Abstract
YknXYZ is the ATP-binding cassette export complex from Bacillus subtilis, where YknX is a membrane fusion protein, YknY is an ATPase, and YknZ is a permease. The yknXYZ genes are arranged into an operon that also includes yknW, encoding a membrane protein with four putative transmembrane segments. Previous studies suggested that the yknWXYZ operon belongs to the σw regulon and protects cells from the endogenous toxin SDP (sporulation-delaying protein) encoded by sdpC. In this study, we investigated the composition and function of YknW and YknXYZ. We report that the yknWXYZ operon is constitutively expressed in growing B. subtilis cells independently from sdpC. Chemical cross-linking in vivo and copurification approaches established that YknX interacts with YknYZ, whereas YknW binds YknXYZ, indicating that all four proteins form a complex in vivo. The complex assembly is modulated by YknW but proceeds in the absence of SdpC. When overproduced alone, YknW provides partial protection against SDP toxin, but all four Ykn proteins are required for full protection against both endogenous and exogenous SDP. We conclude that YknWXYZ is an unusual four-component transporter with a role in the starvation-induced killing of B. subtilis cells.
INTRODUCTION
The yknWXYZ operon was identified as a part of the σw regulon and the antimicrobial stress response in Bacillus subtilis cells (3). Sequence analyses show that the first gene of the operon, yknW, is a putative membrane protein which belongs to the Yip1 family of proteins interacting with Rab GTPases in eukaryotic cells. In yeasts, Yip1 is an essential gene required for vesicular transport (4). The bacterial YknW is highly conserved in Bacillus species and their close relatives but absent from genomes of many Gram-positive and Gram-negative bacteria. The function of YknW remains unknown.
The three other genes in the operon, yknXYZ, encode homologs of macrolide efflux transporters, such as MacAB from Escherichia coli, which are broadly distributed in various bacteria (11). In these transporters, YknX and MacA belong to the membrane fusion protein (MFP) family of proteins (6), whereas YknYZ and MacB are ABC-type transporters (5). Secondary-structure predictions indicate that YknX is a typical MFP containing a single N-terminal transmembrane segment (TMS) and a large extracytoplasmic domain. MFPs are abundant in Gram-negative bacteria, where they associate with various efflux transporters (18). Their proposed function is to facilitate transport of various substrates across the outer membrane by establishing a direct functional link between transporters and outer membrane channels. Although MFPs are present in almost all Gram-positive bacteria, their function in transport across a single membrane envelope remains unclear.
The ABC transporters are a ubiquitous family of proteins involved in various transport functions ranging from the secretion of toxins in bacteria to antigen processing in humans (5). Driven by ATP hydrolysis, all ABC transporters contain a highly conserved nucleotide-binding domain (NBD), which could be encoded either in the same polypeptide (MacB) or as an individual protein (YknY) which associates with the respective permease (YknZ). Based on their unusual membrane topology, MacB and its homologs define a distinct subfamily of ABC transporters: the macrolide exporter family (MEF). Macrolide exporters contain only four TMSs with the NBDs located at their N termini (10). In contrast, a typical ABC transporter contains at least six TMSs with the NBD attached to the C terminus.
The physiological substrates of macrolide exporters remain largely unknown. E. coli MacAB provides moderate protection against macrolide antibiotics when overproduced in E. coli cells lacking the major multidrug efflux pump AcrAB—hence the name of the family (11). MacAB is also implicated in secretion of heat-stable toxin II in enteropathogenic E. coli strains (16). In Pseudomonas aeruginosa, the MacAB homolog is involved in the secretion of the siderophore pyoverdine (9). B. subtilis cells lacking the yknWXYZ operon were found to be more susceptible to killing by the endogenously produced toxin SDP (sporulation-delaying protein) (3). SDP is the product of sdpC, the third gene of the sdpABC operon, which is expressed as a part of the Spo0A regulon in response to starvation (7). The starvation-induced activation of Spo0A takes place in only half of the cells in the population. Cells with activated Spo0A produce antimicrobial toxins, including SDP, that kill cells that have not activated Spo0A. It remains unclear how YknWXYZ protects cells from SDP killing.
Secondary-structure analyses suggested that SdpC contains a typical N-terminal signal peptide, which is followed by a 110-residue (E34 to Y144) hydrophilic extracytoplasmic domain. Its C-terminal domain contains a putative transmembrane α-helix (TMS) and a 37-residue cytoplasmic domain. Processed SdpC is found in the medium, suggesting that its C-terminal domain is not inserted into the membrane (13).
Initially, the toxicity of SDP was attributed to a 63-amino-acid peptide derived from the C-terminal portion of SdpC (8). However, recent analyses of the structure and activity of SDP showed that this toxin is a 42-residue peptide corresponding to C141 to S182 of SdpC containing one intrasubunit disulfide bond (14). SDP treatment of B. subtilis cells delayed growth in a concentration-dependent manner, collapsed the proton motive force, and ultimately caused cell lysis (12). The immunity factors SdpI and YfhL coexpressed with SDP have been shown to bind and provide immunity to SDP, whereas YknWXYZ might be responsible for exporting this toxin into the extracellular milieu (3).
In this study, we analyzed the expression, composition, and function of YknWXYZ. Our results show that this transporter is constitutively produced by growing B. subtilis cells, which contrasts with the transient expression of SdpC at the onset of the stationary phase. Although YknWXYZ expression protects B. subtilis from SDP, neither the amount nor the activity of SDP is dependent on the presence of YknWXYZ. Unlike other MFP-dependent transporters, the activity and assembly of YknXYZ require YknW but proceed in the absence of SDP. Taken together, our results demonstrate that YknWXYZ is an unusual four-component transporter with a role in the starvation-induced killing of B. subtilis cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. B. subtilis strains were a generous gift from John Helmann. Cells were grown in Luria-Bertani (LB) medium (10 g/liter Bacto tryptone, 5 g/liter Bacto yeast extract, and 5 g/liter NaCl, pH 7.0), modified Difco Sporulation Medium (DSM) [5 g/liter Bacto tryptone, 2.5 g/liter Bacto yeast extract, 2.5 g/liter NaCl, 1 g/liter KCl, 120 mg/liter MgSO4 · 7H2O, 1 mM Ca(NO3)2, 10 μM MnCl2, 1 μM FeSO4], or LB agar (LB medium containing 15 g/liter agar) at 37°C. Ampicillin (100 μg/ml) was used for the selection of E. coli. Chloramphenicol (10 μg/ml), kanamycin (10 μg/ml), and tetracycline (10 μg/ml) were used for the selection of B. subtilis.
Table 1.
Strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm | Novagen |
| DH5α | supE44 ΔlacU169 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | |
| B. subtilis | ||
| 168 | trpC2 | P. Klebba |
| Cu1065 | trpC2 attSPβ | 3 |
| HB6098 | Cu1065 Pspac-hy-sdpABC (Cm) | 3 |
| HB6121 | Cu1065 sdpABC-sdpRI∷tet yknW∷Kan | 3 |
| HB6127 | Cu1065 yknWXYZ∷Kan | 3 |
| HB6129 | Cu1065 sdpABC-sdpRI∷tet yknWXYZ∷Kan | 3 |
| HB6178 | Cu1065 sdpABC-sdpRI∷tet yknYZ∷Kan | 3 |
| HB6182 | Cu1065 sdpI∷mls yknWXYZ∷Kan | 3 |
| HB6186 | Cu1065 Pspac-hy-sdpABC (Cm) sdpI∷mls yknWXYZ∷Kan | 3 |
| HB6349 | Cu1065 yknW∷Kan | 3 |
| Plasmids | ||
| pET21d(+) | E. coli cloning vector | Novagen |
| pDG1514 | Contains tetracycline resistance gene (tet) | BGSC ECE100 |
| pEPR | pET21d(+) carrying processed SdpC | This study |
| pETXhis | pET21d(+) carrying yknXhis | This study |
| pETWhis | pET21d(+) carrying yknWhis | This study |
| pHCMC04 | E. coli and B. subtilis shuttle vector | BGSC ECE189P |
| pHTCMC04 | pHCMC04 carrying tet from pDG1514 | This study |
| pHX | pHCMC04 carrying yknX | This study |
| pHXHis | pHCMC04 carrying the 6His-tagged yknX | This study |
| pHWX | pHCMC04 carrying yknWX | This study |
| pHWXHis | pHCMC04 carrying yknW and the 6His-tagged yknX | This study |
| pHW | pHCMC04 carrying yknW | This study |
| pHWHis | pHCMC04 carrying the 6His-tagged yknW | This study |
| pHTWX | pHTCMC04 carrying yknXYZ | This study |
| pHXYZ | pHCMC04 carrying yknXYZ | This study |
| pHXYZHis | pHCMC04 carrying yknXY and the 6His-tagged yknZ | This study |
| pHTXYZ | pHTCMC04 carrying yknXYZ | This study |
| pHYZ | pHCMC04 carrying yknYZ | This study |
| pHYZHis | pHCMC04 carrying yknY and the 6His-tagged yknZ | This study |
| pHWXYZ | pHCMC04 carrying yknWXYZ | This study |
| pHWXYZHis | pHCMC04 carrying yknWXY and 6His-tagged yknZ | This study |
| pHTWXYZ | pHTCMC04 carrying yknWXYZ | This study |
For PCR cloning, genomic DNA from B. subtilis 168 was used as a template. PCR products encoding YknX, YknW, and the whole-length SdpC and its domains were cloned into pET21d(+) vector using the following restriction sites: NcoI and XhoI for YknX and YknW and NcoI and EcoRI for a processed SdpC. For expression in B. subtilis, yknW, yknX, yknWX, yknWXYZ, and yknXYZ and their variants encoding 6His-tagged proteins were cloned into pHCMC04 (B. subtilis Genetic Stock Center [BGSC]) by the use of SpeI and BamHI restriction sites. The tetracycline resistance marker was excised with XbaI from pDG1514 (BGSC) and religated into NheI sites of pHCMC04, pHWX, pHXYZ, and pHWXYZ. No undesired substitutions were detected by DNA sequencing (Oklahoma Medical Research Foundation). As a result of cloning into pETXhis, there is one extra alanine in the second position of YknX.
Spot-on-lawn assay.
This assay was carried as described by Butcher and Helmann (3). Briefly, lawns were created by inoculating 0.7 ml of B. subtilis cells grown to an optical density at 600 nm (OD600) of ∼0.4 into 14 ml of LB containing 0.7% agar, which was kept molten at 50°C. This was poured into a plate and allowed to solidify. Samples were spotted onto plates and dried. Inhibition zones were detected after 10 h of incubation at 37°C. When needed, 1 mM IPTG and 0.5% xylose were added for induction of sdp and ykn operons, respectively.
Immunoblotting analysis.
To generate polyclonal antibodies, E. coli BL21(DE3) harboring one pETXhis, pEPR, or pETWHis was inoculated into 100 ml of LB broth supplemented with 100 μg/ml ampicillin. When the OD600 reached 0.6, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce expression of the proteins. After 2 h of incubation at 37°C, cells were harvested and lysed by sonication in the presence of 100 μg/ml lysozyme and 10 mM EDTA. SdpC was purified from soluble fractions by using His-Bind Resin (Novagen) charged with Ni2+ and standard protocols. For purification of YknX and YknW, unbroken cells were removed and membranes isolated by ultracentrifugation at 72,000 × g for 40 min at 4°C. Membranes were washed, resuspended in 1.5 ml of 20 mM Tris-HCl (pH 8.5), 0.5 M NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5% Triton X-100 and incubated on ice overnight. Solubilized proteins were diluted 5 times by adding same buffer containing 0.2% Triton X-100 and 5 mM imidazole. YknXhis and YknWhis were purified by using His-Bind Resin charged with Ni2+. After purification, the respective protein bands were excised from gels and sent to Covance for production of polyclonal antibodies by the use of standard protocols.
For immunoblotting, samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride membranes (Millipore) in 25 mM Tris base–192 mM glycine–10% methanol. YknX, YknW, and SdpC were detected using standard immunoblotting protocols with a secondary alkaline phosphatase-conjugated anti-rabbit antibody (Sigma). The 5-bromo-4-chloro-3-indoyl phosphate–Nitro Blue Tetrazolium substrates were used to visualize protein bands.
Fractionation of supernatants and MALDI-TOF analysis.
B. subtilis HB6098 (Pspac-hy-sdpABC) was grown in minimal medium containing 10 mM ammonium sulfate, 5 mM potassium phosphate (pH 7.0), 100 mM morpholinepropanesulfonic acid-NaOH (MOPS-NaOH) (pH 7.0), 2 mM MgCl2, 0.7 mM CaCl2, 50 μM MnCl2, 5 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 14 mM d-glucose, 10 mM l-glutamate, and 50 μg/ml l-tryptophan (8). SdpC expression was induced by addition of 1 mM IPTG. When the OD600 reached 1.0 to 1.2, cells were removed by using centrifugation and filtration. The supernatant was applied to a Sep-Pak C-18 cartridge (Waters). Samples were eluted with methanol as described in reference 8. Fractions were lyophilized and reconstituted in 20 μl of 1:1 acetonitrile/water with 0.1% trifluoroacetic acid. Samples (1 μl) were mixed with 1 μl of matrix (sinapinic acid at 10 mg/ml in 1:1 acetonitrile/water) and analyzed with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) set to linear/positive mode (Voyager DE Pro; Applied Biosystems, Molecular Biology-Proteomics Facility at the University of Oklahoma).
RT-PCR.
Reverse transcriptase PCR (RT-PCR) was performed by using a OneStep RT-PCR kit (Qiagen). Cells were grown in the LB medium supplemented with 0.5% xylose until the OD600 reached 0.7. Total RNA was isolated using an RNeasy kit (Qiagen). To detect the yknY transcript, the forward primer 5′-GCTCTAGATGGCCATTCAGCTTTCTAA-3′ and the reverse primer 5′-GAATTCTCGAGTTCTCCCACACTCC-3′ were used to yield a product of 697 bp. To detect the yknZ transcript, the forward primer 5′-GACGGCGTCGACTTGGAAAACATCAG-3′ and the reverse primer 5′-CGCGGATCCCTACTCATAACGCAGCGCTTCAA-3′ were used to yield a product of 1,206 bp.
Chemical cross-linking in vivo and miniscale purification of Ykn proteins from B. subtilis.
B. subtilis HB6127 cells transformed with pHCMC04, pHWHis, pHXHis, pHWXHis, pYZHis, pHXYZHis, and pHWXYZHis plasmids were inoculated into 5 ml of LB medium supplemented with 5 μg/ml chloramphenicol and incubated at 37°C until cells reached the stationary phase. Then, cells were reinoculated into 500 ml of LB medium supplemented with chloramphenicol and incubated until the OD600 reached ∼0.5. Protein expression was induced using 0.5% xylose for 3 h. Cells were collected by centrifugation at 3,440 × g at room temperature for 20 min and resuspended in 20 ml of 0.1 M sodium phosphate buffer (pH 7.0). Each sample was divided into two tubes, and dithiobis(succinimidyl propionate) (DSP) in the final concentration of 0.4 mM was added to one of the tubes, whereas the same volume of dimethyl sulfoxide was added to the other tube as a solvent control. Cross-linking was carried out for 30 min at 37°C. The cross-linking reaction was stopped by addition of 1 M Tris-HCl (pH 8.0). Cells were centrifuged at 3,440 × g at 4°C for 20 min, resuspended in buffer containing 10 mM Tris-HCl (pH 8.0), 5 mM EDTA, and 1 mM PMSF, and treated with 1 mg/ml lysozyme for 30 min on ice. Cells were broken by 3 or 4 rounds of 30-s sonication pulses on a Branson Sonifier 450 apparatus. Unbroken cells were removed by low-speed centrifugation at 3,220 × g at 4°C for 20 min. Total membrane fractions were isolated by ultracentrifugation at 100,000 × g for 1 h at 4°C. Membrane proteins were solubilized in 5 ml of 20 mM Tris-HCl (pH 8.0)–100 mM NaCl–1 mM PMSF–5 mM imidazole–5% Triton X-100 for 5 h on ice. After removal of the insoluble fraction by ultracentrifugation, solubilized proteins were loaded onto a 0.4-ml column packed with Cu2+-charged His-Bind resin (Novagen). Ykn proteins were purified using a step gradient of 5, 15, 30, 100, and 500 mM imidazole. The 6His-tagged proteins were present in 100 mM and 500 mM imidazole fractions. Purified proteins were then concentrated using YM10 centrifugal devices (Millipore) and analyzed by SDS-PAGE and immunoblotting.
For a membrane-mixing experiment, 12.5 mg of total membrane proteins isolated from cells producing YknXHis, YknYZHis, or YknXYZHis was mixed with an equal amount of membrane proteins obtained from B. subtilis HB6127 cells carrying either pHCMC04 vector or pHW plasmid, producing YknW without an affinity tag. After mixing, membrane proteins were solubilized, purified, and concentrated as described above.
RESULTS
YknWXYZ and SdpC are produced independently.
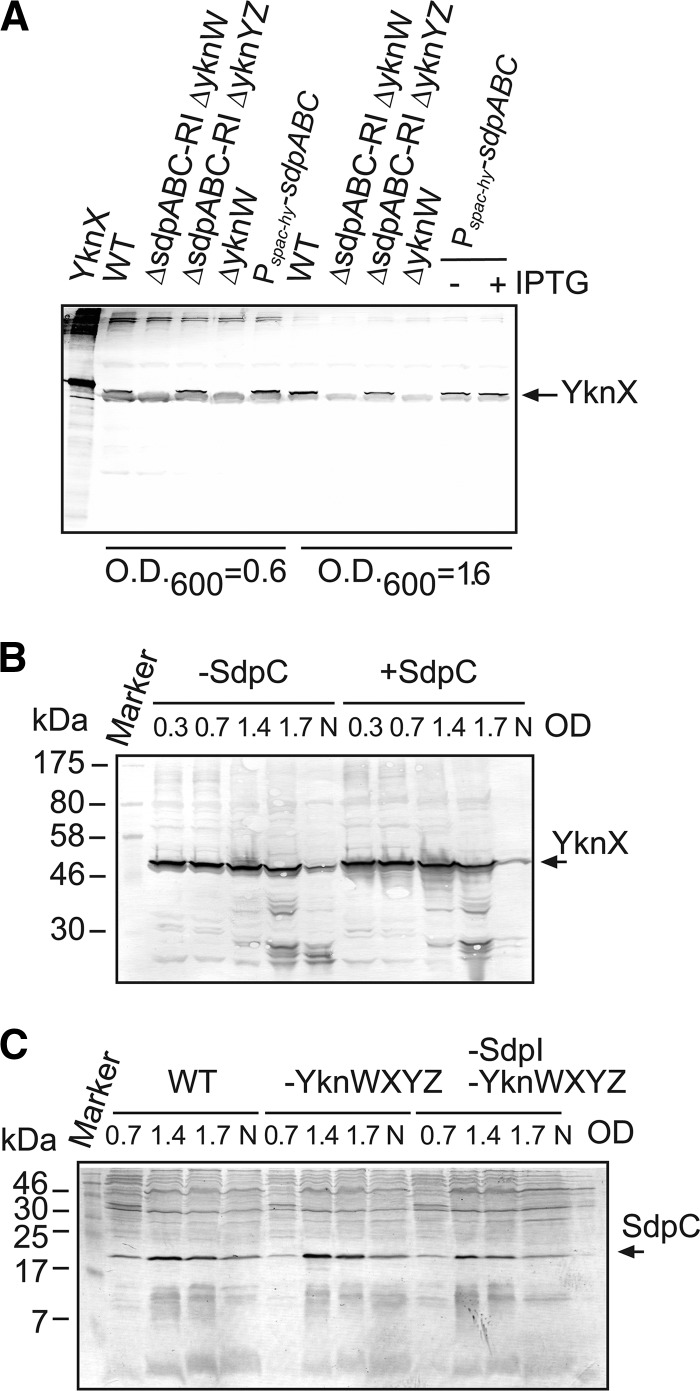
Previous studies suggested that yknWXYZ belongs to the σw regulon, which controls both the production of and defense against antimicrobial compounds (3). Among various inducers of the σw regulon is SDP toxin. To investigate a possible role of SDP in expression of yknWXYZ, we analyzed production of SdpC and YknX in B. subtilis cells with different genetic backgrounds. Immunoblotting analysis of whole-cell extracts with an anti-YknX antibody showed that exponentially growing wild-type (WT) Cu1065 cells produce YknX in the absence of the known σw regulon inducers, such as SDP and antibiotics (Fig. 1A). The time-resolved analysis of expression showed that the amounts of YknX changed very little during growth and the onset of the stationary phase, but some degradation of the protein was observed after transition into the stationary phase (Fig. 1B). Expression levels of YknX were unaffected by the sdpC genotype, even when the expression of Pspac-sdpABC was induced by addition of IPTG (Fig. 1A and B). Similar results were obtained for the expression of YknW (data not shown).
Fig 1.
Growth-phase-dependent expression of YknX and SdpC in B. subtilis cells. (A) Immunoblotting analysis of whole B. subtilis cells with various genetic backgrounds. Cells were grown in LB medium and collected at OD600s of 0.6 and 1.6. Total proteins (5 μg) were separated by 12% SDS-PAGE and analyzed by immunoblotting with polyclonal anti-YknX antibodies. Purified YknXHis (lane 1) was loaded as a positive control. A cross-reacting band with SDS-PAGE mobility slightly faster than that of YknX was more abundant in exponential- than in stationary-phase cells. (B) HB6098 (Pspac-sdpABC) cells were grown in the presence (+SdpC) and absence (-SdpC) of 1 mM IPTG to indicated ODs. A 10-μg volume of total protein was separated by 10% SDS-PAGE and probed with anti-YknX antibodies. N, cells grown overnight. (C) B. subtilis strains Cu1065 (WT), HB6127 (-YknWXYZ), and HB6182 (-SdpI and -YknWXYZ) were grown to indicated ODs. A 10-μg volume of total protein was separated by 16% SDS-PAGE and probed with anti-SdpC antibody. N, cells grown overnight.
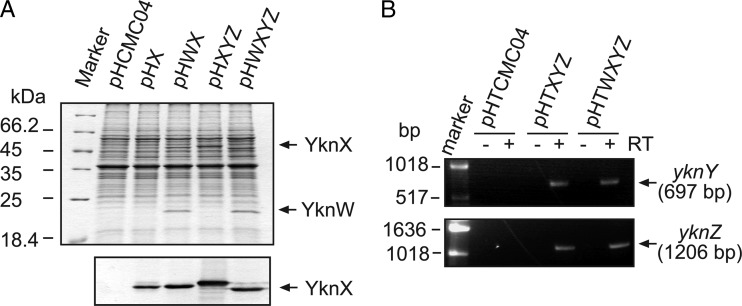
YknX was produced at the same levels in the wild type and cells lacking yknYZ genes encoding the cognate transporter. Thus, the expression and stability of YknX do not require YknYZ. In contrast, no expression of YknX was detected in cells lacking yknW (Fig. 1A). This result suggested either that deletion of yknW has a polar effect on the downsteam genes or that YknW is needed for YknX expression. Since YknX was effectively produced from plasmids lacking yknW (Fig. 2A), it is likely that deletion of yknW disrupted expression of the downstream genes.
Fig 2.
Overproduction of YknW, YknX, and YknYZ in B. subtilis. (A) Membrane fractions isolated from HB6127 (yknWXYZ∷Kan) carrying the indicated plasmids were separated by 12% SDS-PAGE and stained with CBB (top panel) or electroblotted onto a polyvinylidene difluoride (PVDF) membrane and probed with anti-YknX antibodies (bottom panel). The expression of yknWXYZ was induced by addition of 0.5% xylose for 1 h at 37°C. (B) RT-PCR analysis of total mRNA isolated from HB6186 [Pspac-hy-sdpABC (Cm) sdpI∷mls yknWXYZ∷Kan] carrying pHTXYZ or pHTWXYZ with primers complementary to yknY and yknZ transcripts.
To determine whether the expression and processing of SdpC are affected by Ykn proteins, exponential- and stationary-phase B. subtilis cells with various genetic backgrounds were probed with anti-SdpC antibody. In agreement with previous studies (7), the SdpC expression peaked at the onset of the stationary phase and then slowly decreased over time (Fig. 1C). The lack of YknWXYZ and the immunity factor SdpI did not affect the expression of this protein. In whole cells, SdpC was detected as a single band with an estimated molecular mass of ∼22 kDa, which is in good agreement with the predicted product of the sdpC gene (Fig. 1C; see also Fig. S1 in the supplemental material). In agreement with previous data (13, 14), two forms of SdpC were present in culture supernatants: the processed SdpC lacking its signal peptide with an apparent MW corresponding to a molecular mass of ∼19 kDa and the mature SDP toxin corresponding to the C-terminal 42-amino-acid-residue fragment of SdpC (see Fig. S1 in the supplemental material).
Taken together, these results show that SdpC and YknX are expressed independently from each other. Furthermore, the peaks of expression of SdpC and of YknX occur at different times. SdpC is limited to the stationary phase, whereas YknX is expressed in the exponential phase and degraded in the stationary phase. The whole-length SdpC is present in B. subtilis cells, but its processed form is secreted into the medium. The formation of SDP toxin in cells and supernatants proceeds without accumulation of stable SdpC intermediates and without YknWXYZ.
Plasmid-encoded YknWXYZ protects cells from endogenously produced SDP.
Previous studies implicated YknWXYZ in protection against endogenous SDP (3). To gain further insight into a possible functional link between Ykn and SDP, we constructed expression plasmids producing YknX alone and in combination with YknW or YknYZ or both under the control of the tightly controlled xylose-inducible promoter (Table 1). Protein bands corresponding to both YknW and YknX were readily detected in membranes isolated from B. subtilis cells carrying plasmids encoding the respective genes (Fig. 2A). However, we were not able to identify bands specific for YknY or YknZ. To confirm that yknY and yknZ are expressed from the plasmids, we used RT-PCR. As shown in Fig. 2B, both yknY and yknZ transcripts were present in cells carrying the pHTXYZ and pHTWXYZ plasmids. In addition, expression of the yknZ gene was confirmed by attaching a C-terminal 6His affinity tag and protein purification (see Fig. 6).
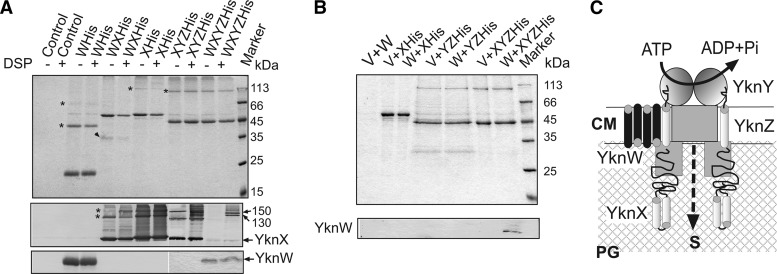
Fig 6.
Composition and assembly of YknWXYZ complex. (A) Combinations of YknWHis, YknXHis, and YknZHis proteins were expressed in B. subtilis HB6127 cells. Control cells carried an empty vector. Miniscale purifications using 6His tags of proteins were carried out from DSP-treated and untreated cells. Purified proteins (0.1 to 0.3 μg) were loaded onto 12% SDS-PAGE gels. Heat denaturation and the reducing agent in the sample buffer were omitted. After separation, proteins were detected using Coomassie brilliant blue (top panel) or transferred onto a PVDF membrane for immunoblotting with anti-YknX (middle panel) or anti-YknW (bottom panel). Oligomers are indicated by stars. A 35-kDa protein present in YknXHis purified from cells overproducing YknW is indicated by an arrow. (B) Total membranes isolated from cells producing YknXHis, YknYZHis, and YknXYZHis were mixed with membranes of cells containing either an empty vector (V) or YknW (W) before solubilization in 5% TX-100. Purification of His-tagged proteins was carried out as described for panel A. Purified proteins (0.4 μg) were analyzed by 12% SDS-PAGE followed by Coomassie staining (top panel) or by immunoblotting with anti-YknW antibodies (bottom panel). For immunoblotting analysis, protein samples were denatured by boiling in SDS sample buffer. (C) Schematic representation of YknWXYZ complex. YknW possesses partial activity and modulates the assembly of the complex. PG, peptidoglycan; CM, cytoplasmic membrane; S, substrate.
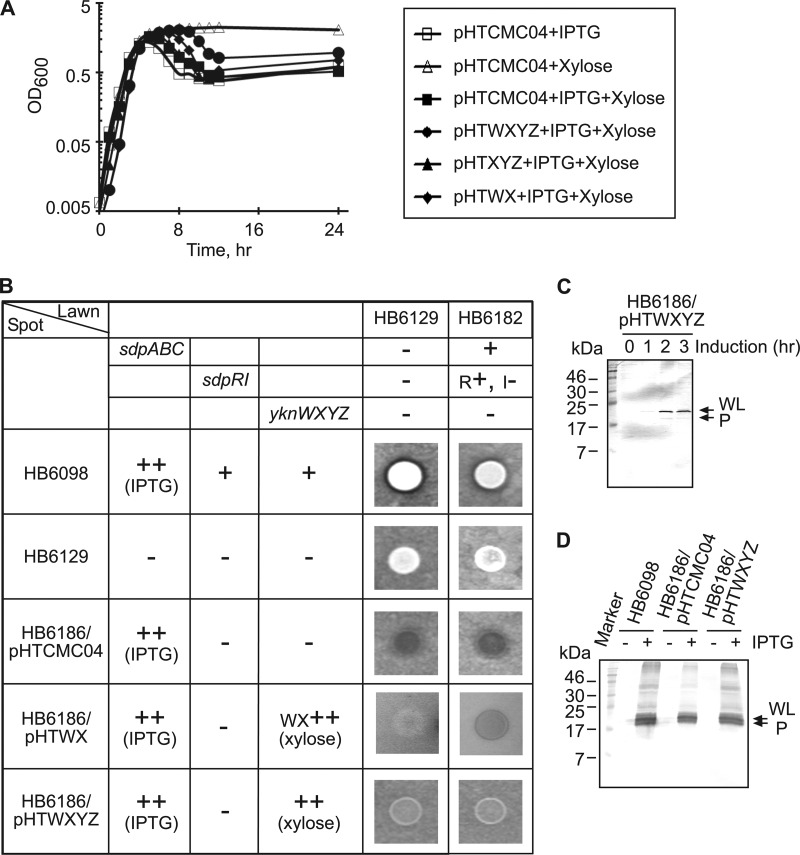
To investigate the role of YknWXYZ in protection against SDP, plasmids carrying yknWX, yknXYZ, and yknWXYZ were introduced into strain HB6186 [Pspac-hy-sdpABC (Cm) sdpI∷mls yknWXYZ∷Kan]. Since SdpI protein provides the primary immunity to SDP toxin, HB6186 cells lyse in a liquid medium and do not grow well on a solid medium when the sdpABC operon is induced by IPTG (3). In agreement, we found that in LB medium, the addition of IPTG induced lysis of HB6186 cells carrying the empty pHTCMC04 vector (Fig. 3A). The plasmid-borne YknXYZ did not rescue this phenotype, as HB6186 cells carrying pHTXYZ plasmid lysed after addition of IPTG as well. In contrast, the overexpression of the complete yknWXYZ operon delayed the IPTG-induced cell lysis for at least 6 h. A partial lysis of YknWXYZ-overproducing cells late in the stationary phase was likely caused by depletion of the xylose inducer and degradation of YknX (Fig. 1B). Surprisingly, the expression of YknWX produced an intermediate phenotype by delaying cell lysis for 2 to 3 h. This result suggested that YknWX possesses partial anti-SdpC activity.
Fig 3.
Protection of B. subtilis cells from the SDP-dependent self-lysis. (A) B. subtilis HB6186 (Pspac-hy-sdpABC sdpI∷mls yknWXYZ∷Kan) carrying pHTCMC04 vector or its derivatives containing yknWXYZ (pHTWXYZ), yknWX (pHTWX), and yknXYZ (pHTXYZ) were grown to mid-exponential phase and diluted 1:50 into DSM either with or without 1 mM IPTG and 0.5% xylose. (B) B. subtilis cells were spotted onto agar plates containing either HB6129 (sdpABC-sdpRI∷tet yknWXYZ∷Kan) or the HB6182 (sdpI∷mls yknWXYZ∷Kan) lawn strain, and plates were supplemented with 1 mM IPTG and 0.5% xylose to induce the production of SDP and Ykn (indicated by ++), respectively. Inhibition zones were detected after 10 h of incubation at 37°C. +, present; −, absent; R+, sdpR present; I−, sdpI absent. (C) Expression of SdpC in cells overproducing Ykn proteins. HB6186 cells carrying the pHTWXYZ plasmid (HB6186/pHTWXYZ) were supplemented with 1 mM IPTG and 0.5% xylose and incubated for 3 h. Protein (10 μg total) was separated by 16% SDS-PAGE and analyzed by immunoblotting with anti-SdpC antibody. WL, whole length; P, processed. (D) Effect of YknWXYZ overproduction on the secretion of SdpC. Secreted proteins were precipitated by 10% trichloroacetic acid (TCA) from 10 ml of culture supernatants of indicated cells. Proteins were separated by 16% SDS-PAGE and analyzed by immunoblotting with anti-SdpC antibody. WL, whole length; P, processed.
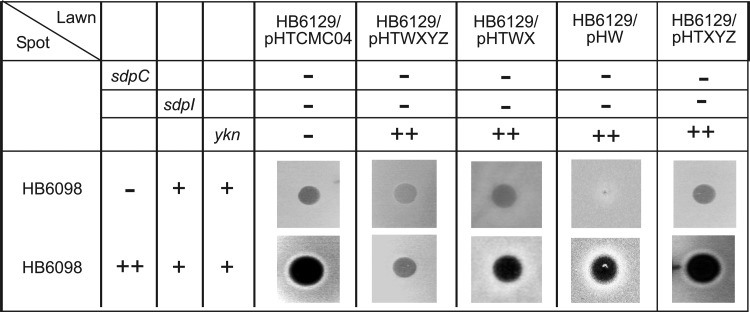
To test whether overproduction of YknWX or YknWXYZ enables growth on solid medium, we spotted HB6186 cells transformed with various plasmids onto lawns of HB6129 (sdpABC-sdpRI∷tet yknWXYZ∷Kan) or HB6182 (sdpI∷mls yknWXYZ∷Kan) cells (Fig. 3B). The HB6129 lawn did not produce its own SDP and was unable to kill the spot but was susceptible to killing by exogenous SDP, whereas the HB6182 lawn was susceptible to killing by both the endogenous toxin and SDP produced by the spotted cells. As expected, HB6098 cells producing the IPTG-induced SDP in the presence of the chromosomal sdpRI and yknWXYZ copies were able to grow on the solid medium and inhibited growth of the lawn cells. No inhibition of growth or clearance of the lawn was observed for the HB6129 ΔsdpABC-sdpRI ΔyknWXYZ cells. In contrast, the IPTG-induced overproduction of SDP in HB6186 [Pspac-hy-sdpABC (Cm) sdpI∷mls yknWXYZ∷Kan] carrying the empty vector led to lysis of both the spot and the lawn cells, confirming that these cells undergo lysis and release the endogenous SDP. This lysis of HB6186 cells was prevented by expression of the plasmid-borne YknWXYZ, albeit the density of the HB6186/pHTWXYZ spot was somewhat lower than that of HB6129 or HB6098 cells. Thus, YknWXYZ protects HB6186 cells from SDP-induced lysis in both liquid and solid media. Consistent with the partial activity, the overproduction of YknWX enabled growth of HB6186 but the density of the spot was even less than that of HB6186/pHTWXYZ (Fig. 3B).
No inhibition of the lawn cells was observed for the HB6186/pHTWXYZ spots (Fig. 3B). Immunoblotting analysis with anti-SdpC antibody showed that overproduction of YknWXYZ does not affect the induction of SdpC (Fig. 3C). At 2 h after addition of IPTG, the whole-length SdpC was clearly detected in HB6186/pHTWXYZ cells. SdpC expression remained at the same level 3 h after addition of IPTG, and supernatants of HB6186/pHTWXYZ cells contained the same amounts of SdpC as HB6098 cells and HB6186 cells carrying the empty vector (Fig. 3D). Hence, the lack of an inhibition zone was likely a consequence of the low density of HB6186/pHTWXYZ cells in the spot and small amounts of SDP released into the medium by surviving cells.
YknWXYZ provides resistance to exogenous SDP.
To investigate whether YknWXYZ provides resistance to exogenous SDP, we first constructed E. coli expression plasmids producing the whole-length SdpC, its processed variant lacking the N-terminal signal peptide sequence, the soluble extracytoplasmic domain, and the C-terminal domain containing the active SDP sequence (see Fig. S1 in the supplemental material). However, none of the recombinant purified SdpC variants killed susceptible B. subtilis cells in a spot-on-lawn assay (data not shown). Therefore, as a source of exogenous SDP, we used concentrated supernatants of HB6098 (Pspac-sdpABC) cells that had been incubated in the presence or absence of IPTG (Fig. 4). In this assay, the SDP-containing and control supernatants were spotted onto lawns of HB6129 (sdpABC-sdpRI∷tet yknWXYZ∷Kan) cells carrying pHCMC04 vector or its derivatives producing YknW, YknWX, YknXYZ, or YknWXYZ, respectively. As seen from the large inhibition zones on the SDP-susceptible HB6129/pHTCMC04 lawn, induction of Pspac-sdpABC in HB6098 cells led to accumulation of SDP in the supernatant and killing of HB6129 (Fig. 4). Interestingly, the lawn HB6129 cells carrying the pHTWXYZ plasmid but not those with the pHTXYZ plasmid were partly resistant to SDP-containing supernatants. In addition, cells producing either YknW alone or both YknW and YknX were slightly less susceptible to SDP than cells carrying the empty vector or those producing YknXYZ.
Fig 4.
Role of Ykn proteins in protection against exogenous SdpC. HB6098 (Pspac-sdpABC) cells were grown in minimal medium in the presence or absence of IPTG. At OD600 ∼ 1.0 to 1.2, cells were removed by centrifugation and ultrafiltration and ice-cold TCA was added to supernatants for a final concentration of 10%. The TCA precipitates were solubilized and neutralized with 0.1 M NaOH and spotted onto an 0.7% LB agar plate containing 0.5% xylose and the indicated lawn strains. Inhibition zones were detected after 10 h of incubation at 37°C. In spot cultures, 1 mM IPTG was used for the induction of SdpC. Xylose at a final concentration of 0.5% was added to all lawn plates to induce expression of the ykn operon.
Taken together, these results show that YknWXYZ protects B. subtilis cells from both endogenous and exogenous SDP toxin. Overproduction of YknW provides weak protection, whereas the YknXYZ transporter does not protect against SDP in the absence of YknW.
YknW but not SdpC modulates the assembly of the YknXYZ complex.
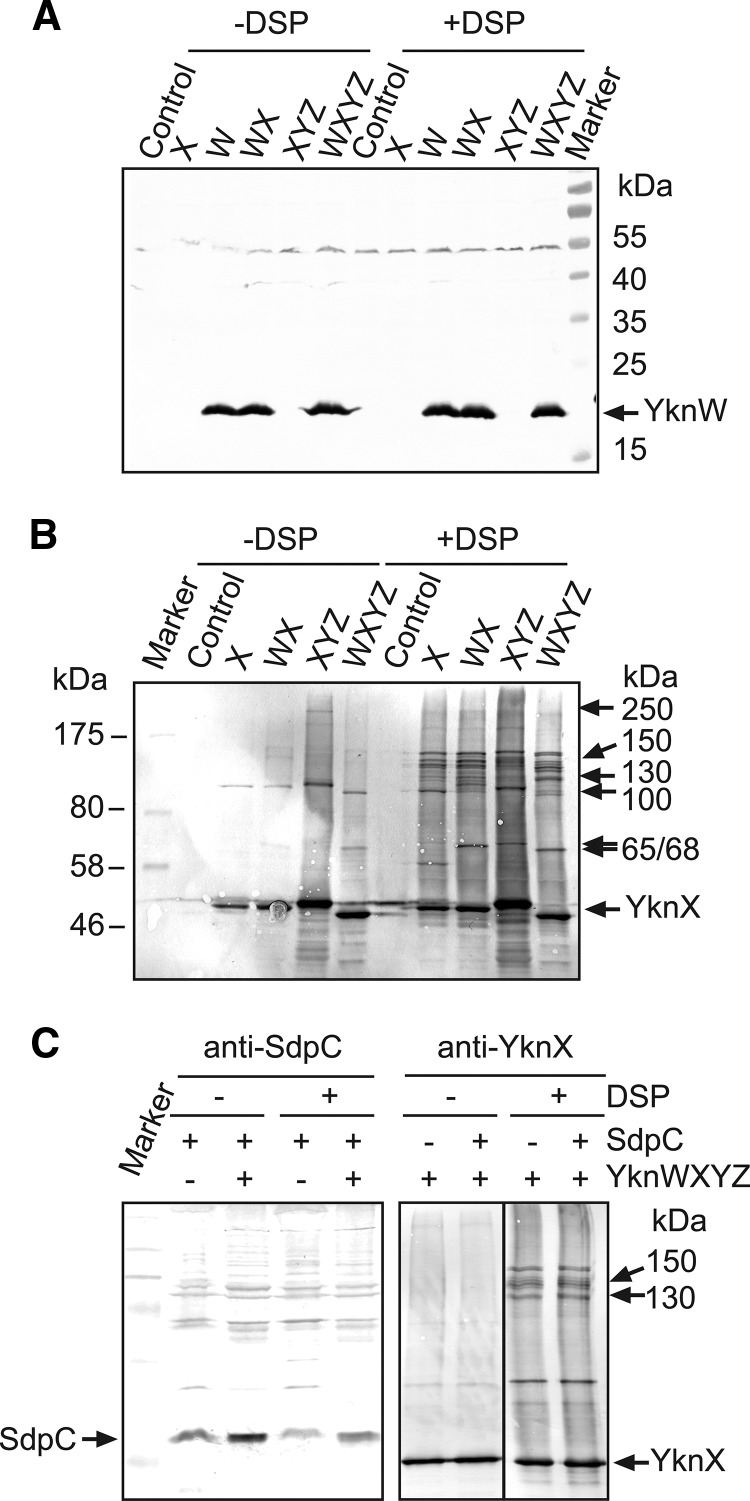
The experiments described above showed that the function of YknXYZ in SDP resistance depends on the presence of YknW. We next used chemical cross-linking of cells producing Ykn proteins separately and in combination to visualize complexes assembled in B. subtilis membranes (Fig. 5). The anti-YknW immunoblotting of membrane fractions isolated from cells carrying various constructs showed a single YknW-specific band (Fig. 5A). Cross-linking with the amine-reactive DSP did not stabilize any YknW-containing complexes. In contrast, even when produced alone, YknX was cross-linked into high-molecular-weight (HMW) species, which, by their masses of ∼100 kDa and ∼150 kDa, could correspond to YknX oligomers (Fig. 5B). Surprisingly, the coexpression of YknW and YknX changed the cross-linking profiles of YknX, with the appearance of additional ∼65-kDa and ∼120-kDa bands. However, neither of these bands cross-reacted with anti-YknW antibodies. It is possible that YknW amounts in these complexes are too small for immunodetection or, alternatively, that YknW promotes formation of YknX-containing complexes without establishing a stable association with them.
Fig 5.
In vivo chemical cross-linking of B. subtilis cells with various ykn and sdpC genotypes. (A and B) In vivo DSP cross-linking of HB6127 (yknWXYZ∷Kan) cells carrying plasmids producing the indicated combinations of Ykn proteins. Membrane fractions were separated by 10% SDS-PAGE followed by immunoblotting with anti-YknW (A) and anti-YknX (B) antibodies. Molecular masses of high-molecular-weight cross-linked complexes are indicated. (C) HB6186 (Pspac-hy-sdpABC sdpI∷mls yknWXYZ∷Kan) cells carrying pHTWXYZ were grown in different combinations of 1 mM IPTG and 0.5% xylose to induce the expression of sdp or ykn. Cells were treated with DSP, and membrane fractions were analyzed by immunoblotting with the indicated polyclonal antibodies.
The coexpression of YknX and YknYZ led to formation of ∼250-kDa species, which were seen in both the untreated and DSP-treated samples (Fig. 5B). Assuming that YknYZ is a dimer, its calculated mass should be close to 135 kDa (YknY is 25,272 Da and YknZ is 42,125 Da). Even with 2:2 YknX:YknYZ stoichiometry, the mass of the complex is expected to be about 220 kDa. Thus, the ∼250-kDa species could represent YknXYZ complexes. An additional ∼68-kDa band in the DSP cross-linked sample could be a complex between YknX and YknY (calculated mass of 66,979 Da).
When all four YknWXYZ proteins were coexpressed, two unique ∼130- and ∼150-kDa bands were clearly seen in cross-linked cell membranes. Since these bands reacted with anti-YknX antibody, all these HMW species contain YknX. Although no YknW could be detected in these complexes by anti-YknW immunoblotting (Fig. 5B), their size is close to that of a four-component YknWXYZ complex assembled in a stoichiometry at 1:1:1:1 (133,279 Da). This result suggests that YknW contributes to assembly of YknX-containing complexes. But whether YknW is a component of these complexes remains unclear.
Cross-linking profiles of YknX were not affected by the presence or absence of SdpC (Fig. 5C). Furthermore, immunoblotting with anti-SdpC antibody did not visualize any HMW bands containing SdpC. This result suggests that SdpC and YknWXYZ do not interact appreciably with each other.
YknWXYZ proteins assemble in a four-component complex.
To investigate the composition of YknX-containing complexes, 6His affinity tags were attached to YknX, YknW, and YknZ and the tagged proteins were produced from plasmids carrying different combinations of ykn genes. The 6His-tagged proteins were indistinguishable from nontagged versions in complementation of the SDP susceptibility phenotype (data not shown). To stabilize protein complexes, B. subtilis HB6127 (yknWXYZ∷Kan) cells carrying various plasmid constructs were treated with a cross-linker DSP and Ykn complexes were purified using metal affinity chromatography. Figure 6 shows analysis of the purified complexes by Coomassie brilliant blue (CBB) staining and by immunoblotting with anti-YknX and anti-YknW antibodies.
All three of YknWHis, YknXHis, and YknZHis were purified as oligomers that were stable even in the absence of DSP (Fig. 6A). In addition to a major ∼20-kDa monomer band, the purified YknWHis sample contained ∼40-kDa and 80-kDa bands, which reacted with both anti-YknW and anti-6His antibodies and therefore correspond to YknWHis dimers and tetramers (Fig. 6A top panel and data not shown). Since no HMW species reacting specifically with anti-YknW antibodies could be seen in B. subtilis membranes (Fig. 5A), these YknWHis oligomers were likely stabilized during purification on a Cu2+ affinity column. The purified YknXHis contained ∼100- to 150-kDa species, which were likely to be its dimers and trimers. Two major bands with MW corresponding to masses of ∼42 kDa and ∼90 kDa were present in purified YknZHis samples. Since both bands were recognized by anti-6His antibodies (data not shown), they corresponded to monomeric and dimeric forms of YknZHis.
In agreement with the in vivo cross-linking result (Fig. 5A), YknW altered the composition of purified YknXHis samples (Fig. 6A). YknXHis oligomers were clearly detected by anti-YknX immunostaining in samples purified from cells overproducing YknXHis alone. Furthermore, both dimers and trimers were stable without cross-linking with DSP. In contrast, in YknXHis samples purified from cells overproducing YknW, YknXHis trimers appeared to be unstable and could be detected only after cross-linking with DSP. In addition, an ∼35-kDa protein was copurified with YknXHis only from membranes containing both YknW and YknXHis. We did not find YknW in purified YknXHis samples. A possible explanation for this result is that most of YknW was integrated into the cytoplasmic membrane, which left no accessible primary amino groups for DSP cross-linking. Alternatively, interactions between YknX and YknW are mediated by another protein.
No YknY monomer could be clearly identified in the purified YknZHis samples. However, a small amount of an ∼49-kDa protein was detected in all YknZHis samples by CBB staining. This band did not react with anti-YknX antibody and was copurified with YknZHis even from cells lacking YknX (Fig. 6B). Given that the MW of YknY monomer corresponds to a molecular mass of 25 kDa, this band could correspond to YknY dimer.
Immunoblotting analysis with anti-YknX antibodies showed that YknX was readily copurified with YknZHis. Cross-linking with DSP was not required to detect this interaction, and roughly the same amounts of YknX were present in YknZHis fractions obtained from the untreated and DSP-treated cells. However, DSP cross-linking stabilized an ∼130-kDa species, which could have represented YknXZHis complexes. Surprisingly, the coexpression of all four Ykn proteins resulted in notably diminished amounts of YknX copurified with YknZHis. Only traces of YknX could be seen in YknZHis samples isolated from DSP-untreated cells. Although DSP cross-linking stabilized YknXZHis complexes (∼130-kDa and 150-kDa bands), no YknX oligomers were detected in these samples. At the same time, small amounts of YknW were present in YknZHis fractions isolated from both the untreated and DSP-treated cells. Since no YknW was detected in YknXhis samples, this result suggests that YknW interacts with YknZ. Alternatively, YknW could specifically bind the tripartite YknXYZ complex.
To determine the interacting partner of YknW, membrane fractions isolated from cells producing untagged YknW were mixed with equal amounts of membranes isolated from cells carrying an empty vector or plasmids producing YknXHis, YknYZHis, or YknXYZHis. After solubilization with detergent, proteins were purified using metal affinity columns. As expected, YknXHis and YknZHis were the major proteins in the isolated fractions. Immunoblotting analysis did not detect YknW in samples containing YknXHis or YknYZHis separately. However, YknW was immunodetected in fractions containing all three YknXYZHis proteins. Thus, YknW forms a four-component complex with YknXYZ (Fig. 6C).
DISCUSSION
Most of the bacterial genomes sequenced to date contain MFP-dependent transporters (18). In Gram-negative bacteria, these transporters are three-component assemblies, which in addition to the MFP and transporter also contain an outer membrane channel (17). Such assemblies span the entire two-membrane envelopes and export drugs and protein toxins directly into the external medium, bypassing the periplasm. In this study, we found that B. subtilis YknWXYZ is also assembled from three components, although here, in addition to a MFP and a transporter, the third component is the membrane protein YknW. Chemical cross-linking and copurification experiments suggested that YknW affects YknX oligomerization, perhaps by changing the conformation of this protein (Fig. 5B and 6A). However, it does not copurify with YknX. Instead, YknW specifically copurifies only with the assembled YknXYZ complex, suggesting that YknX and YknYZ form a composite interface with YknW (Fig. 6A, bottom panel). Furthermore, the amount and the state of YknX pulled from the cells by YknZHis are strongly affected by YknW. It appears that YknX forms a much weaker or a structurally different complex with YknYZ in the presence of YknW. Based on these results, we propose that YknW has a role in the assembly of the functional YknXYZ complex.
Interestingly, YknW, when overproduced from a plasmid, confers a partial protection from SDP toxin (Fig. 4), suggesting additional functions. Multiple sequence alignments indicated that YknW shares a low degree of similarity with transporters belonging to the major facilitator superfamily (data not shown). Hence, the protein could be a transporter, albeit one less efficient in the absence of YknXYZ. On the other hand, YknW and its homologs are restricted to Bacillus species and close relatives and are always located in clusters encoding ABC-type permeases, suggesting a functional link between these proteins (data not shown). The predicted four-TMS topology of YknW and association with ABC proteins are reminiscent of substrate-specific components (S component) of the “energy-coupling factor” (ECF) transporters (15). In ECF transporters, an ABC-type membrane protein functions as an energy subunit that enables active transport of a specific substrate by the S component. Interestingly, two more genes encoding ABC-type permeases are located immediately upstream of yknW and could potentially be functionally linked to YknW.
In agreement with previous studies (3), we found that YknWXYZ is required for protection against SDP-dependent killing of starving B. subtilis cells (Fig. 3 and 4). However, our results also suggest that this transporter has a function independent from SdpC. The Ykn complex is expressed in exponential-phase cells, whereas the production of toxin peaks upon transition into the stationary phase (Fig. 1). In contrast, specific substrates of MFP-dependent transporters involved in secretion of protein toxins (type I secretion) are usually coregulated or coexpressed in a single operon with a transporter. In addition, the assembly of previously characterized type I secretion transporters was shown to be dependent on the presence of a substrate (1, 2). We found no evidence that SdpC interacts with the Ykn transporter or affects its assembly. Perhaps the presence of a different substrate triggers the assembly of Ykn complex in exponential-phase cells, whereas the protection against SDP is inadvertent. If the latter is the case, the Ykn-mediated protection against SDP may not even involve transport of the toxin. Recent studies suggested that exposure of cells to SDP leads to dissipation of the proton-motive force, which in turn triggers autolysis (12). The activity of the Ykn transporter could be important for the integrity of membranes and preservation of the proton-motive force. A protein composition analysis of membranes did not identify notable differences between the Ykn-negative and -positive cells (data not shown). Perhaps the activity of Ykn involves lipids or other small-molecule constituents of the cell envelope. Because the yknWXYZ operon is expressed in growing B. subtilis cells, its physiological substrate is likely to be present in the exponential-growth phase as well.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI052293 to H.I.Z.
We thank Sze Yi Lau for construction of plasmids for recombinant expression of YknX and YknW.
Footnotes
Published ahead of print 15 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Akatsuka H, Binet R, Kawai E, Wandersman C, Omori K. 1997. Lipase secretion by bacterial hybrid ATP-binding cassette exporters: molecular recognition of the LipBCD, PrtDEF, and HasDEF exporters. J. Bacteriol. 179: 4754–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balakrishnan L, Hughes C, Koronakis V. 2001. Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. J. Mol. Biol. 313: 501–510 [DOI] [PubMed] [Google Scholar]
- 3. Butcher BG, Helmann JD. 2006. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60: 765–782 [DOI] [PubMed] [Google Scholar]
- 4. Chen CZ, Collins RN. 2005. Insights into biological functions across species: examining the role of Rab proteins in YIP1 family function. Biochem. Soc. Trans. 33: 614–618 [DOI] [PubMed] [Google Scholar]
- 5. Davidson AL, Dassa E, Orelle C, Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72: 317–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinh T, Paulsen IT, Saier MH., Jr 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 176: 3825–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellermeier CD, Hobbs EC, Gonzalez-Pastor JE, Losick R. 2006. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell 124: 549–559 [DOI] [PubMed] [Google Scholar]
- 8. González-Pastor JE, Hobbs EC, Losick R. 2003. Cannibalism by sporulating bacteria. Science 301: 510–513 [DOI] [PubMed] [Google Scholar]
- 9. Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 106: 20440–20445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi N, Nishino K, Hirata T, Yamaguchi A. 2003. Membrane topology of ABC-type macrolide antibiotic exporter MacB in Escherichia coli. FEBS Lett. 546: 241–246 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi N, Nishino K, Yamaguchi A. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183: 5639–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamsa A, Liu WT, Dorrestein PC, Pogliano K. 2012. The Bacillus subtilis cannibalism toxin SDP collapses the proton motive force and induces autolysis. Mol. Microbiol. 84: 486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linde D, Marischen L, Muller JP. 2003. Characterisation of preYvaY export reveals differences in the substrate specificities of Bacillus subtilis and Escherichia coli leader peptidases. FEMS Microbiol. Lett. 227: 149–156 [DOI] [PubMed] [Google Scholar]
- 14. Liu WT, et al. 2010. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 107: 16286–16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodionov DA, et al. 2009. A novel class of modular transporters for vitamins in prokaryotes. J. Bacteriol. 191: 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamanaka H, Kobayashi H, Takahashi E, Okamoto K. 2008. MacAB is involved in the secretion of Escherichia coli heat-stable enterotoxin II. J. Bacteriol. 190: 7693–7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zgurskaya HI. 2002. Molecular analysis of efflux pump-based antibiotic resistance. Int. J. Med. Microbiol. 292: 95–105 [DOI] [PubMed] [Google Scholar]
- 18. Zgurskaya HI, Yamada Y, Tikhonova EB, Ge Q, Krishnamoorthy G. 2009. Structural and functional diversity of bacterial membrane fusion proteins. Biochim. Biophys. Acta 1794: 794–807 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.