Abstract
Human cytomegalovirus (HCMV) starts its lytic replication cycle only in the G0/G1 phase of the cell division cycle. S/G2 cells can be infected but block the onset of immediate-early (IE) gene expression. This block can be overcome by inhibition of cyclin-dependent kinases (CDKs), suggesting that cyclin A2, the only cyclin with an S/G2-specific activity profile, may act as a negative regulator of viral gene expression. To directly test this hypothesis, we generated derivatives of an HCMV-permissive glioblastoma cell line that express cyclin A2 in a constitutive, cell cycle-independent manner. We demonstrate that even moderate cyclin A2 overexpression in G1 was sufficient to severely compromise the HCMV replicative cycle after high-multiplicity infection. This negative effect was composed of a strong but transient inhibition of IE gene transcription and a more sustained alteration of IE mRNA processing, resulting in reduced levels of UL37 and IE2, an essential transactivator of viral early gene expression. Consistently, cyclin A2-overexpressing cells showed a strong delay of viral early and late gene expression, as well as virus reproduction. All effects were dependent on CDK activity, as a cyclin A2 mutant deficient in CDK binding was unable to interfere with the HCMV infectious cycle. Interestingly, murine CMV, whose IE gene expression is known to be cell cycle independent, is not affected by cyclin A2. Instead, it upregulates cyclin A2-associated kinase activity upon infection. Understanding the mechanisms behind the HCMV-specific action of cyclin A2-CDK might reveal new targets for antiviral strategies.
INTRODUCTION
Human cytomegalovirus (HCMV) is an opportunistic pathogen that peacefully coexists with its host under normal conditions due to its ability to establish a latent, nonproductive infection. In immunocompromised or immunonaive individuals, however, the lytic mode of infection is favored, which, due to the broad cell tropism of HCMV, can result in severe disease.
Lytic replication of HCMV is a highly organized process and occurs in a cascade-like series of events. The starting point and prerequisite for all subsequent steps is the expression of immediate-early (IE) genes. Only a few loci (UL36 to -38, UL115 to -119, UL122 to -123, US3, and IRS1/TRS1) within the ∼240-kbp genome of HCMV are transcribed at IE times of infection, but alternative RNA splicing and translation initiation increase the diversity of the resulting gene products. To facilitate later phases of infection, IE proteins impair many cellular functions, including apoptosis (18, 43, 44, 62), cellular DNA synthesis (48, 72), STAT signaling (51), protein kinase R activity (10, 42), and major histocompatibility complex (MHC) class I-mediated antigen presentation (2, 31). Furthermore, IE proteins are responsible for the activation of viral early genes that encode proteins required for viral DNA replication (70). Most critical in this respect are the 72-kDa IE1 (synonym, IE72) and the 86-kDa IE2 (IE86) nuclear phosphoproteins, both originating from the abundantly transcribed “major IE” (MIE) gene loci UL122 and -123. IE1 derepresses early gene promoters by antagonizing PML-, Sp100-, Daxx/ATRX-, and HDAC3-mediated histone deacetylation (34, 50, 52, 64). It is required, however, only at low multiplicities of infection (MOI), because at high MOI, the increased abundance of incoming viral tegument proteins compensates for the loss of IE1 (20). In contrast, IE2, which contains the same N-terminal 85 amino acids as IE1, is an essential transactivator of early gene transcription (41). Due to IE2-responsive promoter elements within the viral origin of replication (oriLyt), IE2 is also directly involved in the initiation of viral DNA synthesis (77). Therefore, IE2 has attracted considerable interest as an antiviral target (66, 74), and an IE2-specific antisense RNA is already in clinical use for the treatment of HCMV retinitis (26).
Recently, we and others have shown that cells in the S/G2 phase of the cell division cycle exhibit a broad block to HCMV IE gene expression (14, 55, 73). This block acts at the mRNA expression level and affects not only IE1 and IE2, but also other IE gene loci, like US3 and UL36 to -38 (81). Inhibition of cyclin-dependent kinase (CDK) activity by pharmacological (roscovitine, CVT-313, and SU9516) or cellular (p21) inhibitors relieves the block and allows virus replication to proceed with normal kinetics (80). Interestingly, CDK inhibition was also sufficient to overcome the block to IE gene expression in nonpermissive NTera-2 cells (80), which serve as a model system for studying mechanisms of IE gene silencing during quiescent, latent-like HCMV infection (12). These results suggest a general role for CDK activity in the control of lytic gene expression and call for further investigations in this respect. A crucial, as yet unanswered question is which of the numerous different cellular cyclin-CDK complexes (19) are responsible for the CDK-dependent inhibition of IE gene expression. For several reasons, cyclin A2-CDK2 has been suggested as the most likely candidate (80). Due to E2F-dependent regulation of cyclin A2 transcription and anaphase promoting complex/cyclosome (APC/C)-dependent regulation of cyclin A2 protein stability, cyclin A2 expression is low during G0/G1, the HCMV-permissive phases of the cell cycle, and high during S/G2 (6, 78). Although the same holds true for cyclin B1, cyclin B1-CDK1 is kept inactive by S/G2 checkpoint kinases until mitosis. Thus, cyclin A2 and CDK2 constitute the only cyclin-CDK complex whose activity correlates with the cell cycle period of IE gene repression. Furthermore, except for CDK1, CDK2 is the only CDK that is sensitive to both p21 and roscovitine-like inhibitors (80). Finally, cyclin A2 expression is selectively suppressed by viral gene products in the early phase of productive HCMV infection (28, 55, 61, 72), pointing to an incompatibility between cyclin A2 and the progression of the replicative cycle. In line with this, the high cyclin A2 protein levels observed in undifferentiated NTera-2 cells have been suggested to contribute to the silencing of HCMV lytic gene expression in these cells (80).
Here, we show that cyclin A2-CDK indeed has a strong negative influence on lytic HCMV infection. It interferes with the accumulation and accurate processing of IE transcripts, leading to a sustained downregulation of IE2 and, consequently, to a significant delay and reduction of virus replication. Thus, the sensitivity of IE mRNA expression to cyclin A2-CDK seems to determine the long-mysterious cell cycle dependency of HCMV.
MATERIALS AND METHODS
Cells and viruses.
U373-MG cells (ATCC, Manassas, VA), HEK293-T cells (obtained from the DSMZ, Braunschweig, Germany), and mouse 3T3 cells (obtained from Addenbrooke's Hospital, Cambridge, United Kingdom) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMax (Life Technologies), 100 U/ml penicillin, and 100 μg/ml streptomycin. Human embryonic lung (HEL) fibroblasts (Fi301; obtained from the Institute of Virology, Charité, Berlin, Germany) were cultured in Eagle‘s minimum essential medium supplemented with Eagle's balanced salt solution, 25 mM HEPES, 10% FBS, 0.75‰ (wt/vol) sodium bicarbonate, nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-alanyl-l-glutamine, and 50 μg/ml gentamicin. Where indicated (see Fig. 4B), cells were synchronized in G0 by lowering the serum content for 48 h to 0% (HEL fibroblasts) and 0.2% (3T3 fibroblasts). HCMV strain AD169 (ATCC) and murine cytomegalovirus (MCMV) strain Smith (ATCC) were propagated on the above-mentioned fibroblasts. Viral titers were determined by expression of IE1/IE2, as described previously (80). When not otherwise indicated, an MOI of 5 to 10 IE-protein-forming units per cell was used for infection of cells. An HCMV-AD169 stock with 5-bromo-2′-deoxyuridine (BrdU)-labeled viral DNA was prepared essentially as described previously (54).
Fig 4.
MCMV is insensitive to cyclin A2 overexpression and induces cyclin A2-associated kinase activity after infection of quiescent cells. (A) Cyclin A2ΔD and cyclin A2ΔD(R211A)-expressing U373 derivatives (see the legend to Fig. 1), as well as control cells, were infected with MCMV. Five hours postinfection, the cells were harvested and analyzed for IE1 protein expression and DNA content by flow cytometry. (B, C, and D) Mouse 3T3 fibroblasts were lentivirus transduced with expression vectors for human and mouse cyclin A2. (B) Flow cytometric analysis of EGFP marker expression was performed to document homogeneously transduced cell pools. (C) The expression of exogenous cyclin A2 protein was checked by HA immunoblot analysis. A nonspecific band is indicated by an asterisk. (D) Cyclin A2-overexpressing and control 3T3 fibroblasts were infected with MCMV and analyzed at 5 h postinfection for IE1 protein expression and cellular DNA content by flow cytometry. (E) Fibroblasts of human (HEL) and mouse (3T3) origin were made quiescent by 48 h of serum starvation. Then (at 0 h), the cells were either restimulated by 10% fetal calf serum containing medium (+ serum), CMV infected in the presence of medium without serum (+ HCMV/MCMV), or both restimulated and infected by adding virus and serum containing fresh medium simultaneously (+ HCMV/MCMV, + serum). Cells were harvested at regular intervals and prepared for analysis of their endogenous cyclin A2-, B1-, and E1-associated kinase activities. Shorter intervals were chosen for the murine system, because both the 3T3 cell division and the MCMV replication cycle proceeded faster than for their human counterparts. Cyclin-CDK complexes were isolated from cell extracts by immunoprecipitation (IP) using antibodies that are proven to specifically bind the indicated cyclins without destroying the associated kinase activity. To measure this kinase activity, in vitro kinase assays were performed using histone H1 as a substrate. Shown are autoradiographic images of histone H1 phosphorylation. The results are representative of more than three independent experiments.
Flow cytometry.
Cells were harvested by trypsinization and permeabilized by incubation in ice-cold phosphate-buffered saline (PBS)/80% ethanol. Afterward, the cells were stained with virus-specific antibodies and propidium iodide, as described previously (73, 80). The following mouse monoclonal antibodies were used: anti-IE1/IE2 (clone E13; Argene), anti-gB (1-M-12; Santa Cruz Biotechnology), anti-pp28 (CH-19; Santa Cruz Biotechnology), and anti-ie1 (CroMA101; generously provided by Stipan Jonjic, Rijeka, Croatia). Alexa Fluor 488- or 647-conjugated goat anti-mouse IgG antibodies (Life Technologies) were used as secondary reagents. The cells were analyzed with a FACSCanto II flow cytometer (BD Biosciences) using FACSDiva and CellQuest software (BD Biosciences). Cellular debris and cell doublets and aggregates were gated out of analysis. All experiments were performed at least three times, and only representative data are shown.
Immunoblot analysis.
Whole-cell lysates were prepared by sonication in Laemmli buffer, as described previously (80), and were adjusted to equal protein concentrations using the Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories). Samples were then resolved by SDS-polyacrylamide gel electrophoresis and blotted to polyvinylidene fluoride membranes according to standard protocols. After blocking nonspecific binding sites in Tris-buffered saline/0.1% Tween 20 (TTBS)/5% skim milk, the blots were incubated with the following primary antibodies: anti-hemagglutinin (HA) (clone 12CA5; Roche); anti-IE1/2 (E13; Argene); anti-IE1 (6E1) and anti-IE2 (12E2) (both from Vancouver Biotech); anti-pUL22 (6F12), anti-pUL44 (CH16), anti-pUL84 (Mab84), anti-gB (CH28), anti-pp65 (CH12), anti-pp28 (CH19), anti-cyclin A2 (C-19), and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (6C5) (all from Santa Cruz Biotechnology); and anti-pp150 (XP1; generously provided by Bodo Plachter, Mainz, Germany). The blots were then incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibodies (sc-2054/2055; Santa Cruz Biotechnology) and developed with the chemiluminescence substrate SuperSignal West Dura (Thermo-Fisher Scientific).
Immunofluorescence microscopy.
Cells were grown on glass coverslips and infected with BrdU-labeled HCMV, as described previously (81). At 3 h postinfection, cells were fixed, permeabilized, and sequentially stained for viral gene expression and BrdU localization, exactly as described previously (81). The following antibodies were used: mouse anti-IE1/IE2 (clone 8B1.2; Merck-Millipore), mouse anti-BrdU (clone 3D4; Becton, Dickinson), and Alexa Fluor 647-conjugated goat anti-mouse IgG2a and Alexa Fluor 594-conjugated goat anti-mouse IgG1 (Life Technologies). Coverslips were mounted in 4′,6-diamidino-2-phenylindol (DAPI)-containing Fluoromount G medium (Southern Biotech). Images were acquired with an Eclipse A1 laser scanning microscope using NIS-Elements software (Nikon Instruments). Equal microscope settings and exposure times were used to allow direct comparison between samples.
Subcellular fractionation.
Cells were harvested by trypsinization and washed with PBS. Then, the cells were incubated in hypotonic buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 10% glycerol, 0.2% Igepal CA-630, 1 mM EDTA, 1 mM dithiothreitol [DTT], 0.1 mM Na3VO4, 0.1 mM Pefabloc, 2 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μM pepstatin) for 5 min and centrifuged for 15 s at 15,000 × g. The supernatants were further clarified by 10 min of centrifugation at 15,000 × g (4°C) and saved as cytoplasmic extracts. The pelleted nuclei were washed once with PBS and then either processed for immunoblot analysis (lysis in Laemmli buffer [see above]) or used to prepare DNA for quantitation of nuclear-localized viral genomes by real-time PCR analysis.
Kinase assays.
The following antibodies were used, together with protein A/G Sepharose (GE Amersham) to immunoprecipitate cyclin-CDK complexes from cell extracts: cyclin A2 (H-432), cyclin B1 (GNS1), human cyclin E1 (HE111), and mouse cyclin E1 (M-20) (all from Santa Cruz Biotechnology). Immunoprecipitations, as well as subsequent kinase assays, were carried out as described previously (72).
Plasmids.
Cyclin A2ΔD and cyclin A2ΔD(R211A) were kindly provided by Anindya Dutta (University of Virginia) and subcloned by us into the lentiviral expression vector pCDH-CMV-MCS-EF1-GFP-T2A-Puro (System Biosciences) in frame to an N-terminal triple-hemagglutinin (3HA) epitope tag. The resulting plasmids were named pCDH-3HA-cyclinA2-ΔD and pCDH-3HA-cyclin A2ΔD(R211A), and their correctness was confirmed by sequencing. Wild-type coding sequences of human and mouse cyclin A2 were amplified from cDNA libraries (made from RNA preparations from primary fibroblasts of the corresponding species) and cloned into the same lentiviral vector (pCDH-3HA) context. All plasmids were purified by CsCl ethidium bromide equilibrium centrifugation prior to transfection.
Lentiviral transduction.
For production of lentivirus particles, the cyclin A2 expression vectors were cotransfected with packaging (psPAX2) and envelope (pMD2.G) vectors (both obtained from Addgene) into HEK293-T cells. Lipofectamine 2000 (Life Technologies) was used as a transfection reagent. Lentiviruses were harvested from the supernatant 2 to 3 days posttransfection. Viral titers were determined by infecting U373 cells with diluted virus stocks and analyzing the number of green fluorescent protein (GFP)-positive cells by flow cytometry. For experiments, cells were infected at an average MOI of 3 to 5 infectious units (IU) per cell. Stably transduced cells were selected by adding puromycin (4 μg/ml) to the culture medium 72 h postinfection.
Quantitative real-time PCR.
Total cellular RNA was prepared using TRIzol (Life Technologies), and its integrity was checked by agarose gel electrophoresis. RNA (1 μg) from each sample was reverse transcribed to cDNA using the QuantiTect Reverse Transcription Kit from Qiagen. Prior to PCR, cDNA concentrations were adjusted to 2.5 ng/μl. Total DNA of cells and purified nuclei was prepared according to the method of Sambrook et al. (56). Briefly, cells/nuclei were incubated in 10 mM Tris-Cl, pH 8.0, 0.1 M EDTA, 0.5% SDS, 20 μg/ml RNase A for 1 h at 37°C. Then, proteinase K was added to a final concentration of 100 μg/ml, and the incubation was continued for 12 h at 50°C. This was followed by successive phenol and 50% phenol-50% chloroform extraction steps. Finally, DNA was precipitated and washed with 70% ethanol. Quantitative real-time PCR was performed on a 7500 Fast Real-Time PCR System (Life Technologies) using the SYBR green method. Gene-specific primers are listed in Table 1. All samples were analyzed in triplicate. Analysis of data was performed using the ABI 7500 software v2.0.5 (Life Technologies) and Excel (Microsoft). The mRNA expression levels were normalized by subtraction of ribosomal protein L32 threshold cycle (CT) values, resulting in ΔCT values of the analyzed samples. ΔΔCT values represent the difference between two samples, and the fold change of these two values can be calculated as 2−ΔΔCT. Viral DNA was normalized to the cellular DNA input using primers specific for the genomic POLR3C locus (35).
Table 1.
Primers used for quantitative real-time PCR
| Primer name | Primer sequence (orientation 5′–3′) |
|---|---|
| IE1 forward | GCCTTCCCTAAGACCACCAAT |
| IE1 reverse | ATTTTCTGGGCATAAGACATAATC |
| IE2 forward | TGACCGAGGATTGCAACGA |
| IE2 reverse | CGGCATGATTGACAGCCTG |
| IE ex3 forward | TCTGCCAGGACATCTTTCTCG |
| IE ex3 reverse | GGAGACCCGCTGTTTCCAG |
| UL112/113 forward | CAGCAGGGCTTCATGTCTATT |
| UL112/113 reverse | CGCGTACAAAGAGGTGCTC |
| UL37x1 forward | TGCGAAGCCCTCAAAAAG |
| UL37x1 reverse | ATCCCGGAGTCTGTGGTTTT |
| UL37 forward | GCGAAGCCCTCAAAAAGG |
| UL37 reverse | CAGCCACACAGAAACCTGAA |
| US3 forward | GGATGGACTATAGCTCTCAGACC |
| US3 reverse | AGAGAACAGATATACGAGCAGGG |
| US3–SS forward | CTCTCAATCTTACATGGACAGAC |
| US3–SS reverse | GGTTCGTACCTGAGGAACAC |
| US3–DS forward | CTGGATGTGGTGACTGCAGG |
| US3–DS reverse | CCTCGTAGACAACGTGATGTCC |
| UL111A (ORF79) forward | CGATAAAGAATACAAAGCCGCA |
| UL111A (ORF79) reverse | AAAGACCGTCGCAATAAACC |
| UL111A–SS (LAcmvIL–10) forward | CCTACGTTGCAACGTGAGGA |
| UL111A–SS (LAcmvIL–10) reverse | CGTGCTATGAACACGTTGTTACC |
| UL111A–DS (cmvIL10) forward | GGCAATGTCCTCTGTTAGGT |
| UL111A–DS (cmvIL10) reverse | CCGTTATCCGATTTCCTTTCC |
| TRS1 forward | CTGTGCAAATGTGGAAGATACCT |
| TRS1 reverse | GTCCAGTCCCAGAGCTTGAG |
| L32 forward | CAAGGAGCTGGAAGTGCTGC |
| L32 reverse | CAGCTCTTTCCACGATGGC |
| POLR3C forward | GGAAAATGGTAAAGGGTGGTG |
| POLR3C reverse | CATCTTCCCTAACCTGGGAGA |
RESULTS
Cyclin A2 interferes with HCMV gene expression in a CDK-dependent manner.
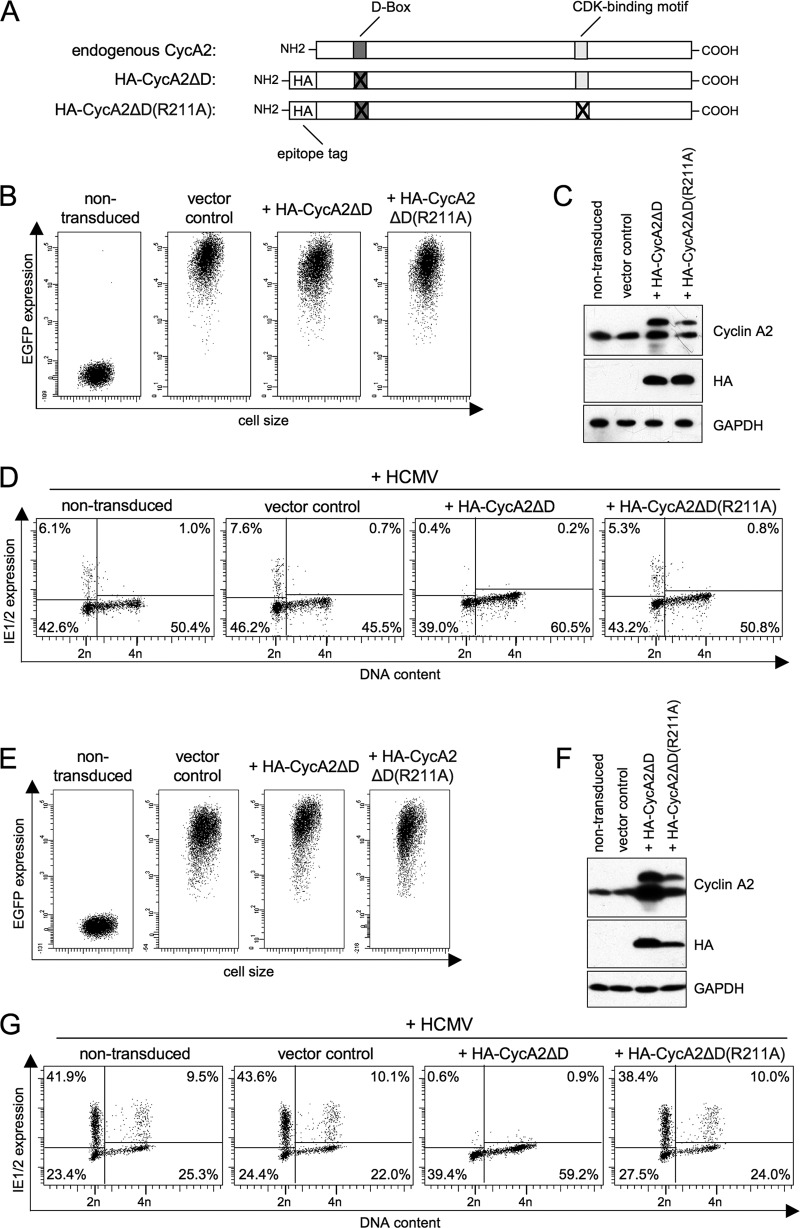
To investigate the influence of cyclin A2 on the HCMV lytic cycle, we created cell lines with stable, cell cycle-independent expression of cyclin A2 and tested if they still supported viral gene expression in G1. Constitutive cyclin A2 expression was achieved by lentiviral transduction of an expression cassette that provides cell cycle-independent transcription of a cyclin A2 mutant (cyclin A2ΔD) that is resistant to APC/C-dependent protein degradation (40). As a control, we transduced cyclin A2ΔD(R211A) containing an additional point mutation that impairs CDK binding (37). Both mutated forms of cyclin A2 were HA tagged to enable discrimination from endogenous cyclin A2 (Fig. 1A). Because primary cells react to aberrant cyclin A2 expression with p53-dependent checkpoint activation and induction of premature senescence (68), we had to rely on transformed cells for our analysis. We chose the fully permissive glioblastoma cell line U373 and the human embryonic kidney carcinoma cell line HEK293, which is nonpermissive for HCMV but allows IE gene expression in a strictly G1-dependent manner, thereby faithfully reflecting the situation in primary cells (80). Lentivirus infection and the following puromycin selection step resulted in homogeneous populations of transduced cells (Fig. 1B and E). Due to moderate virus input levels, the stable exogenous forms of cyclin A2 were found to be expressed under proliferative conditions to overall degrees similar to those of endogenous cyclin A2 (Fig. 1C and F). This was important to minimize unwanted negative effects of cyclin A2 overexpression on cell cycle progression in G2/M. After HCMV infection, cells transduced with an empty lentiviral vector showed the same G1-specific pattern of IE1/IE2 expression at 5 h postinfection (p.i.) as nontransduced control cells, demonstrating that the process of lentiviral infection and genomic integration itself had no influence on the block in S/G2 (Fig. 1D and G). For U373, an additional subpopulation of IE-positive cells with a 4n-DNA content (n is the haploid number of chromosomes) was consistently observed. This population appears to consist of nonproliferating cells that, probably as a consequence of checkpoint activation, remain G2 arrested even under conditions of contact inhibition or serum starvation (see Fig. 5A and data not shown) and therefore, with respect to CDK1/2 activity, more closely resemble G1 cells. Expression of cyclin A2ΔD almost completely abolished IE1/IE2 expression in any cell cycle phase. This effect was dependent on CDK binding, as the cyclin A2ΔD(R211A) mutant behaved like negative controls (Fig. 1D and G). These results suggest that cyclin A2-associated kinase activity is a powerful negative regulator of HCMV lytic gene expression.
Fig 1.
Cyclin A2 overexpression is sufficient to block the onset of IE gene expression in HCMV-infected cells. (A) Schematic overview of lentivirus-expressed cyclin A2 mutants and the endogenous wild-type form. The D box of cyclin A2 was destroyed by the double point mutation R47A/L50V (ΔD). An arginine residue essential for CDK binding was eliminated by the R211A mutation. HA, hemagglutinin tag. (B to G) HEK293 (B to D) and U373 (E to G) cells were transduced with expression vectors for the indicated cyclin A2 mutants or with an empty expression vector (vector control) or were left untransduced. (B and E) The efficiency of transduction was controlled by flow cytometry of the vector-encoded enhanced green fluorescent protein (EGFP) marker. (C and F) The relative levels of exogenous and endogenous cyclin A2 protein expression were determined by immunoblot analysis. (D and G) Cells were infected with HCMV and analyzed at 5 h postinfection by flow cytometry for IE1/IE2 expression and DNA content. Shown are dot plots (n = haploid number of chromosomes) in which cells were divided into four subpopulations: IE1/2-negative G0/G1 cells (lower left quadrant), IE1/2-positive G0/G1 cells (upper left quadrant), IE1/2-positive S/G2/M cells (upper right quadrant), and IE1/2-negative S/G2/M cells (lower right quadrant). The proportion of each subpopulation is indicated as a percentage of the total cells.
Fig 5.
Cyclin A2-CDK activity has a long-lasting negative effect on the early and late phases of HCMV infection. U373 cells were lentivirus transduced as indicated and enriched in G1 phase by density arrest before HCMV infection. (A) The cell cycle distributions at the time of infection and at the indicated time points postinfection were analyzed by flow cytometry. Shown are DNA histograms of propidium iodide-stained cells (n is the haploid number of chromosomes). (B) After infection, cells were harvested at regular intervals and analyzed for expression of immediate-early (IE1/2), early (gB), and late (pp28) proteins by flow cytometry. (C) In parallel, virus growth curves were obtained by determining the number of infectious particles in the cell culture supernatants. (B and C) Means and standard deviations of three independent experiments (until 96 h p.i.), all of them using an MOI of approximately 10. From 120 to 240 h p.i., the values represent averages from two of these experiments. (D) The experiment was repeated using an MOI of approximately 0.1. Shown are growth kinetics, where the data points show the mean values of biological triplicates and the error bars indicate standard deviations.
As cyclin A2-CDK complexes shuttle between the nucleus and the cytoplasm (27) and can be found in both cellular compartments in U373 cells (Fig. 2B), we wanted to know whether cyclin A2 is inhibitory for cytoplasmic or nuclear events during the pre-IE phase of HCMV infection. To this end, we analyzed the subcellular localization of viral genomes at 3 h p.i., a time point at which, in control cells, the newly produced major IE proteins were already detectable (Fig. 2A). Using confocal microscopy and subcellular fractionation methods, it became clear, at both the single-cell and population levels, that cyclin A2 has no influence on the number of viral genomes reaching the nucleus within this time (Fig. 2A and C). This is consistent with our previous analyses of S-phase cells (81) and suggests that neither the processes of cellular and nuclear entry nor the cytoplasmic transport of viral particles is targeted by cyclin A2-dependent regulation.
Fig 2.
Nuclear entry of viral genomes is unaffected by cyclin A2. Cyclin A2-overexpressing cells and the indicated control U373 cells were grown to confluence and infected with BrdU-labeled HCMV. (A) At 3 h postinfection, cells were fixed and analyzed for IE1/IE2 protein expression and subcellular localization of viral genomes by confocal immunofluorescence microscopy. DAPI was used as a nuclear counterstain. (B and C) In a parallel approach, cells were harvested at 3 h postinfection and fractionated into nuclei (nu) and cytoplasm (cy) by hypotonic lysis. (B) The purity of fractions was confirmed and compared to that of whole-cell lysates (wh) by immunoblot analysis of β-tubulin (cytoplasmic marker) and lamin A/C (nuclear marker). In addition, the nucleocytoplasmic distribution of exogenous cyclin A2 was controlled using HA and cyclin A2 antibodies. (C) Total DNA isolated from whole cells and from purified nuclei was analyzed for the relative amount of viral DNA by quantitative real-time PCR using UL112/113-specific primers. The ratios of nuclear and whole-cell viral DNA contents are given as mean values of two independent experiments.
To determine if the antiviral effect of cyclin A2 overexpression is specific to the type of cyclin used, we also tested cyclin B1, the other major cyclin that is expressed with S/G2 kinetics in somatic cells. For this experiment, we transduced U373 cells with the HA-tagged wild-type (wt) forms of both cyclins (Fig. 3), as it turned out during the course of the study that the lentiviral transcription unit was sufficient to drive cell cycle-independent expression of vector-encoded cyclin A2 (data not shown). The transduction efficiencies and expression levels of the two cyclins were very similar (Fig. 3A and B), allowing direct comparison of their influence on HCMV infection. Whereas cyclin A2-wt caused a 10-fold decrease in the number of IE1/IE2-positive cells at 5 h p.i., cyclin B1-overexpressing cells supported viral gene expression to the same extent as vector-transduced control cells (Fig. 3C). This argues that the cell cycle-dependent inhibition of HCMV gene expression is mediated by a cyclin A2-specific mechanism.
Fig 3.
Cyclin B1 overexpression does not interfere with IE1/IE2 gene expression. U373 cells were stably transduced with lentiviral expression vectors for HA-tagged wt forms of cyclin A2 and cyclin B1, as indicated. (A) After puromycin selection, the expression of cotransduced EGFP was controlled by flow cytometry and compared to that of nontransduced cells. (B) Protein levels of exogenous HA-tagged cyclins and the corresponding endogenous cyclins were compared by immunoblot analysis. (C) Five hours after HCMV infection, cells were analyzed for IE1/IE2 protein expression and DNA content as described in the legends to Fig. 1D and G.
If such a cyclin A2-CDK-dependent mechanism blocks the onset of HCMV gene expression in normal S/G2 cells, then MCMV, which is known to be cell cycle independent (73), should prove to be insensitive to cyclin A2 overexpression. To test this prediction, we repeated our analysis with MCMV, using cyclin A2-overexpressing U373 cells and 3T3 (mouse) fibroblasts for infection. In accordance with our prediction, cell cycle-independent MCMV IE1 protein expression was detected not only in control cells, but also in cells that were stably transduced with either human cyclin A2ΔD (Fig. 4A), human cyclin A2-wt, or mouse cyclin A2-wt (Fig. 4B to D). This was independent of the host cell species and demonstrates that the cyclin A2-CDK-dependent blockade of IE gene expression is HCMV specific and correlates with the cell cycle-dependent nature of the virus.
Having shown that MCMV, unlike HCMV, fails to be negatively regulated by cyclin A2-CDK, we asked whether this difference may also be reflected at the level of virus-mediated regulation of cyclin A2-CDK activity during the course of lytic infection. To address this point, we used an approach that has been successfully employed in a number of studies to investigate the influence of HCMV on cyclin-dependent kinases and other cell cycle-related activities (5, 28, 71, 72, 75). The main principle of this approach is to infect cells (mostly nontransformed fibroblasts) that have been synchronized in G0/G1 by growth factor deprivation or contact inhibition. This treatment results in a general downregulation of cyclin-CDK activities (0-h time point in Fig. 4E) and allows the measurement of either the positive or the negative viral impact on such activities in the absence and presence of a cyclin-CDK-activating serum stimulus. In our setting, both HCMV and MCMV were able to induce cyclin E1- and cyclin B1-associated kinase activity in a growth factor-independent manner (Fig. 4E). In contrast, whereas MCMV infection also leads to constitutively high levels of cyclin A2-CDK activity, HCMV lacks this ability and, in addition, strongly delays serum-dependent cyclin A2 induction, consistent with previous observations (28, 72). These findings support the view that cyclin A2-CDK activity is incompatible with the early phase of HCMV infection, and therefore, HCMV has evolved mechanisms to downregulate it.
We then decided to further exploit the U373 system to analyze the long-term effects of cyclin A2 overexpression on HCMV replication. To ensure comparable starting conditions, this time, we allowed the U373 cultures to grow to confluence, so that the majority of cells had accumulated in G0/G1 before infection (Fig. 5A). Strikingly, HCMV infection stimulated confluent cyclin A2ΔD-expressing cells to reenter the cell cycle and undergo an additional cell division during the first 48 h postinfection (Fig. 5A). This was most probably a direct consequence of deficient IE gene expression in these cells, as the cell cycle arrest activity of the IE1 and IE2 proteins (8, 72) is known to counteract potent S-phase-inducing virion proteins, like pp71 and pUL97 (23, 33). In contrast, the increase of DNA content seen in HCMV-infected control cells from 24 to 48 h p.i. (Fig. 5A) is a typical consequence of the accumulation of newly replicated viral DNA (11, 39).
To monitor the progression of the viral replicative cycle in more detail, we analyzed the expression of representative IE, early, and late genes and the release of virus progeny on a daily basis. We first used flow cytometry to determine the percentages of cells that had reached the different stages of infection. It became obvious that the strong CDK-dependent block of IE gene expression seen at 5 h p.i. could not be maintained in all cells over the full observation period (Fig. 5B). At 24 h p.i., the number of cyclin A2ΔD-expressing cells staining positive with the IE1/IE2 antibody had already reached one-third of control values and increased further to one-half at 48 h p.i. and two-thirds at 168 h p.i. Interestingly, the adverse effects of cyclin A2ΔD on the early and late phases of infection were much more robust. In more than 80% of cells, the onset of gB and pp28 expression was delayed by at least 3 to 4 days compared to controls. Even after 10 days the numbers of gB- and pp28-positive cells remained below 50% of control values. The sustained repression of early/late gene expression was reflected by a similar delay and attenuation in production of new virus (Fig. 5C). All these effects depend on the ability of cyclin A2 to bind to its catalytic subunit, as they were prevented by the R211A mutation. Cyclin A2ΔD expression also strongly retarded, but did not permanently suppress, virus growth under low-MOI conditions (Fig. 5D). Thus, the general effectiveness of the CDK-mediated block seems to be MOI independent.
IE1 and IE2 are differentially regulated by cyclin A2-CDK at the level of mRNA processing.
We next tried to explain the finding that the effect of cyclin A2 on early and late viral genes was seemingly more sustained than that on IE1/2 expression. As IE2 is known to be essential for the start of early, and hence also late, gene expression, we considered the possibility that in the above-described setting we had overlooked a stronger effect on IE2. This was not unlikely, in that IE1 is the dominantly expressed protein in the early phase of infection and the antibody we had used does not allow us to distinguish between IE1 and IE2 by flow cytometry. Therefore, we reanalyzed the previous experiments by Western blotting and discriminated IE1 and IE2 by size (Fig. 6A and B) or by using exon 4- and 5-specific antibodies (Fig. 6B). The results were remarkably clear cut: expression of both IE1 and IE2 was completely blocked by cyclin A2-CDK at 5 h p.i., as expected from the flow cytometry data. However, whereas the blocking of IE1 was only transient and the IE1 72-kDa protein had almost reached control levels after 1 to 2 days, it took at least 4 days before the 86-kDa and 55-kDa forms of IE2 became weakly detectable in cyclin A2ΔD-expressing cells (Fig. 6B). This stronger delay of IE2 expression correlated well with the late appearance of early and late gene products (Fig. 6A). One might speculate that the effects on viral gene expression would be even more sustained if the levels of exogenous cyclin A2 did not decrease over time in density-arrested infected cells (HA immunoblot in Fig. 6A).
Fig 6.

Cyclin A2 has a stronger effect on IE2 than on IE1 expression. The indicated U373 derivatives were grown to confluence and infected with HCMV using an MOI of approximately 10. (A) The expression of selected viral gene products and of exogenous, HA-tagged cyclin A2 was monitored by immunoblot analysis from 5 to 96 h p.i. (B) IE1 and IE2 protein expression was analyzed in more detail by using exon 4 (IE1 [top])-, exon 5 (IE2 [second panel from top])-, and exon 3 (IE1 and IE2 [third panel from top])-specific antibodies. The relative molecular masses of the different splice variants are indicated. As the 55-kDa form of IE2 lacks the exon 3-encoded N terminus of IE1 and IE2 full-length proteins, it appears only in the exon 5-specific blot. (A and B) Equal protein content of samples was controlled by Coomassie staining (bottom gels). Shown are representative results from three independent experiments.
Since IE1 and IE2 arise from the same pre-mRNA, IE2-specific long-term repression by cyclin A2 must be a posttranscriptional event. To determine whether it occurs at the level of mRNA processing, we analyzed IE1 and IE2 mRNA expression by real-time PCR using exon-specific primers. Consistent with the protein data, we found that cyclin A2ΔD-CDK was responsible for an almost complete lack of IE1 and IE2 mRNAs at 5 h p.i. In control cells, transcription from the MIE locus had already reached peak levels at this time (Fig. 7, left). After 24 h p.i., IE1 mRNA expression in cyclin A2ΔD-positive cells was still moderate compared to the initial boost in control cells but had increased to steady-state levels that were in the range of control values during late stages of HCMV infection. In contrast, IE2 mRNA expression was more severely affected by cyclin A2-CDK and never reached control levels during the first 96 h of infection (Fig. 7, upper right). This was mirrored by a strongly delayed and attenuated induction of the bona fide IE2 target gene UL112/113 (3, 53) (Fig. 7, lower right). Thus, one can conclude that cyclin A2-CDK, besides inhibiting IE1/IE2 transcription, has an additional effect on IE1/IE2 pre-mRNA splicing or IE2 mRNA stability that is responsible for the long-lasting reduction of HCMV early and late gene expression.
Fig 7.
Sustained inhibition of IE2 mRNA accumulation by cyclin A2-CDK. U373 parental cells and the indicated lentivirus-transduced derivatives were density arrested and HCMV infected. At the indicated times postinfection, total RNA was prepared from infected cells and analyzed for IE1, IE2, and UL112/113 mRNA expression by quantitative real-time PCR. The results are shown as fold change of mRNA expression compared to the values of nontransduced cells at 5 h p.i. Presented are the means and standard deviations of technical triplicates. Where no standard deviation is indicated, the means of technical duplicates are shown.
To determine whether IE1 and/or IE2 mRNA stability is affected by cyclin A2 overexpression, we analyzed the rate of IE1/IE2 mRNA decrease following inhibition of de novo mRNA synthesis by actinomycin D. Actinomycin D treatment was started at 72 h p.i., when, in cyclin A2ΔD-transduced cells, IE1 mRNA expression had reached control levels while IE2 was still repressed (Fig. 8A). After 24 h of actinomycin D treatment (which corresponds to 96 h p.i.), both IE1 and IE2 mRNA levels had fallen to about one-fifth of their initial values in control cells (Fig. 8B). In the presence of cyclin A2ΔD, both mRNAs appeared to be slightly more stable, with no evidence of any differential regulation of either IE1 or IE2. This suggests that cyclin A2-CDK shifts the normal IE1/IE2 balance toward IE1 by negatively affecting IE2 splicing, and not mRNA stability.
Fig 8.
Reduced IE2 mRNA levels in cyclin A2-overexpressing cells are not due to decreased IE2 mRNA stability. The indicated stably transduced U373 derivatives were first infected with HCMV and then, at 72 h p.i., treated with 5 μg/ml actinomycin D or dimethyl sulfoxide (DMSO) as a solvent control. At the beginning of actinomycin D treatment (0 h) and at the indicated intervals thereafter, cells were harvested and analyzed for IE1 and IE2 mRNA content by quantitative real-time PCR. (A) Shown are the relative IE1 (left) and IE2 (right) mRNA expression levels at 0 h posttreatment (72 h p.i.). The values of empty-vector-transduced cells (vector control) were used as a reference and set to 1. (B) Profiles of IE1 (left) and IE2 (right) mRNA expression over the actinomycin D/DMSO treatment period of 24 h. The results are shown as fold change of the corresponding initial values of each cell line at 0 h (72 h p.i.). The means and standard deviations of technical triplicates are presented. Where no standard deviation is indicated, only means of technical duplicates are shown. The data are representative of two independent experiments.
The CDK-dependent effect on mRNA processing is gene specific and also influences the UL37 locus.
To investigate whether expression from other IE gene loci is also impaired by cyclin A2 at the transcriptional and/or posttranscriptional level, we extended our analysis to UL37 and US3. Like IE1/IE2, both genes are known to be repressed in S/G2 (81) and to give rise to different splice products in the productive phase of infection (15). US3 transcripts are subject to internal splicing and exist as unspliced, singly spliced, and doubly spliced isoforms (60, 65). Transcription from the UL37 promoter leads to the abundant unspliced UL37x1 gene product and to two differentially spliced UL37 isoforms that can be distinguished from UL37x1 by the presence of exon 2 (1, 63). All three US3 mRNA species showed expression kinetics that were very similar to that of IE1 (see above). The pronounced peak of US3 transcription at 5 h p.i. was completely prevented by cyclin A2ΔD expression, whereas at later time points, US3 levels recovered and reached normal steady-state levels between 24 and 48 h p.i. (Fig. 9). In contrast, spliced and unspliced UL37 transcripts markedly differ in their responses to cyclin A2ΔD. UL37x1 mRNA expression was strongly but transiently blocked in a CDK-dependent manner, as seen before for US3 and IE1. UL37 splice products, on the other hand, remained almost undetectable in cyclin A2ΔD-expressing cells until 96 h p.i. This suggests that UL37, but not US3, mRNA processing is under the control of cyclin A2-CDK activity. Another IE gene, TRS1, which is not subject to alternative splicing, showed an intermediate phenotype. Its transcription, as for all other IE gene loci, was found to be strongly repressed at 5 h p.i. At later times of infection, it recovered to a certain extent, but in contrast to IE1, US3, and UL37x1, it never reached control levels (Fig. 9).
Fig 9.
Suppression of UL37 splicing by cyclin A2-CDK. Samples already tested for IE1/IE2 mRNA content (see the legend to Fig. 5) were analyzed for relative mRNA expression levels of TRS1; unspliced UL37x1; spliced UL37 (using an exon 1- and 2-specific primer pair); and unspliced, single-spliced (SS), and double-spliced (DS) US3 and UL111A, as indicated. The results are depicted as fold change of mRNA expression compared to the values of nontransduced cells at 5 h p.i. Shown are the means and standard deviations of technical triplicates. Where no standard deviation is indicated, means of technical duplicates are shown. The data are representative of two independent experiments.
We also included UL111A in our analysis to address the question of whether CDK-dependent regulation of viral mRNA processing could have an impact on HCMV latency-associated gene expression. UL111A encodes a viral interleukin 10 homologue (cmvIL-10) and has a splicing pattern that closely mimics that of US3 (30), with the interesting difference that the singly spliced UL111A variant (LAcmvIL-10) can also be found in latently infected cells (29). Using suitable intron- and exon-exon junction-specific primers for quantitative real-time PCR analysis, we found all UL111A isoforms to accumulate with early-late kinetics in control cells and to react to cyclin A2ΔD expression with similar delays of 2 to 3 days (Fig. 9). This finding argues against a particular role of cyclin A2 in the control of LAcmvIL-10 expression and further supports the notion that the prolonged downregulation of IE2 and UL37 mRNA expression by cyclin A2-CDK is a gene-specific phenomenon.
DISCUSSION
The onset of IE gene expression as the critical first step of the HCMV lytic cycle is under the control of cell type-, cell differentiation-, and cell cycle-dependent mechanisms that are poorly understood at the molecular level. Here, we provide, for the first time, direct evidence that cyclin A2 in complex with its catalytic CDK subunit is a potent negative regulator of HCMV IE gene expression and that the absence of cyclin A2 expression in G0/G1 phase is a prerequisite for efficient virus replication in this cell cycle compartment. Cyclin A2-induced inhibition of HCMV shows key features of the well-documented blockade of lytic gene expression in S/G2 (80, 81): (i) it is CDK dependent (Fig. 1), (ii) it occurs after nuclear entry of viral DNA (Fig. 2), (iii) it acts at the mRNA level (Fig. 7), and (iv) it has a general effect on IE transcription, compromising not only the major IE genes IE1 and IE2, but also others, like US3, UL37, and TRS1 (Fig. 9). In addition, the closely related but cell cycle-independent MCMV proved to be inert to cyclin A2 overexpression (Fig. 4). Collectively, this suggests that cyclin A2 is the main cellular determinant of HCMV cell cycle dependency.
Cyclin A2 recruits CDK2 (during S/G2) and CDK1 (at the G2/M transition) into active complexes (45, 46). The same CDKs can be bound and activated by E- and B-type cyclins and by the germ cell-specific cyclin A1. At present, with the exception of cyclin B1 (Fig. 3), we cannot exclude the possibility that one (or several) of those cyclins exerts an antiviral function similar to that of cyclin A2 in the pre-IE phase of HCMV infection. In particular, cyclins E1 and E2 are known to have a substrate spectrum overlapping with that of cyclin A2 and are able to compensate for its loss in cyclin A2 knockout fibroblasts (32). For the following reasons, however, it appears unlikely that cyclin E-associated kinase activity has the same negative effect on HCMV as cyclin A2-CDK. First, it has been demonstrated by Fortunato and coworkers that HCMV IE gene expression can be initiated in late G1 (14), which is the cell cycle period where cyclin E-CDK2 activity sharply peaks and controls S-phase entry (24, 47). Second, cyclin E1 and E2 expression, as well as cyclin E1-CDK2 activity, has been found to be upregulated by the virus shortly after the start of IE gene expression (7, 21, 28, 72). The same is true for the expression and activity of cyclin B1, but not those of cyclin A2 (28, 72), even though the cyclin A2 promoter is a natural downstream target of cyclin E (36). This indicates that cyclin A2 expression is actively suppressed by HCMV and that this suppression is sufficient for the virus to escape any negative control by CDKs. If this is true, one could restrict the search for potential CDK substrates mediating the inhibition of IE gene expression in S/G2 to those candidates that are phosphorylated by cyclin A2 but not cyclin E-CDK complexes. Examples of such substrates are the cellular replication factors MCM2, MCM4 (69), and RPA (17). Our view that the substrate specificity of the cyclin subunit is decisive for the CDK-dependent blockade of HCMV is further supported by the fact that the virus itself encodes a kinase (pUL97) that functions as a cyclin-independent CDK1 and -2 orthologue (22, 23). As a component of the viral tegument (9, 67, 76), pUL97 is already present at pre-IE times of infection. However, it is unlikely to be involved in the CDK-dependent block of IE gene expression, since the CDK1/2 inhibitors that have been used to overcome this block do not inhibit pUL97 (23, 80).
The block of viral gene expression in HCMV-infected S/G2 cells is not permanent, as it is relieved after the cells have completed the division cycle and entered G1 (55). This is in accord with the mitotic shutdown of cyclin A2 expression, which is destroyed in prophase by the 26S proteasome (16) and whose de novo synthesis in G1 is prevented by E2F-mediated promoter repression (59). Therefore, stable ectopic cyclin A2 expression in one of the few virus-permissive cell lines allowed us for the first time to study the long-term effects of this S/G2-specific CDK activity on the infectious cycle of HCMV. Our analysis revealed a bimodal inhibitory effect of cyclin A2-CDK on IE gene expression, consisting of a general but transient blocking of transcript accumulation and a more sustained but gene-specific change in transcript processing. The latter effect leads to a pronounced downregulation of IE2 and UL37 mRNAs, a finding that is strikingly reminiscent of previous results of Sanchez et al. (57). These authors showed that roscovitine treatment during the first 6 h of HCMV infection changes the ratio of IE1 to IE2 and of unspliced (UL37x1) to spliced UL37 transcripts toward higher IE2 and UL37 levels. Thus, CDK inhibition seems to have exactly the inverse effect on mRNA processing of these genes as cyclin A2 overexpression. This suggests that the long-lasting suppression of IE2 and UL37 mRNA accumulation we observed (Fig. 5 and 6) is directly due to a CDK-specific regulatory mechanism and not a mere consequence of inefficient transcription elongation or of delayed entry into the late phase of infection, as has been described for, e.g., a virus with UL21a deleted (13). We provide evidence that such CDK-dependent regulation acts on the level of splicing rather than mRNA stability (Fig. 8), an issue that has been discussed in depth by Sanchez et al. (57). In this respect, our observation that cyclin A2 overexpression does not affect the relative amounts of differentially spliced products from the US3 and UL111A gene loci is interesting (Fig. 6). This argues against a global effect of cyclin A2-CDK on the splicing of viral genes. However, US3 and UL111A transcripts are subject to internal splicing and have the same 3′ ends (30, 38, 65), whereas the ratio of IE1 to IE2 and of UL37x1 to UL37 mRNA expression is governed by the alternative use of cleavage-polyadenylation sites and adjacent downstream 3′ splice acceptor sites (57, 63). Thus, our data would be consistent with a model in which CDK activity controls the balance between IE1/UL37x1 3′-end formation and the generation of IE2/UL37 splice products (57).
Two other experimental situations have been reported in which IE1 and IE2 expression are uncoupled at the mRNA level and consequently also at the protein level. First, the antiviral action of cyclooxygenase 2 inhibitors leads to transient IE1 and prolonged IE2 repression, closely resembling the cyclin A2-induced phenotype (79). Although, to our knowledge, a molecular link between cyclin A2 and cyclooxygenase 2 is not described in the literature, it would be interesting to test in the future if these two phenomena are related. Second, a defect in IE1 sumoylation causes a substantial delay and reduction of IE2 accumulation (49). As our immunoblot analysis of HCMV protein expression was not carried out under conditions that are required to preserve the generally labile SUMO-conjugated products (25), we cannot yet rule out the possibility that a negative effect of cyclin A2-CDK on IE1 sumoylation plays a role in the long-term repression of IE2.
Another unsolved question is why the cyclin A2-CDK-dependent inhibition of IE transcription, which is fully in place at 5 h p.i., cannot be maintained in most cells. One possible explanation is the progressive decline of the exogenous cyclin A2 expression level in density-arrested U373 cells (Fig. 6). This would imply that the transcriptional effect of cyclin A2-CDK has a higher dose sensitivity than the more sustained effect on mRNA processing and that the 20% of cells remaining IE1/IE2-negative even 10 days after high-MOI infection were still above a critical threshold of cyclin A2 kinase activity. Another possibility is that cyclin A2-CDK activity does not block but instead only slows down transcriptional initiation or the preceding steps in the pre-IE phase of infection. This would be consistent with a study by Fortunato et al. showing that after HCMV infection of cells undergoing DNA replication, a few cells become IE positive even before they enter mitosis (14). A severe general slowdown of HCMV transcription in cyclin A2-expressing cells would also provide an alternative and unifying scenario to explain the observation that neither IE1 mRNA nor any other viral transcript in our analysis ever reached the peak levels seen in control cells (at 5 h p.i. for IE1 and US3 variants, at 96 h p.i. for UL37/UL37x1 and UL11A variants, at 48 to 96 h p.i. for IE2, and at 72 to 96 h p.i. for UL112/113) (Fig. 7 and 9).
A possible caveat to all our interpretations of the long-term effects of cyclin A2-CDK on HCMV gene expression results from the observation that cyclin A2 overexpression prevents HCMV from imposing a G1 block (Fig. 5A). In consequence, S/G2-specific activities that are not directly related to cyclin A2-CDK activity may contribute, at least temporarily, to the sustained repression of IE2 and UL37. This influence, however, should not be overestimated, given that also in HCMV-infected control cells, despite the inhibition of cellular DNA synthesis, a multitude of S/G2-specific activities and metabolic processes are likely to be induced by the actions of viral gene products (4, 58).
Work is in progress to identify the CDK substrate(s) that mediates the inhibitory effects of cyclin A2 on viral gene expression. This knowledge will be key for understanding the working mechanisms and kinetics of this inhibition in more detail. Hopefully, it will also help to shed light on perhaps the most relevant question: why HCMV has evolved this cyclin A2-CDK dependency of its lytic cycle.
ACKNOWLEDGMENTS
This work was supported by grants WI2043/2-2 and WI2043/3-1 from the Deutsche Forschungsgemeinschaft (DFG) to L.W. and C.H. J.D.O. was supported by the Studienstiftung des deutschen Volkes.
We are grateful to Anindya Dutta, Stipan Jonjic, and Bodo Plachter for their generous supply of reagents.
J.D.O., R.U., and L.W. performed experiments. J.D.O. and L.W. analyzed data. L.W. conceived experiments and wrote the manuscript. C.H. and L.W. supervised research.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Adair R, Liebisch GW, Colberg-Poley AM. 2003. Complex alternative processing of human cytomegalovirus UL37 pre-mRNA. J. Gen. Virol. 84:3353–3358 [DOI] [PubMed] [Google Scholar]
- 2. Ahn K, et al. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. U. S. A. 93:10990–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arlt H, Lang D, Gebert S, Stamminger T. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bain M, Sinclair J. 2007. The S phase of the cell cycle and its perturbation by human cytomegalovirus. Rev. Med. Virol. 17:423–434 [DOI] [PubMed] [Google Scholar]
- 5. Biswas N, Sanchez V, Spector DH. 2003. Human cytomegalovirus infection leads to accumulation of geminin and inhibition of the licensing of cellular DNA replication. J. Virol. 77:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blanchard JM. 2000. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem. Pharmacol. 60:1179–1184 [DOI] [PubMed] [Google Scholar]
- 7. Bresnahan WA, Albrecht T, Thompson EA. 1998. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J. Biol. Chem. 273:22075–22082 [DOI] [PubMed] [Google Scholar]
- 8. Castillo JP, et al. 2005. Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J. Virol. 79:11467–11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chevillotte M, et al. 2009. Major tegument protein pp65 of human cytomegalovirus is required for the incorporation of pUL69 and pUL97 into the virus particle and for viral growth in macrophages. J. Virol. 83:2480–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Child SJ, Hakki M, De Niro KL, Geballe AP. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dittmer D, Mocarski ES. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dosa R, Burian K, Gonczol E. 2005. Human cytomegalovirus latency is associated with the state of differentiation of the host cells: an in vitro model in teratocarcinoma cells. Acta Microbiol. Immunol. Hung. 52:397–406 [DOI] [PubMed] [Google Scholar]
- 13. Fehr AR, Yu D. 2011. Human cytomegalovirus early protein pUL21a promotes efficient viral DNA synthesis and the late accumulation of immediate-early transcripts. J. Virol. 85:663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fortunato EA, Sanchez V, Yen JY, Spector DH. 2002. Infection of cells with human cytomegalovirus during S phase results in a blockade to immediate-early gene expression that can be overcome by inhibition of the proteasome. J. Virol. 76:5369–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gatherer D, et al. 2011. High-resolution human cytomegalovirus transcriptome. Proc. Natl. Acad. Sci. U. S. A. 108:19755–19760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geley S, et al. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibbs E, Pan ZQ, Niu H, Hurwitz J. 1996. Studies on the in vitro phosphorylation of HSSB-p34 and -p107 by cyclin-dependent kinases. Cyclin-substrate interactions dictate the efficiency of phosphorylation. J. Biol. Chem. 271:22847–22854 [DOI] [PubMed] [Google Scholar]
- 18. Goldmacher VS, et al. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536–12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gopinathan L, Ratnacaram CK, Kaldis P. 2011. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl. Cell Differ. 53:365–389 [DOI] [PubMed] [Google Scholar]
- 20. Greaves RF, Mocarski ES. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72:366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grey F, et al. 2010. A viral microRNA down-regulates multiple cell cycle genes through mRNA 5′UTRs. PLoS Pathog. 6:e1000967 doi:10.1371/journal.ppat.1000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamirally S, et al. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275 doi:10.1371/journal.ppat.1000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hume AJ, et al. 2008. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science 320:797–799 [DOI] [PubMed] [Google Scholar]
- 24. Hwang HC, Clurman BE. 2005. Cyclin E in normal and neoplastic cell cycles. Oncogene 24:2776–2786 [DOI] [PubMed] [Google Scholar]
- 25. Hwang J, Kalejta RF. 2011. In vivo analysis of protein sumoylation induced by a viral protein: detection of HCMV pp71-induced Daxx sumoylation. Methods 55:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jabs DA, Griffiths PD. 2002. Fomivirsen for the treatment of cytomegalovirus retinitis. Am. J. Ophthalmol. 133:552–556 [DOI] [PubMed] [Google Scholar]
- 27. Jackman M, Kubota Y, den Elzen N, Hagting A, Pines J. 2002. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol. Biol. Cell 13:1030–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jault FM, et al. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkins C, Abendroth A, Slobedman B. 2004. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J. Virol. 78:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jenkins C, Garcia W, Abendroth A, Slobedman B. 2008. Expression of a human cytomegalovirus latency-associated homolog of interleukin-10 during the productive phase of infection. Virology 370:285–294 [DOI] [PubMed] [Google Scholar]
- 31. Jones TR, et al. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. U. S. A. 93:11327–11333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalaszczynska I, et al. 2009. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell 138:352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalejta RF, Bechtel JT, Shenk T. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YE, et al. 2011. Human cytomegalovirus infection causes degradation of Sp100 proteins that suppress viral gene expression. J. Virol. 85:11928–11937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klopocki E, et al. 2007. Complex inheritance pattern resembling autosomal recessive inheritance involving a microdeletion in thrombocytopenia-absent radius syndrome. Am. J. Hum. Genet. 80:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knudsen KE, Fribourg AF, Strobeck MW, Blanchard JM, Knudsen ES. 1999. Cyclin A is a functional target of retinoblastoma tumor suppressor protein-mediated cell cycle arrest. J. Biol. Chem. 274:27632–27641 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi H, et al. 1992. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell 3:1279–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Z, Winkler M, Biegalke B. 2009. Human cytomegalovirus: host immune modulation by the viral US3 gene. Int. J. Biochem. Cell Biol. 41:503–506 [DOI] [PubMed] [Google Scholar]
- 39. Lu M, Shenk T. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850–8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Machida YJ, Dutta A. 2007. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 21:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marchini A, Liu H, Zhu H. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marshall EE, Bierle CJ, Brune W, Geballe AP. 2009. Essential role for either TRS1 or IRS1 in human cytomegalovirus replication. J. Virol. 83:4112–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCormick AL, Roback L, Livingston-Rosanoff D, St Clair C. 2010. The human cytomegalovirus UL36 gene controls caspase-dependent and -independent cell death programs activated by infection of monocytes differentiating to macrophages. J. Virol. 84:5108–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCormick AL, Roback L, Mocarski ES. 2008. HtrA2/Omi terminates cytomegalovirus infection and is controlled by the viral mitochondrial inhibitor of apoptosis (vMIA). PLoS Pathog. 4:e1000063 doi:10.1371/journal.ppat.1000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merrick KA, et al. 2008. Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells. Mol. Cell 32:662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Merrick KA, et al. 2011. Switching Cdk2 on or off with small molecules to reveal requirements in human cell proliferation. Mol. Cell 42:624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moroy T, Geisen C. 2004. Cyclin E. Int. J. Biochem. Cell Biol. 36:1424–1439 [DOI] [PubMed] [Google Scholar]
- 48. Murphy EA, Streblow DN, Nelson JA, Stinski MF. 2000. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J. Virol. 74:7108–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nevels M, Brune W, Shenk T. 2004. SUMOylation of the human cytomegalovirus 72-kilodalton IE1 protein facilitates expression of the 86-kilodalton IE2 protein and promotes viral replication. J. Virol. 78:7803–7812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nevels M, Paulus C, Shenk T. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. U. S. A. 101:17234–17239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paulus C, Krauss S, Nevels M. 2006. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. U. S. A. 103:3840–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reeves M, Woodhall D, Compton T, Sinclair J. 2010. Human cytomegalovirus IE72 protein interacts with the transcriptional repressor hDaxx to regulate LUNA gene expression during lytic infection. J. Virol. 84:7185–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodems SM, Clark CL, Spector DH. 1998. Separate DNA elements containing ATF/CREB and IE86 binding sites differentially regulate the human cytomegalovirus UL112-113 promoter at early and late times in the infection. J. Virol. 72:2697–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosenke K, Fortunato EA. 2004. Bromodeoxyuridine-labeled viral particles as a tool for visualization of the immediate-early events of human cytomegalovirus infection. J. Virol. 78:7818–7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salvant BS, Fortunato EA, Spector DH. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning—a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 57. Sanchez V, et al. 2004. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J. Virol. 78:11219–11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanchez V, Spector DH. 2008. Subversion of cell cycle regulatory pathways. Curr. Top. Microbiol. Immunol. 325:243–262 [DOI] [PubMed] [Google Scholar]
- 59. Schulze A, et al. 1995. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc. Natl. Acad. Sci. U. S. A. 92:11264–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shin J, et al. 2006. A short isoform of human cytomegalovirus US3 functions as a dominant negative inhibitor of the full-length form. J. Virol. 80:5397–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shlapobersky M, Sanders R, Clark C, Spector DH. 2006. Repression of HMGA2 gene expression by human cytomegalovirus involves the IE2 86-kilodalton protein and is necessary for efficient viral replication and inhibition of cyclin A transcription. J. Virol. 80:9951–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Skaletskaya A, et al. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. U. S. A. 98:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Su Y, Adair R, Davis CN, DiFronzo NL, Colberg-Poley AM. 2003. Convergence of RNA cis elements and cellular polyadenylation factors in the regulation of human cytomegalovirus UL37 exon 1 unspliced RNA production. J. Virol. 77:12729–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tavalai N, Adler M, Scherer M, Riedl Y, Stamminger T. 2011. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J. Virol. 85:9447–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tenney DJ, Santomenna LD, Goudie KB, Colberg-Poley AM. 1993. The human cytomegalovirus US3 immediate-early protein lacking the putative transmembrane domain regulates gene expression. Nucleic Acids Res. 21:2931–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Trang P, Liu F. 2004. RNase P ribozyme as an antiviral agent against human cytomegalovirus. Methods Mol. Biol. 252:437–450 [DOI] [PubMed] [Google Scholar]
- 67. van Zeijl M, Fairhurst J, Baum EZ, Sun L, Jones TR. 1997. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology 231:72–80 [DOI] [PubMed] [Google Scholar]
- 68. Vaziri C, et al. 2003. A p53-dependent checkpoint pathway prevents rereplication. Mol. Cell 11:997–1008 [DOI] [PubMed] [Google Scholar]
- 69. Wheeler LW, Lents NH, Baldassare JJ. 2008. Cyclin A-CDK activity during G1 phase impairs MCM chromatin loading and inhibits DNA synthesis in mammalian cells. Cell Cycle 7:2179–2188 [DOI] [PubMed] [Google Scholar]
- 70. White EA, Spector DH. 2007. Early viral gene expression and function, p 264–294 In Arvin A, et al. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 71. Wiebusch L, Bach M, Uecker R, Hagemeier C. 2005. Human cytomegalovirus inactivates the G0/G1-APC/C ubiquitin ligase by Cdh1 dissociation. Cell Cycle 4:1435–1439 [DOI] [PubMed] [Google Scholar]
- 72. Wiebusch L, Hagemeier C. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wiebusch L, Neuwirth A, Grabenhenrich L, Voigt S, Hagemeier C. 2008. Cell cycle-independent expression of immediate-early gene 3 results in G1 and G2 arrest in murine cytomegalovirus-infected cells. J. Virol. 82:10188–10198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wiebusch L, Truss M, Hagemeier C. 2004. Inhibition of human cytomegalovirus replication by small interfering RNAs. J. Gen. Virol. 85:179–184 [DOI] [PubMed] [Google Scholar]
- 75. Wiebusch L, Uecker R, Hagemeier C. 2003. Human cytomegalovirus prevents replication licensing by inhibiting MCM loading onto chromatin. EMBO Rep. 4:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wolf DG, Honigman A, Lazarovits J, Tavor E, Panet A. 1998. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch. Virol. 143:1223–1232 [DOI] [PubMed] [Google Scholar]
- 77. Xu Y, Cei SA, Rodriguez Huete A, Colletti KS, Pari GS. 2004. Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt. J. Virol. 78:11664–11677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yam CH, Fung TK, Poon RY. 2002. Cyclin A in cell cycle control and cancer. Cell Mol. Life Sci. 59:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhu H, Cong JP, Yu D, Bresnahan WA, Shenk TE. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. U. S. A. 99:3932–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zydek M, Hagemeier C, Wiebusch L. 2010. Cyclin-dependent kinase activity controls the onset of the HCMV lytic cycle. PLoS Pathog. 6:e1001096 doi:10.1371/journal.ppat.1001096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zydek M, et al. 2011. General blockade of human cytomegalovirus immediate-early mRNA expression in the S/G2 phase by a nuclear, Daxx- and PML-independent mechanism. J. Gen. Virol. 92:2757–2769 [DOI] [PubMed] [Google Scholar]