Abstract
Cysteine is potentially toxic and can affect diverse functions such as oxidative stress, antibiotic resistance, and swarming motility. The contribution of cysteine catabolism in modulating responses to cysteine has not been examined, in part because the genes have not been identified and mutants lacking these genes have not been isolated or characterized. We identified the gene for a previously described cysteine desulfhydrase, which we designated cdsH (formerly STM0458). We also identified a divergently transcribed gene that regulates cdsH expression, which we designated cutR (formerly ybaO, or STM0459). CdsH appears to be the major cysteine-degrading and sulfide-producing enzyme aerobically but not anaerobically. Mutants with deletions of cdsH and ybaO exhibited increased sensitivity to cysteine toxicity and altered swarming motility but unaltered cysteine-enhanced antibiotic resistance and survival in macrophages.
INTRODUCTION
Cysteine desulfhydrase (CDS) degrades cysteine to pyruvate, ammonia, and sulfide (19, 26, 43). The major CDS in Escherichia coli appears to be tryptophanase (TnaA), which has an apparent Km for cysteine of 11 mM (56). TnaA primarily catabolizes tryptophan, which appears to be abundant in the intestinal tract (29). In E. coli, TnaA can become 10% of soluble protein, which suggests the potential to also degrade cysteine in vivo, despite an unfavorable Km (56). Several factors regulate TnaA expression. TnaA is repressed by glucose, pyruvate, and acetate (6, 9, 10, 31). Expression requires cyclic AMP and is induced by tryptophan, cysteine, indole, growth in alkaline broth, depletion of heme and phosphatidyl glycerol, and anaerobic growth with an alternate electron acceptor (9, 51, 52). From these factors, it could be speculated that TnaA contributes to energy generation by degrading tryptophan and possibly cysteine to pyruvate.
Salmonella enterica does not have TnaA, yet cysteine induces a powerful CDS (19, 26, 43). This CDS has been purified from S. enterica, and similarly to TnaA, it contains pyridoxal-5′-phosphate (42). The kcat/Km ratio for l-cysteine, a measure of catalytic efficiency, is 10,340 mM−1 s−1 for S. enterica CDS (calculated from values reported by Kredich et al. [42, 43]) and 6.7 mM−1 s−1 for E. coli TnaA (calculated from values reported by Snell [56]). In other words, the S. enterica CDS appears to be more than three orders of magnitude more efficient than TnaA. To explore the function of the S. enterica CDS and cysteine catabolism, we identified the gene for the major CDS and characterized its regulation and the phenotype of mutants lacking this enzyme.
MATERIALS AND METHODS
Strains and plasmids.
Table 1 lists the strains and plasmids used in this study. All gene deletions and replacements were performed originally into the TT22971 background strain as described previously (21), followed by P22 transduction into the appropriate genetic background, and the products were ensured to be phage free by cross-streaking against P22-H5 on green plate agar (15). The following codons were deleted: ΔcdsH, 8 to 321; ΔybaO, 15 to 446; ΔmetC, 14 to 386; Δsbp, 11 to 317; ΔcysJ, 11 to 587; ΔasrA, 11 to 335; ΔphoP, 1 to 224; ΔcydD, 2 to 574; and ΔcysB, 11 to 315.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or phenotype | Source and/or reference |
|---|---|---|
| Strains | ||
| TR10000 | Wild-type S. enterica serovar Typhimurium LT2 | 47 |
| TO1 | TR10000 ΔcdsH | This study |
| TO2 | TR10000 ΔcdsH ΔmetC∷cat | This study |
| TO3 | TR10000 ΔybaO | This study |
| TO4 | TR10000 ΔcysB∷cat | This study |
| TO5 | TR10000 ΔasrA∷cat | This study |
| TO6 | TR10000 ΔcysJ∷cat | This study |
| TO7 | TR10000 Δsbp∷cat | This study |
| TO9 | TR10000 ΔmetC∷cat | This study |
| TO10 | TR10000 ΔcydD∷cat | This study |
| TO11 | TR10000 [λ (cdsH-lacZ) Kanr] | This study |
| TO12 | TR10000 ΔcdsH [λ (cdsH-lacZ) Kanr] | This study |
| TO13 | TR10000 ΔybaO [λ (cdsH-lacZ) Kanr] | This study |
| TT22971 | metA22 metE551 trpD2 ilv-452 leu-pro-(leaky) hsdLT6 hsdSA29 hsdB strA120/pDK46 | 47 |
| 14028s | Wild-type virulent strain; B serotype | 8 |
| TO15 | 14028s ΔcdsH | This study |
| TO16 | 14028s ΔybaO | This study |
| TO17 | 14028s ΔphoP∷cat | This study |
| SL1433 | S. enterica wild-type virulent strain; B serotype | 61 |
| TO18 | SL1433 ΔcdsH∷cat | This study |
| TO19 | SL1433 ΔybaO∷cat | This study |
| W3110 | E. coli K-12 lacL8 lacIq | Laboratory stock |
| DH5α | E. coli K-12 F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 hsdR17(rK− mK+) λ− | Laboratory stock |
| BW23474 | E. coli K-12 Δ(lacZYA-argF)U169 rpoS(Am) robA1 creC510 hsdR514 ΔuidA(MluI)∷pir-116 endA(BT333) recA1 | Genetic stock center |
| Plasmids | ||
| pMMB190 | Broad-host-range vector, Ampr, lacUV5 promoter, lacZα | 44 |
| pMMB-cdsH | pMMB190 containing cdsH (low copy) | This study |
| pUC18 | Cloning vector | Laboratory stock |
| pUC18-cdsH | pUC18 containing cdsH (high copy) | This study |
| pKD46 | araC bla oriR101 repA101(Ts) λ red (gam+ bet + exo+) | 21 |
| pCP20 | FLP (FRT-specific) recombinase, Ampr Camr | 21 |
| pKD13 | oriRγ bla with the Kanr cassette flanked by FRT sites | 21 |
| pDK13-Cat | pKD13 with the Kanr replaced by Camr | This study |
| pKD4 | oriRγ bla with the Kanr cassette flanked by FRT sites | 21 |
| pINT-s | λ integrase, Ampr, pSC101 origin | 27 |
| pAH125 | lacZ transcriptional fusion vector, Kanr | Genetic stock center |
| pBLS17 | lacZ translational fusion vector (from pAH125) | Laboratory stock |
| pBLS18 | lacZ transcriptional fusion vector (from pBLS17) | This study |
| Empty ASKA | SfiI-cut and religated backbone of JW3582 | This study |
| JW3582 | pCA24N carrying cysE | ASKA library (38) |
| JW0437 | pCA24N carrying ybaO | ASKA library (38) |
| JW2514 | pCA24N carrying iscS | ASKA library (38) |
| JW1670 | pCA24N carrying sufS | ASKA library (38) |
| JW2414 | pCA24N carrying cysM | ASKA library (38) |
| JW2407 | pCA24N carrying cysK | ASKA library (38) |
The cdsH gene and promoter region were amplified from genomic S. enterica TA1650 and cloned into the SmaI site of pUC18. The EcoRI-HindIII fragment of pUC18-cdsH containing cdsH was ligated into EcoRI- and HindIII-cleaved pMMB190. This places the cdsH gene under control of the Ptaclac promoter in a low-copy vector. The cdsH promoter fusions were constructed as described previously (27). A PCR product from 57 bases downstream to 138 bases upstream of the cdsH start codon was cloned into translational fusion vector pBLS17 and transcriptional fusion vector pBLS18, a derivative of pBLS17 containing a trp terminator-deleted lacZ from pAH125 (36). The cdsH-lacZ fusion plasmids were integrated into the λ site of TT22971 and P22 transduced into TR10000 for study. Strains were verified with P2 and P3 CRIM primers (27) and S. enterica-specific primers P1 (5′-GGCATAATAGCAATGTACTGG-3′) and P4 (5′-GCGTTCTGGCACGATAT-3′). For ybaO complementation, the ASKA vector containing ybaO was used (38), and its corresponding empty ASKA vector was constructed by an SfiI cut/religation of the 4,510-bp backbone of ASKA-cysE.
Growth of bacterial cells.
Cells were grown in minimal medium containing W salts (10.5 g/liter K2HPO4, 4.5 g/liter K2HPO4, and 0.05 g/liter MgSO4), 0.4% of a carbon source, and 0.2% (NH4)2SO4 as a nitrogen source, when appropriate. For determination of anaerobic growth rates, 8.5 ml medium was placed in a 9-ml screw-cap tube, and cells were incubated without shaking in a 37°C water bath.
Enzyme assays.
Detection of l-cysteine desulfhydrase was performed using two assays. Assay 1 measured methylene blue formation at 670 nm (58). Methylene blue forms when N′,N′-dimethyl-p-phenylenediamine reacts with sulfide in the presence of FeCl3. One unit is defined as the amount catalyzing the formation of 1 μmol sulfide min−1. Assay 2 was an activity stain for proteins subjected to electrophoresis in a nondenaturing gel (3). In this assay, bismuth reacts with sulfide to form a black precipitate at the site of a cysteine catabolic protein. The assay for β-galactosidase has been previously described (36), with specific activity units defined as nanomoles of product per minute per milligram of protein.
Sulfide detection assays.
Sulfide detection was performed in four ways. Assay 1 involved stabbing cells into LB containing 0.6% agar, 0.1% FeSO4 · 7H2O, and 5 mM cysteine. Assay 2 was an anaerobic test with filter discs. Four-section plates contained the following layers from bottom to top: 1 ml LB containing 0.6% agar, a 6-mm Whatman no. 1 paper filter disc with 15 μl of a chemical solution affixed with 200 μl soft agar, 0.5 ml overnight culture cells mixed with 1 ml LB containing 0.6% agar, and 6 ml LB containing 1.5% agar to the top of the plate. Discs contained 5 μl 1 M cysteine, 5 μl 1 M NaNO3, 5 μl 40% glucose, or 5 μl 10% FeSO4 · 7H2O when indicated. The assay was specific for cysteine, since methionine, glutathione, cystine, and dithiothreitol (DTT) did not generate sulfide. Only FeSO4, and not FeCl3 or FeCl2, generated sulfide. Sulfide did not result from sulfate reduction, since mutants blocked in the reduction pathways (TO5 [ΔasrA], TO6 [ΔcysJ], and TO7 [Δsbp]) still generated sulfide. Assay 3 detects sulfide from silver nitrate-impregnated strips (45). Microcentrifuge tubes (1.5 ml) contained 1.3 ml LB, 0.1% Na2SO4 · 10H2O, and 5 mM cysteine. The strips were sealed within the caps above the liquid cultures. A small hole was pierced in the caps to allow air to escape, and tubes were incubated at 37°C without shaking. Assay 4 used less-reactive lead acetate strips (Fisher Scientific). Silver nitrate and lead acetate strips produce a black stain directly proportional to the amount of volatile sulfide formed. Photographs were taken with the Multi-Doc-It digital imaging system using Launch Doc-It LS image acquisition software.
Swarming.
Swarming agar was prepared as described previously (37) (1% Fisher tryptone, 0.25% NaCl, 0.5% Bacto agar, and 0.5% glucose or xylose). One microliter of an overnight culture grown in LB was spotted in the center of each plate section and was dried for 5 min in a flow hood with the lids removed. To ensure consistent inoculum densities, cell counts were determined by the drop plate method (16) and swarm plates were accepted only if the inoculum size started within 4 mm in diameter. Plates were placed on a tray covered in Saran Wrap to maintain humidity and incubated at 37°C before time point photos were taken with the same imaging system.
Antibiotic resistance.
MICs were determined by the broth microdilution method (63) in LB with 0.1% Na2SO4 · 10H2O. Plates were sealed with AeraSeal breathable film covers and incubated at 37°C. After 14 h of incubation, final optical densities at 600 nm (OD600) were measured using an Infinite M200 Tecan reader and iControl version 1.2.7.0 software.
Survival in macrophages.
The bacterial survival assay was modified from previous methods (13, 39, 53); details are provided in the supplemental material. At various time points, macrophages were washed 3 times with phosphate-buffered saline (PBS) and lysed with 1 ml 0.5% deoxycholate–PBS, and surviving bacteria were enumerated by serial dilutions using the drop plate method (16).
RESULTS
Bioinformatics of the cysteine desulfhydrase gene.
The major CDS in S. enterica has been purified and characterized (42). From the published amino acid composition, we used the AACompIdent tool of the ExPASy Bioinformatics Resource Portal and the TrEMBL database to identify candidate genes. The tool provides a least-squares score using the differences in moles percent for each amino acid. The website indicates a likely identification if (i) the same protein has the lowest score on three different lists, including one which compares all proteins in all species, (ii) the score is under 30, and (iii) there is a large difference (e.g., 2-fold) between proteins in the same species with the lowest two scores. STM0458 has the lowest score on all three lists (see Fig. S1 in the supplemental material). The lowest-scoring proteins in S. enterica are STM0458 and the phosphate-starvation inducible PhnW (2-aminoethylphosphonate aminotransferase), which have scores of 5 and 12, respectively (see Fig. S1 in the supplemental material). The only proteins in Salmonella species with a score of less than 30 that are involved in cysteine metabolism are MetC (a cystathionine lyase) and IscS (a cysteine desulfurase), which have scores of 21 and 26, respectively.
We also compared other properties of the purified CDS with several proteins that could conceivably generate sulfide: MetC, IscS, and SufS (cysteine desulfurases) and CysK and CysM (cysteine synthases A and B, respectively). The amino-terminal residue of purified CDS is serine. Of the potential sulfide-producing enzymes considered, only STM0458 and CysK have a serine after the amino-terminal methionines. The reported mass of the purified CDS subunit (37,000 Da) is closer to the deduced mass of STM0458 (38,889 Da) than to the mass of CysK (34,535 Da), CysM (32,645 Da), or any cysteine desulfurase (≥44,500 Da) (25, 32, 42, 68). These findings strongly suggest that the purified CDS from S. enterica is the product of STM0458, and biochemical evidence presented below confirmed this conclusion. We designate this gene cdsH (cysteine desulfhydrase).
The BLAST algorithm indicates the presence of four S. enterica proteins with ≥20% amino acid identity with STM0458: CysM (cysteine synthase B, 28%), CysK (cysteine synthase A, 24%), STM1002 (a putative diaminopropionate ammonia lyase, 24%), and IlvA (threonine dehydratase, 23%). Due to this homology, STM0458 (cdsH) is annotated as a putative cysteine synthase/cystathionine β-lyase.
Biochemical evidence that CdsH is the predominant cysteine-inducible CDS.
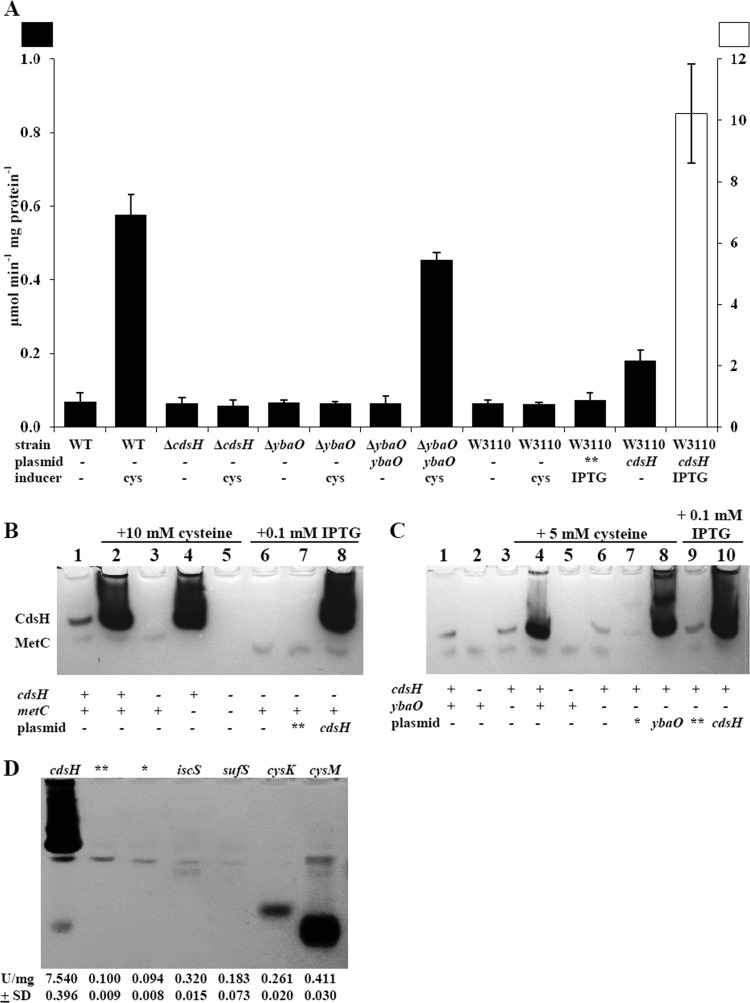
Deletion of cdsH reduced CDS activity by 10-fold (Fig. 1A). This result was observed for cells grown aerobically or anaerobically, in broth or minimal medium (data not shown). The major CDS activity is eliminated from an extract of a ΔcdsH strain subjected to electrophoresis in a nondenaturing gel (Fig. 1B, lane 3, and C, lanes 2 and 5). Plasmid pMMB-cdsH restored this activity in a ΔcdsH strain (Fig. 1D, lane 1). To further confirm that cdsH specifies a CDS, we transformed plasmid pMMB-cdsH into E. coli W3110, which lacks a prominent cysteine-inducible CDS. These cells acquired CDS activity that was 100-fold over the background level and 17-fold over the induced level in S. enterica (Fig. 1A and B, lanes 6 to 8).
Fig 1.
L-Cysteine desulfhydrase activity of crude extracts. (A) Cells were grown in LB to stationary phase at 37°C and assayed for CDS activity by the methylene blue assay. **, empty pMMB190 vector; *, empty ASKA plasmid. (B and C) Activity staining of cysteine desulfhydrase on a nondenaturing 10% polyacrylamide gel. (B) Lanes 1 to 5, activity from strains in an S. enterica TR10000 background: lane 1, TR10000 (wild type); lane 2, TR10000; lane 3, lane TO1 (ΔcdsH); lane 4, TO9 (ΔmetC); lane 5, TO2 (ΔcdsH ΔmetC). Lanes 6 to 8, activity from E. coli W3110: lane 6, W3110; lane 7, W3110 containing pMMB190 (empty vector); lane 8, W3110 containing pMMB190-cdsH. (C) Extracts from derivatives of S. enterica TR10000: lane 1, TR10000; lane 2, TO1 (ΔcdsH); lane 3, TO3 (ΔybaO); lane 4, TR10000; lane 5, TO1 (ΔcdsH); lane 6, TO3 (ΔybaO); lane 7, TO3 (ΔybaO) with empty ASKA vector; lane 8, TO3 (ΔybaO) with ASKA-ybaO; lane 9, TO3 (ΔybaO) containing pMMB190; and lane 10, TO3 (ΔybaO) with pMMB190-cdsH. (D) Extracts from derivatives of S. enterica TR10000 ΔcdsH, with vectors carrying genes indicated above each lane and respective CDS activity units measured by the methylene blue assay below each lane.
A distinctive feature of the cysteine-inducible CDS activity in S. enterica is its high catalytic activity. We observed 600 mU/mg protein of inducible activity in a crude extract (Fig. 1A), which is comparable to the previously reported 260 mU/mg protein (42). The purified S. enterica CDS has a specific activity of about 450,000 mU/mg protein (42). In contrast, purified NifS, a cysteine desulfurase, has a specific activity of 90 mU/mg, which is less than the CDS activity in a crude extract (69).
A second distinguishing feature of the major CDS in S. enterica is its induction by cysteine (26). We confirmed this result from assays from a crude extract (Fig. 1A) and a cell extract subjected to native gel electrophoresis (Fig. 1B, lanes 2 and 4, and C, lane 4). To investigate whether expression of cdsH matched the enzyme activities, we constructed strains with cdsH-lacZ transcriptional and translational fusions. Cysteine induced transcriptional activity in broth (Fig. 2A) and glucose minimal medium (Fig. 2B). The translational fusion showed the same trend (data not shown). Induction of cdsH occurred with cysteine concentrations greater than 200 μM. Expression appeared sooner and was consistently higher in TO12 (ΔcdsH), which might suggest a higher intracellular level of cysteine without CdsH (Fig. 2B).
Fig 2.
Cysteine-inducible regulation of cdsH using cdsH-lacZ transcriptional fusions. (A) TR10000 (wild type) was grown in LB. The arrow indicates addition of 10 mM cysteine. The dotted line represents cell growth in Klett units (100 units is an A600 of ∼0.6), and the solid line represents β-galactosidase activity from a strain with a transcriptional cdsH-lacZ fusion. (B) Strains with a transcriptional cdsH-lacZ fusion, i.e., TO11 (WT), TO12 (ΔcdsH), TO13 (ΔybaO), and TO13 with pYbaO (JW0437), were grown in glucose-ammonia minimal medium with increasing concentrations of cysteine. For TO11, number of determinations (n) = 5; for TO12 and TO13 with pYbaO, n = 2; and for TO13, n = 3.
Other sulfide-producing enzymes.
Native gels revealed a faster-migrating CDS activity, which generated sulfide hours after the predominant activity. We suspected that this other protein was MetC, which can degrade cystine as a side reaction (22). The lag before sulfide detection presumably results from the time required to generate cystine from cysteine oxidation. Strains with a deletion of ΔmetC did not have the lower band (Fig. 1B, lanes 4 and 5).
Ten percent residual CDS activity was found in TO1 (ΔcdsH) and TO2 (ΔcdsH ΔmetC∷cat) (not shown), suggesting the presence of other enzymes with CDS activity that were not cysteine inducible. We did not genetically analyze the enzymes that contribute to this basal activity. Instead, we examined the ability of two cysteine desulfurases, SufS and IscS, and both cysteine synthases, CysK and CysM, to generate sulfide by our assays. These four genes were expressed from high-copy ASKA plasmids. Quantitative assays indicated activity 2- to 4-fold above background for all four enzymes in a ΔcdsH strain (Fig. 1D). However, this activity was at least 20-fold less than that in a strain with pMMB-cdsH, which is a low-copy-number plasmid (Fig. 1D). These results were expected, since CysM and CysK can catalyze the cleavage of cysteine but at only about 1% of the level for the forward reaction (25). The same samples subjected to electrophoresis in a nondenaturing gel detected IscS (just below MetC) but not SufS. The two cysteine synthases, CysK and CysM, generated sulfide, although with substantially less activity and at entirely different positions than the predominant CDS. These results were also expected, since the cysteine synthases are dimeric, while the previously characterized CDS is hexameric (40, 42). We did not further examine whether these enzymes contribute to the basal CDS activity in a ΔcdsH strain.
ybaO regulates cdsH.
The ybaO gene, which is just upstream of cdsH, specifies a putative Lrp/AsnC family transcriptional regulator (67). Lrp (leucine-responsive regulatory protein) binds several amino acids, e.g., leucine and alanine (50). Therefore, we examined whether ybaO controls cdsH expression. A ybaO deletion reduced cysteine-inducible CDS activity 10-fold (Fig. 1A and C) and essentially eliminated β-galactosidase activity from a transcriptional cdsH-lacZ strain (Fig. 2B). Finally, all deficiencies in a ybaO mutant were restored by a plasmid carrying ybaO (Fig. 1A and C [lanes 7 and 8] and 2B). We conclude that YbaO mediates induction by cysteine.
Growth defects of ΔcdsH and ΔybaO mutants.
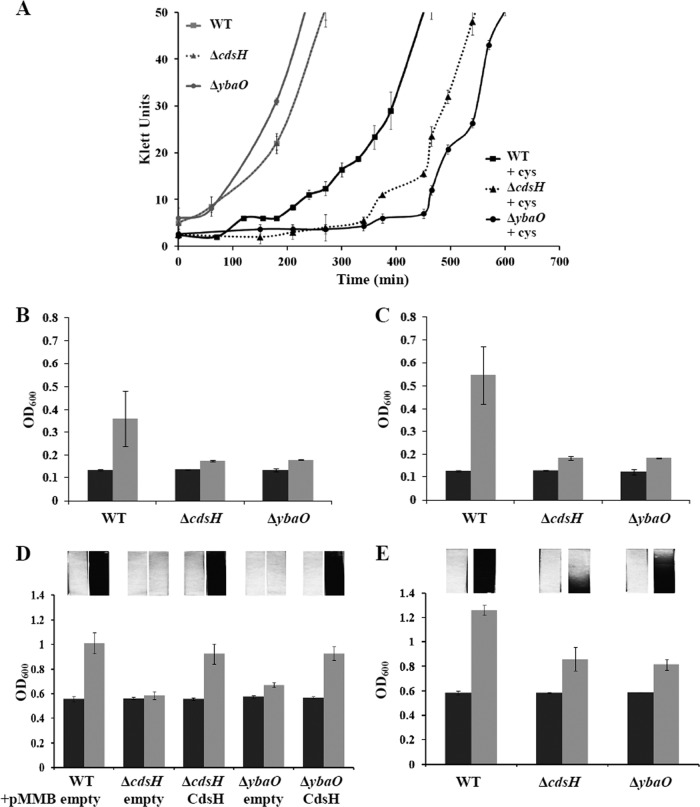
In E. coli, exogenous cysteine transiently causes amino acid starvation, which is overcome by addition of leucine, isoleucine, valine, and threonine (20, 33). The toxicity is only transient, possibly because of induction of cysteine catabolic enzymes (2, 57). If CdsH degrades cysteine in vivo, then mutants with defects in cysteine catabolism might respond poorly to exogenous cysteine. We examined the effect of cysteine in different media. First, 1 mM cysteine in glucose-ammonia minimal medium increased the lag phase before rapid growth for both TO1 (ΔcdsH) and TO3 (ΔybaO) (Fig. 3A). (TO1 and TO3 are here designated the ΔcdsH and ΔybaO mutants, respectively.) Cells were grown anaerobically to minimize the complication of cysteine oxidation to cystine. Second, we grew cells with cysteine as the sole nitrogen source, which requires cysteine catabolism. If the nitrogen from 1 mM cysteine can be utilized, then we would expect growth equivalent to that supported by 1 mM NH4Cl (A600 of ∼0.6) (65). However, TR10000 (wild type [WT]) did not grow with cysteine as the sole nitrogen source, possibly due to toxicity (33). To overcome this inhibition, either 0.2 mM (NH4)2SO4 or 0.02% Casamino Acids was added with either glucose or xylose, respectively, as the carbon source. Cysteine utilization as a nitrogen source with 0.2 mM (NH4)2SO4 was apparent for the wild-type strain but was severely diminished for both the ΔcdsH and ΔybaO mutants (Fig. 3B and C). Since other sulfide-producing enzymes, and possibly cysteine catabolic enzymes, exhibit glucose repression (18), cells were also grown with xylose as a carbon source. Similar results were seen for cells grown with xylose (Fig. 3D and E). This growth defect was reversed by complementation with plasmid pMMB-cdsH (Fig. 3D). Finally, in glucose-ammonia minimal medium with 5 mM cysteine, cells of the ΔcdsH and ΔybaO strains, but not the wild type, turned the culture yellow (spectral peak at around 380 nm) and aggregated. The basis for these properties is unknown.
Fig 3.
Cysteine toxicity and sulfide generation. (A) Anaerobic growth of cells with or without cysteine in glucose-ammonia minimal medium at 37°C. Note that the WT and ΔcdsH curves overlap. (B to E) Growth measurements (A600) were recorded for cells grown with cysteine as the major nitrogen source. (B and C) With glucose as the carbon source and 0.003% (NH4)2SO4 as a trace nitrogen source, growth was measured for 18 h (B) and 24 h (C) without cysteine (black bars) and with 2 mM cysteine (gray bars). (D and E) With xylose as a carbon source and 0.02% Casamino Acids as a trace nitrogen source, growth was measured at 18 h (D) and 24 h (E) without cysteine (black bars) and with 2 mM cysteine (gray bars). Lead acetate strips were affixed to the inner wall of tubes and are shown above the graphs. Blackening of the strip is proportional to the sulfide generated.
Sulfide generation in vivo.
Impaired growth with cysteine as the major nitrogen source suggests that ΔcdsH and ΔybaO mutants might have diminished sulfide production in vivo. Lead acetate strips sealed above a culture with cysteine as the major nitrogen source were used to detect sulfide. The mutants had diminished sulfide generation with cysteine as the major nitrogen source compared to the wild-type strain, which was restored by cdsH complementation (Fig. 3D). For extended incubations, both mutants eventually generated sulfide (Fig. 3E), which suggests the presence of an additional cysteine catabolic, sulfide-producing enzyme(s).
Sulfide production distinguishes S. enterica from related enteric bacteria and is often detected using sulfide-indole motility (SIM) agar (1). SIM medium contains hydrolyzed peptides, iron, and thiosulfate (23). Cells are stabbed into this medium, and sulfide production results in FeS, which forms a black precipitate (14). In modified SIM medium without thiosulfate (cysteine becomes the only source of sulfide), the ΔcdsH mutant and TO2 (ΔcdsH ΔmetC) produced as much sulfide as TR10000 (wild type), whereas the ΔybaO mutant produced little sulfide (see Fig. S2A in the supplemental material). These results suggest YbaO-dependent, CdsH-independent sulfide production from whole cells. This is consistent with the possibility of a second YbaO-regulated cysteine catabolic enzyme. Further qualitative observations are consistent with this possibility. Wild-type and ΔcdsH strains, unlike a ΔybaO mutant, produced a detectable sulfide odor and a grayish-green cell pellet (see Fig. S2B in the supplemental material). A plausible explanation for this observation is that sulfide precipitates trace metals in the medium. Finally, to ensure that YbaO-regulated cysteine catabolism was not an artifact of a specific laboratory strain, we tested for and observed YbaO-dependent sulfide generation from cysteine in three different S. enterica strains (see Fig. S2C in the supplemental material).
To confirm CdsH-independent sulfide production and to more easily control the sulfide source, the assay for sulfide was performed in a different way. We grew cells on LB plates containing ferrous sulfate and with a potential sulfide source on a filter disc. Iron sulfide (FeS) will form a black ring around the disc if sufficient sulfide is produced. These assays had to be performed anaerobically (under a thick layer of agar), since there was no detectable precipitate if the discs were affixed to the aerobic side (Fig. 4A). When cysteine was added to the disc, the wild-type and ΔcdsH strains produced sulfide, but again, a ΔybaO strain did not (Fig. 4B). This phenotype was not due to growth differences (see Fig. S3 in the supplemental material). Well-characterized enzymes generate sulfide from thiosulfate and tetrathionate independent of cysteine catabolism (5, 17, 54). When thiosulfate or tetrathionate replaced cysteine on the disc, the ΔcdsH and ΔybaO mutants produced as much sulfide as the wild type (Fig. 4B). These results show that YbaO regulates sulfide generation from cysteine but not from thiosulfate or tetrathionate. Glucose and nitrate repress sulfide generation from thiosulfate and tetrathionate (5, 18). Glucose and nitrate also repressed anaerobic sulfide generation from cysteine (Fig. 4B).
Fig 4.
Anaerobic sulfide detection using cysteine as the sulfur source. (A) Filter discs containing a sulfur source were placed on top of (aerobic) or under (anaerobic) LB agar for S. enterica TR10000. (B) Anaerobic disc assay for various strains with the indicated additions. WT, S. enterica TR 10000; ΔcdsH, TO1; ΔybaO, TO3; E. coli, W3110. (C) Assay of sulfide generation when cdsH is overproduced from a plasmid in S. enterica TO3 (ΔybaO) or E. coli W3110. IPTG, isopropyl-β-d-thiogalactopyranoside.
We examined sulfide production from cells with a plasmid that overexpressed CdsH. Overproduction increased CdsH activity 12-fold (from 0.6 U to 7.5 U) and contributed to aerobic sulfide generation with cysteine as a nitrogen source (Fig. 3D). However, overproduction did not result in detectable sulfide in vivo by the anaerobic disc assay (Fig. 4C). S. enterica containing the wild-type cdsH gene and a cdsH-lacZ transcriptional fusion grown anaerobically with cysteine induced β-galactosidase and CdsH as indicated in activity staining in a native gel (not shown). These results suggest that CdsH is inactive in vivo in either S. enterica or E. coli grown anaerobically.
Swarming.
Cysteine, but not other amino acids, is important for swarming motility. Swarming is a form of motility on semisolid surfaces (34). Cysteine auxotrophs do not swarm on a low-cysteine-containing medium, but additional exogenous cysteine restores swarming (30, 48, 60).
Mutants with defects in cysteine catabolism consistently exhibited a decreased lag phase before swarming (Fig. 5A and B). Once they were swarming, it appeared that both mutants swarmed at the same speed as the wild type. Such a property has been called precocious swarming (7, 35). Swarming lags are poorly understood, but they can be overcome by increasing inoculum density, certain mutations, or hyperflagellation (34). To rule out the first factor, colony counts of the initial inoculum density were measured for each swarm assay to ensure that all strains started with an average of 5 × 109 CFU/ml (Fig. 5C). As negative controls, we constructed TO10 (ΔcydD) and TO4 (ΔcysB), which exhibit defective swarming (30, 48, 60), and verified their impaired swarming (data not shown). To ensure that the mutant phenotype is specific to swarming motility, we inoculated the strains on swimming motility plates containing 0.25% agar. There was no difference in swimming motility between wild-type, ΔcdsH, and ΔybaO strains (Fig. 5D). We also observed precocious swarming on xylose swarm plates (data not shown). We conclude that defective cysteine catabolism affects swarming-specific motility.
Fig 5.
Swarming motility. (A) Swarming motility on 0.5% agar captured at various time points for which the diameters are represented graphically. (B) Swarm diameters. (C) Serial dilutions of the overnight cultures used to inoculate swarm medium to ensure the same initial starting densities. (D) Swimming motility on 0.25% agar.
Antibiotic resistance.
It has been previously shown that cysteine synthesis is required for antibiotic resistance of swarming cells (60). Therefore, we examined the effect of cysteine catabolism on antibiotic resistance. We measured the MICs of various antibiotics in LB medium (63). High exogenous cysteine increased antibiotic resistance (Fig. 6). This effect is specifically seen for aminoglycosides (streptomycin, spectinomycin, gentamicin, and kanamycin) but not for other antibiotic classes (chloramphenicol and tetracycline) (data not shown). However, a minor effect is seen with ampicillin (not shown), which may be consistent with a previous report that cysteine increases resistance to penicillin (49). Defective cysteine catabolism did not affect antibiotic susceptibility with or without cysteine (Fig. 6).
Fig 6.

Cysteine-enhanced antibiotic resistance. Growth with the indicated concentration of gentamicin, with or without 5 mM cysteine, is shown.
Virulence.
Macrophages are monocyte-derived cells that attack phagocytosed bacteria with acidification, reactive oxygen and nitrogen species, antimicrobial peptides, and lysosomal enzymes (62). S. enterica survives and replicates within macrophages. Monocytes increase cysteine uptake when activated by flagellar interaction with pathogen-associated molecular patterns (PAMPS) (24). Gene profiling has shown that both cdsH and ybaO are upregulated at 4 and 8 h postinfection of macrophages (28). Also, cdsH is upregulated in response to reactive nitrogen species (11). It is possible that cysteine contributes to the interaction between bacteria and macrophages. However, assay of S. enterica survival in a macrophage line showed no difference between wild-type and mutant strains after several hours (see Fig. S4 in the supplemental material).
DISCUSSION
CdsH, CutR/YbaO, and cysteine catabolism.
We identified the gene for the major CDS in S. enterica as STM0458 (cdsH) using bioinformatic and biochemical evidence. The evidence included the lowest least-squares score when comparing the amino acid composition of the previously purified CDS to that of all proteins, subunit size, the amino-terminal amino acid, high catalytic activity, loss of activity in a deletion strain, acquisition of activity by a plasmid that contains cdsH in E. coli (which lacks a major CDS), induction of the gene and protein by cysteine, and the observation that several other known sulfide-producing enzymes could not account for significant CDS activity. We proposed the redesignation of STM0458 as cdsH (cysteine desulfhydrase).
The gene adjacent to (but divergently transcribed from) cdsH specifies the major regulator of cdsH expression. Its current designation is ybaO (STM0459). Since this regulator controls multiple enzymes that catabolize cysteine, we propose the designation cutR (cysteine utilization regulator). (The identification and characterization of a second cysteine catabolic enzyme will be the subject of another communication.) CutR is homologous to Lrp, the leucine-responsive regulatory protein, which binds leucine and several other amino acids. It is plausible that CutR binds cysteine and activates transcription. The primary sequence of YbaO from S. enterica has been aligned with those of the three Lrp homologues from E. coli, Lrp, AsnC, and YbaO (66). It has been previously shown that CysB indirectly affects cysteine-inducible CDS activity by affecting transport (4). However, induction of CDS activity, which is presumably CdsH, is normal in various cysB mutants (41). Mutants with a deletion of either cdsH or cutR have distinct phenotypes that distinguish them from each other and the parental strains. Both mutants were more sensitive to cysteine toxicity, had altered sulfide production aerobically, and exhibited precocious swarming. However, only the cutR mutants had reduced sulfide production anaerobically.
The cdsH gene is one of several genes whose products metabolize sulfur-containing compounds in S. enterica but not E. coli. This group includes ttrABC (tetrathionate reductase), phsABC (thiosulfate reductase), and asrABC (sulfite reductase). The products of these operons can accept electrons for anaerobic respiration. The known properties of CdsH do not suggest an obvious relation to anaerobic respiration. Phylogenetic considerations also suggest that cdsH is not linked to these particular genes. For example, within the family Enterobacteriaceae, some genera lack both cdsH and ttrA (Erwinia), some have both (Salmonella, Klebsiella, Serratia, and Yersinia), and some have one but not the other (Citrobacter and Proteus).
The cdsH gene is present in Salmonella, Klebsiella, Serratia, Yersinia, and Citrobacter but not Escherichia, Shigella, Erwinia, and Proteus. In contrast, all genera have cutR (ybaO). It seems likely that CutR/YbaO regulates more genes than cdsH. The widespread distribution of cutR (ybaO) and cdsH suggests that they are not recent acquisitions within the Enterbacteriaceae.
Cysteine toxicity.
High exogenous cysteine is toxic to bacteria and eukaryotes. Several mechanisms have been proposed, including inhibition of anabolic enzymes, reduction of Fe(III) to Fe(II) and subsequent stimulation of the Fenton reaction and hydroxyl radical production, and inhibition of electron transport (46, 49). The greater lag before exponential growth indicated that cdsH and cutR mutants were more sensitive to exogenous cysteine. The cutR mutant has a longer lag than the cdsH mutant, which suggests that a cutR-regulated enzyme(s) contributed to degradation of cytotoxic cysteine.
Sulfide generation in vivo and cysteine catabolism.
A recent study shows that hydrogen sulfide protects a variety of bacteria from antibiotics (55). The basic mechanism appears to involve lowering the concentration of reactive oxygen species. Endogenous sulfide generation is therefore an important cellular reaction. CdsH generates sulfide with cysteine as the major nitrogen source for cells grown aerobically. In contrast, for anaerobic growth in broth with exogenous cysteine, CdsH was not a major contributor to sulfide generation. Instead, a second cysteine catabolic enzyme generated most of the sulfide. This enzyme, like CdsH, was controlled by CutR. Even when CdsH was overproduced 12-fold, CdsH did not detectably generate sulfide in vivo. We propose that some factor inside cells inhibits CdsH activity. A possible candidate is sulfide itself. The Ki for CdsH for sulfide is 10 μM (43). If the second enzyme has a higher Ki, then CdsH will be inactive when sufficient sulfide accumulates.
Our results suggest that at least two enzymes in S. enterica can degrade cysteine and generate sulfide. We suggest that there are several significant sulfide-generating reactions and that which is active will depend on what compounds are available (e.g., cysteine) and the physical environment (the presence of oxygen). In addition to sulfide-generating reactions of cysteine catabolism, S. enterica also possesses enzymes that can generate sulfide from thiosulfate and tetrathionate. Both of the latter compounds are readily available in the intestinal tract during inflammation (64). Their utilization is controlled by glucose or alternate electron acceptors, such as nitrate (5). Such regulation was also apparent for the CdsH-independent, CutR-regulated cysteine catabolic enzyme (Fig. 4B). Figure 7 summarizes the various reactions and pathways that generate sulfide.
Fig 7.
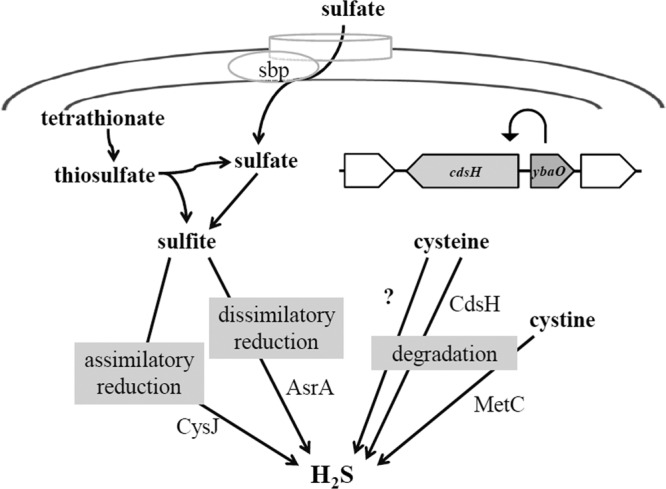
Sulfide-producing pathways. There are several sulfide-generating pathways and enzymes. The assimilatory reduction of sulfite generates sulfide for cysteine synthesis. The dissimilatory pathway of sulfite reduction uses sulfite as an electron acceptor for anaerobic respiration. CdsH is a cysteine catabolic enzyme that is controlled by YbaO. YbaO also controls a second enzyme, indicated by “?,” which will be the topic of another communication. The Sbp protein is the sulfate-binding protein that transports sulfate into the cell. In addition to these enzymes, the cysteine desulfurases and CysK and CysM proteins might provide background sulfide as side reactions.
Cysteine catabolism, swarming motility, and antibiotic resistance.
Mutants impaired in cysteine synthesis had defective swarming, even though cells were tested in a broth that allowed cysteine auxotrophs to grow. It was suggested that swarming requires a higher-than-normal level of intracelluar cysteine and an altered metabolism (30, 49, 60). Our results support this conclusion. Mutants defective in cysteine catabolism, which would be predicted to have higher intracellular cysteine, exhibited precocious swarming. A possible mechanistic link is that high intracellular cysteine inhibits certain enzymes, which may be sufficient to alter metabolism in a way that promotes swarming.
Turnbull and Surette have also shown that swarming cells have elevated resistance to antibiotics and that cysteine enhances antibiotic resistance of swarming cells (60). We confirmed the effect of cysteine on antibiotic resistance, but we observed a cysteine effect for planktonic cells. Loss of cysteine catabolic enzymes did not affect antibiotic susceptibility. Because of the different effects of loss of cysteine catabolism on swarming and antibiotic resistance, we propose that the effect of cysteine occurs by two different mechanisms. A previous study revealed that increasing exogenous cysteine decreases reduced periplasmic cytochrome c levels (49). Since aminoglycoside antibiotic uptake relies on anionic transporters energized mainly by the respiratory chain, impaired electron transport could explain the observed increase in aminoglycoside resistance with cysteine (12, 59). Defects in cysteine catabolism may have little effect on exogenous cysteine under these conditions, and this could explain the lack of effect on antibiotic susceptibility.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants MCB-0323931 from the National Science Foundation and GM085536 from the National Institutes of Health.
We thank Helene Andrews-Polymenis, D. J. Kopecko, and Lora Hooper for strains and the macrophage cell line, Eric Hansen and his lab for training in the use of the macrophage line, Piyush B. Lal for construction of the S. enterica metC and cydD mutants, Juan González and his lab for training and use of equipment, and Jeff DeJong and Santosh D'Mello for the use of tissue culture facilities.
Footnotes
Published ahead of print 8 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Appelbaum PC, et al. 1982. Comparison of three methods for identification of Enterobacteriaceae. Eur. J. Clin. Microbiol. 1: 76–81 [DOI] [PubMed] [Google Scholar]
- 2. Awano N, Wada M, Mori H, Nakamori S, Takagi H. 2005. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 71: 4149–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awano N, et al. 2003. Effect of cysteine desulfhydrase gene disruption on l-cysteine overproduction in Escherichia coli. Appl. Microbiol. Biotechnol. 62: 239–243 [DOI] [PubMed] [Google Scholar]
- 4. Baptist EW, Kredich NM. 1977. Regulation of l-cystine transport in Salmonella typhimurium. J. Bacteriol. 131: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barrett EL, Clark MA. 1987. Tetrathionate reduction and production of hydrogen sulfide from thiosulfate. Microbiol. Rev. 51: 192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beggs WH, Lichstein HC. 1965. Repression of tryptophanase synthesis in Escherichia coli. J. Bacteriol. 89: 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belas R, Schneider R, Melch M. 1998. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J. Bacteriol. 180: 6126–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bogomolnaya LM, Santiviago CA, Yang HJ, Baumler AJ, Andrews-Polymenis HL. 2008. ‘Form variation’ of the O12 antigen is critical for persistence of Salmonella typhimurium in the murine intestine. Mol. Microbiol. 70: 1105–1119 [DOI] [PubMed] [Google Scholar]
- 9. Botsford JL. 1975. Metabolism of cyclic adenosine 3′,5′-monophosphate and induction of tryptophanase in Escherichia coli. J. Bacteriol. 124: 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Botsford JL, DeMoss RD. 1971. Catabolite repression of tryptophanase in Escherichia coli. J. Bacteriol. 105: 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourret TJ, et al. 2008. Nitric oxide antagonizes the acid tolerance response that protects Salmonella against innate gastric defenses. PLoS One 3: e1833 doi:10.1371/journal.pone.0001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bryan LE, Nicas T, Holloway BW, Crowther C. 1980. Aminoglycoside-resistant mutation of Pseudomonas aeruginosa defective in cytochrome c552 and nitrate reductase. Antimicrob. Agents Chemother. 17: 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buchmeier NA, Heffron F. 1989. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 57: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carrington GO, Cleveland P, Jr, von Graevenitz A, Rupp WD. 1975. Biochemically aberrant Salmonella enteritidis ser. Newington from human sources in Connecticut. Yale J. Biol. Med. 48: 83–89 [PMC free article] [PubMed] [Google Scholar]
- 15. Chan RK, Botstein D. 1976. Specialized transduction by bacteriophage P22 in Salmonella typhimurium: genetic and physical structure of the transducing genomes and the prophage attachment site. Genetics 83: 433–458 [PMC free article] [PubMed] [Google Scholar]
- 16. Chen CY, Nace GW, Irwin PL. 2003. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 55: 475–479 [DOI] [PubMed] [Google Scholar]
- 17. Clark MA, Barrett EL. 1987. The phs gene and hydrogen sulfide production by Salmonella typhimurium. J. Bacteriol. 169: 2391–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark MA, Barrett EL. 1987. Catabolite repression of thiosulfate reduction by Salmonella typhimurium. Curr. Microbiol. 16: 27–31 [Google Scholar]
- 19. Collins JM, Monty KJ. 1973. The cysteine desulfhydrase of Salmonella typhimurium. Kinetic and catalytic properties. J. Biol. Chem. 248: 5943–5949 [PubMed] [Google Scholar]
- 20. Cowman RA, Baron SS, Fitzgerald RJ. 1983. Cysteine toxicity for oral streptococci and effect of branched-chain amino acids. Infect. Immun. 39: 1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dwivedi CM, Ragin RC, Uren JR. 1982. Cloning, purification, and characterization of β-cystathionase from Escherichia coli. Biochemistry 21: 3064–3069 [DOI] [PubMed] [Google Scholar]
- 23. Ederer GM, Lund ME, Blazevic DJ, Reller LB, Mirrett S. 1975. Motility-indole-lysine-sulfide medium. J. Clin. Microbiol. 2: 266–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edinger AL, Thompson CB. 2002. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc. Natl. Acad. Sci. U. S. A. 99: 1107–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flint DH, Tuminello JF, Miller TJ. 1996. Studies on the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase in Escherichia coli crude extract. Isolation of O-acetylserine sulfhydrylases A and B and β-cystathionase based on their ability to mobilize sulfur from cysteine and to participate in Fe-S cluster synthesis. J. Biol. Chem. 271: 16053–16067 [DOI] [PubMed] [Google Scholar]
- 26. Guarneros G, Ortega MV. 1970. Cysteine desulfhydrase activities of Salmonella typhimurium and Escherichia coli. Biochim. Biophys. Acta 198: 132–142 [DOI] [PubMed] [Google Scholar]
- 27. Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183: 6384–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hautefort I, et al. 2008. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell. Microbiol. 10: 958–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology 155: 541–550 [DOI] [PubMed] [Google Scholar]
- 30. Inoue T, et al. 2007. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 189: 950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isaacs H, Jr, Chao D, Yanofsky C, Saier MH., Jr 1994. Mechanism of catabolite repression of tryptophanase synthesis in Escherichia coli. Microbiology 140: 2125–2134 [DOI] [PubMed] [Google Scholar]
- 32. Kambampati R, Lauhon CT. 1999. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry 38: 16561–16568 [DOI] [PubMed] [Google Scholar]
- 33. Kari C, Nagy Z, Kovacs P, Hernadi F. 1971. Mechanism of the growth inhibitory effect of cysteine on Escherichia coli. J. Gen. Microbiol. 68: 349–356 [DOI] [PubMed] [Google Scholar]
- 34. Kearns DB. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8: 634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim DJ, Boylan B, George N, Forst S. 2003. Inactivation of ompR promotes precocious swarming and flhDC expression in Xenorhabdus nematophila. J. Bacteriol. 185: 5290–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SH, Schneider BL, Reitzer L. 2010. Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli. J. Bacteriol. 192: 5304–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim W, Surette MG. 2003. Swarming populations of Salmonella represent a unique physiological state coupled to multiple mechanisms of antibiotic resistance. Biol. Proc. 5: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kitagawa M, et al. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12: 291–299 [DOI] [PubMed] [Google Scholar]
- 39. Klumpp J, Fuchs TM. 2007. Identification of novel genes in genomic islands that contribute to Salmonella typhimurium replication in macrophages. Microbiology 153: 1207–1220 [DOI] [PubMed] [Google Scholar]
- 40. Kredich NM. 1996. Biosynthesis of cysteine, p 514–527 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 41. Kredich NM. 1971. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-l-serine on gene expression. J. Biol. Chem. 246: 3474–3484 [PubMed] [Google Scholar]
- 42. Kredich NM, Keenan BS, Foote LJ. 1972. The purification and subunit structure of cysteine desulfhydrase from Salmonella typhimurium. J. Biol. Chem. 247: 7157–7162 [PubMed] [Google Scholar]
- 43. Kredich NM, Foote LJ, Keenan BS. 1973. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J. Biol. Chem. 248: 6187–6196 [PubMed] [Google Scholar]
- 44. Morales VM, Backman A, Bagdasarian M. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97: 39–47 [DOI] [PubMed] [Google Scholar]
- 45. Natusch DF, Sewell JR, Tanner RL. 1974. Determination of hydrogen sulfide in air—an assessment of impregnated paper tape methods. Anal. Chem. 46: 410–415 [DOI] [PubMed] [Google Scholar]
- 46. Park S, Imlay JA. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 185: 1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Penrod JT, Roth JR. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J. Bacteriol. 188: 2865–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pittman MS, Robinson HC, Poole RK. 2005. A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. J. Biol. Chem. 280: 32254–32261 [DOI] [PubMed] [Google Scholar]
- 49. Pittman MS, et al. 2002. Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J. Biol. Chem. 277: 49841–49849 [DOI] [PubMed] [Google Scholar]
- 50. Roesch PL, Blomfield IC. 1998. Leucine alters the interaction of the leucine-responsive regulatory protein (Lrp) with the Fim switch to stimulate site-specific recombination in Escherichia coli. Mol. Microbiol. 27: 751–761 [DOI] [PubMed] [Google Scholar]
- 51. Rompf A, Schmid R, Jahn D. 1998. Changes in protein synthesis as a consequence of heme depletion in Escherichia coli. Curr. Microbiol. 37: 226–230 [DOI] [PubMed] [Google Scholar]
- 52. Saito H, Kobayashi H. 2003. Bacterial responses to alkaline stress. Sci. Prog. 86: 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwan WR, Huang XZ, Hu L, Kopecko DJ. 2000. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect. Immun. 68: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sekowska A, Kung HF, Danchin A. 2000. Sulfur metabolism in Escherichia coli and related bacteria: facts and fiction. J. Mol. Microbiol. Biotechnol. 2: 145–177 [PubMed] [Google Scholar]
- 55. Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334: 986–990 [DOI] [PubMed] [Google Scholar]
- 56. Snell EE. 1975. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 42: 287–333 [DOI] [PubMed] [Google Scholar]
- 57. Sorensen MA, Pedersen S. 1991. Cysteine, even in low concentrations, induces transient amino acid starvation in Escherichia coli. J. Bacteriol. 173: 5244–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soutourina J. 2001. Role of d-cysteine desulfhydrase in the adaptation of Escherichia coli to d-Cysteine. J. Biol. Chem. 276: 40864–40872 [DOI] [PubMed] [Google Scholar]
- 59. Taber HW, Mueller JP, Miller PF, Arrow AS. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51: 439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Turnbull AL, Surette MG. 2008. l-Cysteine is required for induced antibiotic resistance in actively swarming Salmonella enterica serovar Typhimurium. Microbiology 154: 3410–3419 [DOI] [PubMed] [Google Scholar]
- 61. Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. 2008. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U. S. A. 105: 20858–20863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. von Loewenich FD, Scorpio DG, Reischl U, Dumler JS, Bogdan C. 2004. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR) 2 and TLR4, or the TLR adaptor molecule MyD88. Eur. J. Immunol. 34: 1789–1797 [DOI] [PubMed] [Google Scholar]
- 63. Wiegand I, Hilpert K, Hancock RE. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3: 163–175 [DOI] [PubMed] [Google Scholar]
- 64. Winter SE, et al. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467: 426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xi H, Schneider BL, Reitzer L. 2000. Purine catabolism in Escherichia coli and function of xanthine dehydrogenase in purine salvage. J. Bacteriol. 182: 5332–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yokoyama K, Suzuki M. 2005. Orthologous and paralogous FFRPs in E. coli and related proteobacteria. Proc. Jpn. Acad. Ser. B 81: 129–139 [Google Scholar]
- 67. Yokoyama K, et al. 2006. Feast/famine regulatory proteins (FFRPs): Escherichia coli Lrp, AsnC and related archaeal transcription factors. FEMS Microbiol. Rev. 30: 89–108 [DOI] [PubMed] [Google Scholar]
- 68. Zhao C, Kumada Y, Imanaka H, Imamura K, Nakanishi K. 2006. Cloning, overexpression, purification, and characterization of O-acetylserine sulfhydrylase-B from Escherichia coli. Protein Expr. Purif. 47: 607–613 [DOI] [PubMed] [Google Scholar]
- 69. Zheng L, White RH, Cash VL, Jack RF, Dean DR. 1993. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 90: 2754–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.