Abstract
Respiratory epithelial cells and macrophages are the key innate immune cells that play an important role in the pathogenesis of influenza A virus infection. We found that these two cell types from both human and pig showed comparable susceptibilities to initial infection with a highly pathogenic avian influenza (HPAI) H5N1 virus (A/turkey/Turkey/1/05) and a moderately pathogenic human influenza H1N1 virus (A/USSR/77), but there were contrasting differences in host innate immune responses. Human cells mounted vigorous cytokine (tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]) and chemokine (CXCL9, CXCL10, and CXCL11) responses to H5N1 virus infection. However, pig epithelial cells and macrophages showed weak or no TNF-α and chemokine induction with the same infections. The apparent lack of a strong proinflammatory response, corroborated by the absence of TNF-α induction in H5N1 virus-challenged pigs, coincided with greater cell death and the reduced release of infectious virus from infected pig epithelial cells. Suppressor of cytokine signaling 3 (SOCS3), a protein suppressor of the JAK-STAT pathway, was constitutively highly expressed and transcriptionally upregulated in H5N1 virus-infected pig epithelial cells and macrophages, in contrast to the corresponding human cells. The overexpression of SOCS3 in infected human macrophages dampened TNF-α induction. In summary, we found that the reported low susceptibility of pigs to contemporary Eurasian HPAI H5N1 virus infections coincides at the level of innate immunity of respiratory epithelial cells and macrophages with a reduced output of viable virus and an attenuated proinflammatory response, possibly mediated in part by SOCS3, which could serve as a target in the treatment or prevention of virus-induced hypercytokinemia, as observed for humans.
INTRODUCTION
Human cases of highly pathogenic avian influenza (HPAI) virus H5N1 infections carry an alarming mortality rate of 50 to 60%, according to cumulative figures from the World Health Organization (http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html). Despite its high death rate in humans, HPAI H5N1 virus infections are generally confined to wild birds and poultry. However, owing to the inherent nature of the segmented RNA virus to mutate and undergo reassortment, the danger of HPAI viruses gaining the ability to efficiently transmit horizontally between humans, like that of seasonal influenza A virus strains, while retaining high virulence cannot be ignored (5). A commonly cited complication of influenza virus infections in humans is the rapid development of a hyperacute dysregulation of proinflammatory cytokines and chemokines, described as hypercytokinemia or a cytokine storm, which is a self-destructive and often fatal syndrome despite supportive medical interventions (41–43). Prevention by vaccination and treatment by antineuraminidase drugs are the mainstays of influenza management, but they are not without major shortcomings, namely, a long lead vaccine production time and the development of drug resistance (11, 13, 23). A further strategy that is urgently needed to tackle future highly virulent epidemics or pandemics is to develop therapeutic agents that target hypercytokinemia. However, simply blocking proinflammation alone does not improve mortality rates in HPAI H5N1 virus-infected mice (31, 33). There is a great need to understand the host triggers of influenza virus-induced hypercytokinemia, to be able to develop rational interventions to maintain or restore a regulated proinflammatory response during active infection.
In contrast to humans, pigs appear to be largely refractory to contemporary Eurasian HPAI H5N1 virus infections and are highly resistant to the development of any adverse effects. Experimental H5N1 virus challenge studies in pigs found no or only transient and mild clinical symptoms, such as pyrexia, and subsequent seroconversion (6, 12, 19). A retrospective analysis of farm pigs found evidence of previous exposure to HPAI H5N1 virus infection without noticeable clinical signs (8, 27). Recent work showed that the two most important host signaling pathways in response to influenza virus infection that mediate inflammation and an antiviral state (mitogen-activated protein kinase [MAPK] and NF-κB activation) are paradoxically the same pathways that are necessary for virus replication (22, 24), suggesting that the mere detection of a strong host proinflammatory or antiviral response to influenza virus infection does not necessarily imply effective virus control. Therefore, to dissect the molecular controls of effective innate immunity against HPAI H5N1 virus infection, a strategic approach is to establish molecular differences in host innate responses between susceptible (human) and resistant (pig) mammalian species in order to identify critical host factors or cellular responses that could confer host resistance. Key innate immune cells that play an important role in the pathogenesis of influenza A virus infection are respiratory epithelial cells, macrophages (18, 30), and, more recently recognized, endothelial cells (40, 46). By comparing host responses to HPAI H5N1 virus and to other less virulent influenza virus strains in primary respiratory epithelial cells and monocyte-derived macrophages of humans and pigs, we found that innate resistance to HPAI virus infection, as exemplified in pig cells, is characterized by a reduced output of viable virus and an attenuated proinflammatory response, possibly mediated in part by suppressor of cytokine signaling 3 (SOCS3). This raises the possibility of targeting SOCS3 in the treatment of virus-induced hypercytokinemia.
MATERIALS AND METHODS
Primary respiratory epithelial cells and peripheral blood monocytes/macrophages.
Four different batches of primary human respiratory (tracheobronchial) epithelial cells (CC-2540) from Lonza UK were used. Pig respiratory epithelial cells were isolated from stripped tracheobronchial mucosae from eight 3- to 4-month-old pigs. Briefly, washed mucosae were incubated at 4°C overnight with 0.06 U/ml pronase (Sigma) in a 1:1 dilution of Dulbecco's modified Eagle's medium (DMEM)–F-12 medium. Supernatants containing cells were centrifuged and washed in DMEM-Glutamax and cultured in bronchial epithelial growth medium (BEGM) (CC-3170; Lonza UK). Epithelial cells in culture were confirmed by immunocytochemical staining with a mouse monoclonal antibody to the epithelial cell marker pancytokeratin (PCK-26; Abcam), followed by the use of an Envision+ system-horseradish peroxidase (HRP) kit (Dako).
Human and pig peripheral blood monocytes were isolated from human leukocyte-enriched buffy coats derived from collected heparinized blood and from commercial pigs, respectively, by using Histopaque-1077 (Sigma). Cells were washed several times with phosphate-buffered saline (PBS) and cultured (2 × 106 cells/ml) in serum-free RPMI 1640 medium supplemented with 2 mM glutamine, 100 U/ml penicillin–100 μg/ml streptomycin, 1% nonessential amino acids, and 1 mM sodium pyruvate (Invitrogen) at 37°C for 90 min in 6-well plates (Corning). Nonadherent cells were removed with multiple PBS washes. Adherent cells were differentiated into macrophages for 7 days in RPMI 1640 medium supplemented with 10% human AB serum or 10% pig serum, 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems), 100 U/ml penicillin–100 μg/ml streptomycin, 2 mM glutamine, 1% nonessential amino acids, and 1 mM sodium pyruvate. By day 7, cells showed the typical macrophage morphology.
Influenza virus receptor detection and viruses.
Influenza virus receptor distribution on primary cells was determined by lectin cytochemical staining with fluorescein isothiocyanate (FITC)-labeled Sambucus nigra agglutinin (SNA) lectin (Vector Labs), specific for the human influenza virus receptor sialic acid α2,6-galactose (SAα2,6-Gal), and biotinylated Maackia amurensis agglutinin II (MAA II) (Vector Labs), specific for the avian influenza virus receptor sialic acid α2,3-galactose (SAα2,3-Gal), as described previously (17, 25). Clade 2.2.1 HPAI H5N1 (A/turkey/Turkey/1/05) (21) and human H1N1 (A/USSR/77 [USSR]) (38) influenza viruses were used in this study. All the viruses were grown in 10-day-old embryonated chicken eggs by allantoic inoculation. HPAI H5N1 clade 2.2.1 viruses have been associated with the global panzootic since 2003 and continued to dominate human H5N1 cases in Egypt in the last few years. The human USSR H1N1 virus was moderately pathogenic in humans and was responsible for the 1977 epidemic in Russia (38). All HPAI H5N1 virus infection work was carried out in the biological containment level 3 (CL3) Advisory Committee on Dangerous Pathogens containment level 3 [ACDP CL3]/Specified Animal Pathogens Order 1998 containment level 4 [SAPO4]) facility at the Animal Health Veterinary Laboratories Agency (AHVLA).
Infection of epithelial cells and macrophages.
Respiratory epithelial cells and macrophages were infected with specific viruses at the specified multiplicity of infection (MOI), based on virus titration on reference Madin-Darby canine kidney (MDCK) cells, by preincubation with the virus for 2 h in serum-free BEGM and RPMI 1640 medium containing 2% Ultroser G (Pall Biosepra) and 100 U/ml penicillin–100 μg/ml streptomycin, respectively. After 2 h, cells were rinsed three times with PBS and incubated in fresh medium until harvest. For uniformity, l-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK) trypsin (Sigma) at a final concentration of 500 ng/ml (16, 34) was used throughout infections with HPAI H5N1 and USSR H1N1 viruses.
PCR quantification of virus and host genes.
Virus RNA was extracted from culture medium by using a QIAamp viral RNA minikit (Qiagen). A one-step reverse transcription real-time PCR assay was performed to quantify virus matrix gene RNA as previously described (36, 37). The expression of host genes based on cDNA converted from total RNA (SuperScript III first-strand cDNA synthesis kit; Invitrogen) was performed with a LightCycler-480 instrument (Roche), using a relative standard curve approach, normalized to 18S rRNA. Sequence details of human genes are as follows: GGAGAAGGGTGACCGACTCA (forward), TGCCCAGACTCGGCAAAG (reverse), and 5′–6-carboxyfluorescein (FAM)–CGCTGAGATCAATCGGCCCGACTA–6-carboxytetramethylrhodamine (TAMRA)–3′ (TaqMan probe) for the tumor necrosis factor alpha (TNF-α) gene (TNF-α); GCACGATGCACCTGTACGAT (forward), AGACATCACCAAGCTTTTTTGCT (reverse), and 5′-FAM-CTGAACTGCACGCTCCGGGACTC-TAMRA-3′ (TaqMan probe) for the interleukin-1β (IL-1β) gene (IL1β); CCAGGAGCCCAGCTATGAAC (forward), CCCAGGGAGAAGGCAACTG (reverse), and 5′-FAM-CCTTCTCCACAAGCGCCTTCGGT-TAMRA-3′ (TaqMan probe) for the IL6 gene; TaqMan assay identifier Hs01077958_s1 for the beta 1 interferon (IFN-β1) gene (IFNβ1); Hs02330328_s1 for SOCS3; Hs00973637_m1 for OAS1; Hs00895608_m1 for Mx1; Hs00171065_m1 for CXCL9; Hs00171042_m1 for CXCL10; and Hs00171138_m1 for CXCL11. Sequence details of pig genes are as follows: CCCGACTATCTGGACTTTGCT (forward), CCAGCCCCTCATTCTCTTTCT (reverse), and 5′-FAM-CTCCCCTGTCCATCCCTTTATT-TAMRA-3′ (TaqMan probe) for TNF-α; TGCCAACGTGCAGTCTATGG (forward), TGGGCCAGCCAGCACTAG (reverse), and 5′-FAM-TGCAAACTCCAGGACAAAGACCACAAATC-TAMRA-3′ (TaqMan probe) for IL1β; CGCAGCCTTGAGGATTTCC (forward), CAGGTGCCCCAGCTACATTATC (reverse), and 5′-FAM-CAGTTCAGCCTGAGGGCCATTC-TAMRA-3′ (TaqMan probe) for IL6; TCTCTAGCACTGGCTGGAATGA (forward), TCATCCATCTGCCCATCAAG (reverse), and 5′-FAM-ACCGTCATTAAGACTATCCTTGTGGA-TAMRA-3′ (TaqMan probe) for IFNβ1; Ss03387992_u1 for SOCS3; Ss03394660_m1 for OAS1; Ss03393847_m1 for Mx1; Ss03390033_m1 for CXCL9; Ss03391846_m1 for CXCL10; and Ss03648934_m1 for CXCL11 (Applied Biosystems).
Infection of pigs with HPAI H5N1 virus.
All pig work was carried out at the AHVLA using biological containment level 3 facilities (ACDP3/SAPO4) under Home Office license 70/7062. Animal welfare guidelines, protocols, and procedures were approved by the AHVLA Ethics Committee, comprising internal and external members as well as a named veterinary surgeon. Commercial Large White-Landrace-based 4-week-old pigs sourced from a high-health-status farm, prescreened for the absence of exposure to influenza viruses by serology and PCR, were inoculated with A/turkey/Turkey/1/05 virus at 106 median egg infectious doses (EID50) by the upper respiratory tract route, delivered intranasally (2.5 ml) and intratracheally (2.5 ml), and monitored daily for clinical signs (including pyrexia) and nasal virus shedding by real-time PCR (20). Pigs were culled sequentially, and all major tissues were collected for virus detection on days 4, 12, and 30 postinfection. Tissues were stored at −80°C until required.
TNF-α ELISA, proliferation (MTS) assays, propidium iodide flow cytometry, Western blotting, and electroporation.
Human and pig TNF-α enzyme-linked immunosorbent assays (ELISAs) were performed with R&D Systems kits. The determination of the cellular metabolic activity of primary epithelial cells following virus infection was performed by using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay kits (Promega). Following ethanol fixation and propidium iodide staining, the proportion of hypodiploid (nuclear fragmented) cells was quantified with a BD FACS Canto II flow cytometer (BD Biosciences), as previously described (16). Western blotting to detect endogenous SOCS3 using a polyclonal rabbit SOCS3 antibody (2923; Cell Signaling Technology) was performed according to the supplier's protocol. Transfection by electroporation was performed by using a Nucleofector kit (Lonza) designed for human macrophages. Transfected macrophages were allowed to recover for at least 48 h before virus infection.
Quantification of viable virus.
The quantification of infectious virus in a given culture volume was conducted as previously described (16), which was an immunohistochemical focus assay (9) based on infection in MDCK cells for 6 h followed by the immunodetection of influenza virus nucleoprotein expression using a murine antinucleoprotein antibody (Abcam) and an Envision+ system-HRP kit (Dako). Briefly, cells were fixed in acetone methanol for 10 min followed by peroxidase treatment for 10 min and incubation with a 1:1,000 dilution of primary mouse monoclonal antibody to influenza virus nucleoprotein (Abcam) for 40 min at room temperature. The cells were subsequently rinsed with Tris-buffered saline (TBS) and incubated with a horseradish peroxidase-labeled polymer for 40 min. After gentle rinsing with TBS, the cells were incubated with 3,3′-diaminobenzidine (DAB) substrate-chromogen solution for 7 min. Cells positive for viral nucleoprotein were counted with an inverted microscope, and the mean numbers of positive cells in four 96-well plates were used to calculate infectious focus-forming units of virus per microliter of infection volume.
Statistical analysis.
Statistical analysis of the data in Fig. 6 and 8 was performed by a two-sample t test using Minitab 16 software.
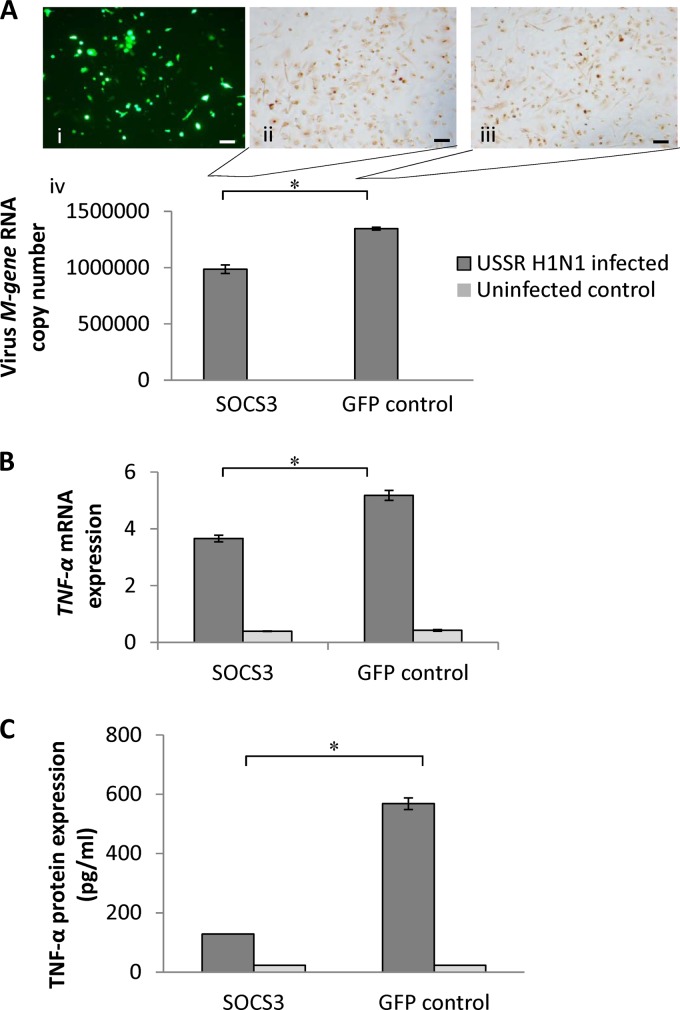
Fig 6.
SOCS3 overexpression in human macrophages reduced USSR H1N1-induced TNF-α production. (A) Human macrophages were transfected with a SOCS3 expression plasmid (pCMV-SOCS3 from Addgene) and a GFP control vector (at a ∼50% transfection efficiency by electroporation) (i); at 48 h posttransfection, cells were infected with USSR H1N1 virus for 24 h at an MOI of 1.0. Antibody detection of virus nucleoprotein showed a similar presence of the protein in SOCS3-overexpressed (ii) and control (iii) macrophages. A significantly lower influenza virus M gene RNA level was detected in culture supernatants from SOC3-overexpressed macrophages than in GFP-transfected macrophages (iv). Scale bars, 100 μm. (B) The TNF-α mRNA expression level in USSR H1N1 virus-infected SOCS3-overexpressed macrophages was significantly reduced in relation to levels in the infected control. (C) Likewise, the corresponding induction of the TNF-α protein by USSR H1N1 virus was significantly reduced in SOCS3-overexpressed macrophages. *, P < 0.05.
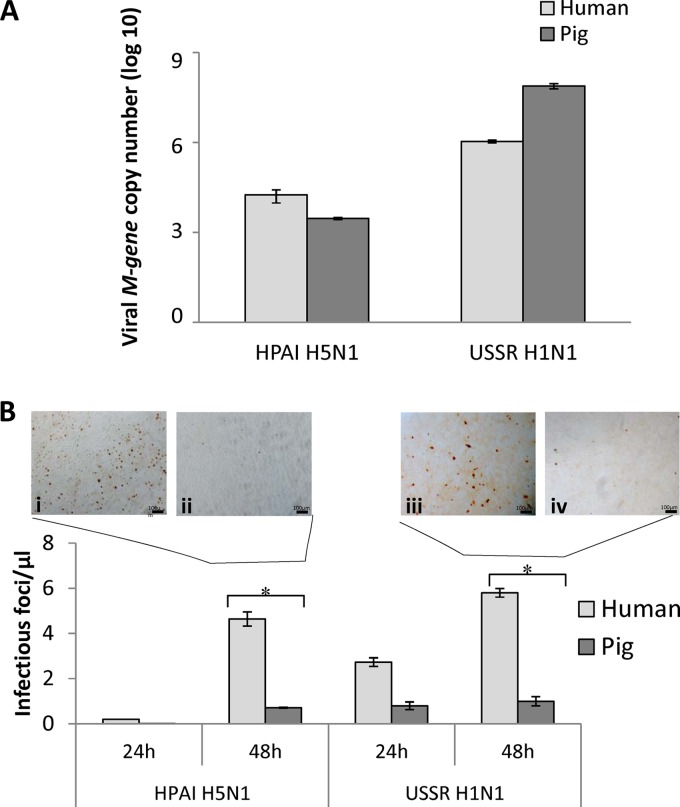
Fig 8.
Pig respiratory epithelial cells released less infectious virus than did the corresponding human cells infected with HPAI H5N1 (A/turkey/Turkey/1/05) and USSR H1N1 (A/USSR/77) viruses. (A) Real-time PCR on 24-h-infected (MOI of 1) culture supernatants showed that the H5N1 M gene RNA level in pig cells was about 10-fold lower than in from human cells. USSR H1N1 virus M gene RNA was more abundant (2 to 3 orders of magnitude) than HPAI H5N1 virus M gene RNA for each mammalian species. Between human and pig cells, USSR H1N1 M gene RNA was more highly expressed in pig cells. Data shown are means of data from three biological replicates. (B) Supernatants of infected human and pig cells collected at 24 h and 48 h postinfection were used to infect MDCK cells for 6 h, followed by immunodetection for virus nucleoprotein. The number of infected MDCK cells per microliter of supernatant was determined. Representative fields of MDCK cells infected with supernatants from 48-h infections of human (i and iii) and pig (ii and iv) cells by HPAI H5N1 (i and ii) and USSR H1N1 (iii and iv) viruses are shown. Pig cells produced significantly less new infectious virus than human cells for both virus subtypes. Data points represent means of data from three biological replicates. Error bars indicate standard errors of the means. *, P < 0.05.
RESULTS
Comparable susceptibilities to initial infection but differences in cytopathogenicity between human and pig cells.
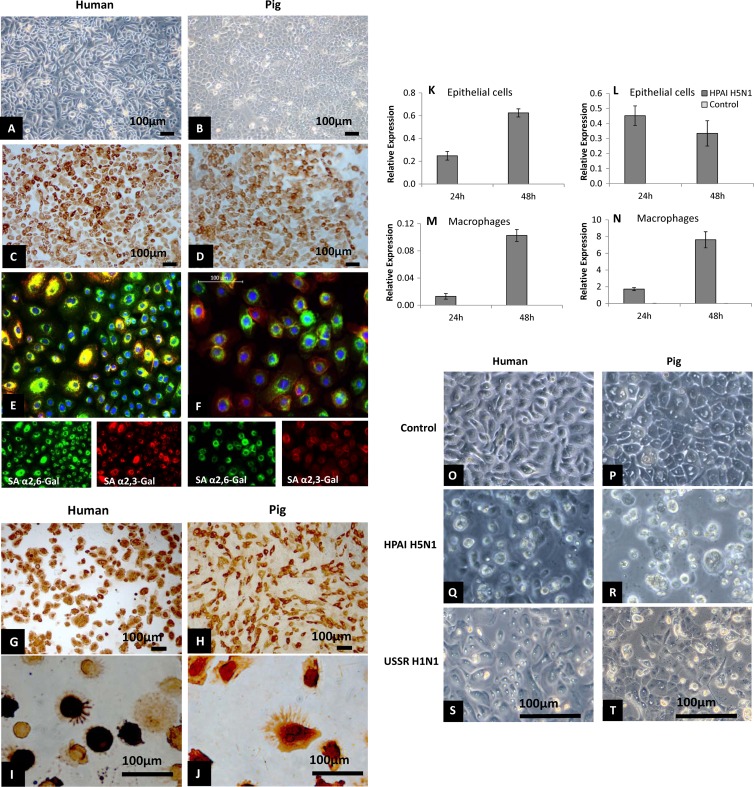
Since humans and pigs show very different clinical outcomes of HPAI H5N1 virus infection, we assessed the relative susceptibilities of virus entry and early infection of the principal primary cell types, respiratory (tracheobronchial) epithelial cells and macrophages, in the two mammalian hosts. Respiratory epithelial cells, as evident by cytokeratin expression (Fig. 1A to D), of human and pig origins possessed sialic acid receptors for human (SAα2,6-Gal) and avian (SAα2,3-Gal) influenza viruses, with the coexpression of both receptors being detected in most cells (Fig. 1E and F). Both receptor types were also detected in human and pig macrophages, with the SAα2,6-Gal receptor type appearing to be more dominant in both mammalian species (data not shown). Respiratory epithelial cells and macrophages of both host species appeared to be equally permissive to virus entry and initial replication with HPAI H5N1 virus, as demonstrated by virus nucleoprotein detection in virtually all cells at 6 h postinfection at an MOI of 1.0 (Fig. 1G to J). Subsequent to the initial HPAI H5N1 infection, epithelial cells and macrophages of both mammalian hosts continued to accumulate viral M gene RNA at 24 h and 48 h of infection, suggesting ongoing virus activity (Fig. 1K to N). However, in pig epithelial cells, there was no increase in M gene expression beyond the first 24 h (Fig. 1L). The M gene expression level in pig macrophages was found to be highly variable between experimental repeats (Fig. 1N). Notably, pig epithelial cells infected with HPAI H5N1 and USSR H1N1 viruses showed more severe cytopathic damage than their human counterparts at 48 h postinfection (Fig. 1O to T).
Fig 1.
Comparable susceptibilities to initial influenza virus infection but differences in cytopathogenicity between human and pig cells. (A to F) Cytokeratin and influenza virus receptor distribution in human and pig respiratory epithelial cells. Human (A and C) and pig (B and D) respiratory epithelial cells were positively immunolabeled (C and D) for cytokeratin (epithelial cell marker), with panels A and B as the negative controls. The host α2,6-galactose (SAα2,6-Gal)-linked sialic acid receptor (associated with human influenza virus binding), shown in green, and the α2,3-galactose (SAα2,3-Gal)-linked sialic acid receptor (associated with avian influenza virus binding), shown in red, were widely distributed and coexpressed in human (E) and pig (F) respiratory epithelial cells. (G to J) Comparable susceptibilities of human and pig respiratory epithelial cells (G and H) and macrophages (I and J) to initial influenza virus infection. Human (G and I) and pig (H and J) epithelial cells and macrophages showed extensive viral nucleoprotein staining after HPAI H5N1 virus (A/turkey/Turkey/1/05) infection (6 h) at an MOI of 1.0. A similar uniform nucleoprotein detection was found following infection with USSR H1N1 (A/USSR/77), avian H2N3 (A/mallard duck/England/7277/06), and classical swine H1N1 (A/sw/Iowa/15/30) (26) viruses (data not shown). (K to N) Relative viral M gene RNA expression in HPAI H5N1 virus infection at an MOI of 1.0 in human (K and M) and pig (L and N) epithelial cells and macrophages indicated an accumulation of viral RNA in the two cell types of human and pig. It should be mentioned that M gene RNA levels in infected pig macrophages (N) were highly variable between experimental repeats. Results shown are for one of three experimental repeats. (O to T) Human respiratory epithelial cells (O, Q, and S) showed less severe cytopathogenicity (cell shrinkage, rounding, and detachment) than pig respiratory epithelial cells (P, R, and T) infected by HPAI H5N1 (Q and R) or USSR H1N1 (S and T) virus at an MOI of 1.0 for 48 h. In comparison with uninfected controls (O and P), pig cells (R and T) showed more severe morphological damage than human cells (Q and S). Results shown are representative of three experimental repeats.
Contrasting proinflammatory responses to H5N1 virus infection between human and pig cells.
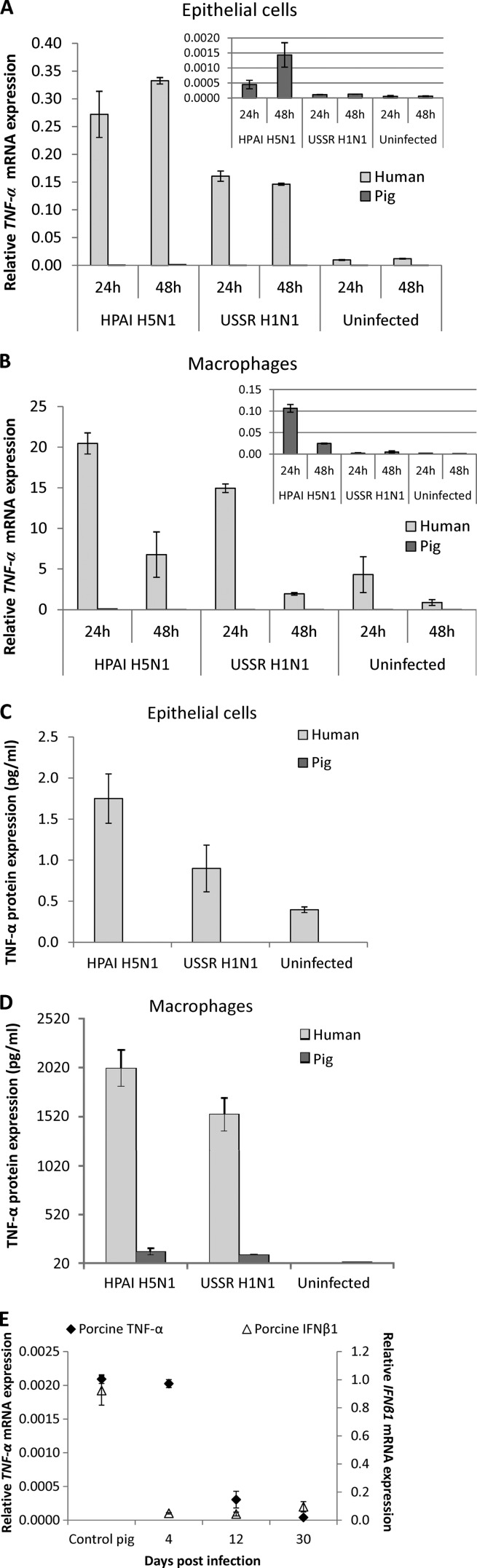
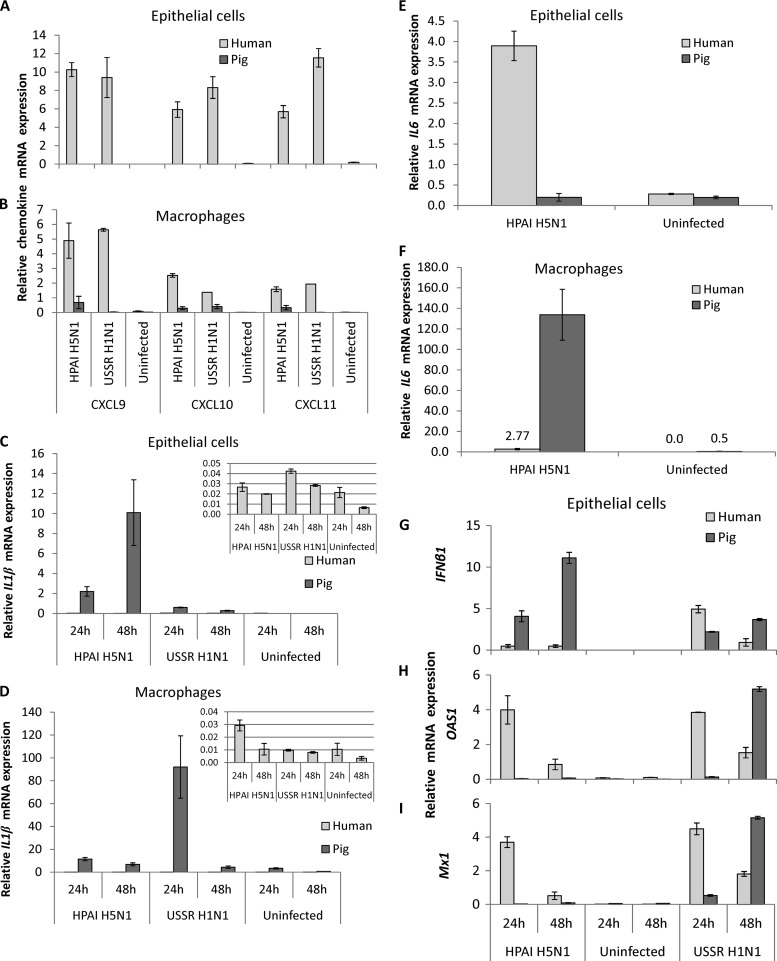
The expression levels of several key proinflammatory cytokines and chemokines linked to the development of hypercytokinemia was determined in human and pig respiratory epithelial cells and macrophages infected with HPAI H5N1 and USSR H1N1 viruses. The TNF-α RNA expression level in human epithelial cells and macrophages was at least 100-fold higher than that in the corresponding pig cells for both viruses (Fig. 2A and B). For each mammalian host, the virus-induced TNF-α expression level in macrophages was similarly many times higher than that in the corresponding epithelial cells from the same host (Fig. 2A and B). The level of TNF-α induction in epithelial cells and macrophages of both host species by HPAI H5N1 virus was consistently higher than that by USSR H1N1 virus, a strain responsible for the severe 1977 epidemic in Russia (38, 45). The quantification of the TNF-α protein in culture supernatants by ELISAs showed a marked contrast between human and pig (Fig. 2C and D) that mirrored the differences in RNA profiles (Fig. 2A and B). TNF-α protein expression was most strongly induced by HPAI H5N1 virus in human macrophages and was around 3 orders of magnitude higher than that in human epithelial cells, indicating that human macrophages are dominant over epithelial cells in TNF-α production. Regardless of the influenza viruses used for infection, pig TNF-α mRNA (Fig. 2A and B) and protein (Fig. 2C and D) were undetectable or barely detectable in epithelial cells and macrophages. This key in vitro observation was corroborated by the in vivo finding that 4-week-old pigs challenged with the same HPAI H5N1 virus showed no apparent induction of the TNF-α or interferon β1 (IFNβ1) gene in their lungs, which coincided with the virtual absence of clinical signs (Fig. 2E). Comparably increased expression levels of three related chemokines, CXCL9, CXCL10 (IP-10), and CXCL11, were detected in human epithelial cells and macrophages infected by HPAI H5N1 and USSR H1N1 viruses (Fig. 3A and B). Again, the expressions of these chemokines were absent or weak in infected pig cells (Fig. 3A and B).
Fig 2.
Contrasting TNF-α responses in human and pig cells. (A and B) TNF-α mRNA expression (normalized to 18S rRNA expression levels) in human and pig respiratory epithelial cells and macrophages following infection by HPAI H5N1 (A/turkey/Turkey/1/05) and USSR H1N1 (A/USSR/77) viruses at an MOI of 1.0. TNF-α RNA expression levels in human epithelial cells (A) and human macrophages (B) were at least 100-fold higher than those in the corresponding pig cells for both viruses and at both time points. Note that for human or pig, the virus-induced TNF-α mRNA expression level in macrophages was many times (1 to 2 orders) higher than that in epithelial cells. (C and D) Detection of the TNF-α protein (ELISA) in culture supernatants was performed on infected human and pig epithelial cells (C) and macrophages (D). (C) TNF-α protein induction in human epithelial cells by HPAI H5N1 virus was higher than that by USSR H1N1. TNF-α was undetectable in pig epithelial cells infected by the same virus subtypes. (D) Macrophages infected for 48 h with HPAI H5N1 and USSR H1N1 viruses showed strong TNF-α induction in human cells, with the highest induction by HPAI H5N1. In contrast, TNF-α was undetectable or barely detectable in pig macrophages infected by all 3 virus subtypes. TNF-α protein production levels from infected human macrophages were about 3 orders of magnitude higher than those from infected human respiratory epithelial cells. All data shown are the means of data from duplicate wells and are representative of data from 3 experimental replicates. Error bars indicate standard errors of the means. (E) Lack of apparent TNF-α and IFNβ1 responses in lungs of 4-week-old pigs challenged with HPAI H5N1 virus. One pig was used per time point. The absence of TNF-α and IFNβ1 after challenge induction coincided with only transient signs of pyrexia in the first 48 h and intermittent nasal virus shedding within the first 4 days of virus challenge (data not shown), and no virus M gene RNA was detected in the lung at all time points. The relative mRNA expression level was determined by real-time PCR and normalized to the 18S rRNA level.
Fig 3.
Relative RNA expression levels of chemokines (CXCL9, CXCL10, and CXCL11), cytokines (IL1β, IL6, and IFNβ1), and IFNβ1-inducible genes (OAS1 and Mx1) in human and pig respiratory epithelial cells and macrophages. Human and pig cells were infected with HPAI H5N1 (A/turkey/Turkey/1/05) and USSR H1N1 (A/USSR/77) viruses at an MOI of 1.0. (A) In human epithelial cells, CXCL9, CXCL10, and CXCL11 were clearly induced by both viruses at comparable levels. However, chemokine induction was absent or barely detected in pig epithelial cells. (B) In human macrophages, all three chemokines were comparably induced by both virus subtypes. Again, in pig macrophages, chemokine induction was absent or weak. (C) IL1β mRNA expression was consistently upregulated in pig epithelial cells infected by both virus subtypes. In infected human epithelial cells, IL1β induction was relatively limited. (D) In pig macrophages, 1L1β mRNA expression was clearly induced by both viruses. IL1β expression in human macrophages, like human epithelial cells, appeared less responsive to HPAI H5N1 and USSR H1N1 infections than their pig counterparts. (E) In human epithelial cells, at 24 h after infection with HPAI H5N1 virus, IL6 induction was consistently pronounced, but in infected pig cells, IL6 expression levels showed a wide variation between experimental repeats. (F) IL6 expression in 24-h H5N1 virus-infected pig macrophages, however, was more highly upregulated than in their human counterparts. (G) In pig epithelial cells, the HPAI H5N1 subtype induced a stronger and increasing IFNβ1 response, but in human HPAI H5N1 virus-infected cells, IFNβ1 induction was less pronounced. USSR H1N1 virus also induced an increasing IFNβ1 response in pig cells; in human USSR H1N1 virus-infected cells, the level of IFNβ1 induction was higher at 24 h but lower at 48 h than those in the corresponding pig cells. (H and I) In both human and pig cells infected with USSR H1N1 virus, the levels of OAS1 (H) and Mx1 (I) expression closely mirrored the relative expression level of IFN-β1. A clear induction of OAS1 and Mx1 by the HPAI H5N1 subtype was found only in human cells at both time points; in HPAI H5N1 virus-infected pig cells, OAS1 and Mx1 were barely detected. Data shown are the means of data from triplicate wells and one representative of three experimental repeats. Error bars indicate standard errors of the means.
Not all major proinflammatory cytokines were dampened in pig cells in response to HPAI H5N1 virus infection. The level of interleukin-1β gene (IL1β) induction in pig epithelial cells and macrophages was manyfold (100-fold or more) higher than that in the corresponding human cells (Fig. 3C and D), which suggests that IL-1β may not be a causal factor of the deleterious effects of hypercytokinemia seen in human cases of H5N1 virus infection. Differences in IL6 responses to HPAI H5N1 virus infection between human and pig cells were less straightforward. In human epithelial cells infected with HPAI H5N1 virus, the level of IL6 induction was consistently high, but the corresponding pig IL6 responses were highly variable between experimental repeats (Fig. 3E). Although IL6 was upregulated in infected human and pig macrophages, induction was much stronger in pig macrophages (Fig. 3F), like the strong induction of IL1β found in both pig cell types, which suggests that IL-6 may not be a precipitating proinflammatory factor responsible for HPAI H5N1 virus-induced hypercytokinemia.
HPAI H5N1 and USSR H1N1 viruses induced an increasing transcriptional response of IFNβ1 in pig but not in human epithelial cells (Fig. 3G). In response to USSR H1N1 virus infection, expression profiles of two IFN-inducible genes examined, OAS1 and Mx1, closely followed the relative expression levels of IFNβ1 in both host species (Fig. 3H and I). However, with HPAI H5N1 virus infection, the relationship between IFNβ1 expression and OAS1 and Mx1 expressions was much less predictable. Human epithelial cells displayed a robust induction of OAS1 and Mx1, but the increasing induction of IFNβ1 transcription in pig cells did not correspond to a similar induction of OAS1 and Mx1 expressions; rather, their expressions remained subdued (Fig. 3H and I), suggesting that HPAI H5N1 virus infection could disrupt the response of IFN-inducible genes to IFNβ1 induction in pig epithelial cells.
SOCS3 appears to be pivotal in moderating an excessive proinflammatory response.
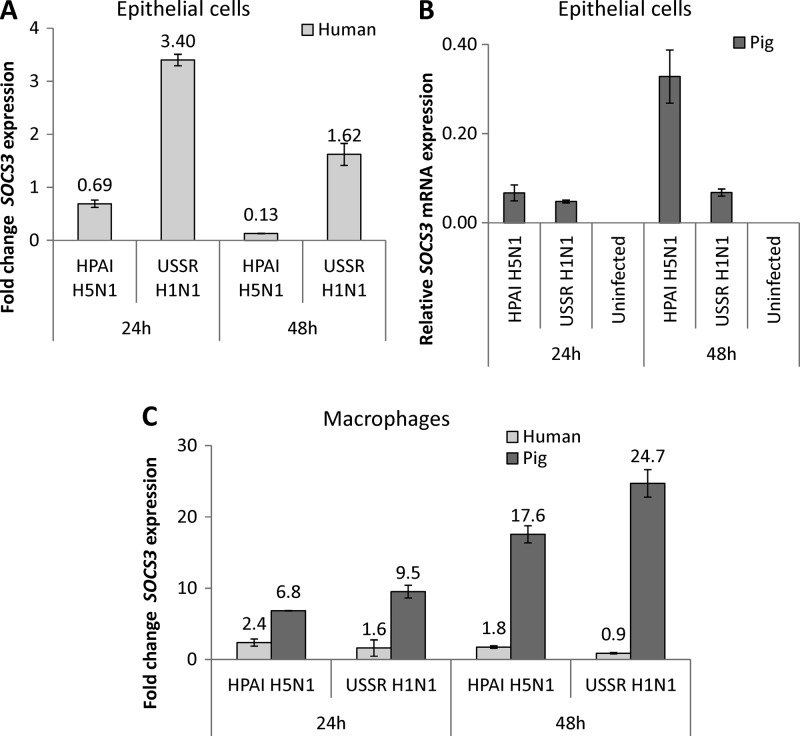
Since the SOCS family of eight genes (28, 44) has been widely recognized to negatively regulate a range of cytokines and growth factors, we compared the relative expression levels of several SOCS members in human and pig respiratory epithelial cells and macrophages infected by HPAI H5N1 and USSR H1N1 viruses. Most SOCS members examined were downregulated in human epithelial cells infected with the HPAI H5N1 subtype (P. X. Chang, personal communication). SOCS3 expression in particular showed a marked contrast between infected human and pig cells (Fig. 4). In human epithelial cells, SOCS3 expression was consistently upregulated (>1.5-fold change) by USSR H1N1 virus but downregulated (<0.7-fold change) by the HPAI H5N1 subtype (Fig. 4A). In pig epithelial cells, however, both viruses upregulated SOCS3 expression; additionally, with H5N1 virus infection in pig epithelial cells, SOCS3 expression levels continued to increase with time of infection (Fig. 4B). For both viruses, pig macrophages also showed an increase in SOCS3 expression levels from 24 h to 48 h postinfection, but the response in human macrophages was much weaker (Fig. 4C). Overall, SOCS3 expression was consistently and highly upregulated in response to HPAI H5N1 virus infection in pig epithelial cells and macrophages but not in human cells. In addition, the SOCS3 protein in pig epithelial cells and macrophages was constitutively expressed and remained strongly expressed postinfection (Fig. 5). In contrast, the SOCS3 protein in human epithelial cells and macrophages was barely or not detected before and after infection (Fig. 5).
Fig 4.
SOCS3 expression in human and pig respiratory epithelial cells and macrophages infected with HPAI H5N1 (A/turkey/Turkey/1/05) and USSR H1N1 (A/USSR/77) viruses for 24 h and 48 h at an MOI of 1.0. (A) In human epithelial cells, SOCS3 expression was downregulated (fold change, <0.7) by HPAI H5N1 virus infection but was upregulated (fold change, >1.5) by the USSR H1N1 subtype. (B) In pig epithelial cells, however, SOCS3 expression was induced by both viruses. Since SOCS3 in uninfected pig cells was barely or not detected, the relative expression level instead of the fold change is presented. (C) Pig macrophages showed higher fold increases of SOCS3 expression levels with both viruses than human macrophages. Overall, SOCS3 in pig epithelial cells and macrophages showed a more vigorous upregulatory response to HPAI H5N1 virus infection than the corresponding human cells. The fold change in the SOCS3 expression level was in relation to levels in uninfected controls. Data shown are from one representative of three experimental repeats. Error bars indicate standard errors of the means.
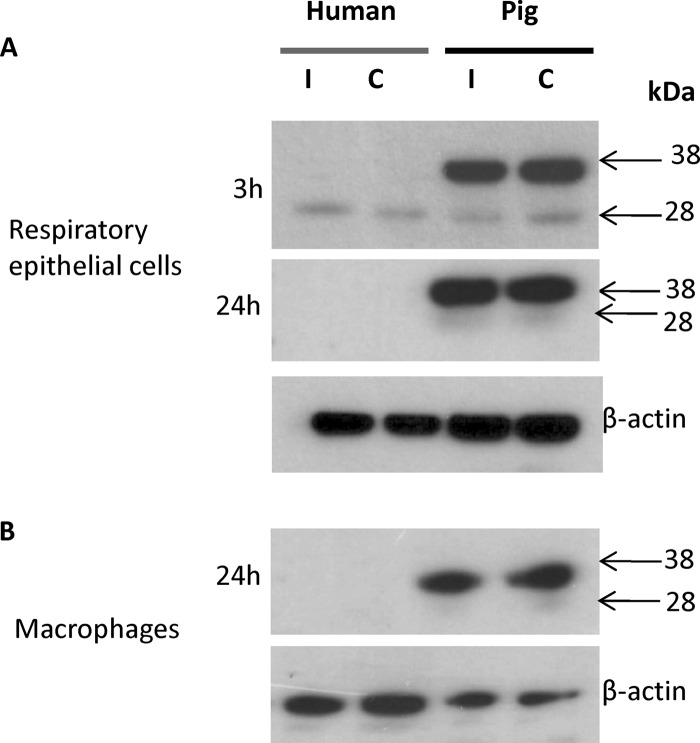
Fig 5.
Contrasting SOCS3 protein profiles between human and pig respiratory epithelial cells and macrophages. (A) Primary epithelial cells were infected (I) with HPAI H5N1 virus at an MOI of 1.0 for 3 h and 24 h, whereupon protein lysates were generated for SOCS3 detection. C, uninfected control. The strong upper band (∼38 kDa) found in each pig sample was likely to be highly phosphorylated SOCS3 but was absent in all human samples. The native SOCS3 protein (∼28 kDa) was weakly expressed (3 h) or absent. Comparable protein loading in each lane was demonstrated by β-actin detection (3 h). (B) The SOCS3 protein was highly expressed in pig macrophages regardless of infection (USSR H1N1 virus at an MOI of 1.0 for 24 h) but was undetected in human macrophages. Protein loading in each lane was exemplified by β-actin detection. In relation to SOCS3 RNA profiles, the detection of high constitutive SOCS3 protein levels in pig cells suggests further differences in posttranscriptional or translational SOCS3 regulation between pig and human cells.
To demonstrate a possible functional role of SOCS3 in moderating proinflammation during virus infection, human macrophages transiently transfected with a SOCS3 expression plasmid were challenged with the USSR H1N1 subtype, which is a robust inducer of TNF-α expression, comparable to the induction by HPAI H5N1 virus infection (Fig. 2A to D). The overexpression of SOCS3 in human macrophages did not appear to affect virus entry and early infection, but the level of virus M gene RNA detected in the supernatants of infected cells appeared to be lower than that of the corresponding control, transfected with a green fluorescent protein (GFP) plasmid (Fig. 6A). Crucially, TNF-α RNA and protein induction by the virus was clearly blunted by the overexpression of SOCS3 (Fig. 6B and C).
Reduced production of infectious virus from pig respiratory epithelial cells.
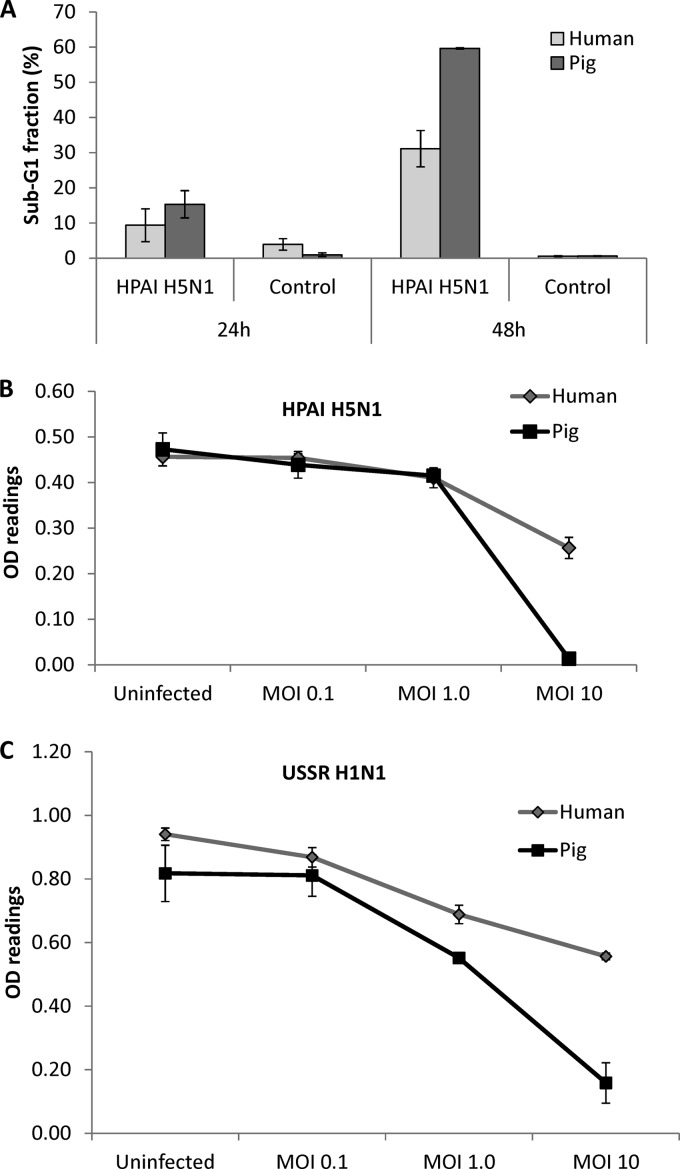
After ascertaining that pig cells displayed an attenuated proinflammatory response to HPAI H5N1 virus infection and high constitutive SOCS3 protein expression levels, we compared the relative viabilities of infected cells and de novo virus production. Coincident with more severe morphological changes (Fig. 1O to T), pig respiratory epithelial cells infected with HPAI H5N1 virus underwent greater nuclear fragmentation at 48 h postinfection (Fig. 7A), as measured by flow cytometry of propidium iodide-labeled cells. Pig epithelial cells also showed a greater reduction in cell viability when infected at an MOI of 10 with HPAI H5N1 (Fig. 7B) and USSR H1N1 (Fig. 7C) viruses than the corresponding human cells, as determined by proliferation (MTS) assays. The virus M gene RNA level found in the supernatants of H5N1 virus-infected human cells was about 10-fold higher than that in the corresponding pig epithelial cells (Fig. 8A). Within each mammalian host species, USSR H1N1 M gene RNA was 2 to 3 orders of magnitude more abundant than H5N1 M gene RNA (Fig. 8A). Importantly, pig epithelial cells infected by HPAI H5N1 or USSR H1N1 virus released significantly less new infectious virus into culture supernatants than the corresponding human cells (Fig. 8B). In summary, a greater resistance of pig epithelial cells to virus infection was manifested as reduced cell viability (Fig. 7) and infectious-virus release (Fig. 8B).
Fig 7.
HPAI H5N1 (A/turkey/Turkey/1/05) and USSR H1N1 (A/USSR/77) virus-infected pig respiratory epithelial cells showed greater reductions in cell viability than the corresponding human cells. (A) HPAI H5N1 virus-infected (MOI of 1.0) human and pig respiratory epithelial cells labeled with propidium iodide were sorted by flow cytometry to determine the proportion of hypodiploid (sub-G1) cells as an indication of cells undergoing nuclear fragmentation. A more severe nuclear fragmentation of pig cells was observed at 48 h infection. Data shown are the means of data from biological replicates. (B and C) Proliferation MTS assays (Promega) were performed on human and pig cells infected for 24 h with HPAI H5N1 virus (B) and USSR H1N1 virus (C) at a range of MOIs, to assess metabolic reductase activity as an indicator of cell viability. At an MOI of 10 for both viruses, much fewer viable pig cells than human cells were detected. Data shown are means of data from four biological replicates. Error bars indicate standard errors of the means. OD, optical density.
DISCUSSION
To our knowledge, this is the first study that links apparent HPAI H5N1 virus resistance in pigs to the innate resistance of key cell types of respiratory epithelial cells and macrophages. This is manifested as attenuated proinflammation and a reduced de novo infectious-virus output. These findings were made possible through a comparative-species approach that examined differences in responses to different influenza viruses, including HPAI H5N1 virus, between human and pig primary respiratory epithelial cells and macrophages. We observed fundamental differences between human and pig cells in the innate response to HPAI H5N1 virus infection after virus entry. The expressions of TNF-α, IL1β, CXCL9, CXCL10, and CXCL11 contrasted sharply between infected human and pig respiratory epithelial cells and macrophages. TNF-α and CXCL10, in particular, have been extensively cited as major agents of proinflammation in human H5N1 cases (15, 41) and in virus challenge studies with HPAI H5N1 subtypes in model species (mice [33], ferrets [3], and macaques [1]) and in in vitro experiments (4, 10). CXCL10 detection in the lung has been proposed to be a poor prognostic indicator for patients with severe acute respiratory syndrome (SARS), a condition that is clinically similar to severe influenza virus infection. Owing to the much higher levels of IL1β induction in pig epithelial cells and macrophages and the strong induction of IL6 in pig macrophages by HPAI H5N1 virus, we suspect that IL1β or IL6 on its own may not be decisive in the induction of hypercytokinemia in human H5N1 virus infections. On the contrary, in mice, the IL-1 response appears to be important for effective HPAI H5N1 virus clearance (39). Based on the contrasting host gene expression levels of H5N1 virus-infected human and pig cells, we speculate that IL-1β may also mediate H5N1 virus clearance in pigs.
The vigorous induction of type I IFNs and IFN-inducible genes in the lungs of HPAI H5N1 virus-infected human patients (41), ferrets (3), and macaques (1) has been well described. It appears that the excessive activation of the well-recognized IFN-mediated antiviral pathway is not effective against such highly virulent viruses and may even be a liability by becoming part of the cascade resulting in hypercytokinemia (3). We report here (Fig. 3G to I) and have also found by separate microarray expression analyses (S. V. Kuchipudi, personal communication) that IFN-inducible genes were strongly upregulated by HPAI H5N1 virus infection in susceptible human but not pig epithelial cells. Unexpectedly, in HPAI H5N1 virus-infected pig epithelial cells, despite strong and increasing IFNβ1 gene expression, a weak IFN-inducible response was found (Fig. 3G to I), suggesting that a vigorous IFN-inducible gene response, as seen for HPAI H5N1 virus-infected human epithelial cells, may not be a good indicator of an effective antiviral response.
SOCS3 attenuates proinflammatory signaling mediated by the signal transducer and activator of transcription (STAT) family of proteins. Pig respiratory epithelial cells and macrophages infected by HPAI H5N1 virus showed a consistent upregulation of SOCS3 (Fig. 4). In contrast, H5N1 virus downregulated SOCS3 expression in human respiratory epithelial cells. Notably, the high constitutive SOCS3 protein expression level detected in pig cells was not found in human cells (Fig. 5). The presumptive phosphorylated SOCS3 protein (38 kDa) was the dominant constitutive form, which is recognized as being active and able to inhibit STAT signaling (2, 7). High constitutive SOCS3 protein levels detected in pig cells (Fig. 5) did not commensurate with low basal levels of SOCS3 mRNA (Fig. 4A and B), suggesting that there are additional regulatory (posttranscriptional or translational) differences in SOCS3 expression between pig and human cells. One such speculation is that the constitutive pig SOCS3 protein is highly stable. The functional importance of SOCS3 was evident by the dampening of a virus-induced TNF-α response in human macrophages (Fig. 6). The anti-inflammatory properties of SOCS3, including the reduction of TNF-α levels, are well recognized (14, 35). It was recently shown that SOCS3 in A549 cells (human alveolar epithelial cell line), induced by the low-pathogenicity PR8 influenza virus, inhibits type I IFN signaling by interfering with the downstream JAK-STAT relay (29). That report and our current findings indicate that there are variations in the SOCS3 response to different influenza viruses and that IFN signaling needs to be appropriately balanced, in that too little (no antiviral response) or too much (complications of hypercytokinemia) can have adverse effects on the host (32). Note that the downregulation of SOCS3 in HPAI H5N1 virus-infected human epithelial cells (Fig. 4A) coincided with a strong induction of IFN-inducible genes (Fig. 3H and I). Conversely, the high constitutive SOCS3 protein expression level in pig epithelial cells (Fig. 5) was associated with a weak expression of IFN-inducible genes (Fig. 3H and I). We surmise that SOCS3 in pig cells is able to confer resistance against the establishment of hypercytokinemia by moderating a proinflammatory response. At present, it is not clear what are the key targets of SOCS in proinflammatory modulation during HPAI H5N1 virus infection. The conspicuous absence of induction of IFN-inducible genes despite clear IFN-β1 activation by HPAI H5N1 virus (Fig. 3) suggests that the JAK-STAT axis could be one such target. Future work will elucidate the interactions of SOCS proteins with such proinflammatory mediators, to gain insights into mechanisms of action and identify therapeutic targets for the prevention or treatment of virus-induced hypercytokinemia.
Paradoxically, HPAI H5N1 and USSR H1N1 virus-infected pig epithelial cells exhibited greater cellular and metabolic damage, accompanied by a significant reduction in the output of viable virus (Fig. 1O to T, 7, and 8B). We recently reported similar findings for resistant duck and susceptible chicken primary cells, where HPAI H5N1 virus-infected duck cells died more rapidly but with a significantly reduced level of production of viable virus (16). At the cellular level, a moderated or downregulated proinflammatory response could conceivably provide a more effective IFN-associated antiviral response and/or facilitate timely apoptotic cell death, which would collectively reduce morbidity and virus replication and improve prognoses. The establishment of pig primary cells as a good mammalian model of host innate resistance against HPAI H5N1 virus infection should greatly accelerate our understanding of the innate mechanisms at the molecular level that moderate proinflammation and inhibit virus production, both of which are of vital clinical importance.
ACKNOWLEDGMENTS
We are grateful to our AHVLA colleagues for their enthusiastic and willing assistance: Stephen Essen (animal study support), Fanny Garcon, and Vivian Coward (technical assistance). We thank Dennis Alexander for helping with the manuscript review. We also thank Pierre-Joseph Royer for his help in generating human macrophages.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Baskin CR, et al. 2009. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. U. S. A. 106:3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cacalano NA, Sanden D, Johnston JA. 2001. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat. Cell Biol. 3:460–465 [DOI] [PubMed] [Google Scholar]
- 3. Cameron CM, et al. 2008. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. J. Virol. 82:11308–11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan MCW, et al. 2005. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen LM, et al. 2012. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 422:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi YK, et al. 2005. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J. Virol. 79:10821–10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohney SJ, et al. 1999. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol. Cell. Biol. 19:4980–4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cyranski D. 2005. Bird flu spreads among Java's pigs. Nature 435:390–391 [DOI] [PubMed] [Google Scholar]
- 9. Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. 2006. Innate defence against human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 176:6962–6972 [DOI] [PubMed] [Google Scholar]
- 10. Hui KPY, et al. 2009. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J. Immunol. 182:1088–1098 [DOI] [PubMed] [Google Scholar]
- 11. Ilyushina NA, Seiler JP, Rehg JE, Webster RG, Govorkova EA. 2010. Effect of neuraminidase inhibitor-resistant mutations on pathogenicity of clade 2.2 A/Turkey/15/06 (H5N1) influenza virus in ferrets. PLoS Pathog. 6:e1000933 doi:10.1371/journal.ppat.1000933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isoda N, et al. 2006. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Arch. Virol. 151:1267–1279 [DOI] [PubMed] [Google Scholar]
- 13. Järhult JD, et al. 2011. Environmental levels of the antiviral oseltamivir induce development of resistance mutation H274Y in influenza A/H1N1 virus in mallards. PLoS One 6:e24742 doi:10.1371/journal.pone.0024742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jo D, Liu D, Yao S, Collins RD, Hawiger J. 2005. Intracellular protein therapy with SOCS inhibits inflammation and apoptosis. Nat. Med. 11:892–898 [DOI] [PubMed] [Google Scholar]
- 15. Korteweg C, Gu J. 2008. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am. J. Pathol. 172:1155–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuchipudi SV, et al. 2012. Rapid death of duck cells infected with influenza: a potential mechanism for host resistance to H5N1. Immunol. Cell Biol. 90:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuchipudi SV, et al. 2009. Differences in influenza virus receptors in chickens and ducks: implications for interspecies transmission. J. Mol. Genet. Med. 3:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. La Gruta NL, Kedzierska K, Stambas J, Doherty PC. 2007. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85:85–92 [DOI] [PubMed] [Google Scholar]
- 19. Lipatov AS, et al. 2008. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog. 4:e1000102 doi:10.1371/journal.ppat.1000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Löndt BZ, et al. 2009. The effect of age on the pathogenesis of a highly pathogenic avian influenza (HPAI) H5N1 virus in Pekin (anas platyrhynchos) ducks infected experimentally. Influenza Other Respi. Viruses 4:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Löndt BZ, et al. 2008. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Ana platyrhynchos) infected experimentally. Avian Pathol. 37:619–627 [DOI] [PubMed] [Google Scholar]
- 22. Ludwig S. 2009. Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J. Antimicrob. Chemother. 64:1–4 [DOI] [PubMed] [Google Scholar]
- 23. Mai LQ, Wertheim HFL, Duong TN, van Doorn HR, Hien NT. 2010. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N. Engl. J. Med. 362:86–87 [DOI] [PubMed] [Google Scholar]
- 24. Mazur I, et al. 2007. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-κΒ-inhibiting activity. Cell. Microbiol. 9:1683–1694 [DOI] [PubMed] [Google Scholar]
- 25. Nelli RK, et al. 2010. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet. Res. 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nerome K, Ishida M, Oya A, Oda K. 1982. The possible origin of H1N1 (Hsw1N1) virus in the swine population of Japan and antigenic analysis of the isolates. J. Gen. Virol. 62:171–175 [DOI] [PubMed] [Google Scholar]
- 27. Nidom CA, et al. 2010. Influenza A (H5N1) viruses from pigs, Indonesia. Emerg. Infect. Dis. 16:1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palmer DC, Restifo NP. 2009. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30:592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pauli E-K, et al. 2008. Influenza A virus inhibits type 1 IFN signaling via NF-κB-dependent induction of SOCS-3 expression. PLoS Pathog. 4:e1000196 doi:10.1371/journal.ppat.1000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peiris JSM, Cheung CY, Leung CYH, Nicholls JM. 2009. Innate immune responses to influenza A H5N1: friend or foe. Trends Immunol. 30:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perrone LA, Szretter KJ, Katz JM, Mizgerd JP, Tumpey TM. 2010. Mice lacking both TNF and IL-1 receptors exhibit reduced inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J. Infect. Dis. 202:1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pothlichet J, Chignard M, Si-Tahar M. 2008. Innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J. Immunol. 180:2034–2038 [DOI] [PubMed] [Google Scholar]
- 33. Salomon R, Hoffmann E, Webster RG. 2007. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. U. S. A. 104:12479–12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shinya K, et al. 2011. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol. J. 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shouda T, et al. 2001. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 108:1781–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slomka MJ, et al. 2009. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza Other Respi. Viruses 3:151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spackman ED, et al. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40:3256–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. 2008. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology 376:53–59 [DOI] [PubMed] [Google Scholar]
- 39. Szretter KJ, et al. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81:2736–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teijaro JR, et al. 2011. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146:980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thitithanyanont A, et al. 2010. Antiviral immune responses in H5N1-infected human lung tissue and possible mechanisms underlying the hyperproduction of interferon-inducible protein IP-10. Biochem. Biophys. Res. Commun. 398:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Ma S. 2008. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 26:711–715 [DOI] [PubMed] [Google Scholar]
- 43. Wareing MD, Tannock GA. 2008. The induction of proinflammatory cytokines in response to avian influenza H5N1 infections and their role in pathogenesis and enhancement of virulence. Antiinflamm. Antiallergy Agents Med. Chem. 7:71–80 [Google Scholar]
- 44. Yoshimura A. 2009. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J. Med. 58:73–83 [DOI] [PubMed] [Google Scholar]
- 45. Zakstelskaja LJ, et al. 1978. Influenza in the USSR in 1977: recurrence of influenza virus A subtype H1N1. Bull. World Health Organ. 56:919–922 [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng H, et al. 2012. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection. J. Virol. 86:667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]