Abstract
The regulation of alternative mRNA splicing factors by extracellular cues and signal transduction cascades is poorly understood. Using an engineered extracellular signal-regulated kinase 2 (ERK2) that can utilize ATP analogs, we have identified the alternative mRNA splicing factor 45 (SPF45), which is overexpressed in cancer, as a novel coimmunoprecipitating ERK2 substrate. ERK2 phosphorylated SPF45 on Thr71 and Ser222 in vitro and in cells in response to H-RasV12, B-RAF-V600E, and activated MEK1. Jun N-terminal kinase 1 (JNK1) and p38α also phosphorylated SPF45 in vitro and associated with SPF45 in cells. SPF45 was differentially phosphorylated in cells by all three mitogen-activated protein (MAP) kinases in response to phorbol myristate acid (PMA), H2O2, UV, and anisomycin stimulation. ERK and p38 activation decreased SPF45-dependent exon 6 exclusion from fas mRNA in a minigene assay in cells. Stable overexpression of SPF45 in SKOV-3 cells dramatically inhibited cell proliferation in a phosphorylation-dependent manner through inhibition of ErbB2 expression. SPF45 overexpression also induced EDA inclusion into fibronectin transcripts and fibronectin expression in a phosphorylation-dependent and -independent manner, respectively, specifically affecting cellular adhesion to a fibronectin matrix. These data identify SPF45 as the first splicing factor regulated by multiple MAP kinase pathways and show effects of both SPF45 overexpression and phosphorylation.
INTRODUCTION
The expression of more than one protein from a single gene is regulated by alternative mRNA splicing, in which the exons from pre-mRNA of a transcribed gene are differentially spliced together (6), affecting the composition of the final protein product. Alternative splicing is thought to regulate between 60 and 74% of the human genome (42, 66), and up to 50% of human genetic diseases arise from changes in alternative splicing (38). Pre-mRNA splicing is regulated by both small nuclear ribonucleoprotein particles (snRNPs) and proteins that function in the stepwise processing of pre-mRNA (29, 65). Alternative splicing is primarily regulated by the hnRNP (heterogenous nuclear ribonucleoproteins) and SR (serine-arginine-rich) families of splicing factor proteins (28, 37, 41). Other alternative splicing factors fall outside these families and contain one or more RNA recognition motifs (RRMs) and protein-protein binding domains. Splice site selection depends on the relative concentrations of these proteins (8, 27) and is regulated by reversible phosphorylation (57). Little is known about how extracellular signals and intracellular signal transduction regulate pre-mRNA splicing.
The alternative mRNA splicing factor SPF45 (splicing factor 45) was identified in mass spectrometry analysis of the spliceosome complex (45) and acts in the second step of splicing, regulating 3′ recognition of alternative splice sites in pre-mRNA of the sxl gene in Drosophila (33). SPF45 regulates alternative splicing of pre-mRNA encoding the death receptor fas in minigene assays in cells, inducing exon 6 skipping, which contains the transmembrane domain (16). Exon 6 skipping has been shown to generate a soluble, dominant negative Fas protein (15). SPF45 consists of an unstructured N-terminal domain, a G-patch motif (2) involved in protein-protein (55) and protein-nucleic acid (26, 58) interactions, and a C-terminal RNA recognition motif (RRM) that is required for mRNA splicing (33). SPF45 also plays a role in DNA repair in Drosophila, associating with RAD201, a member of the RecA/RAD51 family (12). In humans, SPF45 expression is low in most normal tissues but is overexpressed in breast, ovarian, bladder, and prostate cancer (50). Ectopic overexpression of SPF45 in HeLa cervical cancer (50) and A2780 ovarian cancer (48) cell lines induced a multidrug-resistant phenotype, while knockdown of endogenous SPF45 sensitized A2780 cells to etoposide (48).
Mitogen-activated protein (MAP) kinases are a ubiquitous family of serine/threonine, proline-directed protein kinases that regulate many aspects of cell biology, including gene transcription, proliferation, apoptosis, and differentiation. The extracellular signal-regulated kinases (ERKs) are typically associated with cell proliferation, while the Jun N-terminal kinases (JNKs) and p38 MAP kinases are generally associated with apoptosis and inflammation, respectively. ERKs translocate from the cytoplasm to the nucleus upon activation (14, 34), where they phosphorylate nuclear proteins, including transcription factors, to regulate gene expression. MAP kinases are activated downstream of extracellular stimuli and the Ras and Raf oncogenes, regulating cellular processes through phosphorylation of a multitude of substrates. Localized ERK activation within a cell can regulate the timing and choice of substrate phosphorylation (51). Although numerous substrates of ERKs have been identified (35, 36), the diversity of roles of MAP kinases within the cell suggest that there are additional substrates, the identification of which is crucial to the understanding of these important signal transduction kinases.
We previously reported the development of an engineered ERK2 that can uniquely and efficiently utilize an N6-cyclopentyl ATP (cpATP) analog to specifically phosphorylate direct ERK2 substrates (20), aiding in novel substrate identification (9, 20). Using this technique, we show that SPF45 is a novel ERK2-associated substrate. SPF45 was phosphorylated by not only ERK but also p38 and JNK MAP kinases in vitro and in cells, making it the first splicing factor targeted by multiple MAP kinase pathways. We further show that SPF45 overexpression regulates proliferation and cell adhesion and that these effects are dependent upon the MAP kinase phosphorylation sites.
MATERIALS AND METHODS
Cell culture.
COS-1 were grown in Dulbecco's modified Eagle medium (DMEM) (Thermo Scientific). SKOV-3 and ES-2 cells were grown in McCoy's 5A medium (Sigma, St. Louis, MO). IOSE cells were provided by Nellie Auersperg (Univeristy of British Columbia) and were grown in a 1:1 ratio of medium 105 and medium 199 (Sigma). A2780, OVCAR3, OVCAR5, and OV2008 cells were grown in RPMI 1640 medium (Sigma). All cell growth media were supplemented with 10% fetal bovine serum (FBS) (PAA, Dartmouth, MA), and cells were grown at 37°C with 5% CO2. Transient plasmid transfections were performed using Lipofectamine 2000 (Invitrogen). SKOV-3 vector, Myc-SPF45, Myc-SPF45-TASA, Myc-SPF45-TDSD, and FLAG-ERK2-Q103G stable cells were generated by retroviral transduction. Briefly, pAMPHO, VSV-G, and Gag/Pol and either pQCXIP-Myc-SPF45 or pQCXIP-FLAG-ERK2-Q103G were transfected into 293T cells using Fugene (Roche, Mannheim, Germany). Conditioned media containing virus were mixed with Polybrene to a final concentration of 8 μg/ml before infecting SKOV-3 cells. Cellular clones expressing FLAG-ERK2-Q103G and cellular populations expressing Myc-SPF45 proteins or vector were selected with 1.5 μg/ml puromycin for 2 weeks and maintained in 0.75 μg/ml. For suspension cell studies, cells were trypsinized and incubated on agar-coated 6-well dishes for 3 or 24 h before harvest (1).
Antibodies and recombinant proteins.
Generation of peptides and affinity-purified polyclonal antibodies to SPF45 and phospho-SPF45 were performed at Pacific Immunology (Ramona, CA). Peptides used were as follows: SPF45, PYEEDSRPRSQSSKAC; phospho-SPF45-Thr71, SDDRQIVD-T(PO4)-PPHVAAGC; phospho-SPF45-Ser222, YEEQDRPR-S(PO4)-PTGPSNSFC. All antibodies were affinity purified. Anti-phospho-ERK2, phospho-JNK, phospho-p38, JNK, p38, ErbB2, and actin antibodies were purchased from Cell Signaling (Danvers, MA). Anti-SF1 and SF3b155 antibodies were from Abcam (Cambridge, MA). Anti-Myc and antifibronectin antibodies were from Sigma. Anti-ERK antibody 1B3B9 was kindly provided by Michael Weber (University of Virginia). Partially activated His-ERK2 was generated from pETHis-ERK2/MEKR4F from Melanie Cobb (31), and His-SPF45 or a mutant thereof was generated from pET15b-His-SPF45 that we generated. Plasmids were transformed into Escherichia coli BL21 followed by induction with 0.25 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h at 30°C for SPF45 and 12 h for ERK2. Bacteria were lysed in lysis buffer (50 mM phosphate buffer, pH 8.0, 300 mM NaCl, 2 mM phenylmethylsulfonyl fluoride [PMSF], 1 mg/ml lysozyme, and DNase I) for 1 h at 4°C. The lysates were sonicated and centrifuged for 20 min at 15,000 rpm at 4°C. The supernatants were filtered through 0.45-μm-pore-size filters and incubated with nickel beads (Qiagen, Valencia, CA) for 1 h at 4°C with rotation. After incubation, the resin was centrifuged at 3,000 × g for 7 min and the pellets were washed twice with wash buffer (50 mM phosphate buffer, pH 8.0, 300 mM NaCl, 2 mM PMSF, and 15 mM imidazole). His-tagged proteins were eluted with wash buffer containing 150 mM imidazole at 4°C before dialysis of the eluate in 25 mM Tris-HCl, pH 7.5, 1 mM dithiothreitol (DTT), 1 mM EGTA, 1 mM EDTA, 20% glycerol, and 75 mM NaCl. Proteins were stored at −80°C until use.
Nuclear extracts and pulldowns.
HeLa cells were trypsinized and incubated in two volumes of hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl) for 20 min before lysis by Dounce homogenization. Nuclei were pelleted by centrifugation at 3,000 × g for 5 min, and the supernatant (cytoplasm/membranes) was removed. Nuclei were washed once in hypotonic buffer followed by centrifugation. Nuclear proteins were extracted for 30 min in 1 M KCl buffer (20 mM HEPES, pH 7.9, 1 M KCl, 25% glycerol, 0.2 mM EDTA) at 4°C with rotation. Insoluble nuclei were pelleted at 19,000 × g for 30 min, and the supernatant containing the nuclear extract was snap-frozen in liquid nitrogen and stored at −80°C until use. For pulldowns, after preclear with nickel beads, His-ERK2 or buffer was added to nuclear extract and incubated overnight with constant rotation at 4°C in hypotonic buffer containing 0.5% Triton X-100 and 10% glycerol. The following day nickel agarose was added and incubated at 4°C for 1 h. The pellets were washed 3 times in hypotonic buffer containing 0.5% Triton X-100 and 10% glycerol. Bound proteins were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies to SPF45 and ERK2.
Immunoprecipitations and Western blotting.
Cells were lysed in M2 lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5 mM EDTA, 0.5 mM EGTA, 10% glycerol, 50 mM NaF, 40 mM beta-glycerophosphate, 5 mM Na4P2O7, 100 μM Na-orthovanadate, aprotinin, and 2 mM phenylmethylsulfonyl fluoride) followed by sonication. Protein concentrations were determined using the bicinchoninic acid (BCA) kit (Pierce, Rockford, IL). Immunoprecipitations were performed with anti-Myc antibodies followed by protein G-agarose (Roche, Mannheim, Germany). For Western blotting of cell lysates, typically, 25 to 100 μg of protein was run on an SDS-PAGE gel and transferred to a nitrocellulose membrane. The membranes were blocked in 5% milk and were probed with the indicated antibodies. Secondary antibodies conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA) were followed by enhanced chemiluminescence (Pierce). Results were confirmed by at least three independent experiments.
Kinase assays, phosphoamino acid analysis, and mass spectrometry.
Coimmunoprecipitating ERK2 substrates were labeled as described previously (20, 22, 32). Briefly, SKOV-3 cells stably expressing FLAG-ERK2-Q103G were lysed in M2 cell lysis buffer and clarified by centrifugation at 15,000 × g for 15 min, and FLAG-ERK2 was immunoprecipitated with M2 anti-FLAG agarose (Sigma) for 1 h. The immunoprecipitated protein was washed twice in kinase buffer (20 mM HEPES, pH 7.4, 10 mM Mg acetate, 1 mM dithiothreitol) and incubated for 3 min at 30°C in kinase buffer with [γ-32P]N6-cyclopentyl-ATP. [γ-32P]N6-cyclopentyl-ATP was generated as described previously (20, 21). A parallel, nonradioactive kinase reaction was performed on a FLAG-ERK2 immunoprecipitated from six 150-mm dishes of SKOV-3-QG cells put in suspension culture for 3 h (1) and run on the same gel. The gel was silver stained using the Vorum method (43). The gel was cut and a portion exposed for autoradiography, or the corresponding band excised from the gel, trypsin digested, and microsequenced using a Finnigan LCQ ion trap mass spectrometer with a Protana nanospray ion source. ERK2 in vitro kinase assays were performed with 0.5 μg of His-SPF45 or His-SPF45-T71A/S222A and in 40 μl of kinase buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, 100 μM ATP, and 10 μCi of [γ-32P]ATP) (for radioactive reactions) at 30°C for 10 min. Equimolar amounts of His-SPF45, myelin basic protein (Sigma), or lysozyme (Sigma) were also used in a radioactive ERK2 kinase reaction. Reactions were resolved by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) (Pierce) or Immobilon (Millipore, Billerica, MA) membrane, followed by autoradiography and/or Western blotting with the indicated antibody. Radioactive bands were excised, quantitated by Cerenkov counting, and analyzed for phosphorylated residues by phosphoamino acid analysis as described previously (7).
Construction of Δfas minigene and splicing assays.
Genomic DNA from the IOSE cell line was isolated by phenol-chloroform extraction followed by ethanol precipitation. Exon 5 through nucleotide 44 of exon 7 of fas, including introns, was amplified with the primer pair of 5′-AGA TGT GAA CAT GGA ATC ATC AAG GA-3′ and 5′-TTT CCT TTC TGT GCT TTC TGC ATG TT-3′ and ligated into the TOPO vector (Invitrogen). The resulting plasmid was then cut with HindIII and XbaI and subcloned into pcDNA3.1. A 1.1-kb fragment of intron 6 was deleted by PCR with primer pairs of 5′-GTA AGA ATG AGG CAA ATC TTT GTG A-3′ and 5′-CCT TCT TAT ATT TCT CTT AGT GTG AAA GTA-3′, and the PCR product was circularized by blunt-end ligation. For Δfas splicing assays, COS-1 cells were plated at 2.5 × 105 cells/well on six-well dishes and transfected the following day with Δfas, Myc-SPF45, Myc SPF45-TASA, MEK1-DD, ERK2, MKK3-EE, p38α, or empty vector as indicated using Lipofectamine 2000 (Invitrogen). Cells were harvested in TRIzol (Invitrogen) after 24 h, and RNA was isolated by chloroform extraction and isopropanol precipitation. Total RNA (2.0 μg) was reverse transcribed using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). PCR was performed with Δfas-specific primers (forward, 5′-AGA TGT GAA CAT GGA ATC ATC AAG GA; reverse, 5′-AGA TGT GAA CAT GGA ATC ATC AAG GA) using a Mycycler thermal cycler (Bio-Rad) and run on 2% agarose gels. PCR products were quantified using Gel-Pro Analyzer 3.1 across three independent experiments. The results were expressed as a relative ratio, which was calculated by comparing the ratios of the lower band to the upper band in the Δfas and SPF45 groups, which was set to 1. Ratios in the other groups were then compared to this group. Statistical comparisons were performed using analysis of variance (ANOVA) followed by a Tukey post hoc test with statistical significance determined by P < 0.05.
Real-time quantitative reverse transcription (qRT)-PCR and Northern blotting.
Total cellular RNA was extracted from SKOV-3 stable cell lines and reverse transcribed as described above. cDNA was subjected to real-time PCR using RT2SYBR green dye (Qiagen, Valencia, CA) and prevalidated primers for ErbB2, fibronectin 1, and 18s RNA (Qiagen). Each sample was run in duplicate in three independent experiments. The fold change in gene expression was calculated by the ΔΔCT method. For Northern blotting, total RNA was harvested from SKOV-3 stable cell lines in TRIzol (Invitrogen) followed by chloroform-ethanol extraction. Total RNA (5 μg per sample) was electrophoresed on a 1.2% denaturing agarose gel, transferred overnight to Hybond-N+ membrane (GE Healthcare), and subjected to baking and UV cross-linking. Hybridizations were performed in UltraHyb (Applied Biosystems) using a [32P]dCTP single-stranded DNA (ssDNA) probe (1 × 106 cpm/ml) spanning nucleotides 155 to 271 and nucleotides 948 to 1112 in genomic FN (GenBank accession no. M12549.1), corresponding to a region spanning exons 6 and 7, generated by PCR using the following probes: FN6-7 Fwd, 5′-GAG GGA CCT GGA AGT TGT TG-3′; FN6-7 Rev, 5′-GGC CAG TGA CAG CAT ACA CA-3′, followed by asymmetric PCR using FN6-7 Rev and purified using Illustra ProbeQuant G-50 microcolumns (GE Healthcare).
Proliferation assay.
SKOV-3 stable cell lines (1 × 105) were plated on six-well plates on day 1 and counted at 24-h intervals. Briefly, the cells were gently washed with PBS, trypsinized in 100 μl, and collected with 100 μl McCoy's medium supplemented with 50% FBS. Cells were homogenously suspended and manually counted using a hemocytometer.
Adhesion assay.
Tissue culture dishes (96 well) were coated with fibronectin (20 μg/ml), laminin-1 (10 μg/ml), poly-l-lysine (10 μg/ml), or nothing overnight at 4°C. Plates were washed twice with washing buffer (0.1% BSA in PBS) and blocked with blocking buffer (0.5% BSA in PBS) at 37°C for 60 min, followed by washing buffer, and chilled on ice. Stable SKOV-3 cells were suspended at 4 × 105 cells/ml, and 50 μl of the suspension was aliquoted into the wells. Plates were incubated at 37°C and 5% CO2 for 30 min, then placed on a plate shaker at 2,000 rpm for 15 s. Unattached cells were removed by aspiration, and the wells were gently washed with washing buffer three times. Adherent cells were fixed with 4% formaldehyde, washed with washing buffer, stained with crystal violet (5 mg/liter in 2% ethanol) for 10 min, washed gently three times, and dried. Crystal violet stain was solubilized in 2% SDS, and plates were read at 550 nm.
RESULTS
SPF45 is a novel ERK2 binding partner and substrate.
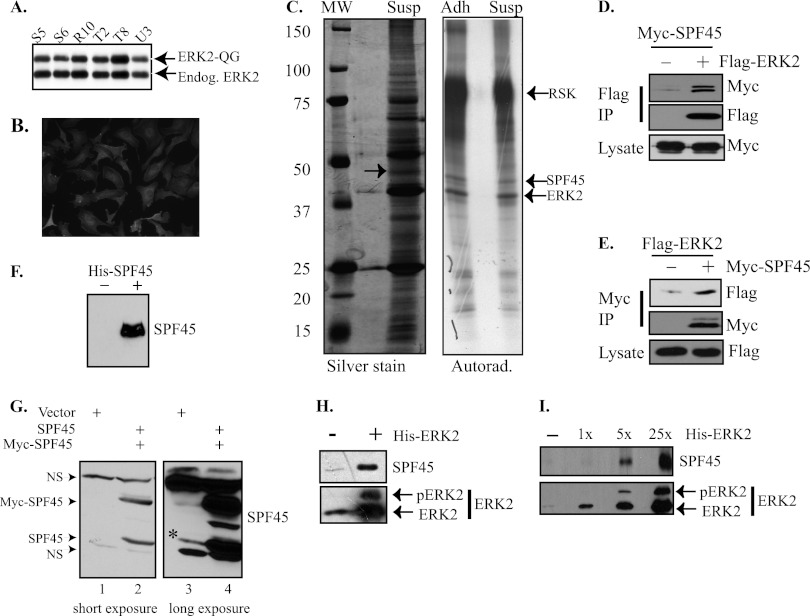
We previously generated an engineered ERK2 mutant, ERK2-Q103G, that we demonstrated can uniquely utilize N6-cyclopentyl ATP (N6-cpATP) to phosphorylate substrates (20). N6-cpATP is a poor substrate for cellular protein kinases (53), and the use of radiolabeled N6-cpATP allows for the specific labeling of ERK2-Q103G substrates in a mixture of cellular proteins, including those that coimmunoprecipitate (20, 22, 23). We generated SKOV-3 ovarian cancer cell lines that stably express FLAG-ERK2-Q103G. There were several clones that expressed FLAG-ERK2-Q103G at endogenous ERK2 levels (Fig. 1A) and expressed the exogenous protein in the entire clonal population (Fig. 1B). Clone T8 was used further and named SKOV-3-QG. We recently demonstrated an enhancement of ERK activation in detached SKOV-3 cells, which induced ERK2 nuclear signaling (1). Therefore, SKOV-3-QG cells were either grown adherent (Adh) or trypsinized and put into suspension culture (Susp) for 3 h. The cells were then lysed, and FLAG-ERK2-Q103G was immunoprecipitated under mild conditions to preserve protein-protein interactions and incubated in kinase buffer containing [γ-32P]N6-cpATP. The kinase reaction mixtures were then run on a gel, silver stained, and processed for autoradiography (Fig. 1C, right panel). We observed labeling of a 47-kDa protein in FLAG-ERK2-Q103G immunoprecipitates under both conditions. A parallel, nonradioactive kinase reaction was performed on a FLAG immunoprecipitate from six 15-cm dishes of suspended SKOV-3-QG cells and run in parallel on the same gel. A silver-stainable band at the same molecular weight as the radioactive band was observed (Fig. 1C, left panel, arrow). Mass spectrometry analysis of this protein band included several proteins, including the mRNA alternative splicing factor SPF45. Other proteins were also detected in this band, and we are determining if they are also substrates of ERK2 (data not shown). Cotransfection of COS-1 cells confirmed that Myc-SPF45 specifically coimmunoprecipitated with Flag-ERK2 (Fig. 1D). Myc-SPF45 isolated from cells ran as a doublet, suggesting modification, possibly by phosphorylation. When the assay was performed in reverse, Flag-ERK2 also coimmunoprecipitated with Myc-SPF45 (Fig. 1E). We were unable to coimmunoprecipitate endogenous ERK and SPF45 (data not shown). However, to further test binding between ERK2 and endogenous SPF45, we first generated a polyclonal anti-SPF45 antibody and histidine-tagged SPF45 (His-SPF45). The anti-SPF45 antibody was able to specifically immunoblot His-SPF45 (Fig. 1F). In addition, this antibody immunoblotted transfected, untagged SPF45 and Myc-SPF45 from cell lysates of transfected COS-1 cells (Fig. 1G, lanes 2 and 4). A longer exposure of the immunoblot demonstrated that the antibody also recognized endogenous SPF45 (Fig. 1G, band beside * in lane 3), which is expressed at low levels in COS-1 cells (data not shown). This antibody was not useful for immunoprecipitation or immunofluorescence (data not shown). To examine endogenous SPF45 association with ERK2, recombinant, partially activated His-ERK2 was incubated overnight with nuclear extracts from HeLa cells, which express higher levels of endogenous SPF45 than COS-1 cells (data not shown). His-ERK2 was reisolated with nickel agarose, and bound proteins were immunoblotted for SPF45. SPF45 was specifically pulled down from nuclear extracts with His-ERK2 and not with nickel agarose alone (Fig. 1H). The small amount observed in the control lane was due to spillover when loading the gel. SPF45 bound His-ERK2 in a dose-dependent manner when increasing amounts of His-ERK2 were incubated with the same amount of nuclear extract (Fig. 1I). These results showed specific association between SPF45 and ERK2.
Fig 1.
SPF45 is a novel ERK2 coimmunoprecipitating substrate. (A) Cell lysates from SKOV-3 clones expressing FLAG-ERK2-Q103G were immunoblotted for ERK2. (B) Cells from clone T8 (designated SKOV-3-QG) from panel A were immunostained with antibodies to FLAG. (C) Anti-FLAG immunoprecipitates from adherent or suspended SKOV-3-QG cells were incubated in an in vitro kinase assay with [γ-32P]N6-cyclopentyl-ATP. The reaction mixtures were run on a gel, silver stained, and processed for autoradiography (right panel). A parallel reaction from six 15-cm dishes of suspended cells was run on the same gel and silver stained (left panel). The arrow indicates the band excised from the gel and processed for mass spectrometry. (D) Myc-SPF45 coimmunoprecipitates with Flag-ERK2. COS-1 cells were transfected with Myc-SPF45 and Flag-ERK2 and Flag immunoprecipitates, and cell lysates were immunoblotted as indicated. (E) Similar to panel D, but Myc immunoprecipitates were immunoblotted. (F) Validation of a novel SPF45 antibody. Either buffer or His-SPF45 was run on a gel and immunoblotted with anti-SPF45 antibody. (G) COS-1 cells were transfected with either empty vector or untagged SPF45 and Myc-SPF45. Cellular lysates were immunoblotted with anti-SPF45 antibody with a short (left panel) or a long (right panel) exposure, the latter of which shows recognition of endogenous SPF45 (lane 3, band next to *). NS, nonspecific band. (H) Partially activated His-ERK2 or buffer was incubated overnight with a nuclear extract from HeLa cells. His-ERK2 was reisolated, run on a gel, and immunoblotted with anti-SPF45 and anti-ERK2 antibody. (I) Same as in panel H, but increasing amounts of His-ERK2 were incubated with the same amount of HeLa nuclear extract per sample.
ERK2 phosphorylates SPF45 on threonine 71 and serine 222.
To confirm that SPF45 is a substrate for ERK2, active His-ERK2 was shown to strongly phosphorylate His-SPF45 in vitro with [γ-32P]ATP (Fig. 2A) on serine and threonine (Fig. 2B). To determine specificity, we performed a kinase reaction using equimolar amounts of myelin basic protein (MBP), a known in vitro ERK2 substrate, His-SPF45, and lysozyme, a negative control. ERK2 phosphorylated SPF45 to an extent equivalent to that of MBP (Fig. 2C). MAP kinases phosphorylate only serine and threonine residues that are followed by a proline, allowing only two potential ERK phosphorylation sites in SPF45, threonine 71 and serine 222, both of which are evolutionarily conserved (Fig. 2D). His-ERK2 phosphorylated His-SPF45 but not His-SPF45-TASA in vitro (Fig. 2E), demonstrating that these are the sites of phosphorylation.
Fig 2.
ERK phosphorylates SPF45 on Thr71 and Ser222. (A) His-SPF45 was phosphorylated by His-ERK2 in vitro using [γ-32P]ATP. The reaction mixtures were run on a gel, transferred, exposed for autoradiography, and immunoblotted for SPF45. (B) Phosphoamino acid analysis of the SPF45 from panel A. (C) His-ERK2 was used to phosphorylate equimolar amounts of myelin basic protein (MBP) (positive control), His-SPF45, or lysozyme (negative control) using [γ-32P]ATP. The reaction mixtures were run on a gel, transferred, and exposed for autoradiography. (D) Evolutionarily conserved sequence of the only two potential MAP kinase phosphorylation sites in SPF45, Thr71, and Ser222 in human SPF45. (E) Recombinant His-SPF45 or His-SPF45-T71A/S222A was phosphorylated in vitro by His-ERK2 using [γ-32P]ATP and processed as in panel A. (F) His-SPF45 was phosphorylated in vitro by His-ERK2 using unlabeled ATP. The reaction mixtures were run on a gel and immunoblotted with anti-SPF45, anti-phospho-Thr71-SPF45, and anti-phospho-Ser222-SPF45 antibodies. (G) His-SPF45 was phosphorylated with GST-ERK2 using unlabeled ATP, reisolated with nickel agarose, and treated with calf alkaline phosphatase (Ptase), as indicated. The reaction mixtures were run on a gel and immunoblotted as in panel F. (H) His-SFP45, His-SPF45-T71A, His-SPF45-S222A, and His-SPF45-T71A/S222A were phosphorylated with His-ERK2 and unlabeled ATP. The reaction mixtures were run on a gel and immunoblotted as indicated. (I) COS-1 cells were transfected with MEK1-DD, ERK2 and wild-type or mutant Myc-SPF45, as indicated. Anti-Myc immunoprecipitates were immunoblotted for Myc and phospho-SPF45.
We generated polyclonal phospho-specific antibodies to both phosphorylated Thr71 and Ser222 using singly phosphorylated peptides as antigens. Both antibodies reacted only with recombinant His-SPF45 phosphorylated in vitro with His-ERK2 and unlabeled ATP (Fig. 2F) and had reduced reactivity to His-SPF45 treated with alkaline phosphatase after His-ERK2 phosphorylation (Fig. 2G). To further test specificity, we generated recombinant protein of single phospho-inhibitory point mutants and phosphorylated them and His-SPF45-TASA in vitro with His-ERK2 and unlabeled ATP. Both antibodies showed specificity to SPF45 only when phosphorylated by ERK2 and not when the phosphorylation site was mutated to alanine (Fig. 2H). In addition, COS-1 cells were transfected with HA-MEK1-DD, FLAG-ERK2, and either Myc-SPF45 or a Myc-SPF45 alanine or aspartate mutant. Immunoblotting of anti-Myc immunoprecipitates showed that the phospho-specific antibodies did not recognize SPF45 when the phosphorylation site was mutated. Collectively, these results demonstrate that the phospho-specific SPF45 antibodies recognized SPF45 only when it was phosphorylated on these sites. However, as with our anti-SPF45 antibody, these antibodies were useful only for Western blotting (data not shown).
Mutation of Ras and Raf occurs frequently in cancer, resulting in hyperactivation of ERK (25, 40). Cotransfection of COS-1 cells with H-Ras-G12V, B-Raf-V600E, or MEK1 S218/222D (MEK1-DD) stimulated no induction of SPF45 phosphorylation above basal levels on their own (Fig. 3A). However, we have observed that cotransfection of exogenous ERK with an upstream activator greatly increases phosphorylation of a cotransfected substrate (19, 24). Indeed, Flag-ERK2 cotransfection with H-Ras-G12V or MEK1-DD increased basal Ser222 phosphorylation and strongly induced Thr71 phosphorylation. B-Raf-V600E induced phosphorylation of both sites, albeit to a lesser extent, due to lower induction of ERK2 activation. MAP kinases bind to some substrates through two aspartate residues in the common docking (CD) domain, which can also be required for phosphorylation of a substrate (59). Transfection of ERK2-DDNN, which carries mutations of these residues, showed decreased ability to phosphorylate SPF45, even though ERK2-DDNN was phosphorylated to the same extent as ERK2 by MEK1-DD (Fig. 3B). ERK2-T183A/Y185F (TAYF), a nonactivatable ERK2 mutant that cannot be phosphorylated by MEK, was unable to phosphorylate SPF45. When cells were cotransfected with SPF45 and both ERK2 and ERK2-DDNN, SPF45 showed a preference for association with ERK2 over ERK2-DDNN (Fig. 3C), which mirrored the observed differences in SPF45 phosphorylation (Fig. 3B). To determine if ERK activation enhanced the association with SPF45, we performed coimmunoprecipitation experiments with ERK2 or ERK2-K52R, a phosphorylatable kinase-dead mutant, in the presence or absence of MEK1-DD. MEK1-DD had little effect on SPF45 association with ERK2, whereas SPF45 showed enhanced basal binding to ERK2-K52R that was further enhanced in the presence of MEK1-DD (Fig. 3D). To determine if the ERK2 phosphorylation sites on SPF45 affected binding, coimmunoprecipitations were performed with the SPF45 alanine mutants. No significant differences in the binding of ERK to the mutants were observed (Fig. 3E).
Fig 3.
Oncogenes stimulate ERK2 phosphorylation of SPF45. (A) Myc-SPF45 was transfected into COS-1 cells with MEK1-DD, H-Ras-G12V, or B-Raf-V600E with or without Flag-ERK2. Myc immunoprecipitates were immunoblotted for phospho-SPF45 and Myc. Cell lysates were immunoblotted for Flag-ERK2 and phospho-ERK, identifying exogenous and endogenous ERK2. (B) COS-1 cells were transfected with Myc-SPF45, Flag-MEK1-DD, and Flag-tagged ERK2, ERK2-DDNN, or ERK2-T183A/Y185F. Myc immunoprecipitates were immunoblotted for phospho-SPF45 and Myc. Cell lysates were immunoblotted for Flag and phospho-ERK. (C) Cells were cotransfected with both Flag-ERK2 and Flag-ERK2-DDNN and either empty vector or Myc-SPF45. Myc immunoprecipitates were immunoblotted for Flag and Myc. Cell lysates were immunoblotted for Flag. (D) COS-1 cells were transfected with Myc-SPF45, HA-MEK1-DD, and either Flag-ERK2 or Flag-ERK2-K52R. Anti-Flag immunoprecipitates were immunoblotted for Myc and Flag. Cell lysates were immunoblotted for Myc and p-ERK. (E) COS-1 cells were transfected with Flag-ERK2 and Myc-SFP45 or an SPF45 mutant. Flag immunoprecipitates were immunoblotted for Myc and Flag. Cell lysates were immunoblotted for Myc.
SPF45 is also a substrate for p38α and JNK1.
All MAP kinases are proline-directed kinases that can have overlapping substrate specificity; therefore, we determined whether SPF45 was an in vitro substrate of JNK1 and p38α. His-SPF45 was incubated with [γ-32P]ATP and purified recombinant ERK2, JNK1, or p38α of equal specific activity. All three kinases demonstrated phosphorylation of SPF45 over three independent experiments, with p38 having the highest overall phosphorylation (Fig. 4A and B). Phosphoamino acid analysis demonstrated that both JNK1 (Fig. 4C) and p38α (Fig. 4D) phosphorylated SPF45 on both serine and threonine. Immunoblotting a nonradioactive in vitro kinase assay showed that p38α and JNK1 phosphorylate both Thr71 and Ser222, with p38α having a stronger preference than JNK1 toward Thr71 (Fig. 4E). Cotransfection experiments in COS-1 cells showed that SPF45 coimmunoprecipitated with both p38α and JNK1, with both kinases having significantly higher basal association with SPF45 than ERK2 (Fig. 4F). To compare phosphorylation in cells, COS-1 cells were transfected with mutationally activated forms of MEK1, MKK3, and MKK4 and either Flag-ERK2, Flag-p38α, or Flag-JNK1, respectively. Both p38α and ERK2 stimulated strong SPF45 phosphorylation, with p38α activation stimulating greater Ser222 and Thr71 phosphorylation (Fig. 4G). JNK1 did not express well or stimulate SPF45 phosphorylation with repeated attempts, possibly due to the induction of apoptosis upon JNK1 hyperactivation.
Fig 4.
SPF45 is phosphorylated by p38α and JNK1. (A) His-SPF45 was phosphorylated in vitro using [γ-32P]ATP and recombinant ERK2, p38α, or JNK1 of equal specific activity. Kinase reaction mixtures were run on a gel and exposed for autoradiography. (B) His-SPF45 bands from panel A were cut out of the membrane and quantitated with Cerenkov counting. The results are from three independent experiments. (C and D) Phosphoamino acid analysis of His-SPF45 phosphorylated in panel A by JNK1 and p38α. (E) His-SFP45 was phosphorylated by p38α or JNK1 using unlabeled ATP. The reaction mixtures were immunoblotted for SPF45 and phospho-SPF45. (F) SPF45 associates with JNK1 and p38α in cells. COS-1 cells were transfected with Myc-SPF45 and either empty vector or Flag-tagged ERK2, p38α, or JNK1. Anti-Flag immunoprecipitates were immunoblotted for Myc and Flag. Cell lysate was immunoblotted for Myc. (G) COS-1 cells were transfected with Myc-SPF45 and either mutationally activated MEK1, MKK3, or MKK4 and Flag-tagged ERK2, p38α, or JNK1, respectively. Myc immunoprecipitates were immunoblotted for phospho-SPF45 and Myc. Cell lysate was immunoblotted for Flag.
Phosphorylation of endogenous SPF45 by MAP kinases.
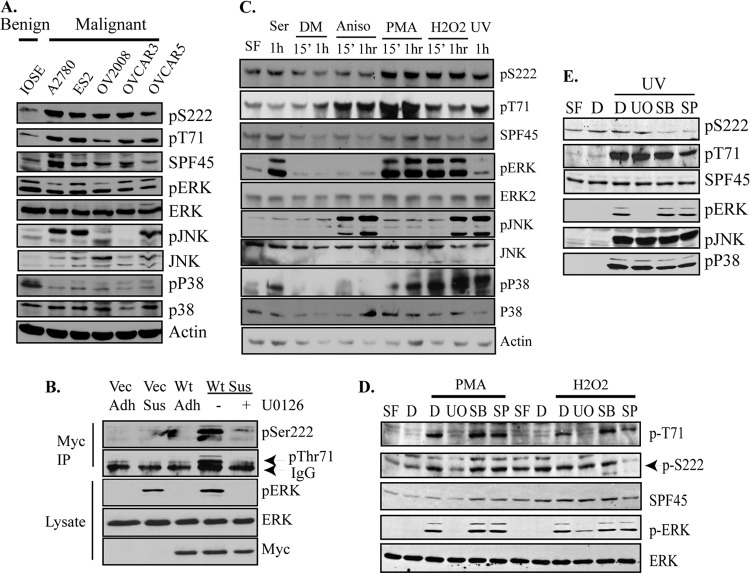
We screened a number of ovarian cancer cell lines for endogenous SPF45 expression, with the benign, immortalized IOSE ovarian cell line serving as a control. IOSE cells contained little endogenous SPF45 expression or phosphorylation (Fig. 5A). A2780, ES-2, OV2008, and OVCAR3 cells had the highest SPF45 expression and OVCAR5 cells had lower expression. Phosphorylation of Ser222 was induced in all the cancer cell lines, while Thr71 phosphorylation was observed in all cells except OV2008. Immunoblotting with anti-phospho-MAP kinase antibodies revealed variable activation of ERK, p38, and JNK. To support our original observation of SPF45 as an ERK2 substrate (Fig. 1C), SKOV-3-Myc-SPF45 cells were detached and incubated in the presence or absence of the MEK inhibitor U0126 to prevent ERK activation. Both Thr71 and Ser222 phosphorylation were induced in suspended cells compared to adherent cells, coincident with ERK activation, and phosphorylation of both SPF45 and ERK was inhibited by U0126 treatment (Fig. 5B). We next attempted to identify extracellular stimuli that induce SPF45 phosphorylation in ovarian cancer cells and to identify the MAP kinase pathway responsible. We chose A2780 cells for these studies due to their high expression of endogenous SPF45 (Fig. 5A). Cells were serum starved overnight and then treated with serum, DMSO control, anisomycin, phorbol myristate acid (PMA), H2O2, or UV light. Anisomycin, PMA, H2O2, or UV light all stimulated Thr71 phosphorylation, while only PMA, H2O2, and UV light stimulated Ser222 phosphorylation (Fig. 5C). Interestingly, serum induced ERK and p38 activation but did not stimulate SPF45 phosphorylation, suggesting that SPF45 phosphorylation may be stimulus specific. To determine the MAP kinase that phosphorylated SFP45 in response to PMA, H2O2 (Fig. 5D), and UV light (Fig. 5E), cells were serum starved as described above and pretreated for 30 min with a specific MAP kinase pathway inhibitor before stimulation. The MAP kinase pathway inhibitors used were U0126 for the ERK pathway, SB203580 for the p38 pathway, and SP600025 for the JNK pathway. SPF45 phosphorylation was ERK dependent in response to PMA, ERK (Thr71) and JNK (Ser222) dependent in response to H2O2, and p38 (Ser222) and JNK (Ser222 and Thr71) dependent in response to UV light. These data demonstrate that SPF45 is phosphorylated by all three kinases in cells by a variety of extracellular stimuli.
Fig 5.
Endogenous SPF45 is phosphorylated by MAP kinases in cells. (A) Cell lysates from ovarian benign and cancer cell lines were immunoblotted as indicated. (B) SKOV-3-Myc-SPF45 cells were either grown adherent (Adh) or put in suspension (Sus) culture in the presence or absence of U0126. Myc immunoprecipitates were immunoblotted for phospho-SPF45 and Myc. Cell lysates were immunoblotted for endogenous ERK and p-ERK. (C) A2780 cells were serum deprived overnight and then treated with either serum, DMSO (DM), anisomycin, PMA, H2O2, or UV light for 15 min or 60 min. Cell lysates were immunoblotted for endogenous proteins with the indicated antibodies. (D) Serum-starved (SF) A2780 cells were pretreated with DMSO (D), U0126 (UO), SB203580 (SB), or SP600025 (SP) prior to stimulation with either PMA or H2O2 for 1 h. Cell lysates were immunoblotted for endogenous proteins as indicated. (E) A2780 cells were put in serum-free medium (SF) overnight and treated with either DMSO (D), U0126 (UO), SB203580 (SB), or SP600025 (SP) for 30 min prior to stimulation with UV light. Cells were harvested 1 h later. Cell lysates were immunoblotted for endogenous proteins as indicated.
Phosphorylation regulates SPF45 alternative splice site utilization toward Fas pre-mRNA.
Splicing factor phosphorylation regulates their splice site utilization, including a requirement for reversible phosphorylation for the catalytic splicing process in some cases (49, 57). Pre-mRNA for the death receptor gene fas is one of only two reported mammalian splicing targets for SPF45, inducing exon 6 skipping/exclusion from Fas mRNA in a minigene assay (16). We used DNA from IOSE cells to perform PCR on a genomic fas sequence consisting of exon 5 through part of exon 7, including introns, and inserted it into pCDNA3.1 downstream of the constitutive CMV promoter, creating a Δfas minigene (30) that can be used to study SPF45 splice site utilization (Fig. 6A), as has been previously reported (16). Transfection of Δfas into COS-1 cells followed by RT-PCR on RNA using vector-specific primers overlapping the Δfas sequence allowed us to readily detect the exogenous normal transcript containing exons 5, 6, and 7 (241 bp) and the alternatively spliced transcript, containing only exons 5 and 7 (178 bp). SPF45 expression increased Δfas alternative splicing (i.e., exon 6 skipping) (16), as measured by an increased ratio of the 178-bp form to the 241-bp form, in a dose-dependent manner, with maximal exon 6 exclusion at 0.8 μg of SPF45 plasmid (Fig. 6B). To determine the effect of ERK signaling on SPF45 splice site utilization in Δfas pre-mRNA, we cotransfected cells with SPF45, Δfas, and either MEK1-DD and ERK2 or vector. MEK1-DD/ERK2 cotransfection inhibited exon 6 exclusion by SPF45 by 40%, an effect that was partially reversed with cotreatment with the MEK inhibitor U0126 (Fig. 6C and D). Similarly, cotransfection of p38α and activated MKK3 inhibited SPF45-dependent splicing 65%, an effect which was completely reversed by the addition of SB203580 (Fig. 6E and F). These data suggest that MAP kinase signaling to SPF45 decreases SPF45 splice site utilization on fas pre-mRNA. We were unable to measure an effect of activated MKK4 and JNK1 on SPF45-regulated splicing, as JNK1 did not express well with MKK4 transfection, likely due to induction of apoptosis (data not shown). In the absence of exogenous MKK and MAP kinases, U0126, SB203580, and SP600025 did not have an effect on SPF45-induced splicing in COS-1 cells (Fig. 6G and H). Mutation of both phosphorylation sites to alanine enhanced splicing by 10.6%, while, surprisingly, mutation to aspartate, which can sometimes act as a phospho-mimetic mutation, enhanced splicing 28.6% (Fig. 6I and J). These results suggest that aspartate mutation does not mimic the effects of phosphorylation by ERK or p38 for one or both phosphorylation sites in regard to Δfas splicing. Similar results were obtained in the presence of MEK1-DD and ERK cotransfection. SPF45 associates with the 3′ splice site recognition factors SF1 and SF3b155 (16). We found that mutation of the phosphorylation sites on SPF45 did not significantly affect association with these proteins (Fig. 6K).
Fig 6.
ERK phosphorylation of SPF45 decreases SPF45 alternative splice site selection in fas pre-mRNA. (A) Diagram of the fas minigene (Δfas) comprised of exon 5 to 44 nucleotides into exon 7 of genomic fas, including introns, with a portion of intron 6 removed. SPF45 enhances exon 6 exclusion from Δfas (16). PCR analysis using vector-specific primers allows for the determination of exon 6 inclusion (241-bp fragment) or exclusion (187-bp fragment) from the mature transcript. (B) SPF45 expression increases exon 6 exclusion. COS-1 cells were transfected with Δfas and increasing amounts of Myc-SPF45. Total mRNA was subjected to PCR using vector-specific primers flanking exons 5 and 7, and reaction products were run on an agarose gel. No other PCR products were detected. (C and D) ERK activation decreases SPF45 alternative splice site utilization. COS-1 cells were transfected with Δfas, Myc-SPF45, MEK1-DD, and ERK2 as indicated. mRNA was isolated and PCR performed as in panel B. A representative gel and Western blot of SPF45 expression is shown in panel C, and the results of three independent experiments are shown in panel D. The ratio of exon 6 exclusion to inclusion for cells transfected with SPF45 was set to 1 in each experiment. (E and F) Same as in panels C and D, but the cells were cotransfected with MKK3-EE and p38α. The results in panel F are a combination of results of three independent experiments. (G and H) COS-1 cells were transfected with Δfas and SPF45 and treated overnight with MAP kinase pathway inhibitors. Splicing products and SPF45 expression were determined as described above. (I and J) COS-1 cells were transfected with Δfas and either empty vector, SPF45, TASA, or TDSD. A representative gel and immunoblot is shown in panel I, and the graph in panel J is the combination of results of three independent experiments. (K) COS-1 cells were transfected with Myc-SPF45 and anti-Myc immunoprecipitates were immunoblotted for Myc and endogenous SF1 and SF3b155. Cell lysates were immunoblotted as indicated.
SPF45 inhibits cell proliferation and ErbB2 expression in a phosphorylation-dependent manner.
SPF45 is overexpressed in several types of cancer (50), but the phenotype of SPF45 overexpression is unknown. We generated stable SKOV-3 cell lines by retroviral transduction that overexpressed Myc-SPF45, Myc-SPF45-TASA, Myc-SPF45-TDSD, or empty vector. Myc-SPF45 and the two mutants were expressed at equal levels (Fig. 7A) and approximately 2-fold more than endogenous SPF45 (Fig. 7B). Immunofluorescence with anti-Myc antibody showed 100% expression of Myc-SPF45 proteins in the populations (Fig. 7C). Cell proliferation assays showed that overexpression of SPF45 and SPF45-TDSD greatly inhibited proliferation compared to vector control cells and cells expressing SPF45-TASA (Fig. 7D). SKOV-3 cells overexpress EGF receptor family members, including ErbB2, which control cell proliferation. Real time qRT-PCR using RT2SYBR green chemistry and prevalidated primers to ErbB2 demonstrated that ErbB2 mRNA expression was dramatically decreased by 69% in SKOV-3-Myc-SPF45 and by 80% in SKOV-3-Myc-SPF45-TDSD cells compared to vector control cells, while SKOV-3-Myc-SPF45-TASA cells showed only a 36% decrease in ErbB2 mRNA (Fig. 7E). The decrease in ErbB2 mRNA expression corresponded to a dramatic decrease in ErbB2 protein levels, while Myc-SPF45-TASA cells had ErbB2 protein levels closer to those seen in SKOV-3 vector cells (Fig. 7F). ErbB2 gene expression is regulated in part by Ets family transcription factors, including PEA3, Elf1, and Elf3 (52, 54). Elf3, a positive regulator of ErbB2 expression, was downregulated at the protein level in SKOV-3-Myc-SPF45 and SKOV-3-Myc-SPF45-TDSD cells compared to vector cells and Myc-SPF45-TASA cells, with the level of expression correlating to relative ErbB2 expression (Fig. 7G). Collectively, these results suggest that SPF45 overexpression decreases cell proliferation through downregulation of Elf3 and ErbB2 expression in a MAP kinase phosphorylation-dependent manner.
Fig 7.
SPF45 overexpression inhibits proliferation and ErbB2 expression in a phosphorylation-dependent manner. (A) SKOV-3 cells were retrovirally transduced with either empty pQCXIP vector (Vec), Myc-SPF45, Myc-SPF45-TASA (TASA), or Myc-SPF45-TDSD (TDSD). Cell lysates were immunoblotted with antibodies to Myc and actin. (B) Lysates from SKOV-3 vector and Myc-SPF45 cells were immunoblotted for SPF45, showing Myc and endogenous SPF45 expression. N.S., nonspecific. (C) SKOV-3 stable cell lines were immunostained with anti-Myc antibody and DAPI stained. (D) SPF45 overexpression inhibits cell proliferation in a phosphorylation-dependent manner. Cell counting of SKOV-3 stable cell lines was performed over 5 days. NS, not significant. (E) mRNA was isolated from the cells in panel A and subjected to real-time qRT-PCR using validated primers to ErbB2. The results are a combination of results of three independent experiments. The level of expression in the vector control cells was set to 1 in each experiment. (F) Cell lysates from the SKOV-3 stable cell lines in panel A were run on a gel and immunoblotted with antibodies to ErbB2 and actin. (G) Cell lysates from the SKOV-3 stable cell lines in panel A were run on a gel and immunoblotted with antibodies to Ets family transcription factors and actin.
SPF45 overexpression regulates cell adhesion, fibronectin expression, and fibronectin splicing through phosphorylation-dependent and -independent mechanisms.
We also determined the effect of SPF45 overexpression and phosphorylation on cell adhesion. The stable cell lines were plated onto four different matrices: tissue culture plastic, poly-l-lysine, laminin, and fibronectin. After 30 min, attached cells were fixed and stained with crystal violet and attachment was quantified by absorbance measurement at 550 nm. All four stable cell lines attached equally well to tissue culture plastic, poly-l-lysine, and laminin within this short time frame (Fig. 8A). An overall increase in attachment was seen for all cell lines plated on fibronectin. However, compared to vector control cells, cells overexpressing wild-type SPF45, TASA, or TDSD had 29.6%, 15.8%, and 19.5%, respectively, less attachment to fibronectin, all of which were statistically significant. Moreover, cells expressing wild-type SPF45 were significantly different than those expressing TASA or TDSD. Real-time qRT-PCR from the SKOV-3 stable cell lines demonstrated that fibronectin (FN1) mRNA expression was upregulated approximately 5-fold in all SKOV-3 stable cell lines that overexpressed SPF45 compared to vector control cells, independent of the MAP kinase phosphorylation sites (Fig. 8B). Northern blot (Fig. 8C) and Western blot (Fig. 8D) analysis confirmed steady-state overexpression of FN1 mRNA and protein in these cells. There are 20 different isoforms of fibronectin mRNA due to alternative pre-mRNA splicing, primarily affecting extra domain 3A (EDIIIA), extra domain 3B (EDIIIB), and the variable region (V or IIICS) (63). Real-time RT-PCR analysis using gene-specific primers for total FN1 mRNA, FN1-EDA, and FN1-EDB using RT2SYBR green chemistry revealed that, again, SPF45 overexpression enhanced the expression of FN1 mRNA, although not to the same extent as in the previous experiments, most likely due to increased cell passage number. Interestingly, Myc-SPF45, but not Myc-SPF45-TASA or Myc-SPF45-TDSD, enhanced the inclusion of the EDA region, but not the EDB region, into FN1 mRNA in a statistically significant manner (Fig. 8E), suggesting that SPF45 enhances EDA inclusion into FN1 mature mRNA dependent upon reversible phosphorylation by MAP kinases.
Fig 8.
SPF45 overexpression increases cell adhesion, FN1 expression, and FN1 splicing in a phosphorylation-dependent and -independent manner. (A) SKOV-3 stable cell lines were plated onto 96-well cell culture dishes (plastic) coated with either poly-l-lysine, laminin, or fibronectin. After 30 min, unattached cells were removed, attached cells were fixed and stained with crystal violet, and adhesion was quantitated by measuring the absorbance at 550 nm. The results are a combination of results of three separate experiments with eight replicates per group in each experiment. (B) mRNA from the SKOV-3–SPF45 stable cell lines, subjected to real-time qRT-PCR using validated primers to FN1. The results are a combination of results of three independent experiments. The level of expression in the vector control cells was set to 1 in each experiment. (C) mRNA from the cells in panel B was isolated, equal amounts were run on an agarose gel, and Northern blot analysis was performed using a [32P]dCTP ssDNA probe to FN1. (D) Cellular lysates from the SKOV-3 stable cell lines were immunoblotted with anti-FN1 and anti-actin antibodies. (E) SPF45 overexpression enhances EDA inclusion into FN1 transcripts in a phosphorylation-dependent manner. mRNA was isolated from the cells in panel B and subjected to real-time qRT-PCR using validated primers to total FN1, the EDA region of FN1, or the EDB region of FN1. The expression of each variant in the vector control cells was set to 1. The results are the combination of results of three independent experiments.
DISCUSSION
Using an engineered ERK2 that can uniquely utilize analogs of ATP to phosphorylate substrates, we have demonstrated that the alternative mRNA splicing factor SPF45 is a novel associated substrate of ERK2, as well as p38α and JNK1. The use of the ERK2-QG/ATP analog system allows one to specifically label ERK2 substrates of ERK2-QG in a mixture of cellular proteins, and we (20) and others (9) have used it previously to identify other novel ERK2 substrates. SPF45 was found to specifically associate with ERK2, p38α, and JNK1 in coimmunoprecipitations from COS-1 cells, with enhanced basal association occurring with p38α and JNK1 compared to ERK. Endogenous SPF45 from HeLa nuclear extracts was pulled down with His-ERK2. Enhanced association of SPF45 with ERK2-K52R compared to ERK2 was likely due to substrate “trapping,” as ERK2-K52R could not phosphorylate SPF45, and this association was enhanced upon ERK2-K52R activation by MEK. Given the reported nuclear localization of SPF45 (50) and the nuclear localization of ERK2 upon activation (14, 34), we presume that ERK2 primarily binds to and phosphorylates SPF45 in the nucleus upon activation of ERK2, which is likely the reason we could not coimmunoprecipitate endogenous proteins. These localization differences may also account for the enhanced binding of p38α and JNK1 compared to ERK2.
The MAP kinase phosphorylation sites on SPF45 are evolutionarily conserved, suggesting an importance in regulating SPF45 function. Thr71 lies in the unstructured N terminus, and Ser222 is near the G-patch domain, a region that is likely involved in protein-protein and protein-RNA interactions (16). Both sites were previously identified as being phosphorylated in a global screen for phosphopeptides from HeLa cells in response to EGF stimulation (46), but to date no kinases have been identified that phosphorylate SPF45 or regulate its function. Our data demonstrate that these two MAP kinase phosphorylation sites on SPF45 are enhanced in response to oncogene activation upstream of ERK and by all three MAP kinases in response to extracellular stimuli. SPF45 has been demonstrated to be overexpressed in a number of tumor types (50), suggesting that SPF45 phosphorylation could be enhanced in these tumors when accompanied by a Ras or Raf hyperactivating mutation. As with ovarian tumors (50), we observed SPF45 overexpression in ovarian cancer cell lines and SPF45 hyperphosphorylation.
Little is known about how MAP kinases regulate alternative splicing, although a few direct splicing factor targets of MAP kinases have been identified. Weg-Remers et al. (62) first showed in primary mouse T cells that signaling through MEK/ERK stimulated inclusion of the variable 5 (v5) exon of CD44, the cellular receptor for hyaluronic acid. The same group later showed that this regulation occurred through direct phosphorylation of Sam68 (Src-associated protein in mitosis 68) by ERK (39). Phosphorylation of heterogenous nuclear ribonucleoprotein (hnRNP) A1, a negative regulator of alternative splicing, in a p38/Mnk1-dependent pathway in response to cellular stress was shown to drive hnRNP A1 cytoplasmic accumulation, thus preventing it from inhibiting alternative splicing of an adenovirus E1A pre-mRNA splicing reporter (60). Our data show additional novel regulation of alternative splicing factors by MAP kinases, with a variety of extracellular stimuli inducing SPF45 phosphorylation by each MAP kinase pathway. ERK and JNK phosphorylate SPF45 in response to H2O2, which suggests that induction of oxidative stress, as often occurs in cancer cells, stimulates phosphorylation of SPF45. Interestingly, while serum stimulation of cells induced strong ERK2 phosphorylation, it did not induce SPF45 phosphorylation, suggesting either that SPF45 phosphorylation is stimulus specific or that phosphorylation is short-lived in serum-stimulated cells due to the action of phosphatases that target SPF45.
The C-terminal UHM domain of SPF45 was shown to be required for SPF45 alternative splicing of fas pre-mRNA in a minigene assay (16), but it has not been reported how SPF45 splice site utilization is regulated by the N-terminal domain or by phosphorylation. ERK and p38 activation negatively regulated SPF45 alternative splicing of Δfas pre-mRNA, the mechanism of which is unknown, as is whether a similar effect would be seen toward other SPF45 splicing targets. Interestingly, mutation of Thr71 and Ser222 to aspartate, which can sometimes mimic phosphorylation, increased basal SPF45 alternative splice site utilization in Δfas pre-mRNA compared to wild-type SPF45, suggesting that in some instances a phosphorylated residue is required. The idea that this may not be the case for all targets of SPF45 is based on our results with FN1 EDA splicing, cell adhesion, Elf-3 and ErbB2 expression, and proliferation. In addition, it has been shown that reversible phosphorylation can be required for efficient splicing, as phosphorylation enhances assembly of spliceosome components, but dephosphorylation is required for the splicing reaction to occur (49, 57). Our results suggest that this may be context specific in regard to the role of SPF45 phosphorylation in different splicing events.
Twenty different splice variants of the extracellular matrix glycoprotein fibronectin have been reported (63). The EDA region of fibronectin is variably expressed in cellular fibronectin (cFN), with inclusion in embryonic tissue, wound healing, and fibrotic liver, ovarian granulosa tumors and little inclusion in normal adult tissues (5). The inclusion of the EDA region has been shown to promote cell proliferation, normal life span (44), and normal brain function (13). Recent work by White et al. (64) demonstrated that transforming growth factor beta (TGFβ) induction of Akt signaling and subsequent phosphorylation of SF2/ASF, the major splicing regulator of the EDA region (17), enhances EDA inclusion in fibroblasts. However, Pelisch et al. (47) have also demonstrated that inhibition of ERK with the specific inhibitor PD098059 inhibits inclusion of the EDA region into fibronectin transcripts. Our data are in agreement with this, suggesting that MAP kinase signaling regulates EDA inclusion through reversible phosphorylation of SPF45. We cannot rule out the possibility that SPF45 stable overexpression regulates the downstream expression of another splicing factor that upregulates EDA inclusion; however, changes in expression of such a factor would be regulated by SPF45 phosphorylation on Thr71 and Ser222.
We observed a significant increase in fibronectin expression upon stable overexpression of SPF45, independent of the MAP kinase phosphorylation sites on SPF45. Fibronectin and beta-1 integrins have been shown to be important in formation of multicellular spheroids in ovarian cancer cell lines (10). In addition, fibronectin overexpression, accompanied with overexpression of several other genes associated with the transforming growth factor beta fibrotic response, enhances the compactness of ovarian cancer multicellular spheroids, enhancing their invasive phenotype (56). These data suggest that SPF45-induced fibronectin expression could enhance multicellular spheroid formation. Multicellular spheroids of cancer cells often have intrinsically higher drug resistance properties than the same cells grown in culture (18), and ovarian cancer is characterized by peritoneal ascites containing multicellular spheroids, which serve as a reservoir of drug-resistant and metastatic cells. SPF45 overexpression has been reported to induce multidrug resistance in both A2780 ovarian cancer (48) and HeLa cervical cancer (50) cell lines grown in monolayers, and the effect in spheroids is unknown. Therefore, SPF45 upregulation of fibronectin expression may have a more pronounced effect in spheroids and, indeed, we have observed a significant overexpression of SPF45 in primary ovarian cancer cells from patient ascites (Y. Liu, unpublished data).
Additionally, our data demonstrate that SPF45 overexpression in ovarian cancer cells strongly decreases cell proliferation and Elf-3 and ErbB2 expression and that these effects were dependent upon the MAP kinase phosphorylation sites on SPF45. Interestingly, the double aspartate mutant of SPF45 behaved like wild-type SPF45, again suggesting that the ability to act in a phospho-mimetic fashion is context specific. Phosphorylation of these sites likely regulates protein-protein interactions within the spliceosome or protein-RNA interactions. ErbB2 protein has been demonstrated to be overexpressed and to correlate with poor prognosis in a number of cancer types, including breast (61) and ovarian (4) cancer. It is interesting that SPF45 is overexpressed in ovarian tumors (50) and cancer cell lines but its overexpression strongly inhibits ErbB2 expression. Transcriptional control of the erbB2 gene has been shown to involve mainly two families of transcription factors. In particular, Ets transcription factors have been shown to regulate erbB2 transcription, particularly by Ets family members PEA3, ESX/Elf-3, and Elf-1 (3, 11, 52), and we observed a decrease in Elf3 protein expression that mirrored the effects on ErbB2 and proliferation, suggesting that SPF45 action downregulates proliferation and ErbB2 expression through an unknown mechanism involving Elf3 downregulation.
In conclusion, we have identified a novel connection between extracellular signals and alternative mRNA splicing and have shown that SPF45 is the first alternative mRNA splicing factor to be targeted by multiple MAP kinase pathways in response to extracellular cues. In addition, we have identified novel biological effects of SPF45 overexpression in cancer cells and have demonstrated that they are regulated in a MAP kinase phosphorylation site-dependent manner.
ACKNOWLEDGMENTS
We thank Michael Weber for ERK antibody, Nellie Auersperg for IOSE cells, Runzhao Li for ES-2 cells, and Roger Davis for MKK3, MKK4, p38, and JNK plasmids. We thank Carola Neumann, Steve Rosenzweig, Dennis Watson, Ken Tew, and Brian Toole for helpful discussions. We also thank Warren Davis for equipment use and experimental expertise.
This work was supported by grants 1R01CA131200 from the National Cancer Institute/National Institutes of Health and W81XWH-07-1-0691 from the Department of Defense, both to S.T.E.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Al-Ayoubi A, Tarcsafalvi A, Zheng H, Sakati W, Eblen ST. 2008. ERK activation and nuclear signaling induced by the loss of cell/matrix adhesion stimulates anchorage-independent growth of ovarian cancer cells. J. Cell Biochem. 105:875–884 [DOI] [PubMed] [Google Scholar]
- 2. Aravind L, Koonin EV. 1999. G-patch: a new conserved domain in eukaryotic RNA-processing proteins and type D retroviral polyproteins. Trends Biochem. Sci. 24:342–344 [DOI] [PubMed] [Google Scholar]
- 3. Benz CC, et al. 1997. HER2/Neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene 15:1513–1525 [DOI] [PubMed] [Google Scholar]
- 4. Berchuck A, et al. 1990. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 50:4087–4091 [PubMed] [Google Scholar]
- 5. Blaustein M, Pelisch F, Srebrow A. 2007. Signals, pathways and splicing regulation. Int. J. Biochem. Cell Biol. 39:2031–2048 [DOI] [PubMed] [Google Scholar]
- 6. Blencowe BJ. 2006. Alternative splicing: new insights from global analyses. Cell 126:37–47 [DOI] [PubMed] [Google Scholar]
- 7. Boyle WJ, van der Geer P, Hunter T. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110–149 [DOI] [PubMed] [Google Scholar]
- 8. Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR. 1997. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J. Cell Biol. 138:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlson SM, et al. Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci. Signal. 4:rs11 doi:10.1126/scisignal.2002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey RC, et al. 2001. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 159:2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang CH, et al. 1997. ESX: a structurally unique Ets overexpressed early during human breast tumorigenesis. Oncogene 14:1617–1622 [DOI] [PubMed] [Google Scholar]
- 12. Chaouki AS, Salz HK. 2006. Drosophila SPF45: a bifunctional protein with roles in both splicing and DNA repair. PLoS Genet. 2:e178 doi:10.1371/journal.pgen.0020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chauhan AK, Moretti FA, Iaconcig A, Baralle FE, Muro AF. 2005. Impaired motor coordination in mice lacking the EDA exon of the fibronectin gene. Behav. Brain Res. 161:31–38 [DOI] [PubMed] [Google Scholar]
- 14. Chen RH, Tung R, Abate C, Blenis J. 1993. Cytoplasmic to nuclear signal transduction by mitogen-activated protein kinase and 90 kDa ribosomal S6 kinase. Biochem. Soc. Trans. 21:895–900 [DOI] [PubMed] [Google Scholar]
- 15. Cheng J, et al. 1994. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263:1759–1762 [DOI] [PubMed] [Google Scholar]
- 16. Corsini L, et al. 2007. U2AF-homology motif interactions are required for alternative splicing regulation by SPF45. Nat. Struct. Mol. Biol. 14:620–629 [DOI] [PubMed] [Google Scholar]
- 17. Cramer P, et al. 1999. Coupling of transcription with alternative splicing: RNA pol II promoters modulate SF2/ASF and 9G8 effects on an exonic splicing enhancer. Mol. Cell 4:251–258 [DOI] [PubMed] [Google Scholar]
- 18. Desoize B, Jardillier J. 2000. Multicellular resistance: a paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 36:193–207 [DOI] [PubMed] [Google Scholar]
- 19. Eblen ST, Catling AD, Assanah MC, Weber MJ. 2001. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol. Cell. Biol. 21:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eblen ST, et al. 2003. Identification of novel ERK2 substrates through use of an engineered kinase and ATP analogs. J. Biol. Chem. 278:14926–14935 [DOI] [PubMed] [Google Scholar]
- 21. Eblen ST, Kumar NV, Weber MJ. 2007. Using genetically engineered kinases to screen for novel protein kinase substrates: generation of [{gamma}-32P]ATP analog from ADP analog. CSH Protoc. 2007:pdb.prot4637. doi:10.1101/pdb.prot4637 [DOI] [PubMed] [Google Scholar]
- 22. Eblen ST, Kumar NV, Weber MJ. 2007. Using genetically engineered kinases to screen for novel protein kinase substrates: phosphorylation of kinase-associated substrates. CSH Protoc. 2007:pdb.prot4638. doi:10.1101/pdb.prot4638 [DOI] [PubMed] [Google Scholar]
- 23. Eblen ST, Kumar NV, Weber MJ. 2007. Using genetically engineered kinases to screen for novel protein kinase substrates: phosphorylation of substrates in cell lysates with exogenous kinase. CSH Protoc. 2007:pdb.prot4639. doi:10.1101/pdb.prot4639 [DOI] [PubMed] [Google Scholar]
- 24. Eblen ST, et al. 2004. Mitogen-activated protein kinase feedback phosphorylation regulates MEK1 complex formation and activation during cellular adhesion. Mol. Cell. Biol. 24:2308–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez-Medarde A, Santos E. 2011. Ras in cancer and developmental diseases. Genes Cancer 2:344–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frenal K, et al. 2006. Structural and functional characterization of the TgDRE multidomain protein, a DNA repair enzyme from Toxoplasma gondii. Biochemistry 45:4867–4874 [DOI] [PubMed] [Google Scholar]
- 27. Hanamura A, Caceres JF, Mayeda A, Franza BR, Jr, Krainer AR. 1998. Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. RNA 4:430–444 [PMC free article] [PubMed] [Google Scholar]
- 28. Hastings ML, Krainer AR. 2001. Functions of SR proteins in the U12-dependent AT-AC pre-mRNA splicing pathway. RNA 7:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hastings ML, Krainer AR. 2001. Pre-mRNA splicing in the new millennium. Curr. Opin. Cell Biol. 13:302–309 [DOI] [PubMed] [Google Scholar]
- 30. Izquierdo JM, et al. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 19:475–484 [DOI] [PubMed] [Google Scholar]
- 31. Khokhlatchev A, et al. 1997. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J. Biol. Chem. 272:11057–11062 [DOI] [PubMed] [Google Scholar]
- 32. Kumar NV, Eblen ST, Weber MJ. 2004. Identifying specific kinase substrates through engineered kinases and ATP analogs. Methods 32:389–397 [DOI] [PubMed] [Google Scholar]
- 33. Lallena MJ, Chalmers KJ, Llamazares S, Lamond AI, Valcarcel J. 2002. Splicing regulation at the second catalytic step by Sex-lethal involves 3′ splice site recognition by SPF45. Cell 109:285–296 [DOI] [PubMed] [Google Scholar]
- 34. Lenormand P, et al. 1993. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 122:1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis TS, et al. 2000. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol. Cell 6:1343–1354 [DOI] [PubMed] [Google Scholar]
- 36. Lewis TS, Shapiro PS, Ahn NG. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49–139 [DOI] [PubMed] [Google Scholar]
- 37. Manley JL, Tacke R. 1996. SR proteins and splicing control. Genes Dev. 10:1569–1579 [DOI] [PubMed] [Google Scholar]
- 38. Matlin AJ, Clark F, Smith CW. 2005. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6:386–398 [DOI] [PubMed] [Google Scholar]
- 39. Matter N, Herrlich P, Konig H. 2002. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature 420:691–695 [DOI] [PubMed] [Google Scholar]
- 40. Maurer G, Tarkowski B, Baccarini M. 2011. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene 30:3477–3488 [DOI] [PubMed] [Google Scholar]
- 41. Mayeda A, Munroe SH, Caceres JF, Krainer AR. 1994. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 13:5483–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Modrek B, Resch A, Grasso C, Lee C. 2001. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 29:2850–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mortz E, Krogh TN, Vorum H, Gorg A. 2001. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics 1:1359–1363 [DOI] [PubMed] [Google Scholar]
- 44. Muro AF, et al. 2008. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neubauer G, et al. 1998. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat. Genet. 20:46–50 [DOI] [PubMed] [Google Scholar]
- 46. Olsen JV, et al. 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648 [DOI] [PubMed] [Google Scholar]
- 47. Pelisch F, Blaustein M, Kornblihtt AR, Srebrow A. 2005. Cross-talk between signaling pathways regulates alternative splicing: a novel role for JNK. J. Biol. Chem. 280:25461–25469 [DOI] [PubMed] [Google Scholar]
- 48. Perry WL, 3rd, et al. 2005. Human splicing factor SPF45 (RBM17) confers broad multidrug resistance to anticancer drugs when overexpressed—a phenotype partially reversed by selective estrogen receptor modulators. Cancer Res. 65:6593–6600 [DOI] [PubMed] [Google Scholar]
- 49. Prasad J, Colwill K, Pawson T, Manley JL. 1999. The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing. Mol. Cell. Biol. 19:6991–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sampath J, et al. 2003. Human SPF45, a splicing factor, has limited expression in normal tissues, is overexpressed in many tumors, and can confer a multidrug-resistant phenotype to cells. Am. J. Pathol. 163:1781–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schaeffer HJ, Weber MJ. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scott GK, et al. 2000. Ets regulation of the erbB2 promoter. Oncogene 19:6490–6502 [DOI] [PubMed] [Google Scholar]
- 53. Shah K, Liu Y, Deirmengian C, Shokat KM. 1997. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc. Natl. Acad. Sci. U. S. A. 94:3565–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shepherd TG, Kockeritz L, Szrajber MR, Muller WJ, Hassell JA. 2001. The pea3 subfamily ets genes are required for HER2/Neu-mediated mammary oncogenesis. Curr. Biol. 11:1739–1748 [DOI] [PubMed] [Google Scholar]
- 55. Silverman EJ, et al. 2004. Interaction between a G-patch protein and a spliceosomal DEXD/H-box ATPase that is critical for splicing. Mol. Cell. Biol. 24:10101–10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sodek KL, Ringuette MJ, Brown TJ. 2009. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 124:2060–2070 [DOI] [PubMed] [Google Scholar]
- 57. Stamm S. 2008. Regulation of alternative splicing by reversible protein phosphorylation. J. Biol. Chem. 283:1223–1227 [DOI] [PubMed] [Google Scholar]
- 58. Svec M, Bauerova H, Pichova I, Konvalinka J, Strisovsky K. 2004. Proteinases of betaretroviruses bind single-stranded nucleic acids through a novel interaction module, the G-patch. FEBS Lett. 576:271–276 [DOI] [PubMed] [Google Scholar]
- 59. Tanoue T, Adachi M, Moriguchi T, Nishida E. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2:110–116 [DOI] [PubMed] [Google Scholar]
- 60. van der Houven van Oordt W, et al. 2000. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varley JM, Swallow JE, Brammar WJ, Whittaker JL, Walker RA. 1987. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene 1:423–430 [PubMed] [Google Scholar]
- 62. Weg-Remers S, Ponta H, Herrlich P, Konig H. 2001. Regulation of alternative pre-mRNA splicing by the ERK MAP-kinase pathway. EMBO J. 20:4194–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White ES, Baralle FE, Muro AF. 2008. New insights into form and function of fibronectin splice variants. J. Pathol. 216:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. White ES, et al. 2010. Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp. Cell Res. 316:2644–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Will CL, Luhrmann R. 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13:290–301 [DOI] [PubMed] [Google Scholar]
- 66. Xing Y, Lee C. 2007. Relating alternative splicing to proteome complexity and genome evolution. Adv. Exp. Med. Biol. 623:36–49 [DOI] [PubMed] [Google Scholar]