Abstract
To identify host genes affecting replication of Tomato bushy stunt virus (TBSV), a small model positive-stranded RNA virus, we overexpressed 5,500 yeast proteins individually in Saccharomyces cerevisiae, which supports TBSV replication. In total, we identified 141 host proteins, and overexpression of 40 of those increased and the remainder decreased the accumulation of a TBSV replicon RNA. Interestingly, 36 yeast proteins were identified previously by various screens, greatly strengthening the relevance of these host proteins in TBSV replication. To validate the results from the screen, we studied the effect of protein kinase C1 (Pkc1), a conserved host kinase involved in many cellular processes, which inhibited TBSV replication when overexpressed. Using a temperature-sensitive mutant of Pkc1p revealed a high level of TBSV replication at a semipermissive temperature, further supporting the idea that Pkc1p is an inhibitor of TBSV RNA replication. A direct inhibitory effect of Pkc1p was shown in a cell-free yeast extract-based TBSV replication assay, in which Pkc1p likely phosphorylates viral replication proteins, decreasing their abilities to bind to the viral RNA. We also show that cercosporamide, a specific inhibitor of Pkc-like kinases, leads to increased TBSV replication in yeast, in plant single cells, and in whole plants, suggesting that Pkc-related pathways are potent inhibitors of TBSV in several hosts.
INTRODUCTION
Replication of plus-stranded-RNA [(+)RNA] viruses requires many components of the host cells, including host proteins and intracellular membranes, which serve as sites of virus replication in infected cells (9, 26, 33, 38, 39, 42, 45, 64). Major advances in cataloging the host factors affecting (+)RNA virus infections have recently been made with several animal and plant viruses (7, 10, 19, 23, 24, 37, 44, 48, 62, 69, 70, 76, 78, 79), yet our knowledge of how many host factors are involved in viral RNA replication is still far from complete.
Tombusviruses, such as Tomato bushy stunt virus (TBSV) and Cucumber necrosis virus (CNV), are single-component RNA viruses with ∼4,800 nucleotides. Only the replication proteins p33 and p92pol, among the five virus-coded proteins, are essential for TBSV replication (41, 87). p92pol is the viral RNA-dependent RNA polymerase (RdRp), whereas replication cofactor p33 (which overlaps the N-terminal prereadthrough segment of p92pol) is an RNA-binding protein and an RNA chaperone (49, 56, 59, 73). Earlier work determined that p33 is involved in template selection and recruitment of viral RNA into replication (34, 46, 56). These proteins interact with each other, with the viral RNA, and with a group of host proteins in cells (25, 27, 32, 46, 51, 60, 61, 67) that leads to the assembly of viral replicase complexes (VRC) on peroxisomal membranes (31, 43, 46).
Systematic genome-wide screens were conducted in yeast (Saccharomyces cerevisiae), a model host, using nonoverlapping gene libraries covering ∼95% of the yeast genome to identify the roles of host genes in TBSV replication (19, 48, 68, 69). These studies led to the identification of ∼150 host genes that either stimulated or inhibited virus replication and RNA recombination. Additional global proteomics approaches, such as protein arrays, a cDNA library screen and mass spectrometry of the purified tombusvirus replicase, have led to the identification of an additional ∼150 host proteins that interact with either p33 and p92 or the TBSV RNA (25, 27, 32, 67). Functions of several of the identified host proteins in TBSV replication have been dissected in yeast and in vitro as well as validated in a native plant host (3, 13, 16, 17, 28, 38, 40, 65, 84, 88).
In spite of the intensive genome-wide and global proteomics screens for TBSV host factors, it seems that previous screens have not reached saturation level, since the overlap among the identified set of host genes from various screens is somewhat low, albeit significant (37, 40). Therefore, we are continuing systematic screening for TBSV host factors in yeast. Accordingly, in this study, we performed a proteome-wide screen with an overexpression library of yeast genes representing over 90% of the yeast proteome to gain further insights into the complexity of TBSV-host cell interaction. Overexpression of 5,500 yeast proteins led to the identification of 141 yeast proteins that affected the accumulation of tombusvirus replicon RNA (repRNA) in yeast. The identified yeast proteins, which either increased or decreased the accumulation of tombusvirus repRNA, are involved in protein metabolism and transport, RNA transcription and metabolism, or other cellular processes. Among these are 36 host proteins that have also been identified in earlier screens, thus validating the idea that these host genes are likely important for TBSV replication.
To further validate the screen results, we chose protein kinase C (Pkc1p), whose overexpression inhibited TBSV replication. The Pkc superfamily of kinases is found exclusively in eukaryotes. They are serine/threonine kinases frequently involved in signal transduction (66). The human Pkc isoenzymes are known to be involved in diseases, such as cancer, diabetes, and Alzheimer's disease (66). While there are four subgroups of Pkc-related kinases in mammals, there is only a single prototypic PKC gene in S. cerevisiae, which greatly facilitates mechanistic and functional studies. Importantly, the yeast Pkc1p contains all the domains identified in the mammalian isoenzymes (66). Pkc1p has been shown to be an essential and multifunctional enzyme regulating cell wall integrity, osmoregulation, conserved MAPK signal transduction, and lipid homeostasis via its effects on the Opi1p repressor of phospholipid biosynthesis, as well as actin filament and cytoskeleton organization and pexophagy (specialized form of autophagy leading to destruction of peroxisomes) (66). In contrast to animal and fungal PKCs, the plant Pkc-like proteins have not yet been characterized in detail (2, 20, 36, 75, 80–83).
In this work, we show that Pkc1p is a potent inhibitor of TBSV replication. Yeast carrying a temperature-sensitive Pkc1p mutant supported TBSV replication at a higher level than wild-type yeast did. Also, an in vitro approach has demonstrated that Pkc1p directly inhibits TBSV RNA synthesis. We also show that cercosporamide, a specific inhibitor of Pkc-like kinases, leads to increased TBSV replication in yeast, in plant single cells, and in whole plants, suggesting that Pkc-related pathways are potent inhibitors of TBSV in several hosts.
MATERIALS AND METHODS
Yeast strains and expression plasmids.
The parental yeast strain (BY4741) was from Open Biosystems. To study the effect of overexpression of selected yeast proteins on TBSV repRNA replication, we used the yeast open reading frame (ORF) collection from Open Biosystems. In this yeast ORF collection, each ORF is expressed from the 2μ plasmid BG1805 under the control of GAL1 promoter and fused to a tandem affinity tag that includes a hemagglutinin (HA) tag and the “zz” domain of protein A at the C terminus. We also used an N-terminally glutathione S-transferase (GST)-tagged ORF library for a limited overexpression screen (obtained from B. Andrews) (72).
The expression plasmid pGAD-His92 (containing CNV p92pol gene and LEU2 marker) (50) and the dual expression plasmid pGBK-His33/DI-72 (co-expressing p33 from the ADH1 promoter and DI-72 RNA from the GAL1 promoter) have been previously described (19). Expression of nonphosphorylatable p33 mutants (A210A211, A205A210A211, and D205) in yeast was done as described previously (71).
Yeast transformation and cultivation.
Yeast strains were cotransformed with different combinations of plasmids using the lithium acetate (LiOAc)–single-stranded DNA (ssDNA)–polyethylene glycol (PEG) method (12), and transformants were selected by complementation of auxotrophic markers. For the replication assay, the parental strain (BY4741) was cotransformed with three separate plasmids: (i) pGAD-His92, (ii) pGBK-His33/DI-72 (19), and (iii) one of the individual yeast ORF clones (Open Biosystems) or the 2μ plasmid pYES-NT-C (Invitrogen) as a control.
Host protein overexpression studies.
Individual colonies of strain BY4741 transformed with plasmids carrying the selected ORFs under the control of the GAL1 promoter along with pGAD-His92 and pGBK-His33/DI-72 were pregrown overnight in SC-ULH− medium (54) containing 2% glucose in 96-deep-well plates to suppress host protein expression and TBSV repRNA replication. To overexpress the particular host protein and launch TBSV repRNA replication, yeast transformants were transferred into 1.5 ml of SC-ULH− plus 2% galactose for 24 h at 29°C in 96-deep-well plates. The final optical density (OD) was ∼0.7 to 1.0. For the detection of the expressed host and viral proteins, we performed Western blotting as described previously (50). We analyzed 9 to 18 independent samples for each host protein. Examination of a large number of samples was important for numerous host proteins that resulted in highly variable effects on TBSV RNA accumulation.
RNA analysis.
Total RNA isolation and Northern blot analysis were performed as described previously (47, 50). Briefly, for extraction of total RNA, yeast cells were broken by shaking for 1 to 2 min at room temperature with equal volumes of RNA extraction buffer (50 mM NaOAc [pH 5.2], 10 mM EDTA, and 1% sodium dodecyl sulfate [SDS]) and water-saturated phenol and then incubated for 4 min at 65°C, followed by ethanol precipitation. The obtained RNA samples were separated on a 1.5% agarose gel and transferred to a Hybond-XL membrane (Amersham) before hybridization with a DI-72-specific probe (47). For detection of plus-strand repRNA, we prepared a 32P-labeled RIII/IV(−) probe with T7 transcription from a PCR product obtained with primers 1165 (AGCGAGTAAGACAGACTCTTCA) and 22 (GTAATACGACTCACTATAGGGCTGCATTTCTGCAATGTTCC) on DI-72 templates.
Protein analysis.
Protein analysis was done as described earlier (47, 50). Briefly, a total of 1 ml yeast culture was harvested, and the pelleted cells were resuspended in 150 μl cold extraction buffer (200 mM sorbitol, 50 mM Tris-HCl [pH 7.5], 15 mM MgCl2, 10 mM KCl, 10 mM β-mercaptoethanol, yeast protease inhibitor mix; Sigma), and 250 μl of glass beads was added to each sample. The cells were broken with a Genogrinder for 2 min at 1,500 rpm. Each sample was further mixed with 600 μl prechilled extraction buffer, and unbroken cells were removed by centrifugation at 100 × g for 5 min. The supernatant was mixed with 0.5 volume of 3× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer followed by SDS-PAGE and Western blot analysis as described previously (47, 50). The primary antibody was anti-His6 (Amersham), and the secondary antibody was alkaline phosphatase-conjugated anti-mouse IgG antibody (Sigma).
In vitro replication assay.
Yeast cell-free extract (CFE) was prepared as described earlier (55). The assay mixture consisted of 2 μl yeast extract, 400 ng of purified p33, 100 ng of p92, and 500 ng of DI-72(+) RNA along with 1× (∼200 ng), 2×, and 4× GST or recombinant PKC1 protein in 20 μl reaction mix. The conditions of the in vitro assay are described elsewhere (55). The reaction mixture was incubated at 25°C for 3 h. RNA was purified by phenol-chloroform extraction, followed by isopropanol-ammonium acetate precipitation. The newly synthesized 32P-labeled repRNAs were analyzed on 5% denaturing PAGE gels as described previously (50).
Use of cercosporamide for Pkc1 inhibition in yeast.
The yeast strain BY4741 was cotransformed with pHISGBK-CUP1-p33ADH-DI-72 and pESC-Ura-CUP1-His-p92 as described above. Transformed yeast cells were inoculated and grown at 28°C in UH− liquid medium without CuSO4. After 24 h of growth at 28°C, each culture was divided into two portions, each of which had an OD at 600 nm (OD600) of approximately 0.2. In one set of cultures, different concentrations of 100% ethanol (0.125, 0.25, and 0.5 μl/ml) were added as a control, while in the other set of cultures, 0.125, 0.25, or 0.5 μg/ml of cercosporamide (Sigma) was added. Yeast cells were grown for another 36 h at 28°C, and total RNA was analyzed as described earlier (6, 48, 50).
Use of cercosporamide for Pkc1 inhibition in protoplasts.
Preparation of Nicotiana benthamiana protoplasts, electroporation with TBSV and Turnip crinkle virus (TCV) RNA, and viral RNA analysis were performed as described previously (49). Cercosporamide was dissolved in ethanol and was added at concentrations of 2 and 4 μM (2- and 4-μg/ml final concentrations) before electroporation to the N. benthamiana protoplasts). Total RNA samples were obtained 40 h postelectroporation.
Plant inoculation with TBSV and treatment with Pkc1 inhibitor.
Lower leaves of 3-week-old N. benthamiana plants were sap inoculated (sap was purified from TBSV-infected leaves). Two days later, ethanol or cercosporamide (2- and 4-μg/ml final concentrations) were infiltrated into the newly emerging top leaves. Total RNA at 4 days postinoculation (dpi) from the infiltrated leaves was isolated and analyzed for TBSV RNA accumulation by Northern blotting as described earlier (50).
RESULTS
Systematic screening of the yeast overexpression ORF collection for host proteins affecting tombusvirus replication.
Using a high-throughput approach (54), we separately overexpressed 5,500 yeast proteins from the galactose inducible GAL1 promoter (11) in yeast cells also replicating TBSV repRNA. Each yeast protein is C-terminally tagged with zz and a His6 tag to aid detection (11). Comparable amounts of yeast cells were harvested 24 h later, followed by Northern blotting to measure the level of TBSV (+)repRNA produced. We used rRNA as a loading control for normalization of data on repRNA accumulation in yeast. The accumulation level of repRNA in yeast carrying pYES plasmid, which expresses only a short peptide, was taken as 100%. As an additional control, we overexpressed a pseudogene (APT2) which has no enzymatic activity when expressed (1) and which failed to interact with p33 (25). Overexpression of Apt2p led to 80% ± 11% repRNA accumulation compared with yeast carrying the pYES control (data not shown) (25). This suggests that protein overexpression in general could reduce the ability of yeast cells to support TBSV repRNA accumulation under the protein overexpression conditions. Based on the pYES and APT2 overexpression controls, we considered overexpression of a protein inhibitory if it significantly reduced repRNA accumulation below 70% and stimulatory if it significantly increased repRNA accumulation above 130% of the wild-type (wt) level (i.e., in comparison with the pYES control).
Of the 5,500 yeast ORFs tested, we found that ∼1,300 had detrimental effects on both yeast cell growth (seen as a low rRNA level) and TBSV RNA accumulation (based on repRNA level). Since these host proteins likely affect TBSV repRNA accumulation indirectly via changing yeast metabolism due to their cytotoxicity when expressed at elevated levels, we did not consider these host proteins among the “winners,” which included only those that showed more selectivity in inhibition of TBSV repRNA accumulation than their effects on yeast cell growth. We also performed a limited screen with a GST-tagged yeast overexpression library (72) to extend the list of host proteins examined for their effects on TBSV replication.
Altogether, the proteome-wide screen led to the identification of 141 host proteins, which affected TBSV replication (Table 1; also, see the supplemental material). Among these, overexpression of 40 host proteins increased and 101 decreased TBSV accumulation in yeast (Table 1). Also, about 26% (36 of the “winner” proteins) have been identified previously by various screens (19, 25, 27, 32, 40, 48, 67–69), greatly strengthening the relevance of these host proteins in TBSV replication. Moreover, the screen also led to the identification of 105 new host proteins affecting TBSV replication.
Table 1.
List and known cellular functions of the identified yeast genes affecting TBSV replication when overexpressed
| Genea | Product and functionc |
|---|---|
| ABP1 | Actin-binding protein of the cortical actin cytoskeleton, important for activation of the Arp2/3 complex, which plays a key role in actin cytoskeleton organization |
| ACF4 | Protein of unknown function; possible role in actin cytoskeleton organization |
| AFG2 | ATPase of the CDC48/PAS1/SEC18 (AAA) family; may be involved in degradation of aberrant mRNAs |
| AFI1 | Arf3p polarization-specific docking factor, required for the polarized distribution of the ADP-ribosylation factor |
| AIM14 | Protein with similarity to iron/copper reductases (FRE1-8), possibly involved in iron homeostasis |
| ALG2 | Presumed early mannosyltransferase involved in the N-linked glycosylation pathway |
| ALK2 | Protein kinase; similar to mammalian haspins |
| APM1b | Mu1-like medium subunit of the clathrin-associated protein complex (AP-1); binds clathrin |
| ARP8b | Nuclear actin-related protein involved in chromatin remodeling; has mRNA binding activity |
| ARR3 | Arsenite transporter of the plasma membrane |
| ASI1 | Putative integral membrane E3 ubiquitin ligase |
| ATG7 | Autophagy-related protein and dual-specificity member of the E1 family of ubiquitin-activating enzymes; mediates the conjugation of Atg12p with Atg5p and Atg8p with phosphatidylethanolamine, required steps in autophagosome formation |
| ATG9 | Transmembrane protein involved in formation of CVT and autophagic vesicles; cycles between the preautophagosomal structure and other cytosolic punctate structures not found in autophagosomes |
| ATG18 | Phosphatidylinositol 3,5-bisphosphate-binding protein of the vacuolar membrane; required for recycling of Atg9p through the preautophagosomal structure |
| AUR1 | Phosphatidylinositol:ceramide phosphoinositol transferase (IPC synthase), required for sphingolipid synthesis |
| BET2 | Beta subunit of type II geranylgeranyltransferase required for vesicular transport between the endoplasmic reticulum and the Golgi |
| BIR1 | Essential chromosomal passenger protein |
| BNI5 | Protein involved in organization of septins at the mother bud neck; may interact directly with the Cdc11p septin; localizes to the bud neck in a septin-dependent manner |
| BRO1b | Cytoplasmic class E VPS factor that coordinates deubiquitination in the MVB pathway by recruiting Doa4p to endosomes |
| BUD21b | UTP16, component of SSU processosome |
| BUL2 | Component of the Rsp5p E3-ubiquitin ligase complex |
| CDC34b | Ubiquitin-conjugating enzyme (E2) and catalytic subunit of SCF ubiquitin-protein ligase complex |
| CDC55 | Nonessential regulatory subunit B of protein phosphatase 2A; actin filament organization |
| CHA4 | DNA-binding transcriptional activator |
| CCZ1 | Protein involved in vacuolar assembly, essential for autophagy and the cytoplasm-to-vacuole pathway |
| CPR1b | Cytoplasmic peptidyl-prolyl cis-trans isomerase (cyclophilin); catalyzes the cis-trans isomerization of peptide bonds N terminal to proline residues |
| CRM1 | Major karyopherin, involved in export of proteins, RNAs, and ribosomal subunits from the nucleus |
| DBP2b | Essential ATP-dependent RNA helicase of the DEAD box protein family, involved in nonsense-mediated mRNA decay and rRNA processing |
| DBP7 | Putative ATP-dependent RNA helicase of the DEAD box family involved in ribosomal biogenesis |
| DDR48b | DNA damage-responsive protein |
| DEG1b | tRNA:pseudouridine synthase |
| DID2b | Class E protein of the VPS pathway; associates reversibly with the late endosome |
| DIE2 | Dolichyl-phosphoglucose-dependent glucosyltransferase of the ER; has a role in regulation of ITR1 and INO1 |
| DTD1 | cm;1>d-Tyr-tRNATyr deacylase; functions in protein translation, may affect nonsense suppression via alteration of the protein synthesis machinery; ubiquitous among eukaryotes |
| EPS1 | Pdi1p (protein disulfide isomerase)-related protein involved in ER retention of resident ER proteins |
| ERB1b | Constituent of 66S preribosomal particles, homologous to mammalian Bop1 |
| ERG13 | HMG-CoA synthase, ergosterol biosynthesis, mevalonate biosynthesis |
| ESS1b | PPIase |
| FEN1 | Fatty acid elongase, involved in sphingolipid biosynthesis |
| FPR4 | PPIase (proline isomerase) localized to the nucleus |
| FSH2 | Serine hydrolase that localizes to the cytoplasm; sequence is similar to Fsh1p and Fsh3p |
| FUN26 | Nucleoside transporter with broad nucleoside selectivity |
| GCD2b | Guanine nucleotide exchange factor for eIF2 translation initiation factor |
| GCN3 | Alpha subunit of the translation initiation factor eIF2B, the guanine-nucleotide exchange factor for eIF2 |
| GEA2 | Guanine nucleotide exchange factor for ARFs, involved in vesicular transport between the Golgi and ER, Golgi organization, and actin cytoskeleton organization |
| GIS4 | CAAX box containing protein of unknown function |
| GGA2 | Golgi-localized protein with homology to gamma-adaptin, interacts with and regulates Arf1p and Arf2p in a GTP-dependent manner in order to facilitate traffic through the late Golgi |
| GLN3 | Transcriptional activator |
| GPI8 | ER membrane glycoprotein subunit of the glycosylphosphatidylinositol transamidase complex that adds GPI anchors to newly synthesized proteins |
| GPM2 | Molecular function unknown |
| GPT2 | Glycerol-3-phosphate acyltransferase located in both lipid particles and the ER, involved in lipid biosynthesis |
| HAA1b | Transcriptional activator involved in the transcription of genes encoding membrane stress proteins |
| HAS1b | ATP-dependent RNA helicase; localizes to both the nuclear periphery and nucleolus |
| HBS1b | GTPase with similarity to translation release factors |
| HOP2 | Meiosis-specific protein that localizes to chromosomes |
| HUL4 | Protein with similarity to hect domain E3 ubiquitin-protein ligases |
| IMP4 | Component of the SSU processome, which is required for pre-18S rRNA processing; member of a superfamily of proteins that contain a sigma-70-like motif and associate with RNAs |
| INO2b | Transcription activator that binds inositol/choline-responsive elements, required for derepression of phospholipid biosynthetic genes |
| IZH4 | Membrane protein involved in zinc and lipid metabolism |
| JJJ1b | Co-chaperone that stimulates the ATPase activity of Ssa1p |
| KEG1b | Integral membrane protein of the ER |
| MAK10 | Noncatalytic subunit of N-terminal acetyltransferase of the NatC type, required for replication of dsRNA virus |
| MAP1 | Methionine aminopeptidase; catalyzes the cotranslational removal of N-terminal methionine from nascent polypeptides |
| MDM38b | Mitochondrial protein; facilitates recruitment of mRNA-specific translational activators to ribosomes |
| MNR2 | Putative magnesium transporter; has similarity to Alr1p and Alr2p, which mediate influx of Mg2+ and other divalent cations |
| MSP1b | Mitochondrial protein involved in sorting of proteins in the mitochondria; putative membrane-spanning ATPase |
| MST28 | Putative integral membrane protein, involved in vesicle formation; forms complex with Mst27p; member of DUP240 gene family; binds COPI and COPII vesicles |
| NGR1 | RNA-binding protein that negatively regulates growth rate; interacts with the 3′ untranslated region of the mitochondrial porin (POR1) mRNA and enhances its degradation; overexpression impairs mitochondrial function |
| NOG1b | Putative GTPase that associates with free 60S ribosomal subunits in the nucleolus |
| NOP53b | Nucleolar protein; involved in biogenesis of the 60S subunit of the ribosome |
| NPL3b | RNA-binding protein that promotes elongation, regulates termination, and carries poly(A) mRNA from nucleus to cytoplasm |
| NRM1 | Transcriptional corepressor of MCB binding factor-regulated gene expression |
| NSP1 | Essential component of the nuclear pore complex, which mediates nuclear import and export |
| NSR1b | Nucleolar protein that binds nuclear localization sequences, required for pre-rRNA processing and ribosome biogenesis; nucleolin |
| NUP1 | NPC subunit, involved in protein import/export and in export of RNAs, possible karyopherin release factor that accelerates release of karyopherin-cargo complexes after transport across NPC |
| OLE1 | Fatty acid desaturase, required for monounsaturated fatty acid synthesis |
| PBP2 | RNA binding protein with similarity to mammalian heterogeneous nuclear RNP K protein |
| OTU2b | member of the ovarian tumor-like superfamily of predicted cysteine proteases |
| PDR17 | Phosphatidylinositol transfer protein; downregulates Plb1p-mediated turnover of phosphatidylcholine, found in the cytosol and microsomes; pdr16 pdr17 double deletion mutants exhibit altered lipid levels |
| PEP7b | Multivalent adaptor protein that facilitates vesicle-mediated vacuolar protein sorting |
| PEP12 | Target membrane receptor (t-SNARE) for vesicular intermediates traveling between the Golgi apparatus and the vacuole; controls biosynthetic, endocytic, and retrograde traffic into the prevacuolar compartment; syntaxin |
| PEX27 | Peripheral peroxisomal membrane protein involved in controlling peroxisome size and no. |
| PEX29 | Peroxisomal integral membrane peroxin, involved in the regulation of peroxisomal size, no., and distribution |
| PEX30 | Peroxisomal integral membrane protein, involved in negative regulation of peroxisome no. |
| PIB1 | RING-type ubiquitin ligase of the endosomal and vacuolar membranes, binds phosphatidylinositol(3)-phosphate; contains a FYVE finger domain |
| PGA2 | Essential protein required for maturation of Gas1p and Pho8p; involved in protein trafficking |
| PIN2 | Protein that induces appearance of PIN+ prion when overproduced |
| PKC1 | Protein serine/threonine kinase essential for cell wall remodeling during growth |
| PLB2 | Phospholipase B (lysophospholipase) involved in phospholipid metabolism |
| POL30b | Proliferating cell nuclear antigen, functions as the sliding clamp for DNA polymerase delta |
| POX1b | Fatty-acyl coenzyme A oxidase, involved in the fatty acid beta-oxidation pathway in the peroxisomes |
| PRE1 | 20S proteasome beta-type subunit; localizes to the nucleus throughout the cell cycle |
| PRO1 | Gamma-glutamyl kinase, catalyzes the first step in proline biosynthesis |
| SPG4 | Protein of unknown function |
| RAD61 | Protein of unknown function |
| RDS1 | Zinc cluster protein involved in conferring resistance to cycloheximide |
| RNY1b | Vacuolar RNase of the T(2) family, relocalizes to the cytosol where it cleaves tRNAs upon oxidative stress |
| ROT1 | Protein that may be involved in cell wall function |
| RPC31 | RNA polymerase III subunit C31; contains HMG-like C-terminal domain |
| RRP4 | Protein involved in rRNA processing; component of the exosome 3→5 exonuclease complex |
| RRP14 | Essential protein, constituent of 66S preribosomal particles |
| RSP5b | E3 ubiquitin ligase of the NEDD4 family |
| RVS161 | Amphiphysin-like lipid raft protein; subunit of a complex (Rvs161p-Rvs167p) that regulates polarization of the actin cytoskeleton, endocytosis, cell polarity |
| SAR1 | GTPase, GTP-binding protein of the ARF family, component of COPII coat of vesicles; required for transport vesicle formation during ER to Golgi protein transport |
| SCP1 | Component of yeast cortical actin cytoskeleton, binds and cross-links actin filaments |
| SEC17 | Peripheral membrane protein required for vesicular transport between ER and Golgi and for the priming step in homotypic vacuole fusion; part of the cis-SNARE complex; has similarity to alpha-SNAP |
| SEC22 | R-SNARE protein; assembles into SNARE complex; cycles between the ER and Golgi complex; involved in anterograde and retrograde transport between the ER and Golgi; synaptobrevin homolog |
| SED5 | cis-Golgi t-SNARE syntaxin required for vesicular transport between the ER and the Golgi complex; binds at least 9 SNARE proteins |
| SPC97 | Component of the microtubule-nucleating Tub4p (gamma-tubulin) complex |
| SRP14 | SRP subunit; interacts with the RNA component of SRP to form the Alu domain, which is the region of SRP responsible for arrest of nascent chain elongation during membrane targeting |
| SPT14 | UDP-GlcNAc-binding and catalytic subunit of the enzyme that mediates the first step in GPI biosynthesis |
| STM1b | Protein required for translation under nutrient stress; binds quadruplex and purine motif triplex nucleic acids |
| SUB1b | Transcriptional coactivator; role in the hyperosmotic stress response |
| SVP26 | Integral membrane protein of the early Golgi apparatus; may function to promote retention of proteins in the early Golgi compartment |
| TIF4631 | Translation initiation factor eIF4G, subunit of the mRNA cap-binding protein complex (eIF4F)) |
| TRM3 | 2′-O-Ribose methyltransferase, catalyzes the ribose methylation of the guanosine nucleotide at position 18 of tRNAs |
| TRZ1b | tRNA 3′-end processing endonuclease tRNase Z; homolog of the human cancer susceptibility gene ELAC2 |
| TRS85 | Subunit of TRAPPIII, a multimeric guanine nucleotide exchange factor for Ypt1p, required for membrane expansion during autophagy and the CVT pathway; directs Ypt1p to the PAS |
| TRS120 | One of 10 subunits of the TRAPP complex of the cis-Golgi which mediates vesicle docking and fusion; involved in ER-to-Golgi membrane traffic |
| UBP8 | Ubiquitin-specific protease that is a component of the SAGA acetylation complex; required for SAGA-mediated deubiquitination of histone H2B |
| UTP7b | Nucleolar protein, component of the SSU processome |
| VCX1 | Vacuolar H+/Ca2+ exchanger involved in control of cytosolic Ca2+ concn |
| VPS3 | Cytoplasmic protein required for the sorting and processing of soluble vacuolar proteins |
| VPS25 | Component of the ESCRT-II complex, which is involved in ubiquitin-dependent sorting of proteins into the endosome |
| VPS27 | Endosomal protein that forms a complex with Hse1p; required for recycling Golgi proteins |
| VPS36 | Component of the ESCRT-II complex; contains the GLUE (GRAM-like ubiquitin binding in EAP45) domain |
| VRP1 | proline-rich actin-associated protein involved in cytoskeletal organization and cytokinesis |
| YBR016W | Plasma membrane protein of unknown function; has similarity to hydrophilins |
| YCK1 | Palmitoylated, plasma membrane-bound casein kinase I isoform; shares redundant functions with Yck2p in morphogenesis, proper septin assembly, endocytic trafficking |
| YCT1 | High-affinity cysteine-specific transporter with similarity to the Dal5p family of transporters |
| YDJ1 | Protein chaperone involved in regulation of the HSP90 and HSP70 functions; involved in protein translocation across membranes; member of the DnaJ family |
| YGL262W | Putative protein of unknown function; null mutant displays elevated sensitivity to expression of a mutant huntingtin fragment or of alpha-synuclein |
| YGR026Wb | Putative protein of unknown function; GFP fusion protein localizes to the cell periphery |
| YGR130C | Putative protein of unknown function |
| YLR126C | Putative protein of unknown function; may be involved in copper and iron homeostasis |
| YMR1 | PI(3)P phosphatase; regulates the localization and levels of PI(3)P; involved in CVT transport |
| YMR259C | Putative protein of unknown function; GFP fusion protein localizes to the cytoplasm |
| YOS1 | Integral membrane protein required for ER to Golgi transport; localized to the Golgi, the ER, and COPII vesicles |
| YPT1 | Rab family GTPase, involved in the ER-to-Golgi step of the secretory pathway |
| YPT52 | GTPase, similar to Ypt51p and Ypt53p and to mammalian Rab5; required for vacuolar protein sorting and endocytosis |
| YSY6 | Protein whose expression suppresses a secretory pathway mutation in E. coli; has similarity to the mammalian RAMP4 protein involved in secretion |
Gene names are based on the Saccharomyces genome database. Downregulators are underlined; upregulators are in bold.
Previously identified.
VPS, vacuolar protein-sorting; MVB, multivesicular body; SSU, small ribosomal subunit; SCF complex, Skp1, Cullins, F-box proteins; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; PPIase, peptidylprolyl cis-trans-isomerase; ARF, ADP ribosylation factor; GPI, glycosylphosphatidylinositol; dsRNA, double-stranded RNA; NPC, nuclear pore complex; SNARE, soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors; SRP, signal recognition particle; TRAPP, transport protein particle; CVT, cytoplasm to vacuole; SAGA, Spt-Ada-Gcn5-acetyltransferase; GFP, green fluorescent protein; PI(3)P, phosphatidylinositol 3-phosphate.
A large number of vesicular transport proteins affect tombusvirus replication.
The 141 host proteins identified in the above screen code for proteins with different molecular functions in various cellular processes (Saccharomyces Genome Database [http://www.yeastgenome.org]). Bioinformatic analysis of the identified host factors in the current proteome-wide screen revealed that, surprisingly, host proteins involved in protein targeting and vesicle-mediated transport are by far the most numerous group of factors (39 host proteins) (Fig. 1). It is currently not known how these proteins could affect TBSV replication, which occurs on the cytosolic surface of peroxisomes (31, 43, 46). It is possible that some of the identified host proteins involved in protein targeting and vesicle-mediated transport might directly affect the peroxisome-to-endoplasmic reticulum (ER) pathway, which has been suggested to be involved in sorting TBSV replication proteins (31). Additional large groups of factors include (i) protein modifying enzymes/factors, (ii) lipid metabolism and membrane biogenesis factors, (iii) RNA-modifying and RNA metabolism factors, (iv) translation factors and ribosomal proteins, and (v) stress-related proteins (Fig. 1). Altogether, the identified host factors could have either direct or indirect effects on tombusvirus replication.
Fig 1.
Grouping of the identified yeast proteins affecting TBSV replication based on their known cellular functions. Systematic screening of the yeast overexpression library resulted in the identification of 141 unique yeast genes that either promoted or inhibited TBSV replication (Table 1). The identified host genes were grouped into 11 categories based on their known cellular and biochemical functions. The number of genes in each category is shown in parentheses. Proteins with multiple functions were placed arbitrarily only in one of the categories, mainly based on their predicted function in TBSV replication.
A temperature-sensitive kinase mutant of Pkc1p supports increased TBSV replication in yeast.
To validate the results from the proteome-wide screen, we chose the highly conserved Pkc1p, which is an essential gene for yeast growth. We studied Pkc1p in detail here, since our previous work showed that recombinant Pkc1p could phosphorylate the replication proteins p33 and p92 in vitro (74). The phosphorylation sites were mapped to serines and a threonine located next to the RNA-binding site of these replication proteins (Fig. 2A and B). Interestingly, phosphorylation of p33 and/or p92 interfered with the abilities of these proteins to bind to the viral RNA in vitro (74). Thus, Pkc1p might be involved in TBSV replication as a regulator of the RNA-binding function of p33. Using phosphorylation-mimicking mutants of p33, we previously showed that reversible phosphorylation affects TBSV replication in plants (71). Nevertheless, critical information regarding the in vivo role of Pkc1p in TBSV replication was lacking. Accordingly, identification of Pkc1p in the proteome-wide overexpression screen (Table 1) provides the first piece of evidence that Pkc1p is a host factor affecting TBSV replication.
Fig 2.
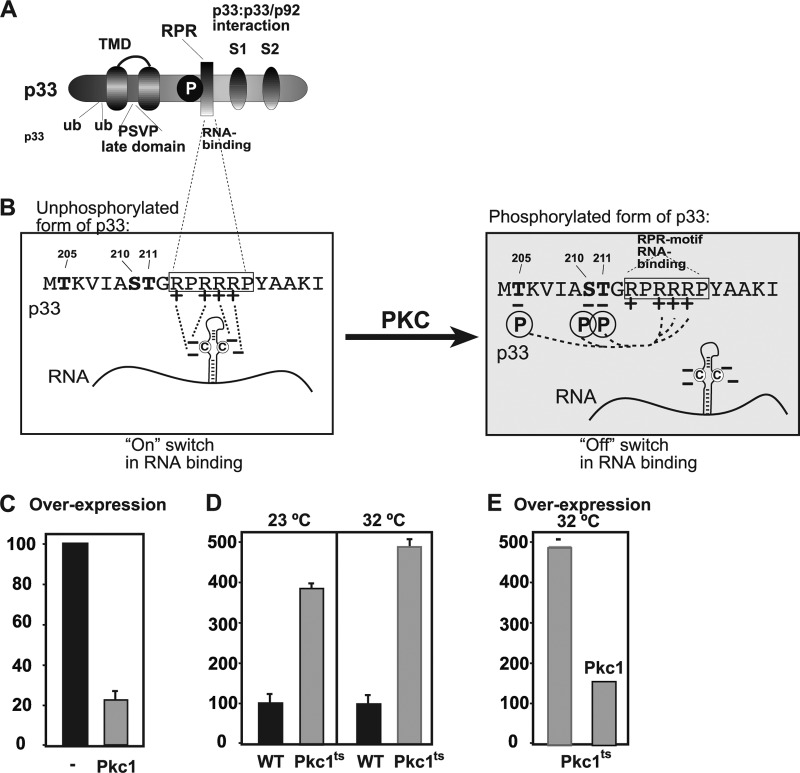
Overexpression of Pkc1p inhibits TBSV repRNA accumulation in yeast. (A) Schematic representation of the known domains in the tombusvirus replication protein p33. TMD, transmembrane domain; P, phosphorylation site; RPR, arginine-proline-rich RNA-binding domain. S1 and S2 are subdomains of the p33:p33-p92 interaction domain. The PSVP late domain, in connection with ubiquitinated lysines, is involved in binding to the host ESCRT components. (B) Binding of the unphosphorylated (left) and phosphorylated (right) forms of p33 to the viral RNA (71, 74). Phosphorylation is performed by Pkc in vitro, and the phosphorylated serine and threonine residues in p33 located in the vicinity of the RPR motif are shown in bold and labeled with the letter P. Note that the positively charged arginines within the RPR motif, critical in binding to the viral RNA, are predicted to be neutralized by the phosphorylated serine and threonine, as shown, resulting in a lack of RNA binding by p33. (C) Reduced TBSV repRNA accumulation in yeast overexpressing yeast Pkc1p. Overexpression was done from the GAL1 promoter. repRNA replication took place for 24 h at 29°C before RNA analysis. The accumulation level of DI-72 (+)repRNA (shown as percentages) was normalized based on that of 18S rRNA. Each experiment was repeated three times. (D) To launch TBSV repRNA replication, we expressed His6-p33 and His6-p92 from the copper-inducible CUP1 promoter and DI-72 (+)repRNA from the constitutive ADH1 promoter in the parental (wt, BY4741) and pkc1ts yeast strains. The yeast cells were cultured for 36 h at either 23°C (permissive temperature) or 32°C (semipermissive temperature) on 2% glucose SC minimal medium. Northern blot analysis was used to detect DI-72 (+)repRNA accumulation. The accumulation level of DI-72 (+)repRNA was normalized based on 18S rRNA. Each experiment was repeated three times. (E) Overexpression of yeast Pkc1p in pkc1ts yeast strains reduced TBSV repRNA accumulation. The yeast cells were cultured for 36 h at 32°C (semipermissive temperature).
To confirm the screen results, first we overexpressed the full-length Pkc1p in the wt yeast strain BY4741. As expected, Pkc1p overexpression reduced TBSV repRNA accumulation ∼4-fold (Fig. 2C). In addition, we used pkc1-4 yeast strain (29), which carries a temperature-sensitive (ts) kinase mutant of Pkc1p (Fig. 2D). We found that the ts mutant of Pkc1p allowed increased TBSV replication in yeast, ∼4-fold at the permissive temperature (23°C) and ∼5-fold at the semipermissive temperature (32°C) (Fig. 2D). Also, complementation with the wt Pkc1p in the pkc1-4 yeast strain led to ∼3-fold less TBSV replication than in the control pkc1-4 yeast strain not expressing wt Pkc1p (Fig. 2E), suggesting that pkc1-4 mutation can be complemented by the plasmid-borne expression of wt Pkc1p. Overall, these results support an inhibitory role for Pkc1p in TBSV replication in yeast.
Treatment with cercosporamide increases TBSV replication in yeast.
To further test if Pkc1p is involved in TBSV replication, we used cercosporamide, which specifically inhibits Pkc-type kinases (21, 77). Treatment of wt yeast with cercosporamide increased TBSV accumulation ∼3-fold (Fig. 3A). This result confirmed that Pkc1p is an inhibitor of TBSV replication in yeast cells.
Fig 3.
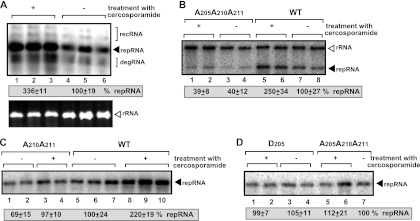
Effect of a Pkc1 inhibitor (cercosporamide) on viral RNA accumulation in yeast. (A) (Top) Northern blot analysis was used to detect DI-72 (+)repRNA accumulation in a yeast strain treated with cercosporamide (0.5 μg/ml) to inhibit Pkc1p function. rec, recombinant RNA; deg, partially degraded. (Bottom) Ethidium-bromide stained gel of total RNA extracts of the samples used for Northern blotting above. (B to D) Northern blot analysis of TBSV repRNA replication in yeast expressing the wt p33 or the nonphosphorylatable p33-A205A210A211 (B) or partially phosphorylatable p33-A210A211 (C) and in p33-D205 mutants from the ADH1 promoter (D), while wt p92 and DI-72 repRNA were expressed from the CUP1 promoter and the GAL1 promoter, respectively. Yeast was cultured for 36 h at 23°C in the presence of cercosporamide (1.0 μg/ml), 2% galactose, and 50 μM CuSO4.
To test if Pkc1p inhibits TBSV replication via phosphorylation of the replication protein p33, we used phosphorylation-deficient mutants of p33, which can be only partially phosphorylatable (A210A211 and D205) or nonphosphorylatable (A205A210A211) by Pkc1p in vitro (71, 74), in untreated yeast or yeast treated with cercosporamide. Interestingly, inhibition of Pkc1p by cercosporamide did not increase the replication of the nonphosphorylatable mutant p33-A205A210A211 (Fig. 3B, lanes 1 and 2 versus lanes 3 and 4, and 3D) or p33-D205 (Fig. 3D, lanes 1 and 2 versus lanes 3 and 4), while replication moderately increased (by ∼30%) when a partially phosphorylatable mutant, p33-A210A211, was used to support TBSV repRNA replication (Fig. 3C, lanes 1 and 2 versus lanes 3 and 4). Altogether, these data support the model that Pkc1p plays a role in TBSV replication via phosphorylation of the viral replication protein p33.
Treatment with cercosporamide also increases TBSV replication in plant protoplasts and whole Nicotiana benthamiana plants.
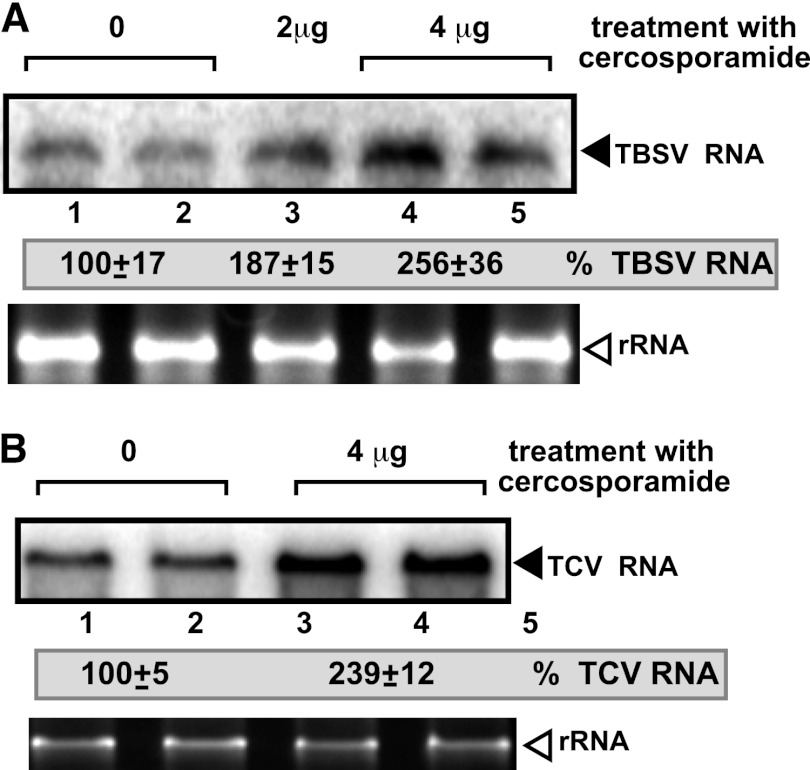
To examine if there is a similar kinase-based regulation of TBSV replication in plant host cells, we treated Nicotiana benthamiana protoplasts (single cells with the cell wall removed) replicating TBSV genomic RNA with cercosporamide at two different concentrations. The higher-concentration cercosporamide treatment increased TBSV genomic RNA accumulation ∼2.5-fold (Fig. 4A). Similar treatment of plant protoplasts with cercosporamide also increased the accumulation of the genomic RNA of TCV, a closely related plant virus to TBSV. Indeed, TCV accumulation increased 2.4-fold compared with that in the ethanol-treated control (Fig. 4B). Thus, it seems that replication of TBSV and TCV is affected by cercosporamide, an inhibitor of Pkc1-like kinases.
Fig 4.
The effect of cercosporamide treatment on TBSV and TCV RNA accumulation in N. benthamiana protoplasts. (A) Northern blot analysis was used to detect genomic (g) TBSV RNA accumulation in protoplasts treated with cercosporamide to inhibit Pkc1-like functions. Protoplasts from N. benthamiana were electroporated with TBSV gRNA and treated with various concentrations of cercosporamide (2- and 4-μg/ml final concentrations). Total RNA samples were obtained 40 h postelectroporation. The ethidium-bromide stained gel at the bottom shows rRNA levels. Note that treatment with ethanol (shown as “0”), which is used to dissolve cercosporamide, was chosen as the control. The accumulation level of TBSV RNA was normalized based on the rRNA. Each experiment was repeated three times. (B) As panel A, except that TCV RNA was used for protoplast electroporation.
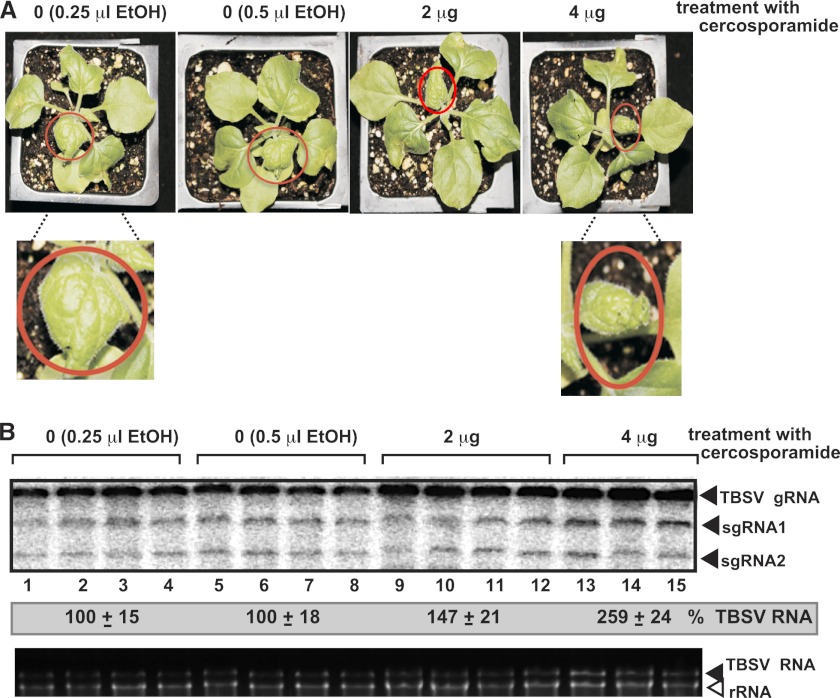
Treatment of N. benthamiana leaves with cercosporamide (at 2 dpi, on newly emerging top leaves) intensified the symptoms caused by TBSV (Fig. 5A) and led to a ∼2.5-fold increase in TBSV RNA accumulation at 4 dpi (Fig. 5B). Thus, it seems that cercosporamide can inhibit the antiviral function of Pkc1-like kinases in whole plants as well.
Fig 5.
The effect of cercosporamide treatment on TBSV RNA accumulation in N. benthamiana leaves. (A) Symptom intensification caused by TBSV infection in plants treated with cercosporamide. Note that leaf curling (the young leaves are circled) is more pronounced in TBSV-infected plants 4 days postinoculation. (B) Northern blot analysis was used to detect gTBSV RNA. Treatment of N. benthamiana leaves with cercosporamide promotes the accumulation of TBSV RNAs. Total RNA samples from the inoculated leaves were obtained 4 days postinoculation and used for Northern blotting (top) and gel analysis (bottom) to show rRNA levels.
Recombinant Pkc1p inhibits TBSV replication in a cell-free replication assay.
Although we previously documented the inhibitory effect of Pkc on the in vitro activity of TBSV RdRp and RNA binding by the replication protein p33 (71, 74), we wanted to obtain further direct evidence that Pkc1p can affect TBSV replication using our recently developed authentic yeast cell-free tombusvirus replication assay (53, 55). In the in vitro TBSV replication assay, we programmed the yeast cell extract (CFE) with the in vitro-transcribed TBSV (+)repRNA and purified recombinant p33 and p92pol obtained from E. coli (schematically shown in Fig. 6A). This led to the in vitro assembly of the viral replicase on the membranes present in CFE and one single cycle of complete TBSV replication, resulting in both negative-stranded and positive-stranded repRNA progeny (53, 55).
Fig 6.
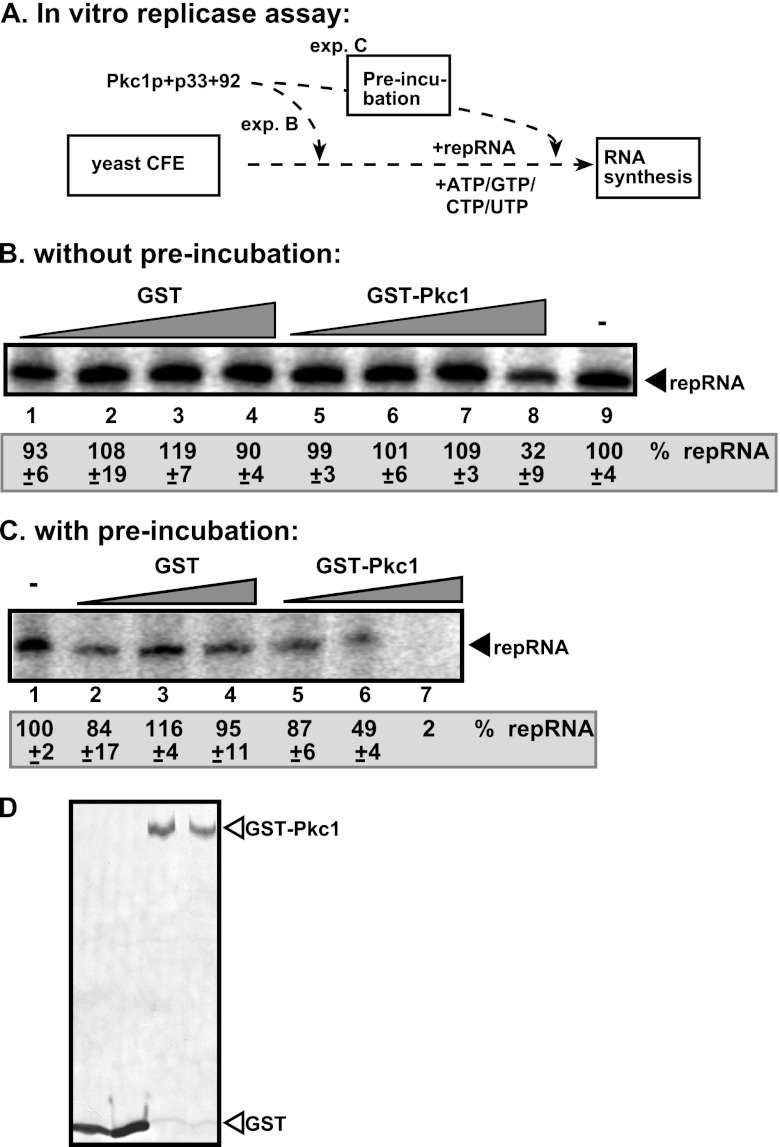
Inhibition of TBSV replication by recombinant Pkc1p in vitro. (A) Scheme of the CFE-based TBSV replication assay. Purified recombinant p33 and p92pol replication proteins of TBSV and in vitro-transcribed TBSV DI-72 (+)repRNA were added to the whole-cell extract prepared from the wt yeast strain. The purified recombinant yeast Pkc1p was added before (exp. C) or during (exp. B) the CFE-based TBSV replication assay. (B) Denaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the in vitro CFE-based TBSV replication assay in the presence of recombinant Pkc1p (1× = 200 ng). Each experiment was repeated three times. (C) CFE-based assay similar to that in panel B, except that Pkc1p was preincubated with p33/p92 in the reaction buffer for 30 min at 25°C. (D) Western blot analysis of purified recombinant GST-Pkc1p with anti-GST antibody.
We expressed and affinity purified recombinant yeast Pkc1p from E. coli (Fig. 6D) and added it to CFE prepared from BY4741 yeast (Fig. 6A). We found that the recombinant Pkc1p inhibited TBSV replication in the CFE-based assay ∼3-fold (Fig. 6B, lane 8 versus 9). Interestingly, preincubation of the recombinant Pkc1p with replication proteins p33 and/or p92 prior to the CFE-based assay led to an almost complete block of TBSV replication in vitro (Fig. 6C, lane 7). This suggests that the recombinant yeast Pkc1p is a potent inhibitor of TBSV replication, likely due to phosphorylation of the replication proteins that inhibits their viral RNA binding abilities, as shown previously for purified Pkc (Fig. 2B) (74).
DISCUSSION
High-throughput overexpression of host proteins is a suitable approach to identify host factors affecting tombusvirus replication.
Most genome-wide screens to identify host factors affecting (+)RNA virus infections are based on RNA interference (RNAi) approaches or the yeast deletion library (7, 19, 23, 24, 39, 44, 48, 62, 69, 70, 76, 78, 79). Additional systematic approaches, such as proteome-wide screens, can also lead to identification of novel host factors, as shown for TBSV by using mass spectrometry, protein arrays, and cDNA library screens (25, 27, 32, 67). In this study, we explored the large yeast ORF expression library (11) that covers over 90% of all known yeast genes for proteome-wide studies of viral host factors. Since we found 36 yeast proteins in the current screen, which were previously identified for TBSV, we suggest that similar proteome-wide approaches could also be useful for other virus-host interactions. Identification of over a hundred novel host proteins affecting TBSV replication in this screen, however, indicates that we still have not reached saturation level in identification of host factors with all the previous screens (38, 40). Bioinformatic analysis (data not shown) suggests that many different host pathways could affect TBSV replication. The largest number of host genes identified affects protein targeting and vesicle-mediated transport (Fig. 1). However, it is unlikely that all the identified host proteins involved in protein targeting and vesicle-mediated transport would directly affect the peroxisome-to-ER pathway involved in sorting TBSV replication proteins (31). Instead, it is possible that overexpression of given proteins would affect vesicle or protein transport in yeast, which might compete with TBSV replication for the transport of viral proteins, host protein factors, or host lipids to the site of tombusviral replication, thus indirectly inhibiting the viral replication process. Indeed, many lipid metabolism and membrane biogenesis genes also affected TBSV replication (Fig. 1), further supporting the idea of competition between the host pathways and TBSV for common host resources, like lipids and host proteins. However, an increasing number of host proteins is known to have direct roles or functions in TBSV replication, as shown for Pkc1p in this work and many others investigated earlier, such as the heat shock protein 70 chaperones (Hsp70) (55, 67, 85, 86), glyceraldehyde-3-phosphate dehydrogenase (13, 84), Cdc34p E2 ubiquitin-conjugating enzyme (25, 67, 84), eukaryotic translation elongation factor 1A (eEF1A) (27, 28), eukaryotic elongation factor 1Bγ (eEF1Bγ) (65), Ded1p RNA helicase (22), Pex19p shuttle protein (52), the Nedd40-like Rsp5p E3 ubiquitin ligase (4, 58), nucleolin (18), cyclophilins (30, 32), and a set of ESCRT proteins (3, 5, 27). Thus, additional in-depth analysis of the identified host factors will be needed to determine whether a given host protein acts directly or indirectly and what this particular host protein's mechanistic role is during viral replication. Altogether, we have already found via multiple screens that more than 400 host genes and proteins affect TBSV replication, indicating the complex nature of RNA virus-host interaction, even in a simple eukaryotic host such as yeast.
Direct inhibition of TBSV replication by Pkc1p.
Previous in vitro work has demonstrated that recombinant Pkc could phosphorylate the serine/threonine residues close to the RPR (arginine-proline-rich) motif in p33 and p92 (Fig. 2A), which inhibits the abilities of p33 and p92 to bind viral RNA and in viral RNA template recruitment to the site of replication (56, 59, 71, 74). This study, which used a CFE-based TBSV replication assay, demonstrates that preincubation of the recombinant yeast Pkc1p with the replication proteins p33 and p92 strongly inhibited TBSV replication in vitro (Fig. 6). Thus, Pkc1p seems to be a negative regulator of TBSV replication, likely due to inhibition of the RNA-binding activity of p33 and p92. Thus, this work in combination with previous in vitro data (56, 59, 71, 74) firmly establishes the inhibitory role of Pkc in the functions of the replication proteins p33 and p92.
Importantly, the current work also provides strong evidence that Pkc1p plays a role in TBSV replication in yeast based on overexpression studies and a ts mutant of Pkc1p. Also, the use of a specific inhibitor of Pkc1p (i.e., cercosporamide) further supports the idea that Pkc1p is a potent inhibitor of TBSV replication. Based on the use of the nonphosphorylatable mutant of p33, it seems that Pkc1p inhibits TBSV replication via phosphorylation of the viral p33 replication protein. Similarly, cercosporamide treatment also increased TBSV replication in plant protoplasts and whole plants, suggesting that Pkc-related host kinases inhibit TBSV in a natural host, too.
Various roles for Pkc in relation to several RNA viruses are emerging. Pkc is known to be a major component of the antiviral response in mammalian cells, as it acts in the signal transduction pathway mediated by human alpha interferon (63). RNA viruses also exploit Pkc during their replication. For example, human parainfluenza virus and Sendai virus use the cellular Pkc to phosphorylate the viral P protein, which is critical for its function as transactivator of the viral RNA polymerase (8, 14, 15). Also, inhibitors of the cellular PKCs are potential agents against human immunodeficiency virus, as they inhibit transcription of viral RNAs (57). In addition, a recent kinome-wide RNAi screen in Drosophila also identified several kinases and phosphatases, including a protein kinase C involved in the poxvirus entry process (35).
Overall, the current work has revealed a role for Pkc1p as an inhibitor of RNA virus replication in vivo. This function for Pkc1p seems to be conserved between yeast and plants, as demonstrated by treatment with the Pkc-specific inhibitor cercosporamide, which resulted in increased TBSV RNA accumulation in yeast, single plant cells, and whole N. benthamiana plants.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brenda Andrews (Banting and Best Department of Medical Research and Department of Molecular Genetics, The Donnelly Centre, University of Toronto, Toronto, Ontario, Canada) for providing the yeast GST-tagged ORF library and Charlie Boone (University of Toronto, Toronto, Ontario, Canada) for providing the pkc1-4 ts-mutant yeast strain. We thank Judit Pogany for critical reading of the manuscript.
This work was supported by NIAID (5R21AI079457-02) and the Kentucky Science Foundation.
Footnotes
Published ahead of print 20 June 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alfonzo JD, Crother TR, Guetsova ML, Daignan-Fornier B, Taylor MW. 1999. APT1, but not APT2, codes for a functional adenine phosphoribosyltransferase in Saccharomyces cerevisiae. J. Bacteriol. 181:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali A, Sivakami S, Raghuram N. 2007. Regulation of activity and transcript levels of NR in rice (Oryza sativa): roles of protein kinase and G-proteins. Plant Science 172:406–413 [Google Scholar]
- 3. Barajas D, Jiang Y, Nagy PD. 2009. A unique role for the host ESCRT proteins in replication of tomato bushy stunt virus. PLoS Pathog. 5:e1000705 doi:10.1371/journal.ppat.1000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barajas D, Li Z, Nagy PD. 2009. The Nedd4-type Rsp5p ubiquitin ligase inhibits tombusvirus replication by regulating degradation of the p92 replication protein and decreasing the activity of the tombusvirus replicase. J. Virol. 83:11751–11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barajas D, Nagy PD. 2010. Ubiquitination of tombusvirus p33 replication protein plays a role in virus replication and binding to the host Vps23p ESCRT protein. Virology 397:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng C-P, Panavas T, Luo G, Nagy PD. 2005. Heterologous RNA replication enhancer stimulates in vitro RNA synthesis and template-switching by the carmovirus, but not by the tombusvirus, RNA-dependent RNA polymerase: Implication for modular evolution of RNA viruses. Virology 341:107–121 [DOI] [PubMed] [Google Scholar]
- 7. Cherry S, et al. 2005. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 19:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De BP, Gupta S, Banerjee AK. 1995. Cellular protein kinase C isoform zeta regulates human parainfluenza virus type 3 replication. Proc. Natl. Acad. Sci. U. S. A. 92:5204–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. den Boon JA, Diaz A, Ahlquist P. 2010. Cytoplasmic viral replication complexes. Cell Host Microbe 8:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gancarz BL, Hao L, He Q, Newton MA, Ahlquist P. 2011. Systematic identification of novel, essential host genes affecting bromovirus RNA replication. PLoS One 6:e23988 doi:10.1371/journal.pone.0023988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gelperin DM, et al. 2005. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 19:2816–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gietz RD, Woods RA. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350:87–96 [DOI] [PubMed] [Google Scholar]
- 13. Huang TS, Nagy PD. 2011. Direct inhibition of tombusvirus plus-strand RNA synthesis by a dominant negative mutant of a host metabolic enzyme, glyceraldehyde-3-phosphate dehydrogenase, in yeast and plants. J. Virol. 85:9090–9102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huntley CC, De BP, Banerjee AK. 1997. Phosphorylation of Sendai virus phosphoprotein by cellular protein kinase C zeta. J. Biol. Chem. 272:16578–16584 [DOI] [PubMed] [Google Scholar]
- 15. Huntley CC, De BP, Murray NR, Fields AP, Banerjee AK. 1995. Human parainfluenza virus type 3 phosphoprotein: identification of serine 333 as the major site for PKC zeta phosphorylation. Virology 211:561–567 [DOI] [PubMed] [Google Scholar]
- 16. Jaag HM, Nagy PD. 2010. The combined effect of environmental and host factors on the emergence of viral RNA recombinants. PLoS Pathog. 6:e1001156 doi:10.1371/journal.ppat.1001156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaag HM, Pogany J, Nagy PD. 2010. A host Ca2+/Mn2+ ion pump is a factor in the emergence of viral RNA recombinants. Cell Host Microbe 7:74–81 [DOI] [PubMed] [Google Scholar]
- 18. Jiang Y, Li Z, Nagy PD. 2010. Nucleolin/Nsr1p binds to the 3′ noncoding region of the tombusvirus RNA and inhibits replication. Virology 396:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD. 2006. Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J. Virol. 80:7394–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasparovsky T, Blein JP, Mikes V. 2004. Ergosterol elicits oxidative burst in tobacco cells via phospholipase A(2) and protein kinase C signal pathway. Plant Physiol. Biochem. 42:429–435 [DOI] [PubMed] [Google Scholar]
- 21. Konicek BW, et al. 2011. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 71:1849–1857 [DOI] [PubMed] [Google Scholar]
- 22. Kovalev N, Pogany J, Nagy PD. 2012. A co-opted DEAD-box RNA helicase enhances tombusvirus plus-strand synthesis. PLoS Pathog. 8:e1002537 doi:10.1371/journal.ppat.1002537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kushner DB, et al. 2003. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc. Natl. Acad. Sci. U. S. A. 100:15764–15769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Q, et al. 2009. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl. Acad. Sci. U. S. A. 106:16410–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Barajas D, Panavas T, Herbst DA, Nagy PD. 2008. Cdc34p ubiquitin-conjugating enzyme is a component of the tombusvirus replicase complex and ubiquitinates p33 replication protein. J. Virol. 82:6911–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Nagy PD. 2011. Diverse roles of host RNA binding proteins in RNA virus replication. RNA Biol. 8:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, et al. 2009. Translation elongation factor 1A is a component of the tombusvirus replicase complex and affects the stability of the p33 replication co-factor. Virology 385:245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Z, et al. 2010. Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthesis. PLoS Pathog. 6:e1001175 doi:10.1371/journal.ppat.1001175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, et al. 2011. Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol. 29:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin JY, Mendu V, Pogany J, Qin J, Nagy PD. 2012. The TPR domain in the host Cyp40-like cyclophilin binds to the viral replication protein and inhibits the assembly of the tombusviral replicase. PLoS Pathog. 8:e1002491 doi:10.1371/journal.ppat.1002491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. 2005. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17:3513–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendu V, Chiu M, Barajas D, Li Z, Nagy PD. 2010. Cpr1 cyclophilin and Ess1 parvulin prolyl isomerases interact with the tombusvirus replication protein and inhibit viral replication in yeast model host. Virology 406:342–351 [DOI] [PubMed] [Google Scholar]
- 33. Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 6:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monkewich S, et al. 2005. The p92 polymerase coding region contains an internal RNA element required at an early step in Tombusvirus genome replication. J. Virol. 79:4848–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. 2010. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 6:e1000954 doi:10.1371/journal.ppat.1000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munnik T, Testerink C. 2009. Plant phospholipid signaling: “in a nutshell.” J. Lipid Res. 50:S260–S265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagy PD. 2011. The roles of host factors in tombusvirus RNA recombination. Adv. Virus Res. 81:63–84 [DOI] [PubMed] [Google Scholar]
- 38. Nagy PD. 2008. Yeast as a model host to explore plant virus-host interactions. Annu. Rev. Phytopathol. 46:217–242 [DOI] [PubMed] [Google Scholar]
- 39. Nagy PD, Pogany J. 2012. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 10:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagy PD, Pogany J. 2010. Global genomics and proteomics approaches to identify host factors as targets to induce resistance against tomato bushy stunt virus. Adv. Virus Res. 76:123–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagy PD, Pogany J. 2008. Multiple roles of viral replication proteins in plant RNA virus replication. Methods Mol. Biol. 451:55–68 [DOI] [PubMed] [Google Scholar]
- 42. Nagy PD, Wang RY, Pogany J, Hafren A, Makinen K. 2011. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 411:374–382 [DOI] [PubMed] [Google Scholar]
- 43. Navarro B, Rubino L, Russo M. 2004. Expression of the Cymbidium ringspot virus 33-kilodalton protein in Saccharomyces cerevisiae and molecular dissection of the peroxisomal targeting signal. J. Virol. 78:4744–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng TI, et al. 2007. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology 45:1413–1421 [DOI] [PubMed] [Google Scholar]
- 45. Novoa RR, et al. 2005. Virus factories: associations of cell organelles for viral replication and morphogenesis. Biol. Cell 97:147–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panavas T, Hawkins CM, Panaviene Z, Nagy PD. 2005. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology 338:81–95 [DOI] [PubMed] [Google Scholar]
- 47. Panavas T, Nagy PD. 2003. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology 314:315–325 [DOI] [PubMed] [Google Scholar]
- 48. Panavas T, Serviene E, Brasher J, Nagy PD. 2005. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. U. S. A. 102:7326–7331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Panaviene Z, Baker JM, Nagy PD. 2003. The overlapping RNA-binding domains of p33 and p92 replicase proteins are essential for tombusvirus replication. Virology 308:191–205 [DOI] [PubMed] [Google Scholar]
- 50. Panaviene Z, Panavas T, Serva S, Nagy PD. 2004. Purification of the cucumber necrosis virus replicase from yeast cells: role of coexpressed viral RNA in stimulation of replicase activity. J. Virol. 78:8254–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pathak KB, Pogany J, Xu K, White KA, Nagy PD. 2012. Defining the roles of cis-acting RNA elements in tombusvirus replicase assembly in vitro. J. Virol. 86:156–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pathak KB, Sasvari Z, Nagy PD. 2008. The host Pex19p plays a role in peroxisomal localization of tombusvirus replication proteins. Virology 379:294–305 [DOI] [PubMed] [Google Scholar]
- 53. Pogany J, Nagy PD. 2008. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. J. Virol. 82:5967–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pogany J, Panavas T, Serviene E, Nawaz-Ul-Rehman MS, DNP 2010. A high-throughput approach for studying virus replication in yeast. Curr. Protoc. Microbiol. 19:16J.1.1-16J.1.15 [DOI] [PubMed] [Google Scholar]
- 55. Pogany J, Stork J, Li Z, Nagy PD. 2008. In vitro assembly of the Tomato bushy stunt virus replicase requires the host Heat shock protein 70. Proc. Natl. Acad. Sci. U. S. A. 105:19956–19961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pogany J, White KA, Nagy PD. 2005. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J. Virol. 79:4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qatsha KA, Rudolph C, Marme D, Schachtele C, May WS. 1993. Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc. Natl. Acad. Sci. U. S. A. 90:4674–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qin J, Barajas D, Nagy PD. 2012. An inhibitory function of WW domain-containing host proteins in RNA virus replication. Virology 426:106–119 [DOI] [PubMed] [Google Scholar]
- 59. Rajendran KS, Nagy PD. 2003. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J. Virol. 77:9244–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rajendran KS, Nagy PD. 2004. Interaction between the replicase proteins of Tomato bushy stunt virus in vitro and in vivo. Virology 326:250–261 [DOI] [PubMed] [Google Scholar]
- 61. Rajendran KS, Nagy PD. 2006. Kinetics and functional studies on interaction between the replicase proteins of Tomato Bushy Stunt Virus: Requirement of p33:p92 interaction for replicase assembly. Virology 345:270–279 [DOI] [PubMed] [Google Scholar]
- 62. Randall G, et al. 2007. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. U. S. A. 104:12884–12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reich NC, Pfeffer LM. 1990. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc. Natl. Acad. Sci. U. S. A. 87:8761–8765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salonen A, Ahola T, Kaariainen L. 2005. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 285:139–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sasvari Z, Izotova L, Kinzy TG, Nagy PD. 2011. Synergistic roles of eukaryotic translation elongation factors 1Bgamma and 1A in stimulation of tombusvirus minus-strand synthesis. PLoS Pathog. 7:e1002438 doi:10.1371/journal.ppat.1002438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmitz HP, Heinisch JJ. 2003. Evolution, biochemistry and genetics of protein kinase C in fungi. Curr. Genet. 43:245–254 [DOI] [PubMed] [Google Scholar]
- 67. Serva S, Nagy PD. 2006. Proteomics analysis of the tombusvirus replicase: Hsp70 molecular chaperone is associated with the replicase and enhances viral RNA replication. J. Virol. 80:2162–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Serviene E, Jiang Y, Cheng CP, Baker J, Nagy PD. 2006. Screening of the yeast yTHC collection identifies essential host factors affecting tombusvirus RNA recombination. J. Virol. 80:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Serviene E, et al. 2005. Genome-wide screen identifies host genes affecting viral RNA recombination. Proc. Natl. Acad. Sci. U. S. A. 102:10545–10550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sessions OM, et al. 2009. Discovery of insect and human dengue virus host factors. Nature 458:1047–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shapka N, Stork J, Nagy PD. 2005. Phosphorylation of the p33 replication protein of Cucumber necrosis tombusvirus adjacent to the RNA binding site affects viral RNA replication. Virology 343:65–78 [DOI] [PubMed] [Google Scholar]
- 72. Sopko R, et al. 2006. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 21:319–330 [DOI] [PubMed] [Google Scholar]
- 73. Stork J, Kovalev N, Sasvari Z, Nagy PD. 2011. RNA chaperone activity of the tombusviral p33 replication protein facilitates initiation of RNA synthesis by the viral RdRp in vitro. Virology 409:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stork J, Panaviene Z, Nagy PD. 2005. Inhibition of in vitro RNA binding and replicase activity by phosphorylation of the p33 replication protein of Cucumber necrosis tombusvirus. Virology 343:79–92 [DOI] [PubMed] [Google Scholar]
- 75. Subramaniam R, Despres C, Brisson N. 1997. A functional homolog of mammalian protein kinase C participates in the elicitor-induced defense response in potato. Plant Cell 9:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Supekova L, et al. 2008. Identification of human kinases involved in hepatitis C virus replication by small interference RNA library screening. J. Biol. Chem. 283:29–36 [DOI] [PubMed] [Google Scholar]
- 77. Sussman A, et al. 2004. Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryot. Cell 3:932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tai AW, et al. 2009. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vaillancourt FH, et al. 2009. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology 387:5–10 [DOI] [PubMed] [Google Scholar]
- 80. Vasconsuelo A, Boland R. 2007. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Science 172:861–875 [Google Scholar]
- 81. Vasconsuelo A, Giuletti AM, Picotto G, Rodriguez-Talou J, Boland R. 2003. Involvement of the PLC/PKC pathway in Chitosan-induced anthraquinone production by Rubia tinctorum L. cell cultures. Plant Science 165:429–436 [Google Scholar]
- 82. Vasconsuelo A, Giulietti AM, Boland R. 2004. Signal transduction events mediating chitosan stimulation of anthraquinone synthesis in Rubia tinctorum. Plant Science 166:405–413 [Google Scholar]
- 83. Vasconsuelo A, Morelli S, Picotto G, Giulietti AM, Boland R. 2005. Intracellular calcium mobilization: a key step for chitosan-induced anthraquinone production in Rubia tinctorum L. Plant Science 169:712–720 [Google Scholar]
- 84. Wang RY, Nagy PD. 2008. Tomato bushy stunt virus co-opts the RNA-binding function of a host metabolic enzyme for viral genomic RNA synthesis. Cell Host Microbe 3:178–187 [DOI] [PubMed] [Google Scholar]
- 85. Wang RY, Stork J, Nagy PD. 2009. A key role for heat shock protein 70 in the localization and insertion of tombusvirus replication proteins to intracellular membranes. J. Virol. 83:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang RY, Stork J, Pogany J, Nagy PD. 2009. A temperature sensitive mutant of heat shock protein 70 reveals an essential role during the early steps of tombusvirus replication. Virology 394:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. White KA, Nagy PD. 2004. Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog. Nucleic Acids Res. Mol. Biol. 78:187–226 [DOI] [PubMed] [Google Scholar]
- 88. Wu B, et al. 2009. A discontinuous RNA platform mediates RNA virus replication: building an integrated model for RNA-based regulation of viral processes. PLoS Pathog. 5:e1000323 doi:10.1371/journal.ppat.1000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.