 2 μg/ml. Unstimulated cells and cells stimulated with CMV-derived peptide (YILEETSVM) served as negative controls. Prior to intracellular cytokine staining, cells were cultured for 5 to 6 h in the presence of 1 μg/ml of anti-CD28 antibody (clone 37.51; BD Pharmingen, Heidelberg, Germany) and 5 μg/ml of brefeldin A (Sigma-Aldrich).
Cell surface and intracellular cytokine staining of murine splenic lymphocytes.
Cell surface staining was performed using anti-CD8 (clone 56.6-7; BD Pharmingen) and anti-CD4 (clone L3T4; BD Pharmingen) T-cell antibodies. Staining of a CD107a molecule (monoclonal anti-mouse CD107a FITC-conjugated antibody, clone GB12, at a dilution of 1:200 [BD Pharmingen]) was performed during a 5-h restimulation of the splenocytes. Dead cells were excluded from analyses using 7-aminoactinomycin D (7AAD) (Becton Dickinson, Heidelberg, Germany). Intracellular cytokine stainings were performed as described elsewhere (82) with the following antibodies: anti-IFN-γ (clone XMG1.2; BD Pharmingen), anti-tumor necrosis factor α ([TNF-α] clone MP6-XT22; eBioscience, Hatfield, United Kingdom), and anti-IL-2 (clone JES6-5H4; eBioscience). Data were acquired on a FACSCalibur or LSR II flow cytometer (Becton, Dickinson, Heidelberg, Germany) from 150,000 to 300,000 lymphocyte-gated events per sample. Analyses were performed using FlowJo software (Tree Star, Ashland, OR).
In vivo cytotoxicity assay.
An in vivo cytotoxic T lymphocyte (CTL) assay was performed as described elsewhere (2), with slight modification, to measure the CTL-mediated cytotoxicity in immunized mice. Briefly, target cells were pulsed with a 1 μM WHcAg-specific epitope consisting of core antigen residues 13 to 21 ([c13-21] YQLLNFLPL) for 2 h at 37°C and afterwards stained with 36 nM carboxyfluorescein succinimidyl ester (CFSE). The unloaded reference cells were stained with 9 nM CFSE. Target and referent cells in a 1:1 ratio were transferred into immunized mice 8 days after the last immunization and into age-matched naïve mice. Recipient mice were sacrificed at 8 h postinjection of the target cells. The splenocytes from these mice were analyzed by flow cytometry for the killing of the target cells. The percent killing was calculated as follows: 100 − [(number of peptide-loaded cells/number of unloaded cells detected for immunized mice)/(number of peptide-loaded cells/number of unloaded cells detected for naïve mice)] × 100%.
CD107a degranulation assay of woodchuck PBMCs.
Woodchuck PBMCs were separated by Ficoll density gradient centrifugation and cultivated as described previously (26). For stimulation, the previously identified WHcAg-derived epitope c96-110 (KVRQSLWFHLSCLTF) and a WHsAg-derived epitope consisting of surface antigen residues 220 to 234 ([s220-234] AGLQVVYFLWTKILT) (26) were added to a final concentration of 2 μg/ml per peptide. Unstimulated cells and cells stimulated with CMV-derived peptide (YILEETSVM) served as negative controls. After 3 days of in vitro stimulation, cells were restimulated and stained for the CD107a molecule with anti-mouse CD107a FITC-conjugated antibody (clone GB12 at a dilution of 1:100; BD Pharmingen) as described previously (26). For CD4 detection anti-human CD4 allophycocyanin-conjugated antibody (clone L200; BD Pharmingen) was used. Dead cells were excluded from analyses using 7AAD.
Proliferation of woodchuck PBMCs.
Antigen-specific proliferation of woodchuck PBMCs was determined by a [2-3H]adenine-based assay as described previously (41). Briefly, 5 × 104 PBMCs were stimulated in vitro with WHcAg-derived peptides (EMC Microcollections) (data not shown) at a concentration of 5 μg/ml for 5 days. Unstimulated cells and cells stimulated with the CMV-derived peptide (YILEETSVM) served as negative controls. Afterwards, cells were labeled with 1 μCi of [2-3H]adenine (Hartmann Analytic, Braunschweig, Germany) for 16 h and collected using a cell harvester (Perkin Elmer, Waltham, MA). Results for triplicate cultures are presented as a mean stimulation index (SI) (mean total absorption for stimulated PBMCs divided by the mean total absorption for unstimulated control). An SI of ≥3.0 was considered significant.
Serology.
Murine WHcAg-specific IgG, IgG1, and IgG2a as well as woodchuck anti-WHc and anti-WHs antibodies were detected by enzyme-linked immunosorbent assay as described previously (41, 42).
Detection of WHV DNA.
WHV DNA was quantified by real-time PCR using a Platinum SYBR green Kit (Invitrogen) as described previously (26).
Evaluation of GOT levels.
The glutamic oxaloacetic transaminase (GOT; also known as aspartate transaminase, or AST) level was quantified according to the standard diagnostic procedure at the Central Laboratory of University Hospital Essen. Values above 50 IU per ml were considered elevated.
In silico prediction of MHC-I-restricted epitopes.
The major histocompatibility complex class I (MHC-I)-restricted epitopes of WHcAg for D and K loci of the mouse haplotype H-2b were predicted by two independent algorithms: SYFPEITHI (http://www.syfpeithi.de) (60) and a bioinformatics and molecular analysis section (BIMAS) MHC peptide binding prediction program (http://www-bimas.cit.nih.gov/molbio/hla_bind/) (49). A score of ≥21 for the SYFPEITHI program and a score of ≥200,000 for the BIMAS algorithm were considered good prediction scores.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism, version 5 (GraphPad Software Inc., San Diego, CA). Statistical differences were analyzed by one-way analysis of variance test using Newman-Keuls multiple comparison posttest. P values of <0.05 were considered significant.
RESULTS
Identification of the CD8+ epitopes of WHcAg in C57BL/6 mice.
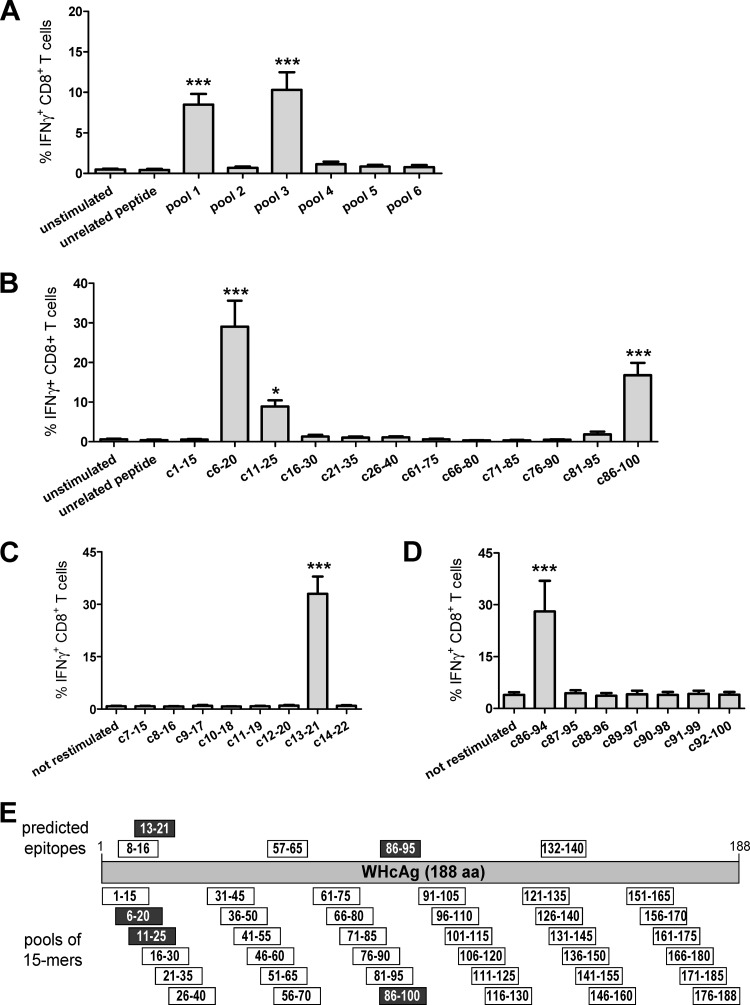
We first identified major histocompatibility complex class I (MHC-I) H-2b-restricted epitopes within the WHcAg to accurately evaluate the CD8+ T-cell response in C57BL/6 mice. We used the classical approach using overlapping peptides and in silico prediction algorithms. Splenocytes of mice immunized two or three times with a DNA plasmid expressing WHcAg were stimulated in vitro for 7 days with six peptide pools containing six overlapping 15-mer peptides, each covering the whole sequence of WHcAg (Fig. 1E) and stained intracellularly for IFN-γ (Fig. 1A). Three 15-mer peptides, c6-20, c11-26, and c86-100, specifically stimulated CD8+ T cells to produce IFN-γ at mean frequencies 29.1%, 8.9%, and 16.8%, respectively (Fig. 1B). As murine MHC class I molecules bind peptides that are 8 or 9 amino acids long (21), a screening with shorter, overlapping 9-mer peptides covering the sequence of WHcAg from amino acids (aa) 7 to 22 and 86 to 100 was performed (Fig. 1C and D). The peptide c13-21 (amino acids YQLLNFLPL) and peptide c86-94 (VNHVNDTWG) were identified as two epitopes of CD8+ T cells. The in silico prediction of potential MHC class I-restricted epitopes of WHcAg for the D and K loci of the mouse haplotype H-2b using two independent algorithms (49, 60) assigned high scores for these two epitopes. Other predicted H2-Db- and H2-Kb-restricted peptides, such as c8-16 (amino acids EFGSSYQLL), c57-65 (QALVCWDEL), and c132-140 (YRPPNAPIL) did not induce any IFN-γ production by CD8+ T cells (data not shown).
Fig 1.
Identification of H-2b-restricted CD8+ T-cell epitopes within WHcAg. Splenocytes of C57BL/6 mice immunized with a DNA plasmid expressing WHcAg were expanded in vitro with six pools containing six overlapping 15-mer peptides covering the sequence of WHcAg. After 7 days of culturing, intracellular IFN-γ was determined (A). Stimulation of the splenocytes with individual peptides from positive pools 1 and 3 determined the 15-mer epitopes' sequences: peptides c6-20 and c11-25 within pool 1 and peptide c86-94 within pool 3 (B). Fine-mapping with overlapping 9-mer peptides pointed out the exact positions of epitopes c13-21 (C) and c86-94 (D). Unstimulated cells and cells stimulated with CMV-derived peptide served as controls. Bars represent the mean values and standard errors of the means obtained from 7 to 10 mice. Asterisks mark the significant difference: *, <0.05; ***, <0.0005. (E) Schematic illustration of WHcAg peptide pools used for stimulation of murine splenocytes and predicted positions of CD8+ T-cell epitopes within the WHcAg sequence. Peptides which gave IFN-γ-positive CD8+ T-cell responses are marked in black.
Improved WHcAg expression from the pCGWHc plasmid induces stronger immune responses in vivo.
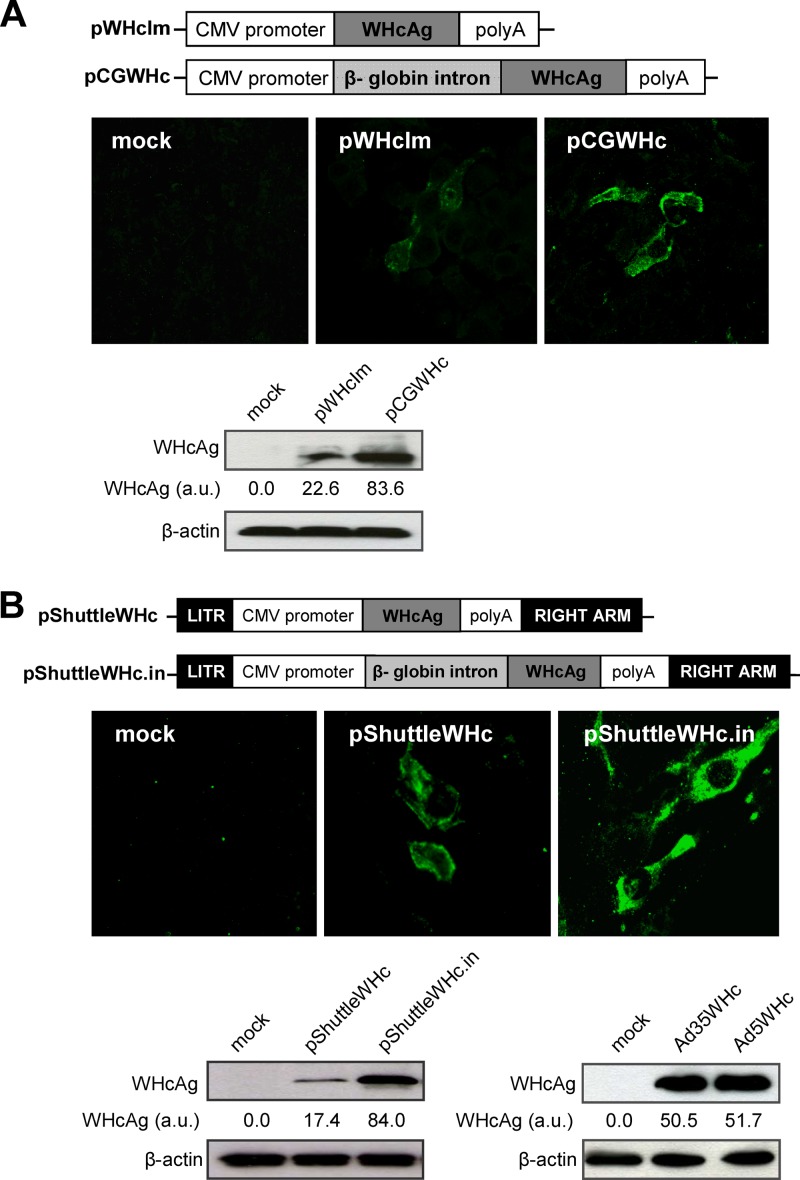
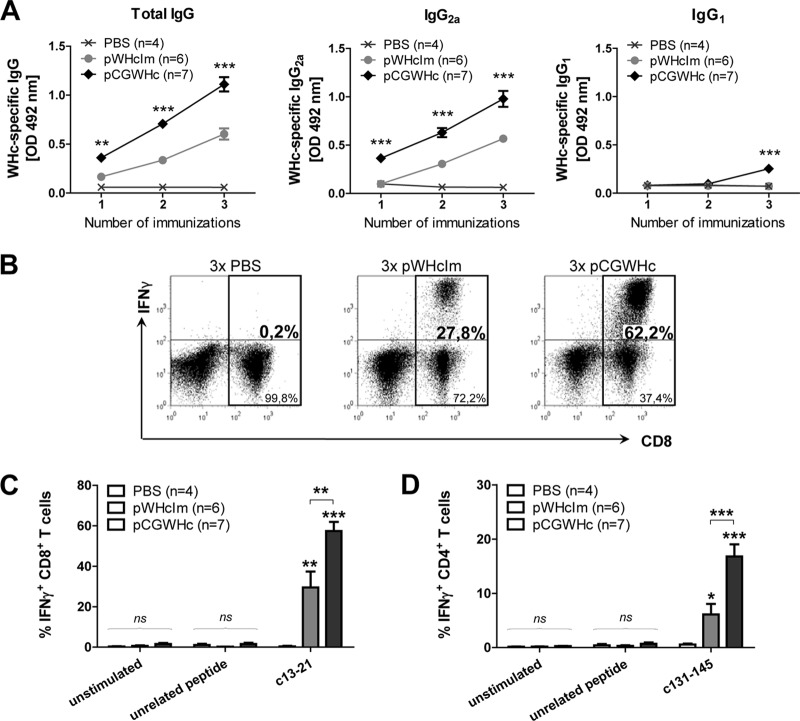
The presence of an intron sequence in the expression cassette of the gene-based vaccines may improve the expression of the encoded protein of interest (32, 40, 68). Therefore, we constructed a new DNA plasmid (pCGWHc) containing a β-globin intron sequence that demonstrated significantly higher WHcAg expression levels than a previously used DNA vaccine, pWHcIm (Fig. 2A). Improved antigen expression may lead to the induction of a more robust and multifunctional immune response in vivo. We immunized C57BL/6 mice three times with an improved pCGWHc plasmid or pWHcIm plasmid at 2-week intervals to verify this hypothesis. The WHcAg-specific antibody (anti-WHc) response was determined in the serum of mice 2 weeks after each DNA immunization (Fig. 3A). The levels of anti-WHc increased significantly in both groups of mice after each plasmid immunization (P < 0.0005). The magnitude of the humoral response induced by immunization with the pCGWHc plasmid was significantly higher (P < 0.005) at every analyzed time point than the response in pWHcIm-vaccinated mice. Evaluation of the subclasses of IgG demonstrated that immunization with both plasmids expressing WHcAg induced predominantly the IgG2a isotype of anti-WHc. The IgG1 isotype of anti-WHc was detectable only after the last vaccination with pCGWHc (P < 0.0005), indicating that immunization with this plasmid induces a very strong TH1 type of response.
Fig 2.
Characterization of WHcAg expression by improved plasmid DNA vaccine (A) and recombinant adenoviral vectors (B). Schematic illustration of the expression cassettes of the pWHcIm plasmid and the newly constructed pCGWHc plasmid containing β-globin intron sequence between the CMV promoter and the WHcAg gene (upper panel). Schematic illustration of the expression cassettes of the adenoviral pShuttle plasmid expressing WHcAg (pShuttleWHc) and the newly constructed pShuttle plasmid containing the β-globin intron (pShuttleWHc.in) (upper panel). The level of WHcAg expression between the plasmids was compared in the BHK cells 24 h posttransfection by indirect immunofluorescence staining (A and B, middle panels) and Western blotting (A and B, lower panels). Expression of WHcAg in HEK-293A cells 36 h after infection with the recombinant adenoviral vectors Ad5WHc and Ad35WHc (MOI of 10) (B, lower panel, right). The IF staining was performed using WHcAg-specific rabbit antiserum and a secondary FITC-conjugated antibody. Western blot analysis was done with the WHcAg-specific antibody. To estimate the variation in the total protein content of the cell lysates, control β-actin immunoblotting was performed. The relative densitometric values shown for WHcAg bands were estimated by ImageJ software. These values were normalized using the respective β-actin controls. au, arbitrary units.
Fig 3.
Analysis of humoral and cellular immune responses induced by immunization with the improved pCGWHc plasmid. C57BL/6 mice were immunized three times (3×) intramuscularly with 100 μg of pWHcIm or pCGWHc plasmid at 2-week intervals. Four mice were injected intramuscularly with 100 μl of PBS and served as controls. (A) WHcAg-specific IgG, IgG2a, or IgG1 antibodies detected in murine serum collected 2 weeks after every immunization (serum dilution, 1:1,000). The asterisks mark the statistically significant difference between pWHcIm- and pCGWHc-immunized mice. (B and C) Representative and summary WHcAg-specific IFN-γ+ CD8+ T-cell responses detected in the splenocytes expanded in vitro for 7 days with CD8+ T-cell epitope c13-21. (D) Summary of WHcAg-specific IFN-γ+ CD4+ T-cell responses in the splenocytes expanded in vitro for 7 days with CD4+ T-cell epitope c131-145. Unstimulated cells and cells stimulated with unrelated CMV-derived peptide served as controls. The bars represent the mean values and standard errors of the means obtained for each group of mice. Asterisks mark s statistically significant differences: *, <0.05; **, <0.005; ***, <0.0005; ns, not significant. The asterisks shown directly above the bars mark the statistically significant difference between plasmid-vaccinated groups and the control group of PBS-injected mice. OD, optical density.
Evaluation of WHcAg-specific CD8+ and CD4+ T-cell responses was performed by intracellular IFN-γ staining of splenocytes stimulated in vitro for 7 days with peptides comprising the CD8+ T-cell epitopes c13-21 and c86-94 or CD4+ T-cell epitope c131-145 (A. D. Kosinska et al., unpublished data). The magnitude of IFN-γ production by CD8+ and CD4+ T cells in the spleens of mice vaccinated with the pCGWHc plasmid was considerably greater than that of the pWHcIm-immunized group (representative data are shown in Fig. 3B). As shown in Fig. 3C, the mean IFN-γ response after stimulation with immunodominant CD8+ epitope c13-21 was 57.4% for pCGWHc-vaccinated mice, which was significantly higher than the 29.5% detected for the pWHcIm-immunized group (P < 0,005). A similar outcome was observed in splenocytes expanded with peptide c86-94 (data not shown). In addition, the mean percentages of IFN-γ+ CD4+ T cells were significantly higher in the group of mice immunized with pCGWHc than in the pWHcIm-immunized group (16.8% and 4.5%, respectively; P < 0.0005) (Fig. 3D).
DNA prime-AdV boost immunization elicits a more robust and functional WHV-specific immune response than DNA immunization alone.
The heterologous DNA prime-AdV boost immunization proved to elicit both robust and effective humoral and cellular immune responses against a variety of pathogens (10, 11, 70, 73, 77, 80). Therefore, we successfully generated replication-defective, WHcAg-expressing adenoviruses, i.e., a serotype 5 adenovirus (Ad5) and a chimeric Ad5 with the fiber of Ad35 (Ad5F35, abbreviated as Ad35). WHcAg expression was also improved by using intron sequences in the expression cassette of the vectors (Fig. 2B). For a detailed analysis of the immune responses after the DNA prime-AdV boost immunization, C57BL/6 mice received the pCGWHc plasmid two times in a 2-week interval and were boosted with Ad5WHc or Ad35WHc or with pCGWHc as a reference.
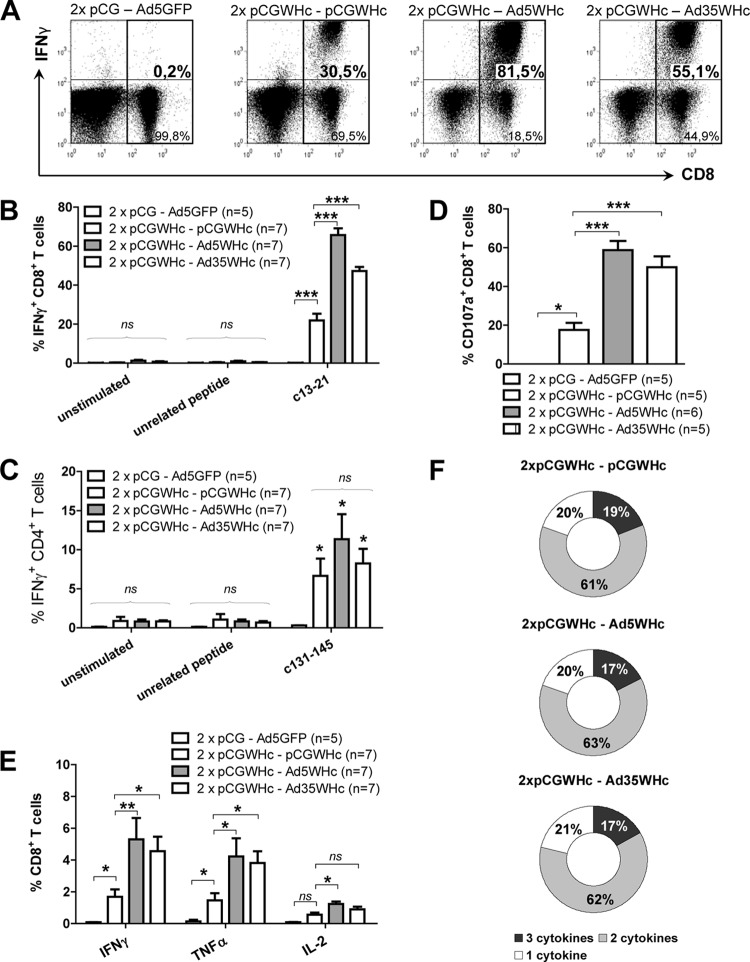
As shown in Fig. 4A, the boosting immunization with adenoviral vectors (Ad5WHc and Ad35WHc) led to the induction of higher levels of anti-WHc than in the group of mice immunized with just the pCGWHc plasmid (P < 0.05). The levels of anti-WHc antibodies were comparable in all mice immunized with pCGWHc plasmid either once or twice (data not shown). Detection of IgG isotypes demonstrated that all tested immunization protocols induced predominantly IgG2a antibodies (Fig. 4B). Nevertheless, the levels of IgG2a were significantly higher in the groups of mice boosted with recombinant adenoviral vectors than in those immunized with the DNA vaccine plasmid pCGWHc (P < 0.005). The presence of low-level IgG1 antibodies in murine serum (Fig. 4C) was detected in DNA prime-AdV boost- as well as in DNA-only-vaccinated groups (P < 0.05 compared to control mice).
Fig 4.
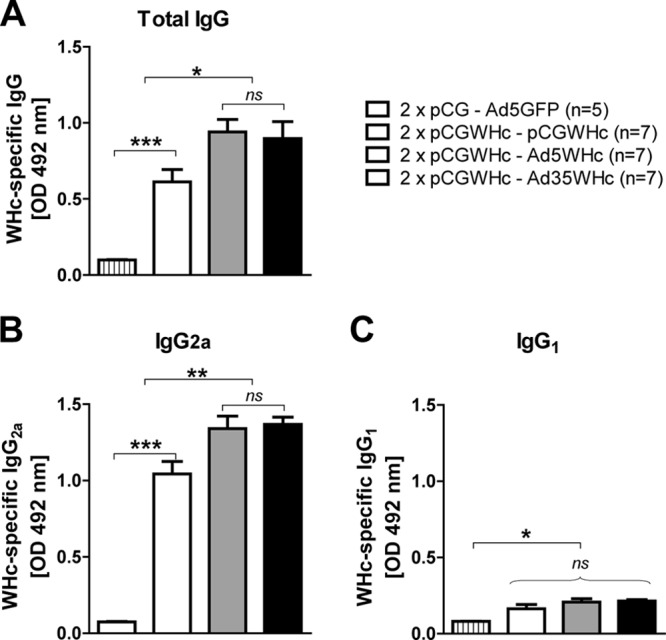
Detection of WHcAg-specific antibodies in the sera of C57BL/6 mice after DNA prime-AdV boost immunization. Mice were primed two times (2×) by immunization with the pCGWHc plasmid. Four weeks later, boosting immunization with Ad5WHc or Ad35WHc or with pCGWHc for reference was performed. Mice immunized with empty pCG and boosted with Ad5 expressing GFP served as controls. WHcAg-specific IgG (A), IgG2a (B), or IgG1 (C) antibodies were detected in sera collected 2 weeks after the last, boosting immunization (serum dilution, 1:5,000). Asterisks mark the significant difference: *, <0.05; ***, <0.0005; ns, not significant).
Comparison of WHcAg-specific CD8+ and CD4+ T-cell responses induced by the heterologous DNA-Ad5WHc or DNA-Ad35WHc regimen with the DNA-only immunization regimen was performed by intracellular IFN-γ staining of splenocytes isolated 2 weeks after the last immunization. The mean percentage of IFN-γ+ CD8+ T cells directed against CD8+ T-cell epitope c13-21 was approximately 21.9% for the pCGWHc-immunized group of mice (Fig. 5A and B) and was significantly lower than the values 65.7% and 47% determined for the pCGWHc-Ad5WHc- and pCGWHc-Ad35WHc-immunized groups, respectively (P < 0.0005). A similar correlation was observed for splenocytes expanded with the peptide c86-94 (data not shown). No statistically significant difference in percentages of IFN-γ+ CD4+ T cells between the groups immunized with WHcAg-expressing vaccines was detected (Fig. 5C).
Fig 5.
Analysis of the cellular immune response induced by DNA prime-AdV boost immunization. C57BL/6 mice were primed two times by immunization with the pCGWHc plasmid. Four weeks later, boosting immunization with Ad5WHc or Ad35WHc or with pCGWHc for reference was performed. Mice immunized with empty pCG and Ad5GFP served as controls. (A and B) Representative and summary WHcAg-specific IFN-γ+ CD8+ T-cell responses detected in the splenocytes expanded in vitro for 7 days with CD8+ T-cell epitope c13-21. (C) Summary of WHcAg-specific IFN-γ+ CD4+ T-cell responses in the splenocytes expanded in vitro for 7 days with CD4+ T-cell epitope c131-145. (D) Degranulation capacity of IFN-γ+ CD8+ splenic T cells expanded in vitro for 7 days with peptide c13-21. (E) Frequencies of IFN-γ-, TNF-α-, and IL-2-producing CD8+ T cells from splenocytes stimulated in vitro for 6 h with the epitope c13-21. The bars represent the mean values and standard errors of the means obtained for each group of mice. Asterisks mark the statistically significant difference: *, <0.05;**, <0.005; ***, <0.0005; ns, not significant. The asterisks shown directly above the bars mark the statistically significant difference between plasmid-vaccinated groups and the control group of mice. (F) Evaluation of multifunctional CD8+ T cells: the percentage of single, double, and triple producers in a cytokine-positive CD8+ T-cell population.
The effector functions of the CD8+ T cells induced by the heterologous prime-boost regimen were further characterized by the ability of the CD8+ T cells to degranulate. The degranulation capacity was measured by flow cytometric detection of the CD107a marker (6, 66) on the splenic lymphocytes expanded in vitro with the CD8+ T-cell epitope c13-21. The percentages of CD107a+ CD8+ T cells were also significantly higher (P < 0.0005) in the groups of mice boosted with Ad5WHc and Ad35WHc (mean values, 58.7% and 49.9%, respectively) than in the pCGWHc-only-immunized mice (mean value, 17.5%) (Fig. 5D).
In addition to the direct cytotoxic activities, CD8+ T cells are able to produce cytokines that exhibit an antiviral activity. We determined the production of TH1 type cytokines by CD8+ T cells, such as IFN-γ, TNF-α, and IL-2, in murine splenocytes stimulated in vitro for 6 h with the peptide c13-21. As presented in Fig. 5E, the cytokine that had the strongest expression within the CD8+ T-cell population in all immunized groups was IFN-γ. TNF-α was produced in slightly lower levels, and the least abundant cytokine was IL-2 (approximately 4-fold lower expression than IFN-γ). Groups of mice primed with pGCWHc and boosted with Ad5WHc exhibited the highest percentages of IFN-γ, TNF-α, and IL-2 (mean values, 5.3%, 4.2%, and 1.2%, respectively). The expression of all analyzed cytokines in this group was significantly higher than the DNA-only-immunized group (P < 0.05). Further, we performed analysis of single-, double-, and triple-positive cells that produce IFN-γ, TNF-α, and IL-2 within the CD8+ T-cell population. As Fig. 5F shows, there was no difference in the quality of cytokine-secreting CD8+ T cells between the heterologous prime-boost regimen groups, using recombinant adenoviral vectors expressing WHcAg, and mice immunized with only WHcAg-expressing plasmid. The average values of single, double, and triple cytokine producers were approximately 18%, 62%, and 20%, respectively.
DNA prime-AdV boost immunization induces CD8+ T cells of stronger cytotoxic potential in vivo than DNA immunization alone.
The cytolytic activity of WHcAg-specific CD8+ T cells was determined in vivo by their ability to eliminate peptide-loaded target cells (2, 19, 82). In the first step, the kinetics of WHcAg-specific CTL-mediated lysis of the target cells at various time points postimmunization (p.i.) were investigated. Briefly, C57BL/6 mice were immunized three times with the pCGWHc plasmid. At day 5, 8, or 14 after the last immunization, mice were intravenously injected with the same number of target cells loaded with the peptide c13-21 corresponding to the CD8+ T-cell epitope and nonloaded cells differently labeled with CFSE dye. After 2 h, immunized mice were sacrificed, and the killing of the target cells was evaluated in the spleen. As shown in Fig. 6A, the most effective elimination of peptide c13-21-loaded cells was observed at day 8 after the last immunization (mean, 13.2%; P < 0.05, compared to naïve mice). At day 5 after the last immunization, the mean killing in the spleen of mice was 7.7%, and at day 14 it was 4.4%. Next, the cytotoxic potential of WHcAg-specific CD8+ T cells elicited by heterologous pCGWHc-Ad5WHc and pCGWHc-only immunizations was evaluated, as described in Materials and Methods. The mice immunized in the DNA prime-Ad5WHc manner showed improved elimination of the peptide c13-21-loaded target cells from the spleen (Fig. 6B). The mean percentage of killing determined for six mice in that group was 43.8% and was significantly higher than the 20.2% obtained for mice immunized with only the pCGWHc plasmid (P < 0.05) (Fig. 6C). The background obtained in mice immunized with vectors that did not express WHcAg was 1.9%. (P < 0.05, compared to immunized mice).
Fig 6.
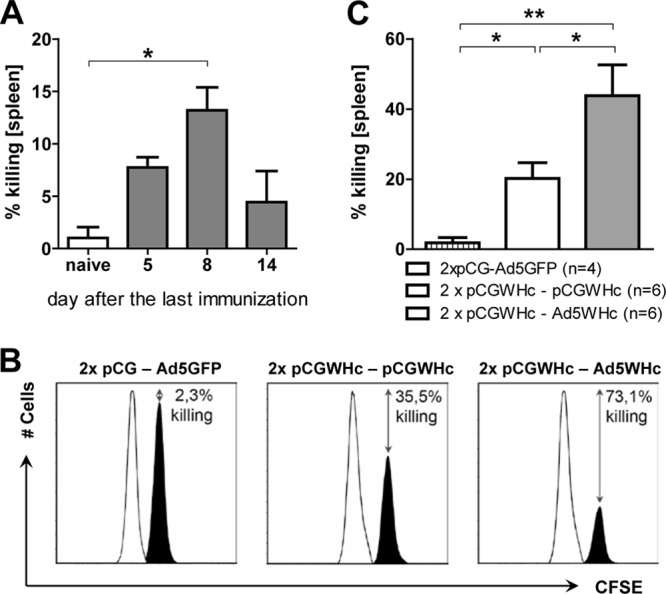
Elimination of the WHcAg epitope-loaded cells from the spleen after DNA prime-AdV boost immunization. (A) The kinetics of WHcAg-specific CTL-mediated elimination of the WHcAg-derived peptide c13-21-loaded target cells at various time points after DNA immunization. (B and C) Comparison of the elimination of the target cells in mice immunized with DNA-AdV or only DNA. Mice were immunized two times with pCGWHc and boosted with Ad5WHc or pCGWHc. Mice immunized with empty pCG and Ad5GFP served as controls. At day 8 after the last immunization, mice were intravenously injected with the same number of target cells loaded with the CD8+ T-cell epitope c13-21 (black peak) and nonloaded cells (white peak) for the reference. After 8 h the mice were sacrificed, and the percentage of target cell killing was investigated in the spleen. Histograms of representative mouse from each immunization group exhibiting the highest killing activity are shown in panel B. In panel C the bars represent the mean values and standard errors of the means obtained from groups of six mice immunized with WHcAg-expressing vaccines and four control mice immunized with empty pCG plasmid in combination with Ad5GFP. The asterisks mark the statistically significant difference: *, <0.05; **, <0.005).
Heterologous Ad5WHc/Ad35WHc immunization in naïve woodchucks protects against infection with WHV.
To determine the potency of recombinant adenoviral vectors to induce protective immune responses in woodchucks, we performed a WHV challenge experiment. It was previously demonstrated that the immunization of woodchucks with a DNA plasmid expressing WHcAg alone is sufficient to achieve protection against WHV infection after challenge (41). Immunization with a heterologous prime-boost regimen would probably lead to the same result. Therefore, we compared the DNA and AdV vaccination regimens separately as immunization of woodchucks with recombinant adenoviral vectors had not been previously tested. Two naïve woodchucks (numbers 46949 and 46957) were immunized with both Ad5WHc and Ad35WHc (Ad5WHc/Ad35WHc). The other two animals (numbers 58063 and 70096) were immunized three times with the pCGWHc plasmid and served as a reference.
The T-cell response after the immunizations was evaluated by a flow cytometric CD107a degranulation assay and [2-3H]adenine-based proliferation assay of woodchuck PBMCs.
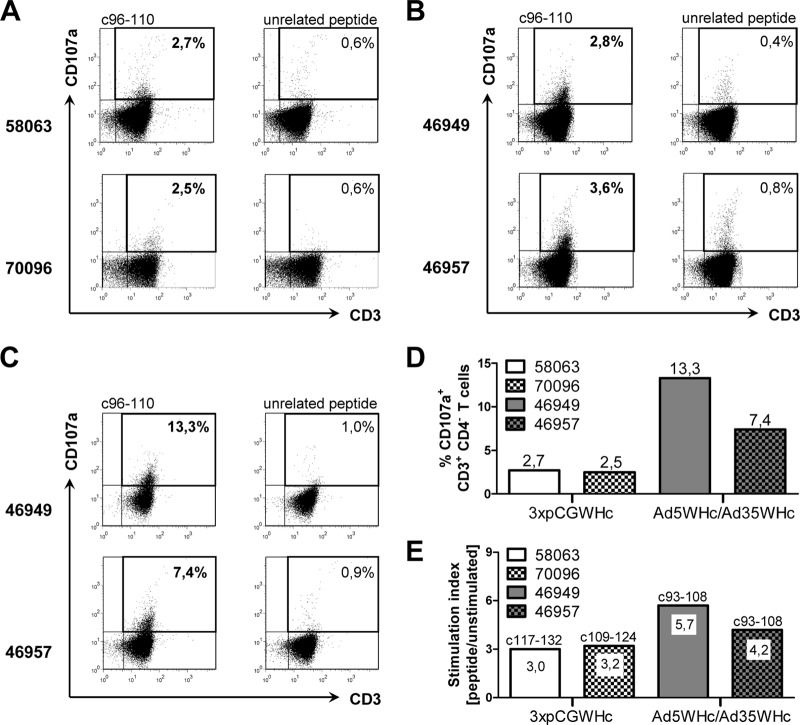
For the detection of WHcAg-specific cytotoxic T cells by the CD107a degranulation assay, stimulation of the PBMCs with the previously characterized WHcAg epitope c96-110 was performed (26). The population of CD3+ CD4[−] lymphocytes was considered to be the CD8+ T cells. Vaccination with pCGWHc plasmid of the naïve woodchucks 58059 and 58063 induced a significant degranulation response directed against WHcAg epitope c96-110 (Fig. 7A). The percentages of 2.7% CD107a+ T cells in the CD3+ CD4[−] population detected for woodchuck 58063 and 2.5% detected for woodchuck 70096 were significantly higher than background values in controls stimulated with unrelated CMV-derived peptide (0.6%). Woodchucks 46949 and 46957 immunized just once with Ad5WHc had a\T-cell responses (2.8% and 3.6%, respectively) of comparable magnitude to the three plasmid DNA immunizations (Fig. 7B and D). After immunization with Ad35WHc, the induced WHcAg-specific CTL response was significantly boosted in both animals. A greater than 4-fold increase in the percentage of WHcAg-specific CD107a+ CD3+ CD4[−] T cells (13.3%) was shown in woodchuck 46949. Woodchuck 46957 demonstrated a 2-fold increase in WHcAg-specific CTLs (7.4%) (Fig. 7C and D). Moreover, we could also detect significant WHcAg-specific proliferative responses (SI of ≥3.0) in all DNA- and Ad5WHc/Ad35WHc-immunized woodchucks (Fig. 7E). Animals immunized three times with pCGWHc plasmid showed the response to two distinct WHcAg-derived peptides: woodchuck 58063 to c117-132 (SI of 3.0) and woodchuck 70096 to c109-124 (SI of 3.2). A proliferation of PBMCs in woodchucks from the Ad5WHc/Ad35WHc vaccination group was seen after stimulation with the same WHcAg-derived peptide c93-108. The detected SI values in these animals were slightly higher (5.7 for 46949 and 4.2 for 46957) than those obtained in the DNA group.
Fig 7.
Cellular immune response in woodchucks immunized with pCGWHc plasmid or adenoviral vectors. (A) Dot plots of PBMCs from woodchucks 58063 and 70096 immunized three times with DNA vaccine, pCGWHc. Dot plots of PBMCs from woodchucks 46949 and 46957 immunized with recombinant AdVs after priming with Ad5WHc (B) and boosting with Ad35WHc (C). CTL response was evaluated by a CD107a degranulation assay in woodchuck PBMCs expanded in vitro for 3 days with WHcAg-derived epitope c96-110 2 weeks after the immunization. Cells stimulated with unrelated CMV-derived peptide served as controls. The cells were gated on the lymphocyte population, 7AAD−, and CD4− cells. Values show the percentages of CD107a+ CD3+ CD4− T cells within the CD3+ CD4− T-cell population. (D) Summary of degranulation responses after immunization with pCGWHc plasmid or with adenoviral vectors at the time point of WHV challenge. (E) The lymphoproliferative responses of PBMCs from immunized woodchucks at the time point of WHV challenge. The PBMCs were stimulated for 5 days with a panel of 10 WHcAg-specific peptides in triplicates. The cells were then pulsed with [2-3H]adenine for 16 h, and the incorporation of [2-3H]adenine was measured. Stimulation index (SI) of ≥3.0 was considered significant. The peptides which gave the positive proliferation responses are shown above the bars.
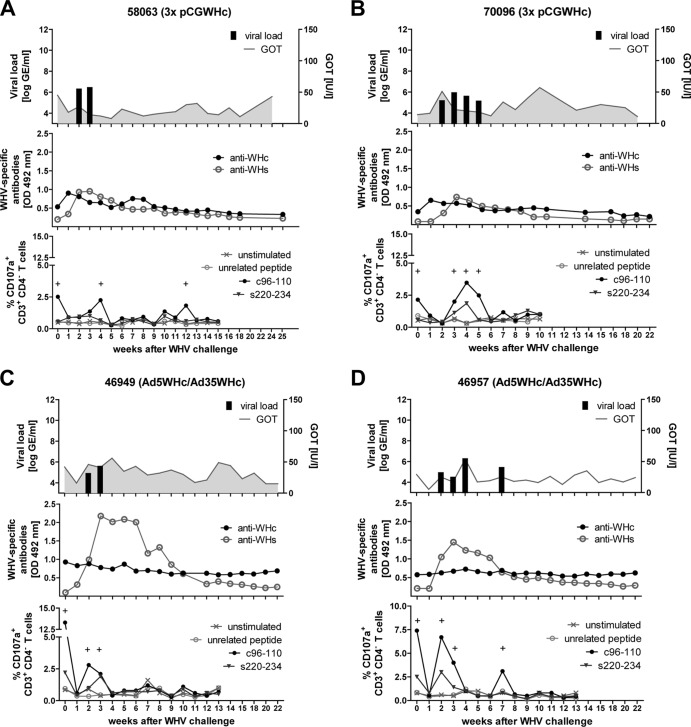
Immunized woodchucks and two untreated control woodchucks were intravenously challenged with 1 × 107 genome equivalents (GE) of WHV and monitored weekly for the markers of infection. As shown for animal 58063 in Fig. 8A and animal 70096 (each of which received three immunizations with pCGWHc) in Fig. 8B, serum WHV DNA was detected at only 2 to 4 weeks after the challenge (weeks 2 to 3 p.i. and weeks 2 to 5 p.i., respectively). Similarly, woodchuck 46949 immunized with Ad5WHc/Ad35WHc was WHV DNA positive at weeks 2 and 3 p.i. (Fig. 8C). Another animal from this vaccination group, 46957, showed viremia from weeks 2 to 4 p.i. and a brief breakthrough at week 7 p.i., accompanied by WHcAg-specific CTL detection (Fig. 8D). All immunized animals demonstrated a 3- to 4-log lower viral load (between 7.3 × 10 4 and 2.5 × 106 WHV GE/ml) than the control group (Fig. 9A and B, upper panels). The anti-WHs antibodies were induced at week 2 p.i. in all vaccinated woodchucks. Interestingly, the levels of anti-WHs were higher in woodchucks vaccinated with recombinant adenoviral vectors (Fig. 8C and D, middle panels) than in DNA-vaccinated animals (Fig. 8A and B, middle panels). The detected CTL responses after WHV infection were predominantly WHcAg specific. The degranulation responses against WHcAg-derived epitope c96-110 were usually present at the end of the viremia period, underlying the importance of CTLs in the resolution of the infection (Fig. 8A to D, lower panels). Only a slight elevation in GOT levels was observed in immunized animals. The values between 51 and 57 were detected for woodchucks 70096 at weeks 2 and 10 p.i., 46949 at week 4 p.i., and 46957 at week 4 p.i., suggesting mild cytotoxic activity in the liver.
Fig 8.
Course of WHV infection of woodchucks immunized three times with pCGWHc plasmid (A and B) or with Ad5WHc/Ad35WHc (C and D). Two weeks after the last immunization woodchucks were intravenously inoculated with 1 × 107 WHV GE (week 0). The viral DNA was quantified by real-time PCR with a detection limit of 103 genome equivalents per reaction. The GOT levels were detected using a standard diagnostic method (upper panels). WHcAg-specific and WHsAg-specific antibodies were (anti-WHc and anti-WHs, respectively) detected in woodchuck sera using protein G coupled to peroxidase (middle panels). Cellular immune responses were determined by a CD107a degranulation assay in woodchuck PBMCs expanded in vitro for 3 days with WHcAg- and WHsAg-derived epitopes c96-110 and s220-234 (lower panels). Unstimulated cells and cells stimulated with CMV-derived peptide served as negative controls. The positive (+) responses are indicated.
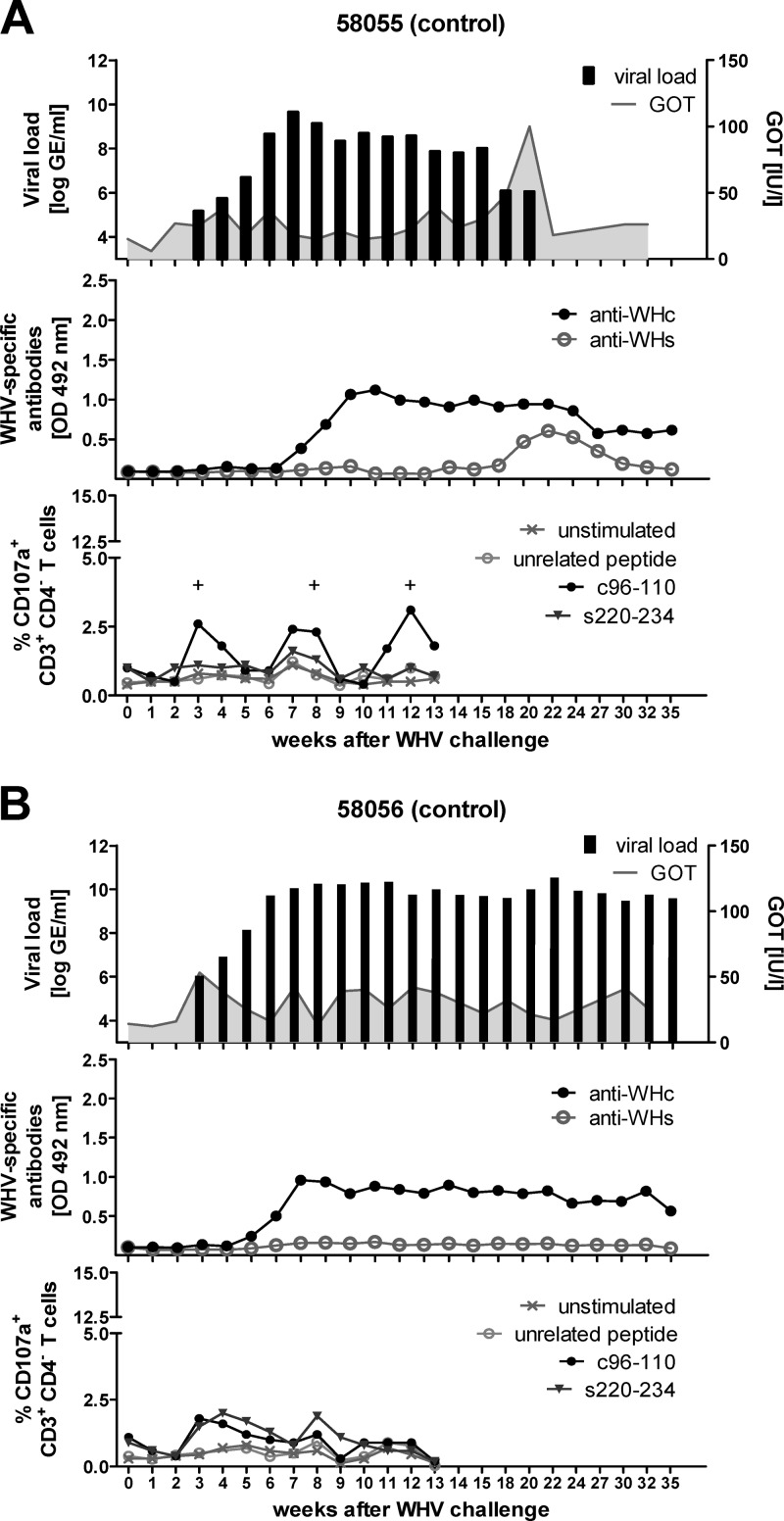
Fig 9.
Course of WHV infection of control woodchucks. Two WHV naïve woodchucks, 58055 (A) and 58056 (B), were intravenously inoculated with 1 × 107 WHV GE (week 0). The viral DNA was quantified by real-time PCR with a detection limit of 103 genome equivalents per reaction. The GOT levels were detected using a standard diagnostic method (upper panels). WHcAg-specific and WHsAg-specific antibodies were (anti-WHc and anti-WHs, respectively) detected in woodchuck sera using protein G coupled to peroxidase (middle panels). Cellular immune responses were determined by a CD107a degranulation assay in woodchuck PBMCs expanded in vitro for 3 days with WHcAg- and WHsAg-derived epitopes c96-110 and s220-234 (lower panels). Unstimulated cells and cells stimulated with CMV-derived peptide served as negative controls. The positive (+) responses are indicated.
The control woodchuck 58055 resolved WHV infection at week 22 (Fig. 9A, upper panel). The high-level viremia, ranging between 6.3 × 107 and 1.3 × 109 WHV GE/ml of serum was observed until week 15 p.i. The resolution of the infection correlated with appearance of anti-WHs from week 18 to 24 p.i. (Fig. 9A, middle panel). The low percentage of WHcAg-specific CTLs (approximately 2.5%) was measurable in the peripheral blood at weeks 3, 8, and 12 p.i. (Fig. 9A, lower panel). The significant elevation of the serum GOT level (100 IU/liter) at week 20 p.i. suggests a massive influx of WHV-specific effector T cells into the liver of woodchuck 58055. The second control animal, 58056, developed a chronic WHV infection and borderline CTL responses and did not develop anti-WHs over the monitoring period (Fig. 9B). No elevation of GOT levels was observed in woodchuck 58056, indicating the lack of WHV-specific T-cell activity in the liver.
DISCUSSION
The induction of a cellular immune response by DNA or recombinant viral vector vaccination has been of major scientific focus to treat chronic viral infections (10, 11, 70) or cancer (13, 27, 53, 59). However, in contrast to the mouse models, the priming of robust and sustained T-cell responses by vaccination in larger animals (e.g., woodchucks and chimpanzees) or patients is particularly difficult. Previously, various ways were tested to induce potent T-cell responses in woodchucks, with only limited success (23, 41–43, 71). Though DNA vaccination provided protection against WHV infection, the induction of detectable T-cell responses was not successful in the previous experiments (41). Excitingly, the new immunization strategy based on the adenoviral vectors primed strong and functional T-cell responses in woodchucks. In the present study, we generated new expression vectors for WHcAg based on the pCG backbone and recombinant adenoviruses, Ad5WHc and Ad35WHc, including an intron sequence in the expression cassette. Clearly, the new vaccines expressed WHcAg at higher levels and induced more potent B- and T-cell responses in the mouse and woodchuck models. In mice, immunizations with the new vaccines induced WHcAg-specific immune responses with improved functionality, such as the increased numbers of cytokine-producing and cytolytic T cells.
The results of our study showed that the improved WHcAg expression by the novel DNA vaccine (pCGWHc) resulted in stronger humoral and cellular immune responses in mice than the previously used DNA vaccine (41, 44). As expected, both plasmids induced a TH1 response, confirmed by the IgG2a subtype of anti-WHc and secretion of IFN-γ by CD8+ and CD4+ T cells. Excitingly, the immunization of mice in the DNA prime-AdV boost regimen further improved the magnitude of the induced immune response. As expected, the strong antibody response correlated with significantly higher frequencies of IFN-γ+ CD8+ T cells detected in both AdV-boosted groups than in DNA-only-immunized mice. A slightly lower percentage of IFN-γ+ CD8+ T cells detected in mice immunized with DNA-Ad35WHc than with DNA-Ad5WHc may be due to the fact that these vectors use different receptors during the internalization process. The recombinant adenoviral vector serotype 5 interacts with coxsackievirus-adenovirus receptor (CAR), and the chimeric Ad35 fiber binds to CD46 (5). Concordant with our results, a slightly less effective performance of Ad5F35 than Ad5 immunization in the mouse model was shown previously (4).
Defects in cytokine secretion and degranulation functions of antigen-specific CD8+ T cells have been associated with chronic HBV (18) and progressive HIV infection (18, 83). Antigen-specific T cells that concurrently present multiple functions, e.g., degranulation and production of various cytokines, play an important role in nonprogressive HIV infection (1, 8) and vaccine-mediated protective immunity against the vaccinia virus (58) and Leishmania major (17). Therefore, the induction of a potent multifunctional CD8+ T-cell response is highly desirable. We could clearly demonstrate that the CD8+ T cells induced by DNA and DNA prime-AdV boost vaccination regimens are highly functional. Mice immunized with DNA-AdV regimens exhibited significantly higher percentages of CD107a+, IFN-γ+, TNF-α+, and IL-2+ CD8+ T cells than the group immunized with DNA only. However, there was no difference in the quality of CD8+ T cells between the groups. This finding can be a result of using the same priming strategy for all immunization regimens. The DNA vaccines proved to be a very potent tool in priming the T-cell response (3). The magnitude of the response might be at a relatively low level; however, it may select the T cells with receptors of high affinity to the antigen of interest (9). The immunization with a very immunogenic recombinant adenoviral vector may result in a significant boost of these antigen-specific memory cells. The results of in vivo cytotoxicity assays proved that the higher magnitude of CTL response induced by the DNA prime-AdV boost regimen was well correlated with the improved killing of WHcAg-derived peptide-loaded target cells.
The immunization of woodchucks with the new pCGWHc plasmid or Ad5WHc/Ad35WHc expressing WHcAg induced a WHcAg-specific immune response and led to the control of a subsequent WHV infection. Previously, we demonstrated that immunization with plasmid with a low expression level of WHcAg could protect woodchucks from WHV infection (41, 71). However, the T-cell response determined by a [2-3H]adenine-based proliferation assay was extremely low and detectable only in several woodchucks (41). The results of this study clearly show that three immunizations with the improved pCGWHc plasmid or heterologous Ad5WHc/Ad35WHc induced significant proliferative responses in all woodchucks prior to the WHV challenge. These proliferative T-cell responses were slightly higher in woodchucks immunized with Ad5WHc/Ad35WHc than in DNA-immunized woodchucks. Nevertheless, the difference in the magnitude of the T-cell response elicited by the two vaccination regimens was well marked in the results of the flow cytometric CD107a degranulation assay (26). This assay allowed the detection, for the first time, of a WHV-specific cytotoxic T-cell response induced by vaccination. The percentages of WHcAg-specific CD107a+ CD3+ CD4− T cells detected after three immunizations with the pCGWHc plasmid were comparable to those induced by just one immunization with Ad5WHc. The high percentages of WHcAg-specific CTLs after boosting immunization with Ad35WHc are comparable to those detected during the acute phase of WHV infection (26). Our results demonstrate the potential of adenoviral vectors to induce a stronger CTL response in woodchucks than “classical” DNA vaccination. This supports the findings obtained in rhesus macaques immunized three times with plasmid DNA or twice with Ad5 expressing the HIV-1 gag gene (10).
The two vaccination regimens induced comparable levels of anti-WHc antibodies. Nevertheless, these antibodies are not able to neutralize the virions of WHV and do not provide sterilizing immunity against WHV infection, as shown previously (76). Therefore, a limited infection of hepatocytes occurred in all vaccinated woodchucks after WHV challenge. In contrast to results in the control animals, the short, low-level viremia and no or low elevation of GOT in immunized woodchucks indicated that only a small number of hepatocytes were infected and effectively eliminated by cytotoxic T cells elicited by vaccination. Despite this T-cell response, the development of neutralizing antibodies directed against the surface antigen is crucial for the resolution of the hepadnaviral infections (12, 14, 35, 41). It was shown previously that core-specific T cells may provide a help to the surface antigen-specific B cells (48). Our results show that the immunization of woodchucks resulted in a rapid and more robust production of anti-surface antibodies after WHV challenge than infected control woodchucks. This observation confirms the “intermolecular help” concept. Anti-WHs were detected in all immunized woodchucks already at week 2 after WHV challenge. Moreover, the levels of anti-WHs correlated with the magnitude of the WHcAg-specific T-cell response after vaccination. The most prominent anti-WHs response was detected in woodchucks immunized with Ad5WHc/Ad35WHc vectors.
Immunization with recombinant adenoviral vectors has already proven to be protective against simian immunodeficiency virus (SIV) (11, 70) and Ebola virus infections (72) in nonhuman primates. Here, we demonstrate the potency of Ad5WHc/Ad35WHc immunization against WHV infection in the woodchuck model.
Taken together, we demonstrate that the improvement of the DNA plasmid and optimization of the vaccination regimen by addition of AdV brought a synergistic effect. The prime-boost regimen examined in this study induced a robust and multifunctional WHcAg-specific T-cell response in mice. Moreover, the vaccination with the new plasmid DNA vaccine and recombinant adenoviral vectors elicited a significant CTL response in naïve woodchucks and led to the rapid development of anti-WHs antibodies and resolution of WHV infection after WHV challenge. A combination of these vaccines could be used as a therapeutic strategy against chronic hepadnaviral infections. In subsequent experiments, we have evaluated the WHcAg-based DNA prime-AdV boost immunization in chronically WHV-infected woodchucks in combination with a potent nucleot(s)ide analogue treatment (Kosinska et al., unpublished results). We could detect the significant WHcAg-specific and WHsAg-specific T-cell responses in all woodchucks that received the vaccination and antiviral treatment but not in control animals. Moreover, two out of four woodchucks from the combination therapy group remained WHV DNA negative from the end of the antiviral treatment up to the end of the monitoring period and developed anti-WHs antibodies.
ACKNOWLEDGMENTS
We are grateful to Wibke Bayer (Institute of Virology, University Hospital of Essen, Germany) for providing the Ad5-expressing GFP, to Ulf Dittmer (Institute of Virology, University Hospital of Essen, Germany) for providing pCG plasmid, and to Xiaolong Fan (Lund University, Lund, Sweden) for providing the plasmid pAdEasy-1/F35. We thank Delia Cosgrove, Jia Liu, and Wibke Bayer for the editorial assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (GK 1045/2 and TRR60) and Wilhelm-Sander Stiftung.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Almeida JR, et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barber DL, Wherry EJ, Ahmed R. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27–31 [DOI] [PubMed] [Google Scholar]
- 3. Barouch DH, et al. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayer W, Schimmer S, Hoffmann D, Dittmer U, Wildner O. 2008. Evaluation of the Friend virus model for the development of improved adenovirus-vectored anti-retroviral vaccination strategies. Vaccine 26:716–726 [DOI] [PubMed] [Google Scholar]
- 5. Bergelson JM. 1999. Receptors mediating adenovirus attachment and internalization. Biochem. Pharmacol. 57:975–979 [DOI] [PubMed] [Google Scholar]
- 6. Betts MR, et al. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65–78 [DOI] [PubMed] [Google Scholar]
- 7. Betts MR, et al. 2005. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 102:4512–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Busch DH, Pamer EG. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casimiro DR, et al. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casimiro DR, et al. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chisari FV, Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29–60 [DOI] [PubMed] [Google Scholar]
- 13. Cho HI, Celis E. 2012. Design of immunogenic and effective multi-epitope DNA vaccines for melanoma. Cancer Immunol. Immunother. 61:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cote PJ, et al. 1986. Protection of chimpanzees from type B hepatitis by immunization with woodchuck hepatitis virus surface antigen. J. Virol. 60:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couillin I, et al. 1999. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis. 180:15–26 [DOI] [PubMed] [Google Scholar]
- 16. Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. 2002. Clinical and immunological efficacy of intradermal vaccine plus lamivudine with or without interleukin-2 in patients with chronic hepatitis B. J. Med. Virol. 66:452–460 [DOI] [PubMed] [Google Scholar]
- 17. Darrah PA, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 18. Das A, et al. 2008. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205:2111–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dietze KK, et al. 2011. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc. Natl. Acad. Sci. U. S. A. 108:2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dikici B, et al. 2003. Failure of therapeutic vaccination using hepatitis B surface antigen vaccine in the immunotolerant phase of children with chronic hepatitis B infection. J. Gastroenterol. Hepatol. 18:218–222 [DOI] [PubMed] [Google Scholar]
- 21. Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. 1991. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351:290–296 [DOI] [PubMed] [Google Scholar]
- 22. Ferrari C, et al. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442–3449 [PubMed] [Google Scholar]
- 23. Fiedler M, Lu M, Siegel F, Whipple J, Roggendorf M. 2001. Immunization of woodchucks (Marmota monax) with hepatitis delta virus DNA vaccine. Vaccine 19:4618–4626 [DOI] [PubMed] [Google Scholar]
- 24. Fitzgerald JC, et al. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 Gag. J. Immunol. 170:1416–1422 [DOI] [PubMed] [Google Scholar]
- 25. Flatz L, et al. 2011. Single-cell gene-expression profiling reveals qualitatively distinct CD8 T cells elicited by different gene-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 108:5724–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frank I, et al. 2007. Acute resolving woodchuck hepatitis virus (WHV) infection is associated with a strong cytotoxic T-lymphocyte response to a single WHV core peptide. J. Virol. 81:7156–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujiwara T. 2011. A novel molecular therapy using bioengineered adenovirus for human gastrointestinal cancer. Acta Med. Okayama 65:151–162 [DOI] [PubMed] [Google Scholar]
- 28. Goonetilleke N, et al. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guidotti LG, et al. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825–829 [DOI] [PubMed] [Google Scholar]
- 30. Guzelbulut F, et al. 2012. Comparison of the efficacy of entecavir and tenofovir in chronic hepatitis B. Hepatogastroenterology 59:477–480 [DOI] [PubMed] [Google Scholar]
- 31. Harari A, et al. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hermening S, Kugler S, Bahr M, Isenmann S. 2004. Increased protein expression from adenoviral shuttle plasmids and vectors by insertion of a small chimeric intron sequence. J. Virol. Methods 122:73–77 [DOI] [PubMed] [Google Scholar]
- 33. Hervas-Stubbs S, et al. 1997. Therapeutic vaccination of woodchucks against chronic woodchuck hepatitis virus infection. J. Hepatol. 27:726–737 [DOI] [PubMed] [Google Scholar]
- 34. Hervas-Stubbs S, et al. 2001. T-helper cell response to woodchuck hepatitis virus antigens after therapeutic vaccination of chronically-infected animals treated with lamivudine. J. Hepatol. 35:105–111 [DOI] [PubMed] [Google Scholar]
- 35. Hoofnagle JH. 1981. Serologic markers of hepatitis B virus infection. Annu. Rev. Med. 32:1–11 [DOI] [PubMed] [Google Scholar]
- 36. Horiike N, et al. 2005. In vivo immunization by vaccine therapy following virus suppression by lamivudine: a novel approach for treating patients with chronic hepatitis B. J. Clin. Virol. 32:156–161 [DOI] [PubMed] [Google Scholar]
- 37. Jung MC, et al. 2002. Immunological monitoring during therapeutic vaccination as a prerequisite for the design of new effective therapies: induction of a vaccine-specific CD4+ T-cell proliferative response in chronic hepatitis B carriers. Vaccine 20:3598–3612 [DOI] [PubMed] [Google Scholar]
- 38. Jung MC, et al. 1991. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J. Hepatol. 13:310–317 [DOI] [PubMed] [Google Scholar]
- 39. Kwak MS, et al. 2011. Long-term efficacy of entecavir therapy in chronic hepatitis B patients with antiviral resistance to lamivudine and adefovir. J. Viral Hepat. 18:e432–e438 doi:10.1111/j.1365-2893.2011.01461.x [DOI] [PubMed] [Google Scholar]
- 40. Li HW, Gao YX, Raizada MK, Sumners C. 2005. Intronic enhancement of angiotensin II type 2 receptor transgene expression in vitro and in vivo. Biochem. Biophys. Res. Commun. 336:29–35 [DOI] [PubMed] [Google Scholar]
- 41. Lu M, et al. 1999. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J. Virol. 73:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu M, Isogawa M, Xu Y, Hilken G. 2005. Immunization with the gene expressing woodchuck hepatitis virus nucleocapsid protein fused to cytotoxic-T-lymphocyte-associated antigen 4 leads to enhanced specific immune responses in mice and woodchucks. J. Virol. 79:6368–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu M, et al. 2003. Induction of antibodies to the PreS region of surface antigens of woodchuck hepatitis virus (WHV) in chronic carrier woodchucks by immunizations with WHV surface antigens. J. Hepatol. 39:405–413 [DOI] [PubMed] [Google Scholar]
- 44. Lu M, et al. 2008. Combination of an antiviral drug and immunomodulation against hepadnaviral infection in the woodchuck model. J. Virol. 82:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maini MK, et al. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mancini-Bourgine M, et al. 2004. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 40:874–882 [DOI] [PubMed] [Google Scholar]
- 47. Menne S, et al. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237–250 [DOI] [PubMed] [Google Scholar]
- 48. Milich DR, McLachlan A, Thornton GB, Hughes JL. 1987. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature 329:547–549 [DOI] [PubMed] [Google Scholar]
- 49. Parker KC, Bednarek MA, Coligan JE. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163–175 [PubMed] [Google Scholar]
- 50. Penna A, et al. 1996. Long-lasting memory T cell responses following self-limited acute hepatitis B. J. Clin. Invest. 98:1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Penna A, et al. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 174:1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Penna A, et al. 1997. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology 25:1022–1027 [DOI] [PubMed] [Google Scholar]
- 53. Pesonen S, Kangasniemi L, Hemminki A. 2011. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol. Pharm. 8:12–28 [DOI] [PubMed] [Google Scholar]
- 54. Petersen J, et al. 2012. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J. Hepatol. 56:520–526 [DOI] [PubMed] [Google Scholar]
- 55. Piccolo P, et al. 2009. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir. Ther. 14:1165–1174 [DOI] [PubMed] [Google Scholar]
- 56. Pol S, et al. 1994. Specific vaccine therapy in chronic hepatitis B infection. Lancet 344:342. [DOI] [PubMed] [Google Scholar]
- 57. Pol S, et al. 2001. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J. Hepatol. 34:917–921 [DOI] [PubMed] [Google Scholar]
- 58. Precopio ML, et al. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J. Exp. Med. 204:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prud'homme GJ. 2005. DNA vaccination against tumors. J. Gene Med. 7:3–17 [DOI] [PubMed] [Google Scholar]
- 60. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219 [DOI] [PubMed] [Google Scholar]
- 61. Ratnam D, et al. 2011. The efficacy and tolerability of pegylated interferon-alpha-2a in chronic hepatitis B: a multicenter clinical experience. J. Gastroenterol. Hepatol. [Epub ahead of print.] doi: 10.1111/j.1440-1746.2011.07051.x [DOI] [PubMed] [Google Scholar]
- 62. Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 [DOI] [PubMed] [Google Scholar]
- 63. Ren F, et al. 2003. Cytokine-dependent anti-viral role of CD4-positive T cells in therapeutic vaccination against chronic hepatitis B viral infection. J. Med. Virol. 71:376–384 [DOI] [PubMed] [Google Scholar]
- 64. Richardson JS, et al. 2009. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One 4:e5308 doi:10.1371/journal.pone.0005308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roggendorf M, Tolle TK. 1995. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology 38:100–112 [DOI] [PubMed] [Google Scholar]
- 66. Rubio V, et al. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 9:1377–1382 [DOI] [PubMed] [Google Scholar]
- 67. Safadi R, et al. 2003. Treatment of chronic hepatitis B virus infection via oral immune regulation toward hepatitis B virus proteins. Am. J. Gastroenterol. 98:2505–2515 [DOI] [PubMed] [Google Scholar]
- 68. Sakurai F, Kawabata K, Yamaguchi T, Hayakawa T, Mizuguchi H. 2005. Optimization of adenovirus serotype 35 vectors for efficient transduction in human hematopoietic progenitors: comparison of promoter activities. Gene Ther. 12:1424–1433 [DOI] [PubMed] [Google Scholar]
- 69. Schlereth B, Germann PG, ter Meulen V, Niewiesk S. 2000. DNA vaccination with both the haemagglutinin and fusion proteins but not the nucleocapsid protein protects against experimental measles virus infection. J. Gen. Virol. 81:1321–1325 [DOI] [PubMed] [Google Scholar]
- 70. Shiver JW, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency virus immunity. Nature 415:331–335 [DOI] [PubMed] [Google Scholar]
- 71. Siegel F, Lu M, Roggendorf M. 2001. Coadministration of gamma interferon with DNA vaccine expressing woodchuck hepatitis virus (WHV) core antigen enhances the specific immune response and protects against WHV infection. J. Virol. 75:5036–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sullivan NJ, et al. 2006. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 3:e177 doi:10.1371/journal.pmed.0030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605–609 [DOI] [PubMed] [Google Scholar]
- 74. Thimme R, et al. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vandepapeliere P, et al. 2007. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 25:8585–8597 [DOI] [PubMed] [Google Scholar]
- 76. Wang J, Gujar SA, Cova L, Michalak TI. 2007. Bicistronic woodchuck hepatitis virus core and gamma interferon DNA vaccine can protect from hepatitis but does not elicit sterilizing antiviral immunity. J. Virol. 81:903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu L, Kong WP, Nabel GJ. 2005. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J. Virol. 79:8024–8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yalcin K, Acar M, Degertekin H. 2003. Specific hepatitis B vaccine therapy in inactive HBsAg carriers: a randomized controlled trial. Infection 31:221–225 [DOI] [PubMed] [Google Scholar]
- 79. Yang PL, et al. 2010. Immune effectors required for hepatitis B virus clearance. Proc. Natl. Acad. Sci. U. S. A. 107:798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang ZY, et al. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zakhartchouk AN, Viswanathan S, Mahony JB, Gauldie J, Babiuk LA. 2005. Severe acute respiratory syndrome coronavirus nucleocapsid protein expressed by an adenovirus vector is phosphorylated and immunogenic in mice. J. Gen. Virol. 86:211–215 [DOI] [PubMed] [Google Scholar]
- 82. Zelinskyy G, Dietze K, Sparwasser T, Dittmer U. 2009. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 5:e1000406 doi:10.1371/journal.ppat.1000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zimmerli SC, et al. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]