Abstract
Efficient cell corpse clearance is critical for health in organisms. Apoptotic cells displaying phosphatidylserine (PS) are recognized by engulfment receptors and ingested through two conserved pathways. In one pathway, engulfment receptor brain-specific angiogenesis inhibitor 1 (BAI-1) or integrin functions upstream of ELMO/DOCK180 and activate the small GTPase Rac1. In the other pathway, engulfment receptor CED-1 or stabilin-2 acts in concert with the adaptor protein GULP to activate Rac1. Stabilin-2, a PS receptor, facilitates phagocytosis of apoptotic cells and mediates the production of anti-inflammatory cytokines. Here, we propose that the stabilin-2 extracellular domain consisting of integrin-binding fasciclin 1 (FAS1) domains coordinates the activities of the two phagocytic pathways via direct interactions with integrin. Interactions between stabilin-2 and integrin were determined using biochemical assays, including coimmunoprecipitation and fluorescence resonance energy transfer (FRET). These interactions appear to have functional relevance, since knockdown of endogenous αvβ5 expression or treatment with a function-blocking αvβ5 antibody significantly decreased stabilin-2-mediated phagocytosis in the absence of soluble factors. Our data collectively suggest that the engulfment receptors of the two phagocytic pathways communicate with each other to orchestrate engulfment of damaged erythrocytes. Coordinated phagocytic signaling would be advantageous for physiological and pathological circumstances that require rapid clearance of abnormal (apoptotic or aged) cells.
INTRODUCTION
More than 1 million cells are recycled per second in the human body. The unwanted cells, including excess cells generated in tissues as part of normal development, aged cells, and damaged cells that arise from disease or infection, undergo apoptosis and are swiftly and safely removed by phagocytes (34). Efficient clearance of apoptotic cells is critical for cellular homeostasis, resolution of inflammation, and the development of multicellular organisms (8, 33). Moreover, inefficient engulfment of apoptotic cells is related to several diseases, including atherosclerosis, chronic inflammation, and autoimmunity (7, 23, 37, 40).
Clearance of apoptotic cells by phagocytes is a complex but highly orchestrated and efficient event that can be divided into several steps (39). First, apoptotic cells release “find-me” signals to attract phagocytes to the site of death within tissues. When phagocytes are in close proximity to apoptotic cells, specific recognition is mediated by interactions between engulfment receptors on phagocytes and “eat-me” signals, such as phosphatidylserine (PS), on the apoptotic cell surface. After subsequent internalization of the corpse, the phagosome undergoes maturation steps, eventually leading to its degradation (19). The final step involves the release of anti-inflammatory cytokines to mediate the immunologically silent removal of apoptotic cells (13, 34).
Specific recognition via PS, which is exposed on early apoptotic cells, is a decisive step for the onset of downstream signaling leading the engulfment process (4). Many receptors function in the tethering of apoptotic cells through two primary mechanisms, specifically, binding either directly to PS or indirectly via soluble bridging molecules. Direct-binding PS receptors include brain-specific angiogenesis inhibitor 1 (BAI-1) (27), T-cell immunoglobulin and mucin domain-containing protein 4 (Tim4) (20, 22), and the atypical epidermal growth factor (EGF) motif containing stabilin-1 and -2 (28, 30). Soluble factors, such as milk fat globule-EGF factor 8 (MFG-E8) and growth arrest-specific gene 6 (Gas6), bind to PS on apoptotic cells and simultaneously engage receptors on phagocytes, such as integrin αvβ3 and the Tyro-3-Axl-Mer (TAM) family of receptors, respectively (3, 11, 36). Other membrane proteins, CD36 and CD68, are additionally capable of binding PS (16, 35). A recent study showed that the receptor for advanced glycation end product (RAGE) binds PS and assists in the clearance of apoptotic cells (12). The answers to the reasons why many receptors and bridging molecules are needed and how different sets of receptors orchestrate phagocytic signals are open to speculation, and these issues require further investigation. The general agreement is that not all receptors are expressed on all phagocytes, and therefore, multiple modes of recognition and coordinated actions of engulfment receptors are involved to contend with different physiological circumstances (14, 33, 34).
While the mechanisms by which numerous cell surface PS receptors activate phagocytic signaling are not fully understood, two conserved signaling pathways have been identified to date. In one pathway, engulfment receptors, such as BAI-1 or integrin αvβ5/αvβ3, function upstream of the CrkII/DOCK180/ELMO complex and, in turn, activate the small GTPase Rac1 (2, 27). Another pathway engages the engulfment receptor CED-1/MEGF10 or stabilin-2, leading to CED-10/Rac1 activation through the adaptor protein CED-6/GULP (18, 19). Recent genetic studies on Caenorhabditis elegans revealed that integrin α functions as an upstream receptor of the CrkII/DOCK180/ELMO complex, supporting the finding that the integrin and its downstream signaling pathway are conserved in C. elegans and mammals (15).
Stabilin-2, one of the PS receptors, mediates rapid cell corpse engulfment (28). The protein recognizes PS through EGF-like domain repeats (EGFrps) in the extracellular region and activates Rac1 via interactions with GULP through the NPXY motif in the cytoplasmic tail (31, 32). In vivo knockdown of stabilin-1 and stabilin-2 confirmed their roles in sequestrating aged red blood cells (RBCs) in the hepatic sinusoid and eventually eliminating aged cells in a mouse model (21). In view of the unique structure of the stabilin-2 extracellular region, which contains both the PS-binding EGF repeat and integrin-binding fasciclin 1 (FAS1) domains, we hypothesized that stabilin-2 communicates with other conserved phagocytic receptors, such as integrin, and coordinates the two phagocytic pathways for rapid and efficient uptake of apoptotic cells. Interestingly, stablin-2 is expressed only in vertebrates, and its interactions with integrin have advantages for complex circumstances requiring the efficient clearance of abnormal (apoptotic or aged) cells.
Here, we present preliminary evidence supporting the coordination of the two conserved phagocytic pathways via direct interactions of the engulfment receptors stabilin-2 and integrin αvβ5. Our findings provide steps toward clarifying fundamental issues, such as why so many receptors are involved and whether they work together to control uptake capacity. The coordinated actions of stablin-2 and conserved integrin may be an evolutionarily adapted mechanism in vertebrates.
MATERIALS AND METHODS
Reagents and antibodies.
A rabbit polyclonal antibody (Ab) against integrin β5 was obtained from Abcam for Western blotting. A mouse monoclonal antibody against integrin αvβ5 was purchased from Millipore (clone P1F6) and used for function blocking and fluorescence-activated cell sorter (FACS) analysis. Polyclonal antibodies against integrins αv and β3 (Cell Signaling) were used for Western blotting, and monoclonal αvβ3 Ab (clone 23C6; Millipore) was used for function blocking and FACS analysis. A monoclonal antibody (MAb) recognizing the FLAG epitope (clone M2) was obtained from Sigma. Anti-Myc antibody (clone 9E10) was purchased from Santa Cruz Biotechnology. A monoclonal antibody against stabilin-2 (5G3) was obtained as previously described (28). Protein A-Sepharose beads were obtained from Upstate. Integrin αvβ5 was purchased from Millipore Biotech.
Plasmids.
The cDNA encoding full-length human stabilin-2 was cloned into pcDNA3.1 with a FLAG tag (pcDNA-Stab2-FLAG). To generate a C-terminal deletion mutant of stabilin-2 (pcDNA-Stab2ΔC-FLAG), the pcDNA-Stab2-FLAG vector was digested with XhoI and PmeI to remove a cDNA fragment containing the C-terminal region, and then the cDNA fragment containing the truncated C-terminal region was amplified by PCR and cloned into the XhoI-PmeI-digested pcDNA-Stab2-FLAG vector. The Stab2-Myc- and Stab2-U4-Myc-expressing vectors have been described previously (32). To generate an expression vector for mutant stabilin-2, in which the extracellular domain was replaced with the fourth EGF-like domain repeat (EGFrp), fragments of stabilin-2 cDNA encoding signal peptide, the fourth EGFrp, and the transmembrane and cytoplasmic region were amplified from full-length stabilin-2 cDNA by PCR, cloned into the pcDNA3.1(−)/Myc-His vector (Invitrogen), and then designated pcDNA-Stab2-E4. To generate an expression vector for mutant stabilin-2, in which the extracellular domain was replaced with the seventh FAS1 domain, the pcDNA-Stab2-Myc vector was digested with BamHI and XhoI to remove the cDNA fragment corresponding to the extracellular region except for the seventh FAS1 domain, and then the N-terminal sequence containing the signal peptide region was amplified by PCR and cloned into the BamHI-XhoI-digested pcDNA-Stab2-Myc vector. All PCRs were performed using Pfu DNA polymerase (Promega). All plasmid constructs were verified by DNA sequencing (Bionics, Seoul, South Korea).
Cell cultures and stable transfection.
L cells that stably expressed stabilin-2 (Stab-2/L cells) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), as previously described (1). To generate L cells expressing Stab2ΔC, the cells were transfected with the pcDNA-Stab2ΔC vector and selected in G418 (400 g/ml). Individual G418-resistant colonies were isolated after 2 weeks of culture. The final clones were designated Stab2ΔC/L cells. 293FT cells were obtained from Invitrogen and maintained in DMEM (high glucose) supplemented with 10% FBS and the appropriate antibiotics. Human monocytes were obtained from healthy donors, isolated using monocyte isolation kit II systems (Miltenyi Biotec), and then cultured in an X-Vivo 10 system (BioWhittaker) containing 10% human serum. The differentiated macrophages, human monocyte-derived macrophages (HMDMs), were utilized at 7 to 10 days of culture.
FACS analysis.
For the analysis of surface staining, the cells were incubated with 1 μg of appropriate monoclonal antibodies on ice for 30 min with gentle agitation every 5 min. Next, the cells were washed twice, resuspended in phosphate-buffered saline (PBS), and incubated with 4 μg of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) antibody/ml for 45 min on ice. After three washes with PBS, the cells were resuspended in PBS and then analyzed via flow cytometry on a FACSCalibur cytometer (BD Bioscience).
RNA isolation and reverse transcription-PCR.
Total RNA was extracted from cells using TRIcol reagent in accordance with the manufacturer's instructions (Invitrogen), and the quantity and quality of the isolated RNA were then determined by measuring the absorbance at 260 and 280 nm. The reverse transcription reaction was performed in a mixture with a final volume of 30 μl that contained 2 μg of total RNA, 200 ng of oligo(dT)15 primer, 1× reverse transcription buffer, 0.5 mM deoxynucleotide triphosphate mixture, RNasin recombinant RNase inhibitor, and 200 units of Moloney murine leukemia virus reverse transcriptase (Promega). After incubation for 50 min at 42°C, the reverse transcription reaction was terminated by heating the reaction mixture for 15 min at 70°C. The newly synthesized cDNA was then amplified by PCR in a reaction mixture comprised of 2 μl of cDNA templates, 1.5 mM MgCl2, 1 unit of Taq polymerase, and 0.3 μM mouse MFG-E8 primers (sense, 5′-TGT TCA ACC CGA CTC TGG AG-3′; antisense, 5′-CTG CTG GAG GCT GAC ATC TG-3′) or mouse Gas6 primers (sense, 5′-ATC CAG GAG ACA GTC AAG GC-3′; antisense, 5′-CCA GGA CCA CCA ACT GCT TC-3′). Mouse β-actin primers 5′-TCA CCC ACA CTG TGC CCA TCT ACG A-3′ (sense) and 5′-GGA TGC CAC AGG ATT CCA TAC CCA-3′ (antisense) were used as positive and internal controls, respectively. The amplification was performed under the following conditions: 94°C for 2 min (initial denaturation); 94°C for 30 s, 58°C for 30 s, and 72°C for 45 s (35 cycles for MFG-E8 and Gas6 and 25 cycles for beta-actin); and 72°C for 7 min (final extension).
Binding and phagocytosis of aged RBCs.
Aged RBCs were prepared via the incubation of cells in PBS (20% hematocrit) at 37°C, as previously described (28). Aged RBCs were added to the transfected L cells and then incubated for 1 h to assess binding and engulfment. To get rid of soluble factors in serum, the Stab-2 cells were washed twice with PBS and incubated with HEPES buffer A (20 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM CaCl2, 0.1% bovine serum albumin [BSA]). To test inhibitory effects, monoclonal antibodies or recombinant stabilin-2 proteins were preincubated with Stab-2 cells for 30 min at 37°C in HEPES buffer A. After the unbound RBCs were washed away, the uningested RBCs were lysed via addition of deionized H2O for 10 s, which was followed by immediate replacement with PBS as described previously (10). The cells were then fixed with methanol and stained by using a DiffQuick staining kit (IMEB, Inc.). The binding and engulfment of aged RBCs was then quantified via light microscopy. Percent phagocytosis was determined as the percentage of phagocytes that were positive for engulfment, and the phagocytic index was determined as the average number of ingested aged RBCs per cell as described previously (32). At least 100 cells were scored per well, and all experiments were repeated at least three times. In certain experiments, the phagocytosis assay was performed in the presence of MAb 5G3 (10 to 20 μg/ml), isotype controls (immunoglobulin G1, 20 μg/ml), or MAb to integrin αvβ3 or αvβ5 (5 to 10 μg/ml).
Coimmunoprecipitation assays.
293FT cells were transiently transfected with integrin β5- and stabilin-2-expressing vectors. The cells were then lysed using immunoprecipitation (IP) buffer (50 mM Tris-HCl [pH 7.4], 1% Triton X-100, 150 mM NaCl, 10 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitors). The lysates were then cleared by centrifugation at 10,000 × g for 10 min at 4°C, which was followed by incubation with anti-Myc, anti-FLAG, or anti-integrin β5 antibody at 4°C overnight. The immunoprecipitates were recovered on protein A-Sepharose beads. After washes four times with IP buffer, the beads were then resuspended in SDS-PAGE sample buffer and boiled for 10 min. Bound proteins were then analyzed by immunoblotting using anti-FLAG, anti-Myc, or anti-integrin β5 antibody.
FRET analysis.
Confocal fluorescence resonance energy transfer (FRET) images were obtained as previously described (31). Briefly, COS7 cells were cultured on a collagen-coated 35-mm glass-base dish (Asahi Techno Glass, Toyko, Japan) and then transfected with pcDNA-stabilin-2-superenhanced cyan fluorescent protein (seCFP) and pcDNA-Venus-integrin β5. Images were obtained using a Leica DM IRB inverted microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Cascade 512B (electron-multiplying charge-coupled-device) camera (Roper Scientific, Trenton, NJ), a CSU-10 spinning Nipkow disk confocal unit (Yokogawa Electric, Toyko, Japan), an emission filter wheel (MAC5000; Lud1 Electronic Products, Hawthorne, NY), and a krypton/argon laser. All systems were controlled using the MetaMorph software program (Universal Imaging, Downingtown, PA). Fluorescent images were acquired sequentially through seGFP, Venus, and FRET filter channels. Images were acquired by using a 2-by-2 binning mode and a 200-ms exposure time. FRET analysis was carried out as described previously (38). Corrected FRET (FRETC) was calculated by using the following formula: FRET − (0.16 × seCFP) − (0.2 × Venus). FRETC images were displayed in the pseudocolor mode.
Binding assay.
In in vitro binding assays, integrin αvβ5 or BSA was incubated with various concentrations of each protein, which was diluted in a binding buffer containing 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20, 1 mM MnCl2, 1 mM MgCl2, and 0.1 mM CaCl2 for 1 h at room temperature, and nonbound proteins were removed by washing with PBS. The total binding of each protein for integrin αvβ5 was detected by His probe-horseradish peroxidase antibody and quantified by measuring horseradish peroxidase activity. Enzyme activity was stopped by adding 8 N H2SO4 buffer and measured using a Tecan model Sunrise basic microplate reader at 490 nm. Specific binding of each protein for αvβ5 integrin was calculated as the difference between total and nonspecific binding, which was measured in every experiment by determining the binding of each protein to BSA-coated wells. All data represent the average of duplicate measurements. All assays were repeated at least three times, yielding identical results.
Inhibition of integrin β5, GULP, and DOCK180.
Small interfering RNA (siRNA) was synthesized by Invitrogen. The target sequences of β5 were 5′-AAG CAA GGC AAG CGA UGG AUA GUC-3′ and 5′-UAC AGC CGC AUG UGC AAU UGU AGG C-3′. The target sequences of DOCK180 were 5′-ACG UGA ACC GGA GUC ACC UUC GAU U-3′ and 5′-AAU CGA AGG UGA CUC CGG UUC ACG U-3′. GULP siRNA was prepared as described previously (31). Stab-2/L cells were transfected with the indicated siRNA for 24 h in the presence of 10% FBS in DMEM using Lipofectamine 2000 following the manufacturer's instructions (Invitrogen). Mock transfection was done in parallel using the Stealth RNA interference negative control with appropriate GC contents (Invitrogen). After 48 h, the cells were subjected to the phagocytosis assays. The inhibition of protein expression was determined by Western blot analysis.
Statistical analysis.
Statistical significance was assessed via analysis of variance (ANOVA). A P value of <0.05 was considered statistically significant.
RESULTS
Stabilin-2 conveys phagocytic signals through both GULP-dependent and -independent mechanisms.
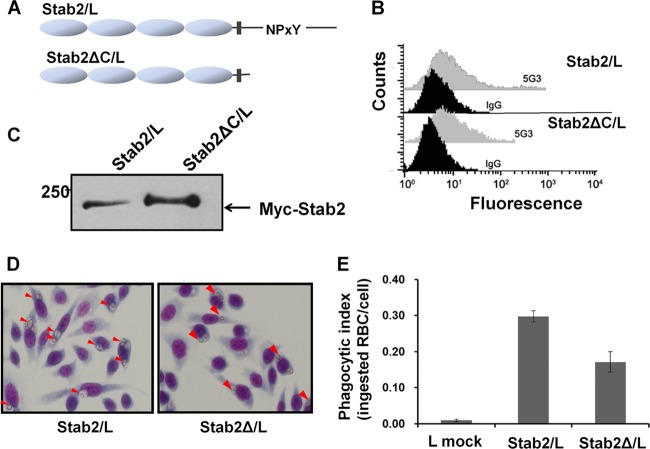
We recently demonstrated that recognition of apoptotic cells by stabilin-2 leads to activation of the GULP-dependent phagocytic pathway (31). Direct physical interactions between the phosphotyrosine-binding (PTB) domain of GULP and the NPXY motif of the stabilin-2 cytoplasmic tail have been reported. Overexpression of GULP enhances uptake of apoptotic cells, whereas knockdown of endogenous GULP expression decreases stabilin-2-mediated phagocytosis. Moreover, the TAT-fused PTB domain of GULP acts in a dominant-negative manner upon transduction into Stab-2/L cells, resulting in impaired engulfment of aged RBCs in stabilin-2-expressing cells. However, the GULP-dependent signaling pathway of stabilin-2 poses a conundrum, in that knockdown of GULP expression or overexpression of dominant-negative TAT-PTB is not sufficient to eliminate total phagocytic activity mediated by stabilin-2. This finding could be explained by a limited transfection efficiency of GULP siRNA or a partial dominant-negative effect of TAT-PTB. To further elucidate this issue, we generated a cell line (Stab2ΔC/L) stably expressing NPXY-deleted stabilin-2 and examined its phagocytic activity (Fig. 1A). Stab2ΔC/L cells expressed truncated stabilin-2 on the cell surface to a similar extent as wild-type Stab-2/L cells, as evident from flow cytometry and the Western blots shown in Fig. 1B and C. Elimination of NPXY and consequent loss of binding to GULP were confirmed via sequence and coimmunoprecipitation analyses. We employed aged RBCs as target cells, since they do not bind to phagocytes without apoptotic signals and bound but not engulfed RBCs are easily eliminated via hypotonic lysis (10). Therefore, all aged RBCs present within Stab-2/L cells are presumably those that are engulfed after hypotonic lysis. Despite the lack of the NPXY motif and the consequent loss of GULP-dependent signaling, Stab2ΔC/L cells were capable of taking up aged RBCs, whereas mock-transfected cells (L cells) were not. Activity was up to 50% that of wild-type Stab-2/L cells (Fig. 1D and E), indicating that the extracellular region of stabilin-2 alone retains about half the phagocytic activity. This observation suggests that the extracellular region of stabilin-2 promotes phagocytic signaling in a GULP-independent manner, possibly by communicating with other receptors. Accordingly, we investigated the molecules participating in GULP-independent phagocytosis in stabilin-2-expressing cells and examined their interactions with the extracellular region of stabilin-2.
Fig 1.
Engulfment of aged RBCs by L cells expressing C-terminus-deleted stabilin-2 (Stab2ΔC/L). (A) Schematic diagram of Stab-2/L and Stab2ΔC/L. (B) Flow cytometry detection of stabilin-2 constructs on Stab-2/L or Stab2ΔC/L cells. Surface expression was detected by stabilin-2 monoclonal antibody (5G3) and compared with that for the mouse IgG control. (C) Total protein levels of Stab-2/L cells and Stab2ΔC/L cells. Total cell lysates were subjected on SDS-PAGE, and stabilin-2 expression was assessed by use of Myc antibody. (D) Aged RBCs were added to parent L/Mock, Stab-2/L, or Stab2ΔC/L cells and then incubated for 1 h to assess engulfment under serum-free conditions. After the unengulfed RBCs were washed away via hypotonic lysis, cells were stained with DiffQuick stain. The engulfment of aged RBCs was measured under a light microscope. (E) The phagocytic indexes were determined as the average number of ingested aged RBCs per cell (phagocytic index = number of ingested aged RBCs/total number of cells). The results are expressed as the mean ± SD from at least three experiments.
Integrin αvβ5 and its phagocytic signaling pathway are involved in stabilin-2-mediated phagocytosis.
The stabilin-2 extracellular region consists of four repeat units, each containing both PS-binding EGF repeat and integrin-binding FAS1 domains (32). FAS1 domains are present in cell adhesion molecules and reported to bind various integrin family members, including αvβ3 and αvβ5 (5). Earlier, we showed that stabilin-2 interacts with αMβ2 integrin through its FAS1 domains and is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium (17). Integrin receptors are conserved phagocytic receptors in a variety of species (from C. elegans to mammals), and αvβ3 and αvβ5 integrins transmit GULP-independent phagocytic signals, which, in turn, recruit the CrkII/DOCK180/ELMO complex and activate Rac1 for phagocytic activity (6).
To address the role of αvβ3 or αvβ5 integrin in stabilin-2-mediated phagocytosis, we initially examined whether these integrins are expressed on the surface of stabilin-2-expressing cells. Parental mouse fibroblast L cells barely express adhesion molecules such as cadherins (25). Thus, L cells were scattered as a single-cell suspension. Previously, we had been interested in the functional roles of stabilin-2 in cell-cell interaction; hence, we generated stabilin-2-overexpressing cell lines from L cells. Stab-2/L cells obtained PS-dependent phagocytic activity (28) and also formed clusters in a time-dependent manner, suggesting surface expression of adhesion receptors in stabilin-2-expressing cells (29). FACS analysis indicated expression of αvβ5 integrin but not αvβ3 integrin in stabilin-2-expressing cells (Stab-2/L). Neither β5 nor β3 integrin was expressed on the surfaces of mock-transfected cells (L) (Fig. 2A). Total protein levels of αv and β3 were not elevated upon stabilin-2 overexpression (Fig. 2B). Only β5 showed a slight but not significant increase. Truncated stabilin-2-expressing cells (Stab2ΔC/L) also expressed αvβ5 integrin on the cell surface.
Fig 2.
Specific expression of integrin αvβ5 in stabilin-2-expressing cells and inhibition of phagocytosis by blocking of the integrin signaling molecule FAK. (A) Surface expression of integrin αvβ3 or αvβ5 on L/Mock, Stab-2/L, or Stab2Δ2 cells. (B) Total protein expression of β5, αv, and β3 in L/Mock and Stab-2/L cells. Total cell lysates were subjected to SDS-PAGE, and expression of integrin subunits was monitored using corresponding antibodies. β-Actin (*) of each cell lysate was assessed as a control. (C) Expression of MFG-E8 and Gas6 monitored by reverse transcription-PCR analysis. Soluble factors MFG-E8 and Gas6 were not expressed in stabilin-2-expressing cells (L/Stab2), whereas Raw264.7 macrophages expressed both MFG-E8 and Gas6. β-Actin expression was measured as a control. (D) After pretreatment with FAK inhibitors, cells were incubated with HEPES-buffered medium (20 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM CaCl2, 0.2% BSA) containing aged RBCs plus PF228, PF271, or dimethyl sulfoxide for an additional 1 h at 37°C for phagocytosis assay as described above. NT, nontreated. The phagocytic indexes were determined as the average number of ingested aged RBCs per cell (phagocytic index = number of ingested aged RBCs/total number of cells). The results are expressed as the mean ± SD from at least three experiments. (E) Decreased FAK activity upon pretreatment of FAK inhibitors (PF228 or PF271). Cells were preincubated with medium containing PF228, PF271, or dimethyl sulfoxide (DMSO) over a range of concentrations for 1 h and lysed for immunoblot analysis. Phosphorylated Tyr397 (pTyr397) was detected by anti-FAK pY397 polyclonal antibody and compared with total FAK. (F) Representative images of engulfment of aged RBCs (red arrowheads) in Stab-2/L cells in the presence of FAK inhibitors (PF228 and PF271) and dimethyl sulfoxide as a control.
Since the β5 integrin subunit mediates activation of focal adhesion kinase (FAK) in the initialization step of phagocytosis and stimulates GULP-independent phagocytic signaling (9), we examined whether specific inhibitors of FAK interfere with stabilin-2-mediated phagocytosis. To eliminate the involvement of phagocytic activity of integrin through soluble factors in serum, we performed all subsequent experiments in the absence of serum. Furthermore, Stab-2/L cells did not express the soluble factor MFG-E8 or Gas6, while Raw264.7 macrophages did (Fig. 2C). This result confirmed that the medium did not contain soluble factors which might be secreted from Stab-2/L cells. Two different FAK inhibitors, PF228 and PF271, which inhibit phosphorylation of FAK Tyr397 reduced uptake of aged RBCs in stabilin-2-expressing cells in a dose-dependent manner (Fig. 2D to F). The putative integrin-binding domain of the stabilin-2 extracellular region, specific expression of αvβ5 in Stab-2/L cells, and impaired phagocytosis by FAK inhibitors led us to hypothesize that stabilin-2 communicates with another phagocytic receptor, αvβ5 integrin, and additionally transmits phagocytic signals through the GULP-independent pathway.
αvβ5 integrin participates in the phagocytic activity of stabilin-2-expressing cells, although soluble factors are not involved.
To determine whether the αvβ5 integrin is functionally related to phagocytosis of stabilin-2-expressing cells, expression of the β5 subunit was inhibited and engulfment of aged RBCs under serum-free conditions was monitored. The number of ingested aged RBCs was significantly but not completely diminished in β5 siRNA-transfected cells compared with that in control siRNA-transfected cells (Fig. 3A and B). Inhibitory effects upon knockdown of β5 expression were also observed in Stab2ΔC/L cells, suggesting that the extracellular region of stabilin-2 is functionally related to αvβ5 integrin. Although we conducted this experiment under serum-free conditions, it is possible that integrin β5 activates an unknown phagocytic pathway which is independent of stabilin-2 as well as soluble factors. To rule out this possibility, we examined whether functional blocking antibodies against stabilin-2 or β5 impede phagocytic activity (Fig. 3C). Pretreatment with antibodies before the addition of aged RBCs revealed significant inhibition of phagocytosis with the stabilin-2 monoclonal antibody (5G3) in dose-dependent manner. Increased amounts of Stab-2 Ab further inhibited phagocytosis under the serum-free condition, whereas the larger amount of αvβ5 monoclonal antibody partially blocked engulfment. The blocking activity of the integrin αvβ5 antibody was confirmed by testing its suppression of adherence of Stab-2/L cells to a vitronectin-coated plate (data not shown). Interestingly, a combination of antibodies against stabilin-2 and αvβ5 inhibited the uptake ability of stabilin-2-expressing cells to a greater extent than αvβ5 antibody but to the same extent as stabilin-2 antibody. The β3 antibody and corresponding IgG control had no inhibitory effects. This finding indicates that the monoclonal antibodies against two engulfing receptors, stabilin-2 and integrin αvβ5, block independent signaling pathways during phagocytosis (Fig. 3C) and suggests that even without soluble factors, integrin αvβ5 can exert phagocytosis by interaction with stabilin-2. Interactions between stabilin-2 and αvβ5 integrin may thus coordinate two independent phagocytic pathways (GULP dependent and GULP independent) for rapid and efficient uptake of apoptotic cells.
Fig 3.
Inhibition of phagocytosis via integrin αvβ5 knockdown. (A) siRNA-induced inhibition of integrin β5 subunit expression in Stab-2/L cells. The cells were transfected with siRNA or control (con) siRNA with similar GC contents. At 60 h after transfection, the suppression of integrin β5 subunit expression was confirmed by Western blotting. β-Actin (*) of each cell lysate was assessed as a control. (B) Inhibition of aged RBC engulfment by integrin β5 pretreatment. The phagocytic indexes were determined as indicated. The results are expressed as the mean ± SD from at least three experiments. **, P < 0.01 (ANOVA). (C) Stab-2/L cells were preincubated with the indicated amount of monoclonal antibody against stabilin-2 (5G3), β5, or isotype-matched control Ab for 1 h at 37°C before addition of aged RBCs. mIgG, mouse IgG. The phagocytic indexes were determined, and the results are expressed as the mean ± SD from at least three experiments.
Stabilin-2 interacts directly with αvβ5 integrin in the phagocytic cup region.
To examine whether stabilin-2 interacts with αvβ5 integrin, we transiently transfected CHO-K1 cells with FLAG–stabilin-2 and the β5 subunit and further examined whether stabilin-2 and αvβ5 integrin form a complex. As shown in Fig. 4A, FLAG–stabilin-2 coimmunoprecipitated with integrin β5 in the presence of an anti-β5 antibody. In a reverse experiment with a FLAG-tagged specific antibody, we observed immunoprecipitation of FLAG–stabilin-2 with integrin β5. Experiments conducted with FLAG–stabilin-2ΔC lacking the cytoplasmic tail disclosed that FLAG–stabilin-2ΔC forms a complex with integrin β5 (Fig. 4B), suggesting that the extracellular or transmembrane region of stabilin-2 is responsible for the interaction.
Fig 4.
Association of stabilin-2 and its cytoplasmic deletion mutant with integrin β5. (A) 293FT cells were transiently transfected with stabilin-2–FLAG- and/or integrin β5-expressing vector. IB, immunoblotting. (B) Stabilin2ΔC-FLAG- and/or integrin β5-expressing vector was transfected into 293FT cells. The lysates were subjected to immunoprecipitation (IP) using either anti-integrin β5 (left) or anti-FLAG antibody (right). The immunoprecipitates were then analyzed by immunoblot analysis using anti-integrin β5 and anti-FLAG antibodies, respectively. Expression of stabilin-2 and integrin β5 proteins in total cell lysates was examined by immunoblot analysis (TCL, total cell lysate). A representative result of three independent experiments is shown. (C) Interaction between stabilin-2 and integrin β5 was assessed by FRET analysis. Three fluorescent images of COS7 cells expressing stabilin-2–seGFP and Venus-β5 were obtained, FRETC was calculated, and the results are displayed using quantitative pseudocolor (see Materials and Methods). In the lower panels, stabilin-2–seCFP and Venus alone were overexpressed in COS7 cells, and their interaction was assessed by FRET analysis as a control. (D) Colocalization of stabilin-2 (red) and endogenous integrin αvβ5 (yellow) in phagocytic cup area. Stab-2/L cells were allowed to ingest NBD-PS-coated beads (green). The cells were fixed and immunostained for Myc–stabilin-2 and αvβ5. (E) Colocalization of endogenous stabilin-2 (red) and integrin αvβ5 (green) in phagocytic cup area of stabilin-2-expressing native cells (human monocyte-derived macrophages). Stab-2/L cells were allowed to ingest NBD-PS-coated beads (blue). The endogenous stabilin-2 and αvβ5 were stained using the corresponding antibodies, as described in Materials and Methods. DIC, differential interference contrast.
To establish whether stabilin-2 and integrin αvβ5 interact directly in cells, we performed fluorescence resonance energy transfer (FRET) analysis using seCFP tagged to the C terminus of the stabilin-2 (stabilin-2–seCFP) as a donor and the Venus-tagged C terminus of integrin β5 (integrin-β5–Venus) as an acceptor. This probe pair was coexpressed in COS7 cells and subjected to confocal microscopic analysis. Digital images were acquired through seCFP, Venus, and FRET channels from a single cell. Since FRET from seCFP to Venus occurs only in cases where the two proteins are in very close proximity (<50 Å), direct interactions of stabilin-2 and integrin αvβ5 should be assessed by measuring a corrected FRET (FRETC) value calculated for the entire image on a pixel-by-pixel basis using a three-filter micro-FRET method and presented as a quantitative pseudocolor image (38). Increased FRETC was detected in COS7 cells expressing both stabilin-2–seCFP and β5-Venus (Fig. 4C). However, this signal was not observed when control Venus was employed as an acceptor. These results collectively indicate that stabilin-2 interacts directly with the αvβ5 integrin in cells.
Next, cellular colocalization of stabilin-2 and endogenous integrin αvβ5 was examined using confocal microscopy (Fig. 4D). Our experiments showed that stabilin-2 and integrin αvβ5 are specifically colocalized in the phagocytic cup area during engulfment of 4-nitrobenzo-2-oxa-1,3-diazole (NBD) PS-coated beads. Experiments were conducted under strict serum-free conditions. Thus, it appears that the localizing effect of integrin αvβ5 surrounding the PS beads is mediated by interactions with stabilin-2 but not soluble factors. This colocalization was observed between C-terminal truncated stabilin-2 and integrin, supporting the theory that an extracellular or transmembrane region is responsible for these interactions. Next we examined colocalization of endogenous stabilin-2 and integrin αvβ5 in HMDMs, which express stabilin-2 (Fig. 4E). Under serum-free conditions, stabilin-2 and integrin αvβ5 are colocalized on the phagocytic cup area during engulfment of NBD-PS-coated beads, suggesting that the interaction of these receptors is expected in native cells as well.
The FAS1 domain of stabilin-2 mediates interactions with integrin αvβ5.
To ascertain the domain of the stabilin-2 extracellular region required for binding to integrin, three different Myc–stabilin-2 deletion mutants were constructed, specifically, one expressing only a single unit as an extracellular region and two others expressing only the EGF domain repeat or FAS1 domain (Fig. 5A). The deletion constructs were transiently transfected into CHO-K1 cells with an integrin β5 subunit, and the specific domain forming an immunocomplex with αvβ5 integrin was identified. The results indicate that FAS1-containing constructs form a complex with αvβ5 integrin but not the EGF domain repeat, suggesting that the FAS1 domain is critical for binding to αvβ5 integrin. To further evaluate the role of the FAS1 domain in αvβ5 integrin binding, we generated recombinant proteins of the FAS1 domain, EGFrp, and a single unit (Stab-U) of stabilin-2 and examined their effects on the phagocytic activity of Stab-2/L cells. Stab-U and FAS1 proteins impeded the engulfment of aged RBCs in a dose-dependent manner, whereas the EGF domain repeats had no effect (Fig. 5D). To further demonstrate that the FAS1 domain interacts directly with αvβ5 integrin, enzyme-linked immunosorbent assay (ELISA) plates were coated with purified αvβ5 integrin and subsequently incubated with stabilin-2 domain derivatives. The Stab-U and FAS1 domain proteins, but not EGFrp, bound efficiently to the αvβ5-coated plates (Fig. 5E). ELISA plates were coated with BSA, and nonspecific binding was subtracted from the total absorbance. Our results indicate that stabilin-2 interacts with αvβ5 integrin via its FAS1 domain in the extracellular region.
Fig 5.
Role of FAS1 domain in association of stabilin-2 with integrin αvβ5. (A) Schematic diagrams of stabilin-2 deletion mutants (Stab2-U4, Stab-F7, and Stab2-E4). S, Signal peptide; E4, fourth EGF-like domain repeat; F7, seventh FAS1 domain; L, link domain. (B) 293FT cells were transiently transfected with Stab2-U4-Myc- and/or integrin β5-expressing vector. The lysates were subjected to immunoprecipitation (IP) using anti-integrin β5, and the immunoprecipitates were then analyzed by immunoblot analysis using anti-integrin β5 and anti-Myc antibodies, respectively. Expression of Stab2-U4 and integrin β5 proteins in total cell lysates were examined by immunoblot analysis (TCL). A representative result of three independent experiments is shown. (C) 293FT cells were transiently transfected with Stab2-E4-Myc-, Stab2-F7-Myc-, and/or integrin β5-expressing vector. The lysates were subjected to immunoprecipitation (IP) using anti-integrin β5, and the immunoprecipitates were then analyzed by immunoblot analysis using anti-integrin β5 and anti-Myc antibodies, respectively. Expression of Stab-E4, Stab2-F7, and integrin β5 proteins in total cell lysates was examined by immunoblot analysis (TCL). (D) Effects of FAS1-containing proteins on the engulfment of aged RBCs by Stab-2/L cells. Stab-2/L cells were preincubated with the indicated stabilin-2 proteins, and then aged RBCs in serum-free buffer were added. After incubation for 1 h, the number of ingested RBCs per cell was counted. (E) The FAS1 domain directly interacts with integrin αvβ5. ELISA plates were coated with integrin αvβ5 or BSA and then incubated with the indicated stabilin-2 proteins. After 1 h, proteins bound to wells were quantified using anti-His antibody. The results are expressed as the mean ± SD from at least three experiments.
Knockdown of the GULP and integrin pathways independently inhibits phagocytosis.
Physical interactions of stabilin-2 and αvβ5 integrin possibly allow cross talk between the two conserved phagocytic signaling pathways, specifically, the GULP-dependent pathway via interactions of GULP and the cytoplasmic domain of stabilin-2 and the GULP-independent, DOCK180/ELMO/CrkII-dependent pathway mediated by αvβ5 integrin (Fig. 6A). Previous blocking experiments indicate that stabilin-2 and αvβ5 integrin are involved in independent phagocytic signaling pathways. To investigate whether there is a functional relationship between these two pathways in stabilin-2-mediated phagocytosis, we examined the effects of GULP siRNA and DOCK180 siRNA on the phagocytic activity of stabilin-2-expressing cells (Stab-2/L cells). Pretreatment with GULP or DOCK180 siRNA partially inhibited the uptake of aged RBCs in stabilin-2-expressing cells (Fig. 6B and C). However, these effects were not observed when Stab-2/L cells were treated with control siRNAs. Upon simultaneous inhibition of GULP and DOCK180 expression, the inhibitory effect was augmented. These results suggest that GULP and DOCK180 play roles in stabilin-2-mediated phagocytosis via independent signaling pathways.
Fig 6.

Two phagocytic signaling pathways (GULP dependent and GULP independent) involved in stabilin-2-mediated phagocytosis. (A) Diagram explaining two phagocytic signaling pathways (GULP dependent and GULP independent) under stabilin-2. (B) Knockdown of GULP and DOCK180 independently inhibits phagocytosis. siRNA-induced inhibition of integrin β5 subunit expression in Stab-2/L cells. The cells were transfected with siRNA. Control siRNAs with similar GC contents were used. At 60 h after transfection, the suppression of GULP or DOCK180 expression was confirmed by Western blotting (WB). β-Actin (*) of each cell lysate was assessed as a control. (C) The inhibition of aged RBC engulfment by integrin β5 pretreatment. The phagocytic indexes were determined as indicated. The results are expressed as the mean ± SD from at least three experiments. **, P < 0.01; *, P < 0.05 (ANOVA).
DISCUSSION
Uncleared cell corpses are related to several diseases, including systematic lupus erythematosus, atherosclerosis, Alzheimer's disease, and Parkinson's disease (7). Thus, rapid and efficient removal of unwanted cells is important for the health of organisms, and clarifying the mechanisms by which apoptotic cells are recognized and removed quickly is important for understanding engulfment machinery for future therapeutic benefit.
The clearance process is rapid and efficient, since very few apoptotic cells are present, even in tissues with high cellular turnover. Multiple distinct PS receptors bind phosphatidylserine displayed as a cluster on the apoptotic cell surface, leading to the hypothesis that PS receptors coordinate to ensure efficient apoptotic cell clearance. Orchestrated actions of different engulfment receptors have been proposed in an engulfment synapse model (33). The synapse model suggests that multiple receptors and coreceptors and their coordinating specific interactions regulate specific tethering and tickling events during cell corpse engulfment. Mer tyrosine kinase and the β5 integrin subunit cooperate to internalize apoptotic cells. These receptors recognize apoptotic cells via soluble factors and amplify only the DOCK180-dependent signaling pathway (42).
The important issues that require clarification are as follows: (i) how do different sets of receptors orchestrate phagocytic signals for efficient uptake of dying cells under certain physiological circumstances, and (ii) is there cross talk of phagocytic signaling via the conserved functional pathways under different sets of PS receptors? There is an apparent redundancy in the receptors required for engulfment in macrophages. Since blocking of individual receptors with antibodies appears to diminish but not completely abrogate engulfment of apoptotic targets, we employed a cell system (Stab-2/L) stably expressing stabilin-2, a PS-recognizing receptor. Stabilin-2 expression confers PS-dependent phagocytic properties to cells that are otherwise unable to recognize and engulf apoptotic cells (28). Among the PS-recognizing receptors, stabilin-2 appears to use the intracellular adaptor GULP, whereas BAI-1 is dependent on the DOCK180/ELMO/Rac1 signaling pathway and Tim4 acts only as a tethering receptor which seems to rely on other receptors for the tickling process (26, 27, 31). Recent studies have suggested that integrin αvβ3 is involved in the tickling process, cooperating with Tim4 only in the presence of MFG-E8 (41).
Despite disruption of binding to GULP upon removal of the cytoplasmic tail of stabilin-2, about 50% of phagocytic activity remained (Fig. 1). This result was consistent with the moderate inhibitory effect observed with the GULP blocking assay using GULP siRNA or a dominant-negative mutant (31). The phagocytic activity of C-terminus-deleted stabilin-2-expressing cells (Stab2ΔC/L) was reliably observed under serum-free conditions with various amounts (50 to 90%) of intact stabilin-2-expressing cells (Stab-2/L). These observations suggest that stabilin-2 signaling is partially independent of GULP and that potential cross talk with other engulfing receptors occurs through extracellular or transmembrane domains. We investigated the involvement of cell adhesion receptors integrin αvβ5 and αvβ3, since integrins play a conserved role in the engulfment of apoptotic cells in a range of species (from C. elegans to mammals) and appear to cooperate with other engulfing receptors (15). Importantly, integrin acts upstream of the DOCK180/ELMO/Rac1 signaling module, which may explain the phagocytic activity of stabilin-2 devoid of interactions with GULP (6). Furthermore, stabilin-2 contains seven repeats of the integrin-binding domain FAS1 in the extracellular region (28).
Our results indicate that integrin αvβ5 functions in stabilin-2-mediated phagocytosis. Interestingly, the surface expression of integrin αvβ5 was specifically upregulated in both wild-type and mutant stabilin-2-expressing cells compared to that in mock-transfected cells (Fig. 2A). The appearance of an elongated cell shape and cluster formation of stabilin-2-expressing cells during culture may be related to surface expression of adhesion receptors, such as integrin αvβ5. Total protein levels of integrin αv, β3, and β5 were not significantly changed upon overexpression of stabilin-2. We are unsure why integrin αvβ5 is specifically expressed on the surface or whether it is a result of stabilin-2 overexpression. One hypothesis would be that integrin αvβ5 is recruited to the surface by specific interaction with overexpressed stabilin-2 receptors, although β5 shares 50% sequence identity with β3. Even though FAS1 domains have been reported to bind several sets of integrins, including β3 and β5, in vitro (24), in vivo interaction could be different by local concentration. Careful examination of sequence similarity between integrins β5 and β3 gave us a hint about the putative specific recognition sites in β5 which are not involved in folding or interaction with αv. The second helical region (positions 196 to 201) of β5 would be one of targeting regions because it is distinct from the main body of folding and consists of a unique sequence compared to that of β3. These possibilities are open to further investigation, however. Another interesting finding is that integrin αvβ5 mediates the engulfment of aged RBCs by stabilin-2-expressing cells without the bridge molecule MFG-E8. MFG-E8 is essential for the phagocytic activity of integrin, even in cooperation with Mer tyrosine kinase or Tim4. The presence of soluble factors in serum confers additional phagocytic activity to stabilin-2-expressing cells. Under serum-free conditions, only about 30% of engulfing activity was observed (data not shown). Our data indicated that the Stab-2 Ab was able to block phagocytic activity to a similar extent as the combination of Stab-2 and αvβ5 Ab under serum-free conditions. Thus, we suggest that the phagocytic activity of integrin αvβ5 observed under serum-free conditions is dependent on stabilin-2.
The integrin αvβ5 participates in engulfing activity via direct interactions with stabilin-2. Interactions between receptors were confirmed by monitoring in vivo interactions between stabilin-2 and integrin via confocal imaging and FRET. Further examination of the interacting domains revealed that the FAS1 domain of stabilin-2 is critical for this interaction. The distinctive structure of stabilin-2, which consists of both PS-binding and integrin-binding domains, may be responsible for its unique function. Interestingly, stabilin-2 is expressed only in vertebrates, and its interactions with integrins are advantageous for complex circumstances in vertebrates.
The caveat is that the interaction between overexpressed receptors is constitutive. In reality, the interactions may be too transient upon activation of stabilin-2 or integrin αvβ5.
Nevertheless, our data provide preliminary evidence for the coordination of the two conserved phagocytic pathways via direct interactions of the engulfment receptors stabilin-2 and integrin αvβ5 and further contribute to our understanding of why so many receptors are involved and whether they work together to control uptake capacity. The coordinated action of stabilin-2 and conserved integrin may be an evolutionarily adapted mechanism in vertebrates.
ACKNOWLEDGMENTS
This work is supported by a National Research Foundation of Korea grant funded by the South Korean government (2009-0076035); the Converging Research Center Program through the Ministry of Education, Science and Technology (2010K001054); the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0720550-2); and a National Research Foundation of Korea (NRF) grant funded by the South Korean government (MEST) (2010-0029206).
We are grateful to David D. Schlaepfer at the University of California, San Diego, for his generous grant of the FAK inhibitors PF228 and PF271.
Footnotes
Published ahead of print 7 May 2012
REFERENCES
- 1. Ahn DC, et al. 2008. Branching pattern of aortic arch in the Korean water deer. J. Vet. Med. Sci. 70:1051–1055 [DOI] [PubMed] [Google Scholar]
- 2. Albert ML, Kim JI, Birge RB. 2000. alphavbeta5 Integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905 [DOI] [PubMed] [Google Scholar]
- 3. Asano K, et al. 2004. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 200:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bratton DL, Henson PM. 2008. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr. Biol. 18:R76–R79 [DOI] [PubMed] [Google Scholar]
- 5. Cho CW, et al. 2002. HPV E6 antisense induces apoptosis in CaSki cells via suppression of E6 splicing. Exp. Mol. Med. 34:159–166 [DOI] [PubMed] [Google Scholar]
- 6. Dupuy AG, Caron E. 2008. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J. Cell Sci. 121:1773–1783 [DOI] [PubMed] [Google Scholar]
- 7. Elliott MR, Ravichandran KS. 2010. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 189:1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fadeel B, Xue D, Kagan V. 2010. Programmed cell clearance: molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation. Biochem. Biophys. Res. Commun. 396:7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finnemann SC. 2003. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 22:4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gigli I, Nelson RA., Jr 1968. Complement dependent immune phagocytosis. I. Requirements for C′1, C′4, C′2, C′3. Exp. Cell Res. 51:45–67 [DOI] [PubMed] [Google Scholar]
- 11. Hanayama R, et al. 2002. Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187 [DOI] [PubMed] [Google Scholar]
- 12. He M, et al. 2011. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 12:358–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henson PM. 2005. Dampening inflammation. Nat. Immunol. 6:1179–1181 [DOI] [PubMed] [Google Scholar]
- 14. Henson PM, Bratton DL, Fadok VA. 2001. The phosphatidylserine receptor: a crucial molecular switch? Nat. Rev. Mol. Cell Biol. 2:627–633 [DOI] [PubMed] [Google Scholar]
- 15. Hsu TY, Wu YC. 2010. Engulfment of apoptotic cells in C. elegans is mediated by integrin alpha/SRC signaling. Curr. Biol. 20:477–486 [DOI] [PubMed] [Google Scholar]
- 16. Imachi H, et al. 2000. Human scavenger receptor B1 is involved in recognition of apoptotic thymocytes by thymic nurse cells. Lab. Invest. 80:263–270 [DOI] [PubMed] [Google Scholar]
- 17. Jung MY, Park SY, Kim IS. 2007. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with alphaMbeta2 integrin. J. Leukoc. Biol. 82:1156–1165 [DOI] [PubMed] [Google Scholar]
- 18. Kinchen JM. 2010. A model to die for: signaling to apoptotic cell removal in worm, fly and mouse. Apoptosis 15:998–1006 [DOI] [PubMed] [Google Scholar]
- 19. Kinchen JM, Ravichandran KS. 2007. Journey to the grave: signaling events regulating removal of apoptotic cells. J. Cell Sci. 120:2143–2149 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi N, et al. 2007. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27:927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SJ, Park SY, Jung MY, Bae SM, Kim IS. 2011. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 117:5215–5223 [DOI] [PubMed] [Google Scholar]
- 22. Miyanishi M, et al. 2007. Identification of Tim4 as a phosphatidylserine receptor. Nature 450:435–439 [DOI] [PubMed] [Google Scholar]
- 23. Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. 2010. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6:280–289 [DOI] [PubMed] [Google Scholar]
- 24. Nam JO, Jeong HW, Lee BH, Park RW, Kim IS. 2005. Regulation of tumor angiogenesis by fastatin, the fourth FAS1 domain of betaig-h3, via alphavbeta3 integrin. Cancer Res. 65:4153–4161 [DOI] [PubMed] [Google Scholar]
- 25. Nose A, Nagafuchi A, Takeichi M. 1988. Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54:993–1001 [DOI] [PubMed] [Google Scholar]
- 26. Park D, Hochreiter-Hufford A, Ravichandran KS. 2009. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 19:346–351 [DOI] [PubMed] [Google Scholar]
- 27. Park D, et al. 2007. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450:430–434 [DOI] [PubMed] [Google Scholar]
- 28. Park SY, et al. 2008. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15:192–201 [DOI] [PubMed] [Google Scholar]
- 29. Park SY, Jung MY, Kim IS. 2009. Stabilin-2 mediates homophilic cell-cell interactions via its FAS1 domains. FEBS Lett. 583:1375–1380 [DOI] [PubMed] [Google Scholar]
- 30. Park SY, et al. 2009. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 122:3365–3373 [DOI] [PubMed] [Google Scholar]
- 31. Park SY, et al. 2008. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 283:10593–10600 [DOI] [PubMed] [Google Scholar]
- 32. Park SY, Kim SY, Jung MY, Bae DJ, Kim IS. 2008. Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol. Cell. Biol. 28:5288–5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ravichandran KS. 2010. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207:1807–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Savill J, Fadok V. 2000. Corpse clearance defines the meaning of cell death. Nature 407:784–788 [DOI] [PubMed] [Google Scholar]
- 35. Savill J, Hogg N, Ren Y, Haslett C. 1992. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90:1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott RS, et al. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211 [DOI] [PubMed] [Google Scholar]
- 37. Sokolowski JD, Mandell JW. 2011. Phagocytic clearance in neurodegeneration. Am. J. Pathol. 178:1416–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorkin A, McClure M, Huang F, Carter R. 2000. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10:1395–1398 [DOI] [PubMed] [Google Scholar]
- 39. Stuart LM, Ezekowitz RA. 2005. Phagocytosis: elegant complexity. Immunity 22:539–550 [DOI] [PubMed] [Google Scholar]
- 40. Thorp EB. 2010. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis 15:1124–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toda S, Hanayama R, Nagata S. 2012. Two-step engulfment of apoptotic cells. Mol. Cell. Biol. 32:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Y, Singh S, Georgescu MM, Birge RB. 2005. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 118:539–553 [DOI] [PubMed] [Google Scholar]