Abstract
Although the three Treponema pallidum subspecies (T. pallidum subsp. pallidum, T. pallidum subsp. pertenue, and T. pallidum subsp. endemicum), Treponema paraluiscuniculi, and the unclassified Fribourg-Blanc treponeme cause clinically distinct diseases, these pathogens are genetically and antigenically highly related and are able to cause persistent infection. Recent evidence suggests that the putative surface-exposed variable antigen TprK plays an important role in both treponemal immune evasion and persistence. tprK heterogeneity is generated by nonreciprocal gene conversion between the tprK expression site and donor sites. Although each of the above-mentioned species and subspecies has a functional tprK antigenic variation system, it is still unclear why the level of expression and the rate at which tprK diversifies during infection can differ significantly among isolates. To identify genomic differences that might affect the generation and expression of TprK variants among these pathogens, we performed comparative sequence analysis of the donor sites, as well as the tprK expression sites, among eight T. pallidum subsp. pallidum isolates (Nichols Gen, Nichols Sea, Chicago, Sea81-4, Dal-1, Street14, UW104, and UW126), three T. pallidum subsp. pertenue isolates (Gauthier, CDC2, and Samoa D), one T. pallidum subsp. endemicum isolate (Iraq B), the unclassified Fribourg-Blanc isolate, and the Cuniculi A strain of T. paraluiscuniculi. Synteny and sequence conservation, as well as deletions and insertions, were found in the regions harboring the donor sites. These data suggest that the tprK recombination system is harbored within dynamic genomic regions and that genomic differences might be an important key to explain discrepancies in generation and expression of tprK variants among these Treponema isolates.
INTRODUCTION
The pathogenic, noncultivable treponemes include Treponema pallidum with its three subspecies (T. pallidum subsp. pallidum, T. pallidum subsp. pertenue, and T. pallidum subsp. endemicum), Treponema carateum, Treponema paraluiscuniculi, and the unclassified Fribourg-Blanc treponeme. T. pallidum subsp. pallidum and T. paraluiscuniculi are the etiologic agents of human and rabbit venereal syphilis, respectively, while T. pallidum subsp. pertenue, T. pallidum subsp. endemicum, and T. carateum cause the endemic treponematoses known as yaws, bejel, and pinta (10, 18). Although regarded as distinct syndromes, these treponematoses share many noteworthy similarities, particularly evident when the clinical manifestations of the diseases are compared. Generally acquired during sexual intercourse, human syphilis alternates episodes of active clinical disease with periods of asymptomatic, latent infection (61). T. pallidum multiplication at the inoculation site induces the appearance of a primary lesion (chancre) (37) that persists for several weeks and then spontaneously heals following treponemal immune clearance. Proliferation of disseminated T. pallidum cells may cause the systemic manifestations of secondary syphilis, such as mucocutaneous lesions and generalized lymphadenopathy (5), that also spontaneously resolve. Following a latency stage, syphilis progression into the tertiary stage may result in gummatous disease, cardiovascular syphilis, general paresis, or tabes dorsalis (27, 28, 54).
Like venereal syphilis, yaws, bejel, and pinta have relapsing courses and prominent cutaneous manifestations but are generally acquired during childhood via skin-to-skin and skin-to-mucous membrane contact (transmission routes that are also similar to those of T. pallidum subsp. pallidum) (2, 49, 50). It has been stated that central nervous system (CNS) and placental invasion do not occur in these infections, although this statement has been disputed (51). The Fribourg-Blanc treponeme was isolated from an African baboon with no signs of clinical infection. Little is known about the pathogenesis of the infection (20); however, experimental inoculations showed that this organism is able to produce an active infection in humans (58, 59). Rabbit venereal syphilis (caused by T. paraluiscuniculi) is a milder infection characterized by slightly elevated, scaly patches or eroded sores in the genitals, the anus, and, less frequently, the eyelids, lips, and paws of the animals (15). Development of a systemic secondary stage preceding latency (15, 57) in T. paraluiscuniculi-infected rabbits has never been properly investigated.
These pathogens are morphologically indistinguishable, as well as antigenically and genetically very similar. Antigenic profile studies showed only minimal differences in antibody reactivity to T. pallidum subsp. pallidum, T. pallidum subsp. pertenue, Fribourg-Blanc, and T. paraluiscuniculi lysates (4, 41, 47, 60). More recently, several comparative genomics studies confirmed the high level of relatedness among these treponemal strains (22, 42, 43, 56).
Given the remarkable relatedness shared by these pathogens and their ability to escape a strong immune response and persist in the host, it is plausible that similar mechanisms are employed by these spirochetes to ensure their survival during infection. Antigenic variation likely plays an important role. In T. pallidum subsp. pallidum, we have demonstrated that the putative outer membrane protein (OMP) TprK undergoes extensive antigenic variation in seven variable (V) regions, called V1 to V7, separated by conserved amino acid sequences (12, 36). Although the location of TprK in the pathogen's OM has been questioned (13, 29), several lines of evidence support TprK as being surface exposed. In rabbits infected with the Nichols strain, TprK variable regions are targets of a strong humoral response (44, 45) and antibodies against TprK were shown to be opsonic and enhance treponemal phagocytosis by macrophages (11). These data indeed agree with current three-dimensional (3D) models that show TprK as a beta-barrel structure with the protein's V regions protruding toward the extracellular milieu (A. Centurion-Lara, unpublished data). Furthermore, immunization of rabbits with recombinant TprK significantly attenuated lesion development following infectious challenge, although it did not provide sterile immunity (11). The role of antigenic variation of TprK in immune evasion during syphilis infection is also supported by the evidence that immunization with a synthetic TprK V6 peptide induces positive selection of new V6 variants upon challenge with the parental isolate (25). Additionally, immunosuppression in the rabbit model results in a significantly lower proportion of V6 variants identified during infection, providing strong evidence that the immune response positively selects for treponemes expressing variant V regions (25).
Using the T. pallidum subsp. pallidum Chicago strain as a model, we have previously shown that generation of tprK variants occurs during experimental infection by nonreciprocal segmental gene conversion, namely, the unidirectional transfer of genetic information from donor sites (DSs), located approximately 130 kbp downstream of the single tprK expression site, into the tprK expression locus (12). In the Chicago strain genome, a total of 52 tprK DSs can be identified; of these, 50 are located in the 3′-flanking region of TP0131, and 2 are in the TP0131 5′-flanking region (12). A large proportion of DSs also show the unique 4-bp terminal repeat sequences defining individual tprK V regions. A variable number of DSs are available for each V region: 5, 6, 10, 4, 6, 13, and 8 DSs for V1, V2, V3, V4, V5, V6, and V7, respectively, in the Chicago strain (12).
LaFond et al. (36) showed that generation of tprK variants occurs at different rates in the Chicago versus the Nichols isolates of T. pallidum subsp. pallidum. tprK diversifies rapidly in the Chicago strain, while diversification is considerably slower in the Nichols strain of T. pallidum subsp. pallidum. The rates of diversification of tprK have not been yet experimentally compared with respect to other T. pallidum subsp. pallidum strains among yaws and bejel isolates or in the Fribourg-Blanc isolate, though sequence heterogeneity in the tprK gene in these isolates suggests a higher baseline diversification rate than in the Nichols isolate of T. pallidum subsp. pallidum.
Because of the apparent importance of TprK in treponemal pathogenesis, we have compared tprK donor and expression sites among these spirochetes. We found sequence diversity of DSs among isolates, as well as the presence of A/T-rich sequences of various lengths, resembling an upstream regulatory (UP) element, in the tprK promoter region. Furthermore, putative cis-acting DNA elements, able to form guanine quadruplexes (G4) and also exhibiting sequence variability among treponemal species and subspecies, were found in proximity to the tprK expression site and within DSs. Similar sequences were shown to be required for PilE antigenic variation by Neisseria gonorrhoeae (8, 9). Our study lays the foundation for further investigations that will elucidate how such differences among strains, subspecies, and species could affect the generation and expression of TprK variants during treponemal infections.
MATERIALS AND METHODS
Treponemal strain propagation and nucleic acid extraction.
Eight T. pallidum subsp. pallidum strains (Nichols Sea, Nichols Gen, Sea81-4, Chicago, Dal-1, Street14, UW126, and UW104) were used in this study. With the exception of the Nichols Gen, Dal-1, and Street14 strains, whose genome sequences are already available (GenBank accession numbers AE000520.1, CP000805.1, and CP003115.1, respectively), all other strains were propagated in our laboratory for sequencing purposes. The Chicago strain genome was previously obtained in our laboratory (22) (GenBank accession number CP001752.1). The Seattle Nichols strain was supplied by James N. Miller (University of California, Los Angeles, CA) in 1979; the Sea 81-4 strain was isolated in Seattle from a primary lesion in 1981; the Chicago strain was initially supplied by Paul Hardy and Ellen Nell (Johns Hopkins University, Baltimore, MD), along with the Cuniculi A, Samoa D, Iraq B, and Fribourg-Blanc strains. Peter Perine (Centers for Disease Control and Prevention, Atlanta, GA) initially provided the Gauthier strain of T. pallidum subsp. pertenue. All T. pallidum subspecies, T. paraluiscuniculi, and the Fribourg-Blanc treponeme were propagated in New Zealand White rabbits by intratesticular inoculation as previously described (39). T. carateum is unable to multiply in rabbits, and no strains or samples of this pathogen are available. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under protocols approved by the University of Washington Institutional Animal Care and Use Committee (IACUC). Before infection, each rabbit had been serologically tested to rule out a naturally occurring infection with T. paraluiscuniculi. Treponemes were extracted from infected rabbit testes at peak orchitis. Collected organisms were separated from host cellular gross debris by low-speed centrifugation (250 × g for 10 min at room temperature); the supernatants were spun in a microcentrifuge for 30 min at 12,000 × g at 4°C. The pellet was suspended in 1 ml of 1× lysis buffer (10 mM Tris, pH 8.0, 0.1 M EDTA, 0.5% sodium dodecyl sulfate) for DNA extraction or in 400 μl of Ultraspec buffer (Biotecx Laboratories, Inc., Houston, TX) for RNA isolation. DNA extraction was performed as previously described with the QIAamp DNA minikit (Qiagen Inc., Chatsworth, CA) (21), taking careful precautions to prevent cross-contamination between samples. RNA extraction was performed according to the manufacturer's instructions followed by treatment with TURBO-DNase (Ambion, Austin, TX). Absence of residual DNA was assessed by PCR as previously described (21). DNase-treated RNA was stored at −80°C until use.
PCR amplification, cloning, sequencing, and sequence analysis of tprK open reading frames (ORFs) and donor regions.
The T. pallidum subsp. pallidum strain Nichols genome sequence (19) was used to design primers (Table 1) targeting the 5′ and 3′-flanking regions of the TP0131 gene, where the tprK donor sites are located. PCR amplification for the TP0131 5′-flanking region using genomic DNA as a template was performed in a 50-μl final volume containing 200 μM deoxynucleoside triphosphates (dNTPs), 1.5 mM MgCl2, and 2.5 U of GoTaq DNA polymerase (Promega, Madison, WI). The cycling conditions were as follows: initial denaturation at 95°C for 5 min, followed by 95°C for 1 min, 60°C for 1 min, and 72°C for 2 min for 45 cycles in total, with a final extension at 72°C for 10 min. The TP0131 3′-flanking region was amplified using a TaKaRa Bio Inc. (Shiga, Japan) LA PCR kit, preferred for targets of >3 kbp. Amplification was performed in a 50-μl final volume containing 200 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 2.5 U of Taq polymerase. The cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 95°C for 1 min, 60°C for 1 min, and 72°C for 3 min for 45 cycles in total, with a final extension at 72°C for 10 min.
Table 1.
Primers used in this study
| Primer | Sequence | Usea | Target |
|---|---|---|---|
| 5DF-S | 5′-CAGTCCAAGAAGCGGAAAAG | 1, 2 | TP0131 5′-flanking region |
| 5DF-As | 5′-AAACGCCAGCAGTTCCAGCG | 1, 2 | TP0131 5′-flanking region |
| TP0136Ins-S | 5′-ACACAACGGCTGCGAATACT | 1 | TP0131 5′-flanking region |
| TP0136Ins-As | 5′-ACCGACCGTGCCCCATACT | 1 | TP0131 5′-flanking region |
| TPrflp-S | 5′-TTTTGTGTGTGGGGAGGAGT | 1, 2 | TP0131 5′-flanking region |
| TPrflpAs | 5′-ATGGCTGGGGGTAGGGCTTGC | 1, 2 | TP0131 5′-flanking region |
| 3DF-S | 5′-ATTGCCGAGAGCATCTGGT | 1, 2 | TP0131 3′-flanking region |
| 3DF-As | 5′-CCGCTCTCCTTCCCATAAGA | 1, 2 | TP0131 3′-flanking region |
| 3DF-S1 | 5′-GCCTCATACCGACTGGGAG | 1, 2 | TP0131 3′-flanking region |
| 3DF-S2 | 5′-CCTGCTCCTGGTACGAAGT | 1, 2 | TP0131 3′-flanking region |
| 3DF-S3 | 5′-CAGCAGGAACTCCCAGCACGT | 2 | TP0131 3′-flanking region |
| 3DF-As1 | 5′-GGGCGGACGTCCCACACC | 2 | TP0131 3′-flanking region |
| 3′DF-1-2-AS | 5′-ATTGCGTCTTTTGCGCGCG | 2 | TP0131 3′-flanking region |
| 3′DF-1-3-AS | 5′-AAACGCGGCGCTGTGGTTT | 2 | TP0131 3′-flanking region |
| 3′DF-1-4-AS | 5′-GGGAGTTGGGCTCGGGC | 2 | TP0131 3′-flanking region |
| 3′DF-1-5-AS | 5′-CTCGGGCTTGGGTGAAGG | 2 | TP0131 3′-flanking region |
| 3′DF-1-6-AS | 5′-ACACCCTGGGTGAAAAGACT | 2 | TP0131 3′-flanking region |
| 3′DF-1-7-AS | 5′-GTACGTGCTGGGAGTTCCT | 2 | TP0131 3′-flanking region |
| 3′DF-1-8-AS | 5′-TGTGCGGTCATGGCCATGT | 2 | TP0131 3′-flanking region |
| 3′DF-1-9-S | 5′-CGCAGGCCAGCAATGTATTT | 2 | TP0131 3′-flanking region |
| 3′DF-1-10-AS | 5′-TTTGGTTTGCCCTCCCAG | 2 | TP0131 3′-flanking region |
| 3′DF-1-11-S | 5′-CAAGCAGGGAGGACGCGT | 2 | TP0131 3′-flanking region |
| 3′DF-1-12-AS | 5′-CTTCGGGAATCGAGTGTAGT | 2 | TP0131 3′-flanking region |
| 3′DF-1-13-S | 5′-TCGTACTGCGCGTCATCGA | 2 | TP0131 3′-flanking region |
| 3′DF-1-14-S | 5′-TGGACTATCCATTCCCCATC | 2 | TP0131 3′-flanking region |
| 3′DF-1-15-AS | 5′-GTTGGGCTGGGGGAAATAG | 2 | TP0131 3′-flanking region |
| 3′DF-1-16-S | 5′-TATCGCCCCAACGCACGC | 2 | TP0131 3′-flanking region |
| 3′DF-1-17-S | 5′-GACGGGGAGGGCAAGTCA | 2 | TP0131 3′-flanking region |
| 3′DF-1-18-S | 5′-TACATGCCCGTCCATTGGAA | 2 | TP0131 3′-flanking region |
| 3′DF-1-19-AS | 5′-CATGGCATTGGTGAGAAAGA | 2 | TP0131 3′-flanking region |
| 3′DF-1-20-S | 5′-CAAAGACAGTCAGGGCAAGG | 2 | TP0131 3′-flanking region |
| 3′DF-1-21-AS | 5′-TGTCCTGTTCTTGTGCTCCA | 2 | TP0131 3′-flanking region |
| 3′DF-1-22-S | 5′-TCTACTTCTCGGCTCGCAGT | 2 | TP0131 3′-flanking region |
| 3′DF-1-23-AS | 5′-TATTGAGTGTGCGCTTACGG | 2 | TP0131 3′-flanking region |
| 3′DF-2-S | 5′-ACAACGAAGTTCCATAGGAC | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS | 5′-TTTAAAGGGTTCGTTCTCGC | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-1 | 5′-ATCCTCTTACGCGTCTCGTC | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-2 | 5′-CACTCCCGCGCATATTCCC | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-3 | 5′-GCCGAAAGGCGTATAGTACT | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-4 | 5′-TGCAAGGTAATAGTACGGAGT | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-5 | 5′-TTGGATGGCGCATCGATTAC | 2 | TP0131 3′-flanking region |
| 3′DF-2-AS-6 | 5′-GCACGGCGCTGTTCAGTAA | 2 | TP0131 3′-flanking region |
| KI-S | 5′-GTGGTGTATCAGCGGGTAGG | 1, 2 | tprK ORF |
| KI-As | 5′-GACATGCCCCTACGAATTG | 1, 2 | tprK ORF |
| K-RFLP-S | 5′-TGGCGTGCAGGAATACATTA | 2 | tprK V3–V7 |
| K-RFLP-As | 5′-TACCCCACACTCGTAATACCC | 2 | tprK V3–V7 |
| K-RT-PCR-S | 5′-AGTTTGCGTCTAACACCGACTG | 2 | tprK V2–V5 |
| K-RT-PCR-As | 5′-TCGCATGGCCATGTTGAGAAAT | 2 | tprK V2–V5 |
| 9V-As | 5′-CCTCAAGGAAAGAAGTATCAGG | 2 | tprK V1–V5 |
| K-RACE-1 | 5′-AGCACACAGACCCCAAAGCTTC | 3 | tprK V3–V4 |
| K-RACE-2 | 5′-TAATGTATTCCTGCACGCCATC | 4 | tprK V4–V3 |
| K-RACE-3 | 5′-CAAACTTAAACGCAATATCAAA | 4 | tprK V2–V1 |
1, amplification (sequence analysis); 2, sequencing; 3, reverse transcription (5′-RACE protocol); 4, amplification (5′-RACE protocol).
Short amplicons from the TP0131 5′-flanking region and long amplicons from the TP0131 3′-flanking region were subsequently cloned into the pCR2.1-TOPO and pCR-XL-TOPO cloning vectors (Invitrogen, Carlsbad, CA), respectively, according to the manufacturer's instructions. Plasmid DNA from colonies containing inserts was extracted with the QIAprep spin miniprep kit (Qiagen), and at least two clones, each one from a different amplification reaction, were sequenced in both directions using a primer-walking approach (primers are listed in Table 1). Sequencing reactions and analyses were performed by the University of Washington Sequencing Facility. Sequences were assembled using the BioEdit sequence alignment editor (http://www.mbio.ncsu.edu/bioedit/bioedit.html) and then aligned using the MAFFT alignment program (http://mafft.cbrc.jp/alignment/software/). Similarly, the tprK gene was amplified from all strains described above using tprK flanking primers and was sequenced using appropriate internal primers (Table 1). Amplification conditions for the tprK gene were previously described (26).
Prediction of G-quadruplex-forming sequence (G4FS) location in T. pallidum subsp. pallidum (Nichols Gen strain) genome and Borrelia burgdorferi (B31 strain) lp28-1.
Composition and distribution of putative quadruplex-forming G-rich sequences in the full-length T. pallidum subsp. pallidum (Nichols strain) genome (GenBank accession number AE000520.1) and full-length Borrelia burgdorferi (B31) lp28-1 plasmid (GenBank accession number AE000794.2) were identified using QGRS (Quadruplex-forming G-Rich Sequences) Mapper (http://miracle.igib.res.in/quadfinder/gquadruplex.html) and QuadFinder (http://bioinformatics.ramapo.edu/QGRS/analyze.php). Parameters for QGRS Mapper were set so that the maximum length of the putative G-quadruplex was 30 nucleotides (nt) with a minimum of 2 G groups. Loop size was set from 0 to a maximum of 15 nt. Parameters for QuadFinder were 3≤ G-Stretch ≤ 5 and 1≤ N-Stretch ≤ 7. Hypothetical G4FS sequences identified by both programs and with G-scores (provided only by QGRS Mapper) equal to or greater than 29 were analyzed in this study. The scoring method uses previously published principles (3, 33, 34), in which G4FS is assigned a score based on a system which rewards an arrangement of G's that is likely to form a unimolecular quadruplex. For calculation of G-scores, the size and distribution of the gaps in the predicted QGFS are taken into account. Genomic coordinates of the putative G4FS, provided by both QGRS Mapper and QuadFinder, were used to determine G4FS location within ORFs or intergenic regions using the annotation of the T. pallidum subsp. pallidum strain Nichols genome or B. burgdorferi B31 plasmid lp28-1 available in GenBank.
Circular dichroism.
DNA oligonucleotides for circular dichroism (CD) were obtained from MWG Biotech (Ebersberg, Germany). CD spectra were obtained as described by Joachimi et al. (32). Briefly, oligonucleotides were resuspended at 5 μM final concentration in diethylpyrocarbonate-treated water buffered with 50 mM Tris-HCl (pH 7.5) and optional addition of 100 mM KCl. Oligonucleotides were denatured by heating to 95°C for 5 min, and renaturation was allowed by slow cooling to a final temperature of 20°C over a period of 16 h. CD spectra were recorded on a Jasco 715 spectrometer in 1-cm-path cuvettes, with 0.5-nm resolution, 1.0-nm bandwidth, and speed of 20 nm/min at 20°C. Each spectrum was accumulated three times and averaged. Buffer alone was used as a negative control.
5′-RACE of tprK gene.
The 5′ rapid amplification of cDNA ends (5′-RACE) system (Invitrogen) was used to determine transitional start sites (TSSs) in the 5′-flanking regions of tprK. 5′-RACE analysis was performed on T. pallidum subsp. pallidum (Nichols Sea strain) and T. paraluiscuniculi (Cuniculi A strain) total RNA following the manufacturer's instructions, except that the SuperScript system for cDNA synthesis was replaced by the ThermoScript reverse transcription (RT) kit (Invitrogen) to increase yield due to the high GC content (52.8%) of treponemal transcripts. A 2.5-pmol sample of gene-specific primer (K-RACE-1; Table 1) and 2 μg of sample RNA were used in each reaction. After the reaction was terminated, 1 μl of RNase H was added to the tube and the cDNA was incubated for 20 min at 37°C. All PCR amplification reactions were performed using 50 μl of dC-tailed cDNA in a 50-μl final volume containing 200 μM each dNTP, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 50 mM KCl, 400 nM each primer, and 2.5 U of GoTaq DNA polymerase (Promega). For nested PCR, 1 μl of the original amplicon (for a 1/500 dilution) was used for the second amplification. Cycling parameters were as follows: initial denaturation for 2 min at 94°C, followed by 1 min at 94°C, annealing for 1 min at 63°C, and extension for 1 min at 72°C, for a total of 45 cycles. The final extension was 10 min at 72°C. PCR products were separated in 2% agarose gels, gel purified, cloned, and sequenced as described above.
Nucleotide sequence accession numbers.
All new sequences obtained in this study were deposited in GenBank. Accession numbers are reported in Table 2.
Table 2.
GenBank accession numbers
| Species and strain | Sequence | Accession no. |
|---|---|---|
| T. pallidum subsp. pallidum | ||
| Nichols Gen | TP0131 5′-flanking region | AE000520.1 |
| TP0131 3′-flanking region | ||
| Nichols Sea | TP0131 5′-flanking region | JX025054 |
| TP0131 3′-flanking region | JX025071 | |
| Chicago | TP0131 5′-flanking region | CP001752.1 |
| TP0131 3′-flanking region | ||
| Sea81-4 | TP0131 5′-flanking region | JX025057 |
| TP0131 3′-flanking region | JX025068 | |
| UW104 | TP0131 5′-flanking region | JX025056 |
| TP0131 3′-flanking region | JX025069 | |
| UW126 | TP0131 5′-flanking region | JX025055 |
| TP0131 3′-flanking region | JX025070 | |
| Street14 | TP0131 5′-flanking region | CP000805.1 |
| TP0131 3′-flanking region | ||
| Dal-1 | TP0131 5′-flanking region | CP003115.1 |
| TP0131 3′-flanking region | ||
| T. pallidum subsp. pertenue | ||
| Gauthier | TP0131 5′-flanking region | JX025058 |
| TP0131 3′-flanking region | JX025064 | |
| CDC2 | TP0131 5′-flanking region | CP002375.1 |
| TP0131 3′-flanking region | ||
| Samoa D | TP0131 5′-flanking region | JX025060 |
| TP0131 3′-flanking region | JX025066 | |
| T. pallidum subsp. endemicum | ||
| Iraq B | TP0131 5′-flanking region | JX025059 |
| TP0131 3′-flanking region | JX025065 | |
| Unclassified | ||
| Fribourg-Blanc | TP0131 5′-flanking region | JX025061 |
| TP0131 3′-flanking region | JX025067 | |
| T. paraluiscuniculi | ||
| Cuniculi A | TP0131 5′-flanking region | JX025062 |
| TP0131 3′-flanking region | JX025063 |
RESULTS
tprK donor sequences in the TP0131 (tprD) 3′-flanking region contain similarities and unique elements among treponemal species, subspecies, and strains.
All species, subspecies, and strains of pathogenic treponemes analyzed in this study (Table 3) show similar architecture in the TP0131 3′-flanking region, which contains the larger group of DSs, contained within the ORFs annotated in the published Nichols genome (Nichols Gen) as TP0126 to TP0130 (with the exception of TP0127, which contains no DSs), as well as within the intergenic regions that separate these ORFs (schematically represented in Fig. 1; chromosomal location 148374 to 151104 in the published Nichols genome [19]). According to our analysis, the genomes of the Fribourg-Blanc isolate and the human treponemes, including the Nichols strain of T. pallidum subsp. pallidum propagated in our laboratory (identified as Nichols Sea), harbor 3′ donor regions of comparable sizes, ranging from 3,931 to 3,988 bp (Table 3). A deletion of 1,204 bp was identified in the TP0126-TP0127 intergenic spacer in the Nichols strain originally used for the first T. pallidum subsp. pallidum genome project (Nichols Gen) (19) (Fig. 1 and Table 3). At the beginning and at the end of this deletion, there are two direct repeats of 24 bp (AATGTATTTCAGGGTGTCTTTCTC), suggesting a loop-out mechanism for this deletion. The deletion decreases the overall number of DSs in the Nichols Gen isolate to 34, compared to the 54 DSs described in the Nichols strain propagated in our laboratory (Nichols Sea) (Table 4). Similarly, in the T. paraluiscuniculi Cuniculi A strain genome, a 1,441-bp deletion of portions of TP0127 and TP0128 reduces these DS-containing regions to only 2,490 bp (Table 3 and Fig. 1), eliminating 20 DSs (Table 4) that in T. pallidum strains overlap the TP0128 sequence and the TP0128-TP0127 intergenic spacer (Fig. 1) (12).
Table 3.
Isolates sequenced in this study and corresponding sizes of DS regionsa
| Species and strain | Length (bp) of genomic region hosting tprK DSs |
|
|---|---|---|
| TP0131(tprD) 3′-flanking region | TP0131(tprD) 5′-flanking region | |
| T. pallidum subsp. pallidum | ||
| Nichols Genb | 2,730 | 239 |
| Nichols Seab | 3,931 | 239 |
| Chicago | 3,931 | 239 |
| Sea81-4 | 3,938 | 239 |
| UW104 | 3,988 | 239 |
| UW126 | 3,988 | 239 |
| Dal-1d | 3,931 | 239 |
| Street14d | 3,988 | 239 |
| T. pallidum subsp. pertenue | ||
| Gauthier | 3,977c | 239 |
| Samoa D | 3,976 | 239 |
| CDC2d | 3,977c | 239 |
| T. pallidum subsp. endemicum | ||
| Iraq B | 3,975 | 239 |
| Unclassified | ||
| Fribourg-Blanc | 3,976 | 239 |
| T. paraluiscuniculi | ||
| Cuniculi A | 2,490 | 400 |
These regions also include TP0127 (687 bp), although this ORF does not contain DSs (Fig. 1).
Nichols Gen refers to the published Nichols strain sequence (19); Nichols Sea is the Nichols strain currently propagated in our laboratory.
This 1-nt difference between Gauthier/CDC2 and Samoa D 3′-flanking regions is due to a indel within a varying homopolymeric G tract.
Sequences from Dal-1, Street14, and CDC2s were obtained from GenBank (accession numbers CP000805.1, CP003115.1, and CP002375.1, respectively).
Fig 1.

Schematic representation of the TP0131(tprD) 3′-flanking genomic region containing tprK DSs of the following treponemal isolates: T. pallidum subsp. pallidum (Nichols Gen, Nichols Sea, Seattle 81-4, and UW126 strains), T. pallidum subsp. pertenue (Gauthier, CDC2, and Samoa D strains), T. pallidum subsp. endemicum (Iraq B strain), the Fribourg-Blanc (simian) isolate, and T. paraluiscuniculi (Cuniculi A strain). The TP0131 3′-flanking regions are identical between T. pallidum subsp. pallidum Nichols Sea, Chicago, and Dal-1 strains, between UW126, UW104, and Street14 strains, and between T. pallidum subsp. pertenue Samoa D, Gauthier, and CDC2 strains. Lengths of the ORFs and intergenic spacers are not in proportion.
Table 4.
Total number of donor sites and distribution by V region
| Species and strain | Total no. of DSs |
Total no. of DSs per tprK V region |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TP0131 (tprD) 3′-flanking region | TP0131 (tprD) 5′-flanking region | V1 | V2 | V3 | V4 | V5 | V6 | V7 | |
| T. pallidum subsp. pallidum | |||||||||
| Nichols Gen | 32 | 2 | 3 | 3 | 6 | 3 | 4 | 9 | 6 |
| Nichols Sea | 50 | 4 | 5 | 6 | 11 | 4 | 6 | 13 | 9 |
| Chicago | 50 | 2 | 5 | 6 | 10 | 4 | 6 | 13 | 8 |
| Sea81-4 | 50 | 2 | 5 | 6 | 10 | 4 | 6 | 13 | 8 |
| UW104 | 50 | 2 | 5 | 6 | 10 | 4 | 6 | 13 | 8 |
| UW126 | 50 | 2 | 5 | 6 | 10 | 4 | 6 | 13 | 8 |
| Street14a | 50 | 2 | 5 | 6 | 10 | 4 | 6 | 13 | 8 |
| Dal-1a | 50 | 4 | 5 | 6 | 11 | 4 | 6 | 13 | 9 |
| T. pallidum subsp. pertenue | |||||||||
| Gauthier | 52 | 2 | 5 | 6 | 10 | 4 | 6 | 14 | 9 |
| Samoa D | 52 | 2 | 5 | 6 | 10 | 4 | 6 | 14 | 9 |
| CDC2a | 52 | 2 | 5 | 6 | 10 | 4 | 6 | 14 | 9 |
| T. pallidum subsp. endemicum | |||||||||
| Iraq B | 52 | 2 | 5 | 6 | 10 | 4 | 6 | 14 | 9 |
| Unclassified | |||||||||
| Fribourg-Blanc | 52 | 2 | 5 | 6 | 10 | 4 | 6 | 14 | 9 |
| T. paraluiscuniculi | |||||||||
| Cuniculi A | 31 | 5 | 3 | 4 | 8 | 5 | 6 | 5 | 5 |
Sequences from these strains were obtained from GenBank (accession numbers CP000805.1, CP003115.1, and CP002375.1 for Dal-1, Street14, and CDC2, respectively).
Among the syphilis-causing pallidum strains, there is sequence conservation in the TP0130, TP0129, TP0128, and TP0127 putative ORFs, but single nucleotide polymorphisms (SNPs) and indels occur in the TP0128-TP0127 and the TP0127-TP0126 intergenic spacers (Fig. 1). In the UW126 and UW104 strains, we identified an insertion of 51 bp located in the TP0127-TP0126 intergenic spacer modifying an already existing donor site for tprK V7; this insertion codes for the peptide sequence GAVPGAVGAVPGAAA (40). TP0130, TP0129, TP0128, and TP0127 putative ORFs are also conserved in non-syphilis-causing strains (T. pallidum subsp. pertenue, T. pallidum subsp. endemicum, and the Fribourg-Blanc isolate).
The most significant change in the sequences of the non-syphilis-causing strains (Gauthier, Iraq B, Samoa D, CDC2, and the Fribourg-Blanc treponeme) is a deletion of 30 bp within the TP0128-TP0127 intergenic region that does not affect any DS identified to date and an insertion of 90 bp (Fig. 1) within the TP0127-TP0126 spacer containing one new DS of 33 nt for V6 (CAAGCCCTACCCCCAGCCATTTACTTCCCCCTG) and a small donor site for V7 (CTGCTCCTG). Two more insertions, although of limited length and not containing any recognizable DS, are located in the TP0130-TP0129 intergenic region (Fig. 1) and in the TP0128-TP0127 intergenic region (Fig. 1) of the non-syphilis-causing strains.
In T. paraluiscuniculi (Cuniculi A strain), the 3′ donor region contains a deletion of 1,441 bp encompassing 96 bp of the 5′ end of the TP0129 open reading frame (ORF), the entire TP0128 ORF, and a large portion of TP0127 (Fig. 1). Interestingly, the 3′ donor region of T. paraluiscuniculi Cuniculi A contains 356 bp that correspond to the V3-V5 regions and intervening conserved regions present in the tprK expression site of this strain. There is no equivalent to this region in the donor sites of any of the other treponemal strains examined to date. The longest DNA region in the human and rabbit treponemes and in the Fribourg-Blanc isolate that contains a sequence corresponding to the tprK ORF conserved portion is only 148 bases that belong to the 3′ end of the gene located immediately downstream of one DS for V7 (44 bp long). The V7 donor site and the conserved tprK region are arranged in the same order as they occur in the tprK expression site. A full-length sequence alignment for the TP0131 3′-flanking regions of all T. pallidum subspecies, T. paraluiscuniculi, and the Fribourg-Blanc isolate is provided in Fig. S1 in the supplemental material. The sequences of all DSs identified in this study are reported in Table 5.
Table 5.
Sequence of all donor sites for tprK variable regions in treponemal species and subspecies studied here
| Region and site | Sequence | Strain(s)d |
|---|---|---|
| V1 DSs | ||
| DS7 | GGGCATTGCATCTGAAACTGGTGGCGCCGGAGCCCTCA | All strains |
| DS15 | GGGGGCATTGCATCCGTGGTGGCGCCATCAAGCA | Nichols Gen, UW104, UW126, Street14, Sea81-4 |
| GGGGCATTGCATCCGTGGTGGCGCCATCAAGCA | Nichols Sea, Chicago, Dal-1 | |
| GGGGGCATTGCATCCGATGGTGGCACCATCAAGCA | Cuniculi A, Gauthier, Iraq B, CDC2, Samoa D, Fribourg-Blanc | |
| DS20 | GGGGCTTGCATCTGAAAAAAATGGTGGCGCCCAACCCCTCAAGCAc | Nichols Sea, Chicago, Dal-1 |
| GGGGCATTGCATCTGAAAAAAATGGTGGCGCCCAACCCCTCAAGCAc | Gauthier, Iraq B, Samoa D, Fribourg-Blanc, Sea81-4, CDC2, UW104, UW126, Street14, Nichols Gen | |
| DS29 | CTGGGGCATTGCATATGAAAATGGTGGCGCCCAACCCCTCAAGCAb,c | All strains |
| DS37 | GGGGCATTGCATCCGAAGATGGTAGCGCCGGAAACCTCAAGCATGGAb | All strains |
| GGGCATTGCATCCGAAGATGGTAGCGCCGGAAACCTCAAGCATGGAb | Cuniculi A | |
| V2 DSs | ||
| DS8 | ACCGACTGGGAGGGCAAAGACAGTCAGGGCAAGGCCCCAGCAGGAACTTCCAGCAC | Cuniculi A |
| ACCGACTGGGAGGGCAAAGACAATCAGGGCAAGGCCCCAGCAGGAACTCCCAGCAC | Iraq B | |
| ACCGACTGGGAGGGCAAAGACAGTCAGGGCAAGGCCCCAGCAGGAACTCCCAGCACGTA | All others | |
| DS16 | ACTGGGAGGGCAAAGACAGCAAGGGCGTCGCCCAAGCAGGAGCAAACCACAGCA | Gauthier, CDC2 |
| ACTGGGAGGGCAAAGACAGCAAGGGCGTCGTCCAAGCAGGAGCAAACCACAGCA | All others | |
| DS21 | CTAACACCGACTGGGAGGGCAAACCAAACGGCAACGTCCCAGCAGGAGTAACCCCCAGCAc | All strains |
| DS30 | ACACCGACTGGGAGGGCAAGTCAAACACGGGCGCCCCAGCAGCAGGb,c | All strains |
| DS38 | GGGAGGGCAAGTCAAACACGGGCGCCGCCCGAGCAGGAAGAAACCACCGCb | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc |
| GGGAGGGCAAGTCAAACACGGGCGCCGCCCGAGCAGGAAGAAACCACAGCb | All others | |
| DS45 | ACATCAACTTCCCGGTGTATGGGGGTGTCTTGCACGCATCGCAGGCTb | All strains |
| V3 DSs | ||
| DS3.1a | TACAATTCCACGCTTTCTGGCGACTATGCCCTACCCCGAGCCCCAGCCCCAATCCTA | Cuniculi A |
| DS3 | TAGCGGCTATGCCGCAGCCCCAGCCATCAACTTCCCGGT | Cuniculi A |
| TAGCAGCTATGCCACAGCCCGAGCCGGAGCCGACATCAACTTCCCGGT | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc | |
| TAGCGGCTATGCCACAGCCCGAGCCGGAGCCGACATCAACTTCCCGGT | All others | |
| DS10 | CCCGAGCCCTACCCGCAGCCGCAGCCGCAGTCAACAACGACATCTTAT | Cuniculi A |
| CCCGAGCCCTACCCGCAGCCGCAGCCGCAGCCGCAGTCAACAACGACATCTTAT | All others | |
| DS12 | CCCCAAGCCCAAGCCGCAGCCAACATCAACTTCCCGGTATGGA | Cuniculi A |
| CCCCAAGCCCCAGCCGCAGCCAACATCAACTTCCCGGTATGGA | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc | |
| CCCCAAGCCCCAGCCGCAGCCAACATCAACTTCCCGGTATGGG | All others | |
| DS18 | GCCCGAGCCGCAGCCCCAGCCGGAGCCAACGACATCTCAT | Cuniculi A |
| GCCCGAGCCCGAGCCGGAGCCGCAGTGCCAGCCGCAGCCGACGACATCTTAT | All others | |
| DS24 | AACTCCACACTGTCTAGCGGCTATGCCCAAGCAGCCGGAGCCGCAGCCCAc | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc, Sea81-4 |
| AACTCCACACTGTCTAGCGGCTATGCCCAAGCAGCCGGAGCCGCAGCCGGAGTCCCAGCCCTACCCGGAGCCGCAGCCCAc | All others | |
| DS26 | AGCCCCAGCCCCAGCCCCAGCCCCAGCCAACAACGCCATCTTATGGGA | Nichols Gen, Nichols Sea, Chicago, Dal-1 |
| AGCCCCAGCCCCAGCCCCAGCCCCAGCCCCAGCCAACAACGCCATCTTATGGGA | Sea81-4 | |
| GCCCTGGCCCGAGCCCGACCCCCAGCCCCAGCCAACAACGCCATCTTATGGGA | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc, UW104, UW126 | |
| AGCCCTGGCCGGAGCCCTACCCCGAGCCCCAGACATCTTATGGGA | Cuniculi A | |
| DS32 | CCCCAGCCCAACCCCCAGCCAACATCTTATGGGb,c | All strains |
| DS34 | GCCCAAGCCCGAGCCCTGGCAGCCGGb,c | All strains |
| DS41 | GCCCTACCCCCAGCCGCAGCCCCAGCCAATGACATCTTATGGGb | Cuniculi A |
| GCCCAACCCCCAGCCGTAGCCCCAGCCAATGACATCTTATGGGAb | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc | |
| GCCCAACTCCCAGCCGTAGCCCCAGCCAATGACATCTTATGGGAb | All others | |
| DS43 | TCCACGCTTTCTGGCGACTATGCCCGACCCGGAGCCGCAGCCGb | Cuniculi A |
| TCCACACTTTCTGGCGACTATGCCCGAGCCGCAGCCGCAGCCGb | Sea81-4 | |
| TCCACGCTTTCTGGCGACTATGCCCGAGCCGCAGCCGCAGCCGb | All others | |
| DS55e | TGCTGCTCCTACGAAGTGGAAGGCAGGATATTGTGGGTAT | Nichols Sea, Dal-1 |
| V4 DSs | ||
| DS6 | TGGGGCATAAGAAAAACGGAGCGAATGGCGACATAGGCGCAGA | All strains |
| DS14 | CGTGGGGCGTAAGAAAGACGGAGCGCAGGGCAATGGCGTAGGCGC | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc |
| CGTGGGGCGTAAGAAAGACGGAGCGCAGGGCACCGTAGGCGC | All others | |
| DS36 | CAGACGTGGGGCATAAGAAAGAGAATGCAGCGAACGTCAATGGCACCGTb | All strains |
| DS36.1 | CAGACGTGGGGCATAAGAGAAACGTCCGAGCCCAAGCCCAAGCCGCAGATGCGTT | Cuniculi A |
| DS47 | CGTGGGGCATAAGAAAAACGCAGCTCCCGATGGCATAGGCGCCTCACGCGCb | All strains |
| V5 DSs | ||
| DS3.2a | TTCCCGGTGTGGGACGTCCGCCCGCATCGAAGGCCAGCAATGTGTTTAAAGATGTCTTTCTCACCGATACCACACCCATGCGGACGCACGACGGTGCAG | Cuniculi A |
| DS5 | CGCATCGAAGGCCAGCAATGTGTTTATAGACGTCTTTCTCACCAATGCCATGGACATGCAG ACGCACGACTG | Cuniculi A |
| CGCATCGGAGGCCAGCAATGTGTTTAAAGACGTCTTTCTCACCAATGCCATGGACATGCAG ACGCACGACTG | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc | |
| CGCATCGAAGGCCAGCAATGTGTTTAAAGACGTCTTTCTCACCAATGCCATGGACATGCAG ACGCACGACTG | All others | |
| DS13 | CGCAGGCCAGCAATGTATTTCAGGGAGTATTTCTCAACATGGCCATGACCGCACACGACTG | All strains |
| DS13.1 | TATTTCGCATCGAAGGCCAGCAATGTGTTTAAAGATGTCTTTCTCGCTAGAAACATGGACAT GCAGACGCACGACTGTGCTACTTATATCAA | Cuniculi A |
| DS28 | AGCAATGTATTTCAGGGTGTCTTTCTCACCACACCCATGCAGAAGGACGACTGb | All strains |
| DS35 | TATTCGGGGGAGTATTTCTCACCAATAACATGCTGCAGCACGACTGb | All strains |
| DS46 | AGCAATGTATTCGAGGGAGTATTTCTCACCACACCCATGCGGACGCACGACATb | Cuniculi A |
| AGTAATGTATTTCAGGGTGTCTTTCTCACCGATACCACACCCATGCGGACGCACGACATb | All others | |
| DS48 | CGCATCGAAGGCCAGCAATGTATTTCAGGGTGTCTTTCTCGCTCGAAACATAGCCATGCG AGAGCACGACTGb,c | Samoa D |
| CGCATCGAAGGCCAGCAATGTATTTCAGGGTGTCTTTCTCGCTAGAAACATAGCCATGCGA GAGCACGACTGb,c | Gauthier, Iraq B, CDC2, Fribourg-Blanc | |
| CGCATCGAAGGCCAGCAATGTATTCGAGGGAGTATTTCTCGCTAGAAACATAGCCATGCGA GAGCACGACTGb,c | All others | |
| V6 DSs | ||
| DS1a | AAGCCCTACCCCCAGCCATTTACTTCCC | All strains |
| DS4 | CCCCAGCCATCAACTTCCCGGTGT | Cuniculi A |
| AGCCCGAGCCGGAGCCGACATCAACTTCCCGGTGT | All others | |
| DS11f | CGTACATGCCCGTCCACTACAAGGTCCTGACCGGACCCCAAGCCCAAGCCGCAGc | Cuniculi A |
| CGTACATGCCCGTCCACTACACGGTCCTGACCGGACCCCAAGCCCCAGCCGCAGc | All strains | |
| DS19f | CGTACATGCCTGTCCATTACAAAGTCCTAAAAGCCCACGCCCGAGCCCCAGCCGACATCCA CTTCCCGGTGTc | All strains |
| DS23f | GCCCGAGCCGTACCCCCAGCCCGAGTTGACATCTACTTCTCGGc | All strains |
| DS25f | CGTACGTACATGCCCGTCCATTGGAAAGCCCCAGCCCCAGCCCCAGCCCCAGCCAACAACGC CATCTTATGGGA | Nichols Gen, Nichols Sea, Chicago, Dal-1 |
| CGTACGTACATGCCCGTCCATTGGAAAGCCCCAGCCCCAGCCCCAGCCCCAGCCCCAGCCAACAACGCCATCTTATGGGA | Sea81-4 | |
| CGTACGTACATGCCCGTCCATTGGAAAGCCCTGGCCCGAGCCCGACCCCCAGCCCCAGCCAACAACGCCATCTTATGGGA | Gauthier, Iraq B, Samoa D, CDC2, Street14, Fribourg-Blanc | |
| TGTACATGCCCGTCCATTGGAAAGCCCTGGCCGGAGCCCTACCCCGAGCC | Cuniculi A | |
| DS33 | TGCCCGTCCACGCCCAAGCCCGAGCCCTGGCAGCCGGAGTCCCACCCGGAGCCCCTG ATAb,c | All strains |
| DS40f | GAGAACGGCATGCCCGTCCATTGGAACGTCAGCGTACAGTCCCACGCCCGAGCCCTACCCCCAGCCGCAGCCCCAGCCAATGACATCb | Cuniculi A |
| CATGCCCGTCTATTACTTCGCAGCCCGAGCCCAACCCCCAGCCGTAGCCCCAGCCAATGACATCTb | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc | |
| CATGCCCGTCTATTACTTCGCAGCCCGAGCCCAACTCCCAGCCGTAGCCCCAGCCAATGACATCTb | All others | |
| DS42f | CGTACATGCCCGTCCATTGGAACGCCTTCACCCAAGCCCGAGCCCTGCCCGGAGCCCCAGTCCCAGCCATTTACTTCCCGGTb | Cuniculi A |
| CGTACATGCCCGTCCATTGGAACGCCTTCACCCAAGCCCGAGCCCTGCCCGGAGCCCCAGTCCCAGCCATCTACTTCCCGGTb | All others | |
| DS44f | GCCCGAGCCGCAGCCGCAGCCGGGGCTGGAGTCGACATCAACTTCCCGGTGTATGGb,c | All strains |
| DS50 | AAAACCCGAGCCCTACCCGCAGCCGCAGCCGCAGCCGCAGTCAACAACGACATCTc | All strains |
| DS51 | AACTCCACACTGTCTAGCGGCTATGCCCAAGCAGCCGGAGCCGCAGCCCAc | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc |
| AACTCCACACTGTCTAGCGGCTATGCCCAAGCAGCCGGAGCCGCAGCCGGAGTCCCAGCCCTACCCGGAGCCGCAGCCCAc | All others | |
| DS52 | CCCGAGCCCTACCCGCAGCCGCAGCCGCAGCCGCAGTCAACAACGACATCTTATc | All strains |
| DS53 | AAGCCCTACCCCCAGCCATTTACTTCCC | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc |
| V7 DSs | ||
| DS2a | CCCCCTGCTGCTCCTGGTACGAAGTGGAGCAAGGAATATTGTGGGTATTACG | Gauthier |
| CCCCCTGCTGCTCCTGCTACGAAGTGGAGCAAGGAATATTGTGGGTATTACG | All others | |
| DS2.1a | CCCGGTTGTAG | Cuniculi A |
| DS2.2a | GACGTGGAGCAAGGAATATTGTGGGTATTACGA | Cuniculi A |
| DS9 | GTACGGCGGTACGAACAAAAAGGCCACGCCCCCTGCTGCTCCTGCTGTTCCTACGAAGTGGAAGGCAGAATATTG | Cuniculi A |
| GTACGGCGGTACGAACAAAAAGGCCACGCCCCCTGCTGCTCCTGCTGCTCCTACGAAGTGGAAGGCAGAATATTG | All strains | |
| DS17 | GTACAGGCGGTACGAACAAAAAAGCTGCTGCTGCAGCCCCTGCTCCTGGTACGAAGTGGAGCA | Cuniculi A |
| GTACGGCGGTACGAACAAAAAAGCTGCTGCTGCAGCCCCTGCTCCTGGTACGAAGTGGAGCA | All strains | |
| DS22 | GTACGGCGGTACGAACAAGCAAGCTGCTGCGGTCTGCTCTTACGAAGc | All strains |
| DS27 | CCCCTGCTGCTGCAGTTCCTGGTACGAAGTc | All strains |
| DS31 | GCGGTACGAACAAGAAAAACGATGCTGCTCCTGGTGCAGTTCCTGGGGCGGTCCCTGGTGCAGTTCCTGGTGCTGCTGCTCTTACGAAGTGGAAGGCAGGATATTGTGGGTATb,c | UW126, Street14 |
| GCGGTACGAACAAGAAAAACGATGCTGCTCCTGGTGCGGTTCCTGGGGCGGTCCCTGGTGCAGTTCCTGGTGCTGCTGCTCTTACGAAGTGGAAGGCAGGATATTGTGGGTATb,c | UW104 | |
| GCGGTACGAACAAGAAAAACGATGCTGCTCCTACGAAGTGGAAGGCAGGATATTGTGG GTATc | All others | |
| DS39 | TGCTCCTGCTCTTACGAAGTGGAGCAAAGGATATTGTGGGTATTACGc | Cuniculi A |
| TGCTCCTGGTACGAAGTGGAGCAAGGGATATTGTGGGTATTACGc | Fribourg-Blanc | |
| TGCTCCTGGTACGAAGTGGAGCAAGGAATATTGTGGGTATTACGc | Gauthier, Iraq B, Samoa D, CDC2 | |
| TGCTCCTGCTACGAAGTGGAAGGCAGAATATTGTGGGTATTACGc | All others | |
| DS49 | GTACGGCGGTACGAACAAGCAAGCTGCTGCGGTc | All strains |
| DS54 | CCCCCTGCTGCTCCTGGTACGAAGTGGAGCAAGGAATATTGTGGGTATTACGA | Gauthier, Iraq B, Samoa D, CDC2, Fribourg-Blanc |
| DS56e | CCCCCAGCCCAACCCCCAGCCAA | Nichols Sea, Dal-1 |
DSs located in the TP0131 (tprD) 5′-flanking region.
DSs absent in the original T. pallidum subsp. pallidum Nichols strain genome sequence (19).
DSs absent in T. paraluiscuniculi (Cuniculi A strain).
Nichols Gen, published Nichols strain sequence (19); Nichols Sea, Nichols strain propagated in our laboratory.
These DSs are located in the Tp0136 insertion.
These DSs are also used to generate variability in V3.
tprK donor sequences in the TP0131 5′-flanking region are highly conserved among treponemal species and subspecies.
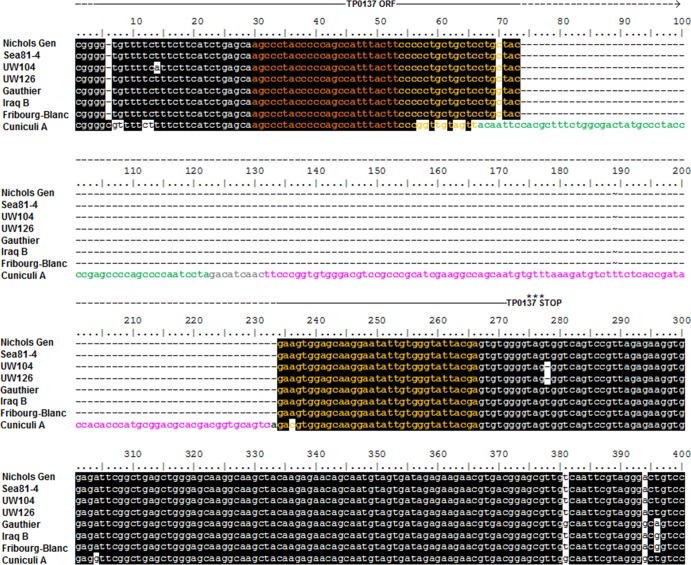
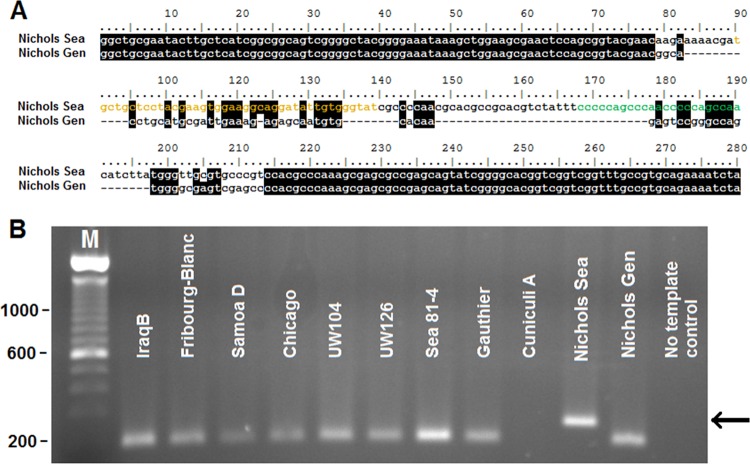
Sequence comparison of the DSs localized in the TP0131 5′-flanking region (chromosomal location 158280 to 158518 in the Nichols Gen strain genome) shows nearly complete sequence identity among all syphilis- and non-syphilis-causing strains, as well as the Fribourg-Blanc treponeme. In contrast, this genomic region is unique in T. paraluiscuniculi in that it contains an insertion of 177 bp (Fig. 2). This insertion has homology with DNA sequences in the TP0131 3′ donor region and contains one duplicate donor cassette for V3 and one for V5 (Fig. 2, in green and pink, respectively). Except for the presence of 22 SNPs in the Cuniculi A strain, and 4 SNPs in the T. pallidum subsp. pertenue strains Gauthier and Samoa D (Fig. 2), as well as in the Fribourg-Blanc treponeme, the DNA sequences flanking the Cuniculi A insertion are identical in all strains. These flanking sequences carry a DS for V6 and one for V7 (Fig. 2, dark and light orange, respectively) and are preceded by the ORF homologous to TP0136 (19), reported to be a surface-exposed, fibronectin-binding protein (7). Additional donor sites were recently identified in Nichols Sea as a 125-bp insertion, containing one DS for V3 (green) and one for V7 (orange) (Fig. 3A). This insertion precisely reproduces a sequence contained in the Nichols Sea TP0131 3′ donor region (see Fig. S1 in the supplemental material, positions 2825 to 3000) that is absent in the Nichols Gen strain. Amplification of the corresponding genomic region in the other strains used in this work (performed with primers TP0136Ins-S and TP0136Ins-As; Table 1) initially suggested that the TP0136 insertion was unique to the Nichols Sea strain (Fig. 3B). Recently, however, Mikalová et al. reported a similar insertion in the T. pallidum subsp. pallidum Dal-1 genome (43). Additionally, this TP0136 insertion is found in the Nichols Farmington, Nichols Dallas, and Nichols UCLA strains while not found in the Nichols CDC, Street14, or Nichols Houston strain (T. B. Reid, unpublished data). Lack of amplification when Cuniculi A DNA is used (Fig. 3B) is due to the marked sequence diversity at the primer binding site between the rabbit treponeme and the Nichols Sea TP0136 ORFs (19).
Fig 2.
Alignment of tprK DSs located in the TP0131 (tprD) 5′-flanking region of treponemal strains. T. paraluiscuniculi (Cuniculi A strain) shows a newly identified insertion containing one donor site for V3 (green) and one donor site for V5 (pink). Three DSs, one for V6 and two for V7, are highlighted in dark and light orange, respectively. Shaded areas indicate regions of sequence identity. Nichols Sea and Dal-1 sequences are identical to the sequence shown for Nichols Gen. Street14 sequence is identical to the sequence shown for UW126. Samoa D sequence is identical to the sequence from the Iraq B strain. CDC2 sequence is identical to the sequence shown for Gauthier. The TP0137 ORF, as annotated in the Nichols Gen (19), is indicated above the alignment.
Fig 3.
(A) In the Nichols Sea strain of T. pallidum subsp. pallidum, a 125-bp-long replacement sequence can be found in the TP0131 5′-flanking region, within TP0136, containing one DS for V3 (green) and one for V7 (orange). (B) PCR amplification of the genomic region encompassing the insertion shows an amplicon of higher size only when Nichols Sea genomic DNA is used as the template. Lane M, size markers (sizes are in bp). Arrow indicates amplicon position.
tprK expression site.
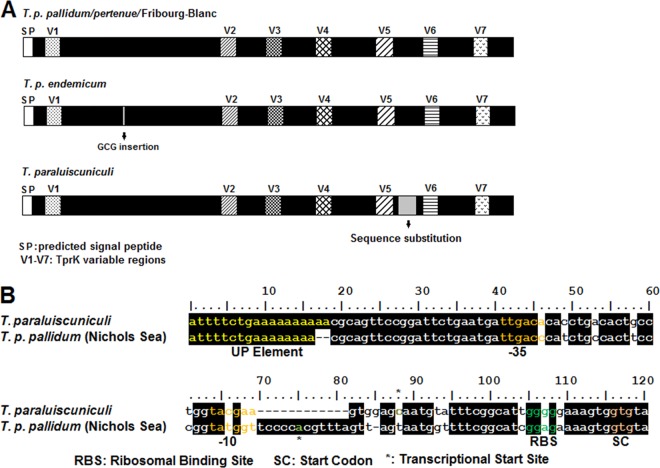
DNA alignments of tprK sequences from the T. pallidum pallidum, T. pallidum pertenue, and T. pallidum endemicum subspecies, as well as the Fribourg-Blanc and Cuniculi A isolates, reveal that the tprK expression site of these strains is characterized by a high degree of sequence identity of the conserved regions, with similar location and sequence anatomy of V regions (12). In the T. pallidum subsp. endemicum isolates, the conserved region between V1 and V2 contains an extra CGC (Ala) codon (Fig. 4A). In T. paraluiscuniculi TprK, a unique sequence (SGDPYTHLLTGLNAGVEARV) previously identified between V5 and V6 (26) is identical to a conserved region of subfamily I and II Tpr antigens (Fig. 4A, sequence substitution). Sequence variability, though limited, is also present in the tprK promoter region (Fig. 4B) when T. paraluiscuniculi and T. pallidum are compared. More specifically, diversity spans from an A/T-rich sequence located 22 nt upstream of the putative tprK −35 sigma70 (σ70) signature, the −35 and −10 σ70 sequences, to the spacer between the putative −10 σ70 sequence and tprK start codon, the region which contains the experimentally identified transcriptional start site (TSS) (Fig. 4B). In prokaryotes, A/T-rich sequences located in close proximity to the −35 σ70 hexamer are also known as UP elements. These elements are sequences that increase transcription by interacting with the C-terminal domain of the RNA polymerase α-subunit (16, 17, 52, 53). Recently, intrastrain variability in the number of A's of this putative UP element was identified by T. B. Reid (unpublished) in the Chicago (7 to 9 A's) and Nichols (7 to 8 A's) strains of T. pallidum subsp. pallidum and in T. paraluiscuniculi, Cuniculi A strain (9 to 10 A's) (L. Giacani, unpublished data). Equally interesting is that a typical ribosomal binding site (RBS) (-GGAG-) is not identifiable in T. paraluiscuniculi as opposed to the remaining treponemal isolates (Fig. 4B). Despite such sequence differences, there is sequence homology of the putative −35 and −10 σ70 sequences from these isolates with the E. coli consensus sequences. The fact that TprK is still expressed in both these isolates despite sequence diversity in the promoter region is supported by the experimentally identified TSSs, the presence of tprK mRNA during infection (Giacani, unpublished), and the fact that antibodies and T-cell responses are generated against TprK during experimental infection with the Cuniculi A strain of T. paraluiscuniculi (26).
Fig 4.
(A) Schematic representation of the tprK ORF in T. pallidum subspecies and T. paraluiscuniculi showing sequence conservation and heterogeneity within the conserved regions. (B) Sequence diversity within the tprK promoter region between T. pallidum isolates and T. paraluiscuniculi. RBS, ribosomal binding site (green); SC, tprK start codon (orange). Yellow, UP element; orange, −10 and −35 σ70 putative consensus sequences. *, experimentally determined transcriptional start sites for tprK.
G4FS identification and circular dichroism spectra.
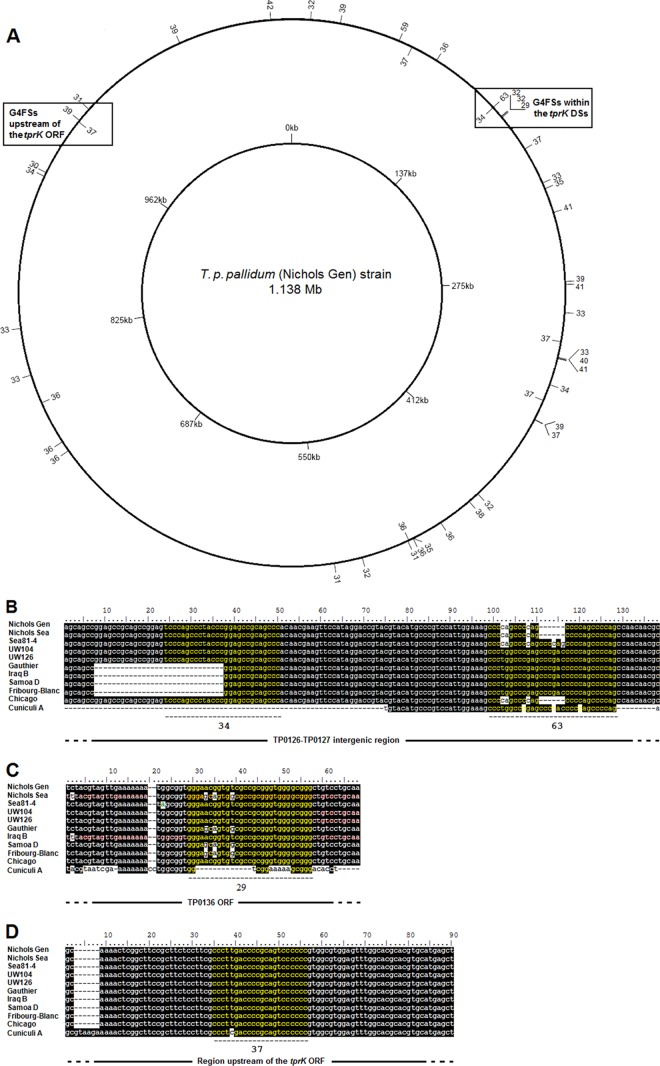
Forty-five putative G4FS with a G-score of ≥29 (approximately 1% of the putative G4FS found in total by QuadFinder and QGRS Finder) were identified in the T. pallidum subsp. pallidum strain Nichols genome (19) (Table 6 and Fig. 5A), almost evenly distributed across the genome, with the exception of the sequence between coordinates 550000 and 733330 (Fig. 5A), where no putative G4FS were detected. Five G4FS are clustered in the large DS-containing genomic region surrounding Tp0131. In more detail, two G4FS (with G-scores of 34 and 63) were identified within the TP0126-TP0127 intergenic region (Table 6 and Fig. 5B), while three G4FS were found 145 bp (with a G-score of 29) (Fig. 5C) and 855,953 bp (both with a G-score of 32) upstream of the TP0131 5′-flanking region with tprK DSs (Table 6). A putative G4FS (G-score of 37) was also found 493 bp upstream of the annotated tprK start codon (Table 6 and Fig. 5D). Different degrees of sequence length and variability were found in these hypothetical G4FS among treponemal species and subspecies, in particular for the sequences located within the TP0131 3′-flanking region (Fig. 5B and C). A total of 15 putative G4Fs with G-scores between 9 and 19 were found in the B. burgdorferi (B31) lp28-1 plasmid, 12 of which lie between coordinates 26997 and 28073 (see Table S1 in the supplemental material).
Table 6.
G4FS identified in the Nichols strain genome of T. pallidum subsp. pallidum (19)
| Position | Location | Length (bp) | Sequencea | G-score | Strand |
|---|---|---|---|---|---|
| 12379b | TP0012 | 29 | GGGAGCCTTCAATAAGGGGGAGGGAAGGG | 32 | + |
| 32161 | TP0025 | 17 | GGGATAGGGGGGATGGG | 39 | + |
| 72671 | TP0067 | 25 | GGGGGGGGGTATGGGGCTTATGGGG | 59 | + |
| 82202 | TP0074 | 30 | GGGGAGTTGGGAACCCTTTTGGGAATTGGG | 37 | − |
| 100635 | TP0091 | 30 | GGGCGGGGGTACCCCGGGTGGCACATCGGG | 36 | + |
| 149328c | IGR104 | 22 | GGGGCTGGGGCTGGGGCTGGGG | 63 | + |
| 149398c | IGR104 | 26 | GGGCTGCGGCTCCGGGTAGGGCTGGG | 34 | − |
| 157297c | TP0136 | 29 | GGGAACTGCGGTTGCGGGGGAGGGGGGGG | 32 | + |
| 157393c | TP0136 | 29 | GGGAACTGCGGTTGCGGGGGAGGGGGGGG | 32 | + |
| 158101c | TP0136 | 29 | GGGAACGGTGTCGCCGCGGGTGGGGCGGG | 29 | + |
| 183960 | TP0162 | 20 | GGGTAAACGGGGGGAGTGGG | 37 | + |
| 209260 | TP0196 | 27 | GGGAGCTGTAAGATGGGTCGGGGTGGG | 33 | + |
| 213318 | TP0207 | 27 | GGGTCGTGGGTCCTCCTCTGGGCGGGG | 35 | + |
| 230028 | IGR175 | 20 | GGGGGAGGGAAGGGTGTGGG | 41 | + |
| 276466 | TP0264 | 24 | GGGTGCACAGGGTGAGGGAGGGGG | 39 | + |
| 278453 | IGR211 | 19 | GGGAAGGGGGAGGGAAGGG | 41 | + |
| 297175 | TP0282 | 24 | GGGGGGCTAGGGCTAAGTGCAGGG | 33 | + |
| 316621 | TP0303 | 29 | GGGTAGAGGGAACAGGGATTCGTACCGGG | 37 | − |
| 327048d | TP0312 | 25 | GGGGGGAATAGTGGTGGGGATAGGG | 33 | + |
| 327636d | TP0312 | 24 | GGGAATGGGGATAGGGTTGCTGGG | 40 | + |
| 327948d | IGR236 | 16 | GGGCCGGGGGGGGGGG | 41 | + |
| 346017 | TP0326 | 27 | GGGTGAACGGGGGGGTGGACTTTCGGG | 34 | + |
| 357406 | TP0334 | 27 | GGGATTCGGGTGTGGGCATCTAACGGG | 37 | − |
| 371115 | TP0345 | 30 | GGGAGGGCACCCCTTGCGGGGCACGCCGGG | 33 | + |
| 371369 | TP0346 | 27 | GGGTATGGGCGCTTTGGGATATATGGG | 39 | + |
| 433549 | TP0408 | 26 | GGGGGGCTGTGGGTCGGTCAAACGGG | 32 | + |
| 441155 | TP0414 | 25 | GGGTGACGGGGATATTCGGGATGGG | 38 | + |
| 464653 | TP0438 | 19 | GGGCGGGGGGTGTGCCGGG | 36 | + |
| 485699 | TP0456 | 30 | GGGTGAGGGCGGTGCATTGGGCGTTCCGGG | 36 | + |
| 485881 | TP0456 | 20 | GGGTGGGGGGAACTGTTGGG | 35 | + |
| 488893 | TP0458 | 26 | GGGTGAACAGAGGGTGCAGGGGAGGG | 36 | − |
| 489727 | IGR335 | 24 | GGGGGGAGGGTGACTGAAGGTGGG | 31 | + |
| 521840 | TP0488 | 26 | GGGATTTTTCTGTAGGGGGGGTGGGG | 32 | + |
| 540710 | IGR364 | 24 | GGGGGGAGGGTGTCGGAGGACGGG | 31 | + |
| 742540 | IGR483 | 30 | GGGCGGGGTATGCGCAGGGTGCACAGCGGG | 36 | + |
| 749636 | TP0685 | 30 | GGGAAAGGGTGCCGTGGTGGGGCTTATGGG | 36 | + |
| 778932 | TP0711 | 30 | GGGCATCGGGTGCGGGGCAGGAGACTCGGG | 36 | − |
| 798620 | TP0733 | 23 | GGGGCGGGGGGTTTCACCTCGGG | 33 | + |
| 830276 | IGR549 | 22 | GGGCCTTTTTGAGGGCGGGGGG | 33 | + |
| 933620 | TP0856 | 30 | GGGTACCGACGTGGGTCTGCAATGGGTGGG | 34 | + |
| 936331 | TP0859 | 27 | GGGTATCGCCACACCCGGGGGGGGGGG | 30 | + |
| 976267e | TP0898 | 22 | GGGGGGACTGCGGGGTCAAGGG | 37 | − |
| 977324e | TP0898 | 16 | GGGGGGTGCGGGTGGG | 39 | + |
| 986979 | TP0905 | 25 | GGGGGGCAGGGCGTCTAGGCAAGGG | 31 | + |
| 1061766 | TP0978 | 24 | GGGATTATCGGGGGAGGGATAGGG | 39 | + |
| 1123979f | TP1030 | 15 | GGGCGGGGGGGGGGG | 42 | + |
Putative G residues involved in formation of the G quadruplex are underlined.
Putative G4FS located downstream of tprB.
Putative G4FS located within the tprD (TP0131) 3′-flanking region (containing tprK DSs). These sequences were tested for ability to form G4 using circular dichroism.
Putative G4FS located upstream of tprE.
Putative G4FS located upstream of tprK expression site. These sequences were tested for ability to form G4 using circular dichroism.
Putative G4FS located upstream of tprL.
Fig 5.
Location of the putative G4FS in the T. pallidum subsp. pallidum (Nichols Gen strain) genome (19). (A) G4FS are mapped using the G-score (ranging from 29 to 63, shown in the outer ring) in the T. pallidum subsp. pallidum Nichols Gen strain. Except for the genomic region encompassed approximately between nt 550000 and 733330, G4FS appear to be evenly distributed throughout the genome. G4FS in proximity to the tprK DSs and tprK gene are indicated. (B) Two of the putative G4FS (yellow) located in the TP0131 3′-flanking region (TP0128-TP0127 intergenic spacer) in T. pallidum subspecies and T. paraluiscuniculi showing sequence diversity among these strains. For each putative G4FS the G-score is indicated below the sequences. The Street14 sequence is identical to the UW126 strain sequence, the Dal-1 sequence is identical to the Chicago strain sequence, and the CDC2 sequence is identical to the Gauthier sequence. (C) One of the putative G4FS (yellow) located in the TP0131 5′-flanking region (within the TP0136 ORF) in T. pallidum subspecies and T. paraluiscuniculi showing sequence diversity among these strains. The G4FS G-score is indicated below the sequence. The Street14 sequence is identical to the UW104 strain sequence, the Dal-1 sequence is identical to the Chicago strain sequence, and the CDC2 sequence is identical to the Gauthier sequence. (D) Putative G4FS located upstream of the tprK ORF in T. pallidum subspecies and T. paraluiscuniculi showing sequence diversity between the rabbit treponeme and the rest of the strains. Street14, Dal-1, and CDC2 sequences are identical to Nichols Gen sequences.
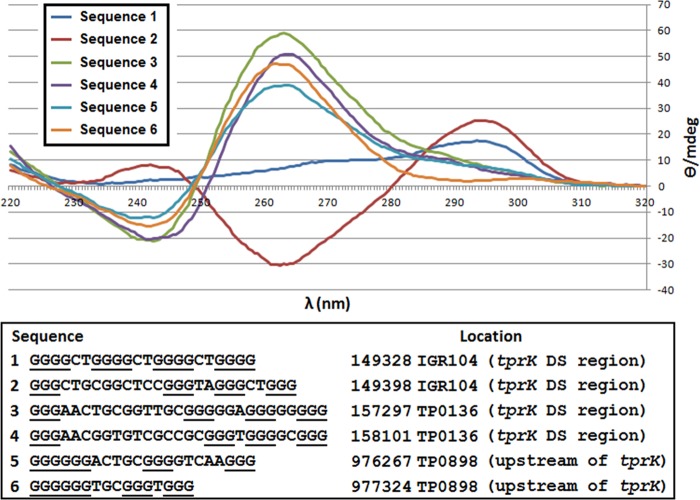
CD analysis of selected putative G4Fs in proximity to the tprK expression and donor sites (marked with an asterisk in Table 6 and reported in Fig. 6) showed that all but one of the analyzed sequences fold into well-defined quadruplexes. CD spectra are compatible with G4 structures with all-parallel strand orientation for sequences 3 and 4 (located within the region harboring the tprK donor sites) and for sequences 5 and 6 (located in proximity to the tprK expression site). Interestingly, both sequence 5 and sequence 6 fold into G4s despite a missing nucleotide in the first loop, in theory required for a perfect three-stack G4 (32). Sequence 1 failed to fold into a G4, despite its high G-score. Sequence 2 generated spectra compatible with a partly folded G4 with antiparallel strand orientation (32). CD spectra are shown in Fig. 6.
Fig 6.
Circular dichroism (CD) spectra of putative G4Fs in proximity to the tprK expression and donor sites. Most of the sequences fold into well-defined quadruplexes, with spectra compatible with G4 with all-parallel strand orientation for sequences 3 to 6. Sequence 1 failed to fold into a stable G4, despite its very elevated G-score. Sequence 2 folded into a structure that resembles a partly folded G4 with antiparallel strand orientation. Buffer alone was used as a negative control (data not plotted).
DISCUSSION
The TprK antigen, a putative OMP and virulence factor, has been shown to undergo antigenic variation (12) by segmental gene conversion during experimental infection in rabbits (25). This antigenic variation system has two components: an expression site that harbors the tprK ORF and donor sites, located at both flanking sides of TP0131, that are copied into the seven tprK V regions. Given the importance of the TprK antigen during syphilis and, likely, during other treponemal infections, it is striking that Treponema isolates like the Chicago strain of T. pallidum subsp. pallidum can diversify the sequence of the tprK gene (before the appearance of TprK-specific antibodies) at a significantly high baseline rate (35, 36), while in other equally virulent syphilis strains, like Nichols, the tprK gene remains virtually clonal during passage at 10-day intervals and varies its sequence only after onset of an adaptive immune response against the initial TprK variant (36). Because no differences were found between the annotated recombinases in these isolates (data not shown), to investigate the reasons behind such dissimilar rates of variation, we comparatively analyzed the anatomy of the tprK expression site and DS regions among treponemal species and subspecies.
There is a very high degree of synteny and sequence conservation (99.57%) among the T. pallidum subspecies and the Fribourg-Blanc isolate in the genomic regions where the tprK DSs are located. This degree of sequence identity suggests a very close evolutionary relationship among these isolates. The ∼1.2-kb deletion observed in the Nichols Gen strain is known today to be carried by a subpopulation of this strain of T. pallidum subsp. pallidum (56) and was likely caused by the loss of genetic material between two direct repeats (highlighted in Fig. S1 in the supplemental material), which may undergo excision due to slipped-strand mispairing during DNA replication (38, 62). In the Nichols Gen strain, the implications of the loss of ∼30% of its donor sites due to the ∼1.1-kb deletion for the physiology of this spirochete and disease progression are currently unclear. Even when a full repertoire of DSs is present (such as in the Nichols Sea strain genome), diversification of the TprK antigen in the different Nichols lineages appears to require an adaptive host immune response against the initial TprK variant (36). Since its isolation in 1912 from the cerebrospinal fluid (CSF) of a patient with secondary syphilis (46), the Nichols strain of T. pallidum subsp. pallidum has been continuously propagated in rabbits, and the hypothesis that slower tprK variability in the Nichols strain could reflect its adaptation to rapid passage in immunologically naive rabbits cannot be excluded.
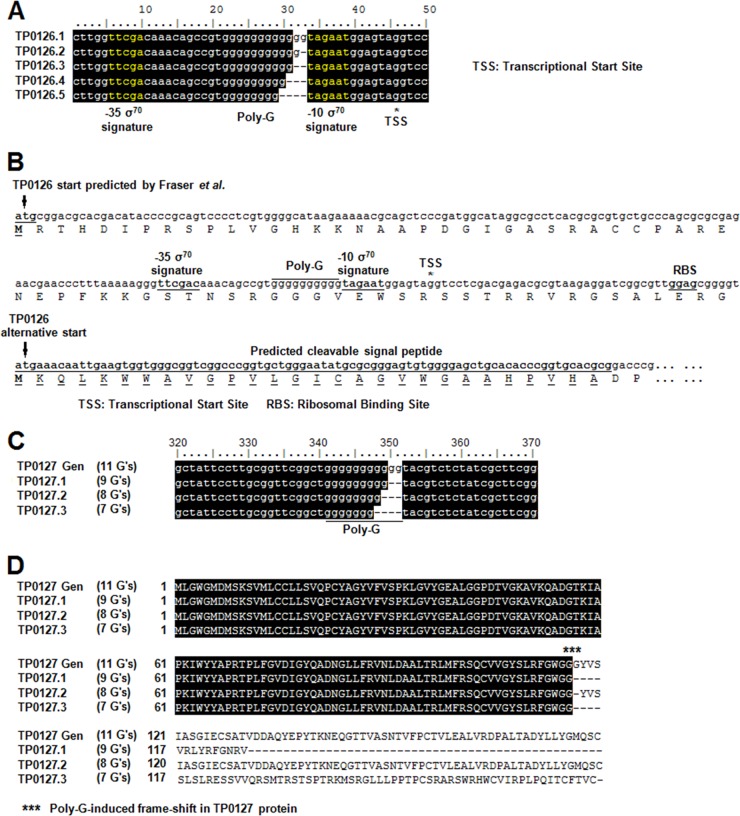
Variability in the TP0131 3′-flanking region also affects the ORF annotation process for the sequences encompassing TP0126-TP0127. This topic was also addressed by Mikalová et al. (43), who predicted a TP126 ORF of 1,374 nt (458 amino acids [aa]) in Samoa D but an ORF of only 669 nt (223 aa) in Gauthier and Fribourg-Blanc. According to this annotation, TP0126 sequence would encompass several tprK DSs in Samoa D but none in Gauthier or Fribourg-Blanc. To further complicate this scenario, in the original T. pallidum subsp. pallidum genome project (19), in the TP0131 −3′-flanking region (Fig. 1, Nichols Gen strain), TP0126 was annotated as a 291-aa putative protein, an intermediate size between those of Samoa D and Gauthier/Fribourg-Blanc TP0126 (43). Based on our study, however, we propose that two elements contribute to the correct annotation of this genomic region: namely, the presence of variable homopolymeric G-tracts (poly-G) both upstream of TP0126 and within the TP0127 ORF (Fig. 7A to C). With regard to TP0126, a poly-G of 10 G residues (like the one reported for Samoa D) (43) extends the TP0126 coding sequence to 1,374 nt, while a poly-G of only nine residues induces the identification of an alternative start for TP0126, located 705 nt downstream of the one identified by Mikalová et al. in the Samoa D strain. The intrastrain variability of this poly-G prompted us to identify the most likely start codon for TP0126. Initial prediction of promoter elements for TP0126 (Fig. 7A) supports our hypothesis that, in all these genomes, TP0126 is actually a 223-aa protein, encoded by an ORF that does not encompass any tprK DS. This shorter TP0126 is predicted to carry a cleavable signal peptide, and it shows structural homology (data not shown) with Escherichia coli OmpW, an OMP involved in transporting small hydrophobic molecules from the extracellular milieu into the periplasm (1, 30). Also, because the poly-G sequence is located between putative classical σ70 signatures, we postulate that TP0126 might undergo phase variation, similar to that described for Nesseria menigitidis porA (63), where the poly-G is also located between the −10 and −35 σ signatures. Changes in the poly-G length in the porA promoter regulate gene transcription by varying the optimal spacing of 17 nucleotides between the consensus sequences. Preliminary results (not shown) using a reporter gene system (23), in which our identified TP0126 promoter drives green fluorescent protein (GFP) transcription, showed that poly-G sequences of various lengths induced different levels of fluorescence reflecting modulation of transcription. This reinforces the hypothesis that, in all isolates studied here, TP0126 might indeed be a shorter ORF than predicted for Samoa D (43) or the Nichols Gen strain (19). While the poly-G associated with TP0126 is located in the putative promoter of this shorter ORF, the poly-G tract associated with TP0127 is located within its coding sequence. Depending upon the number of G's present within the TP0127 ORF, two alternative TP0127 proteins of comparable size but different primary structure can be predicted, as well as a third variant characterized by a premature stop codon that truncates the protein (Fig. 7B). Previously, poly-G tracts were shown in T. pallidum subsp. pallidum to control transcription of a subset of tpr genes (23); however, in the case of TP0127, variation in the poly-G length might also induce the synthesis of different proteins from the same genomic locus. Interestingly, when the sequence unique to the TP0127.3 variant (Fig. 7D) is analyzed for structural homology using the LOMETS metaserver (zhanglab.ccmb.med.umich.edu/LOMETS), a MutS1 homolog is identified. MutS1 homologs are involved in preventing recombination between partially homologous DNA sequences, suggesting a possible role for this DS-neighboring ORF in the generation of tprK variability.
Fig 7.
Length variability of poly-G sequences in the promoter region of TP0126 and predicted effect in translation of TP0127. (A) Variability in the number of G residues in the poly-G upstream of TP0126 ORF. Sequences obtained from amplification of the TP0126 ORF and its newly predicted promoter regions with template DNA from the T. pallidum subsp. pallidum Chicago strain. Putative −10 and −35 σ70 signatures are highlighted in yellow. (B) Comparison of TP0126 start codon identified by Fraser et al. (19) and the alternative TP0126 start downstream of the poly-G sequence. Predicted σ70 signatures, transcriptional start site (TSS), ribosomal binding site (RBS), and putative signal peptide for the alternative ORF are indicated. (C) Variability in the poly-G within the TP0127 ORF. TP0127 Gen refers to the TP0127 ORF identified by Fraser et al. (19); alternative poly-G lengths were isolated from the T. pallidum subsp. pallidum Chicago strain. (D) Effect of different poly-G length on TP0127 predicted proteins. Nine G residues (TP0127.1) cause an early TP0127 truncation; 11 or 8 residues are associated with a 180- or 179-aa-long peptide (TP0127 Gen and TP0127.2, respectively), while 7 residues (TP0127.3) result in a 175-aa-long protein with a different amino acid sequence than TP0127.2.
Upstream of the tprK ORF, where the gene promoter is located, sequence heterogeneity might influence the level of tprK expression, due to (i) the presence of a variable A/T-rich element, similar to an UP element, upstream of the tprK −35 σ70 signature and (ii) the absence of a typical RBS in T. paraluiscuniculi, compared to the other treponemal strains analyzed here. Preliminary quantitative amplification studies targeting tprK mRNA in Nichols Sea, Chicago, Sea81-4, Gauthier, Samoa D, Iraq B, and Cuniculi A strains (24; Giacani, unpublished) show differing levels of expression of the tprK gene in these isolates, which warrants further exploration of the role of sequence diversity in the tprK promoter region among strains.
It is particularly noteworthy that G-rich sequences able to fold into guanine quadruplex (G4) motifs are found upstream of the tprK expression site and within the genomic regions where the tprK DSs are located. Although G4FS (31, 65) have been implicated in many biological processes in eukaryotes, such as mRNA stability (55), transcription pausing (64), Fragile X mental retardation protein binding (14), and translation initiation and repression (6), Cahoon and Seifert (8, 9) recently reported that a G4FS located upstream of the pilE gene, in the intergenic region preceding the ORF, is required for pilin antigenic variation in N. gonorrhoeae. Specifically, this putative N. gonorrhoeae G4FS is hypothesized to be a specialized sequence that favors recombination initiation and gene conversion between pilE and its donor sites. The role of G4FS in the generation of tprK variants is still hypothetical, and it awaits further experimental evidence. It is intriguing that, in treponemal isolates, the location of these G4FS mirrors that reported for N. gonorrhoeae, with the G-rich sequence of the G4FS in proximity to the ORF start codon and in the opposite strand with respect to the expression site (8). The finding of G4FS within the genomic region containing tprK DSs is novel, however, in that there is no parallel finding for N. gonorrhoeae, in which the presence of G4FS near the pilE donor sites was not reported (8). The presence of sequence variability in the DNA regions where these treponemal G4FS are located suggests a possible role for these sequences in the generation of tprK variants in pathogenic treponemes. Interestingly, when G4FS are predicted in Borrelia burgdorferi B31 linear plasmid 28-1 (lp28-1), which contains donor sequences and an expression site for the variable surface-exposed lipoprotein VlsE (48), 12 of 15 predicted G4FS are located within the vlsE expression sites (nt 26997 to 28073; data not shown). Even though the location of these G4FS differs from the one described in both N. gonorrhoeae and T. pallidum subsp. pallidum, the distribution of G4FS on lp28-1 is suggestive of the involvement of these sequences also in the generation of VlsE variants in the Lyme disease spirochete.
Our findings set the stage for future studies in which many aspects of the tprK antigenic variation system may be explored, including DS usage among species and subspecies and the roles of SNPs, G4FS, and small sequence changes in adaptation and pathogenicity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants AI063940 and AI42143 (to S.A.L., A.C.-L., and L.G.) and AI096129 (to L.G.) and the 2010 ASTDA Developmental Award (to L.G.).
We are grateful to Christina Marra for providing us with the T. pallidum subsp. pallidum UW104 and UW126 strains.
Footnotes
Published ahead of print 1 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Albrecht R, Zeth K, Soding J, Lupas A, Linke D. 2006. Expression, crystallization and preliminary X-ray crystallographic studies of the outer membrane protein OmpW from Escherichia coli. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 62: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antal GM, Lukehart SA, Meheus AZ. 2002. The endemic treponematoses. Microbes Infect. 4: 83–94 [DOI] [PubMed] [Google Scholar]
- 3. Bagga PS. 2008. Bioinformatics approaches for studying untranslated regions of mRNAs. Methods Mol. Biol. 419: 1–21 [DOI] [PubMed] [Google Scholar]
- 4. Baker-Zander SA, Lukehart SA. 1984. Antigenic cross-reactivity between Treponema pallidum and other pathogenic members of the family Spirochaetaceae. Infect. Immun. 46: 116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baughn RE, Musher DM. 2005. Secondary syphilitic lesions. Clin. Microbiol. Rev. 18: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonnal S, et al. 2003. A single internal ribosome entry site containing a G quartet RNA structure drives fibroblast growth factor 2 gene expression at four alternative translation initiation codons. J. Biol. Chem. 278: 39330–39336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinkman MB, et al. 2008. A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect. Immun. 76: 1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325: 764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahoon LA, Seifert HS. 2011. Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol. Microbiol. 81: 1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castro LG. 1994. Nonvenereal treponematosis. J. Am. Acad. Dermatol. 31: 1075–1076 [DOI] [PubMed] [Google Scholar]
- 11. Centurion-Lara A, et al. 1999. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189: 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centurion-Lara A, et al. 2004. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol. Microbiol. 52: 1579–1596 [DOI] [PubMed] [Google Scholar]
- 13. Cox DL, et al. 2010. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect. Immun. 78: 5178–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darnell JC, et al. 2001. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107: 489–499 [DOI] [PubMed] [Google Scholar]
- 15. DiGiacomo RF, et al. 1984. Clinical course and treatment of venereal spirochaetosis in New Zealand White rabbits. Br. J. Vener. Dis. 60: 214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estrem ST, Gaal T, Ross W, Gourse RL. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. U. S. A. 95: 9761–9766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estrem ST, et al. 1999. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase alpha subunit. Genes Dev. 13: 2134–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farnsworth N, Rosen T. 2006. Endemic treponematosis: review and update. Clin. Dermatol. 24: 181–190 [DOI] [PubMed] [Google Scholar]
- 19. Fraser CM, et al. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281: 375–388 [DOI] [PubMed] [Google Scholar]
- 20. Fribourg-Blanc A, Niel G, Mollaret HH. 1963. Note sur quelques aspects immunologiques du cynocephale africain. Bull. Soc. Pathol. Exot. 56: 474–485 [PubMed] [Google Scholar]
- 21. Giacani L, Hevner K, Centurion-Lara A. 2005. Gene organization and transcriptional analysis of the tprJ, tprI, tprG, and tprF loci in Treponema pallidum strains Nichols and Sea 81-4. J. Bacteriol. 187: 6084–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giacani L, et al. 2010. Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J. Bacteriol. 192: 2645–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giacani L, Lukehart S, Centurion-Lara A. 2007. Length of guanosine homopolymeric repeats modulates promoter activity of Subfamily II tpr genes of Treponema pallidum ssp. pallidum. FEMS Immunol. Med. Microbiol. 51: 289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giacani L, et al. 2007. Quantitative analysis of tpr gene expression in Treponema pallidum isolates: differences among isolates and correlation with T-cell responsiveness in experimental syphilis. Infect. Immun. 75: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giacani L, et al. 2010. Antigenic variation in Treponema pallidum: TprK sequence diversity accumulates in response to immune pressure during experimental syphilis. J. Immunol. 184: 3822–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giacani L, et al. 2004. Tpr homologs in Treponema paraluiscuniculi Cuniculi A strain. Infect. Immun. 72: 6561–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gjestland T. 1955. The Oslo study of untreated syphilis. Acta Derm. Venereol. Suppl. 35 (Suppl. 34): 3–368 [DOI] [PubMed] [Google Scholar]
- 28. Golden MR, Marra CM, Holmes KK. 2003. Update on syphilis: resurgence of an old problem. JAMA 290: 1510–1514 [DOI] [PubMed] [Google Scholar]
- 29. Hazlett KR, et al. 2001. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193: 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong H, Patel DR, Tamm LK, van den Berg B. 2006. The outer membrane protein OmpW forms an eight-stranded beta-barrel with a hydrophobic channel. J. Biol. Chem. 281: 7568–7577 [DOI] [PubMed] [Google Scholar]
- 31. Huppert JL. 2010. Structure, location and interactions of G-quadruplexes. FEBS J. 277: 3452–3458 [DOI] [PubMed] [Google Scholar]
- 32. Joachimi A, Benz A, Hartig JS. 2009. A comparison of DNA and RNA quadruplex structures and stabilities. Bioorg. Med. Chem. 17: 6811–6815 [DOI] [PubMed] [Google Scholar]
- 33. Kikin O, D'Antonio L, Bagga PS. 2006. QGRS Mapper: a web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res. 34: W676–W682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kostadinov R, et al. 2006. GRSDB: a database of quadruplex forming G-rich sequences in alternatively processed mammalian pre-mRNA sequences. Nucleic Acids Res. 34: D119–D124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LaFond RE, et al. 2003. Sequence diversity of Treponema pallidum subsp. pallidum tprK in human syphilis lesions and rabbit-propagated isolates. J. Bacteriol. 185: 6262–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaFond RE, Centurion-Lara A, Godornes C, Van Voorhis WC, Lukehart SA. 2006. TprK sequence diversity accumulates during infection of rabbits with Treponema pallidum subsp. pallidum Nichols strain. Infect. Immun. 74: 1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. LaFond RE, Lukehart SA. 2006. Biological basis for syphilis. Clin. Microbiol. Rev. 19: 29–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levinson G, Gutman GA. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol. Biol. Evol. 4: 203–221 [DOI] [PubMed] [Google Scholar]
- 39. Lukehart SA, Baker-Zander SA, Sell S. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 124: 454–460 [PubMed] [Google Scholar]
- 40. Marra CM, et al. 2006. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J. Infect. Dis. 194: 1771–1773 [DOI] [PubMed] [Google Scholar]
- 41. Martin PM, Cockayne A, Georges AJ, Penn CW. 1990. Immune response to Treponema pertenue and Treponema pallidum Nichols in patients with yaws. Res. Microbiol. 141: 181–186 [DOI] [PubMed] [Google Scholar]
- 42. Matejkova P, et al. 2008. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikalová L, et al. 2010. Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: most of the genetic differences are localized in six regions. PLoS One 5: e15713 doi:10.1371/journal.pone.0015713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morgan CA, Lukehart SA, Van Voorhis WC. 2003. Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect. Immun. 71: 5605–5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. 2002. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J. Immunol. 169: 952–957 [DOI] [PubMed] [Google Scholar]
- 46. Nichols HJ, Hough WH. 1913. Demonstration of Spirochaeta pallida in the cerebrospinal fluid. JAMA 60: 108–110 [Google Scholar]
- 47. Noordhoek GT, et al. 1990. A new attempt to distinguish serologically the subspecies of Treponema pallidum causing syphilis and yaws. J. Clin. Microbiol. 28: 1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norris SJ. 2006. Antigenic variation with a twist—the Borrelia story. Mol. Microbiol. 60: 1319–1322 [DOI] [PubMed] [Google Scholar]
- 49. Perine PL. 1998. Endemic treponematoses, p 1033–1036 In Isselbacher KJ, et al. (ed), Harrison's principles of internal medicine, 14th ed. McGraw Hill, Inc., New York, NY [Google Scholar]
- 50. Perine PL, et al. 1984. Handbook of endemic treponematoses: yaws, endemic syphilis, and pinta. World Health Organization, Geneva, Switzerland [Google Scholar]
- 51. Roman GC, Roman LN. 1986. Occurrence of congenital, cardiovascular, visceral, neurological, and neuro-ophthalmologic complications in late yaws: a theme for future research. Rev. Infect. Dis. 8: 760–770 [PubMed] [Google Scholar]
- 52. Ross W, Aiyar SE, Salomon J, Gourse RL. 1998. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J. Bacteriol. 180: 5375–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ross W, Ernst A, Gourse RL. 2001. Fine structure of E. coli RNA polymerase-promoter interactions: alpha subunit binding to the UP element minor groove. Genes Dev. 15: 491–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simon R. P. 1985. Neurosyphilis. Arch. Neurol. 42: 606–613 [DOI] [PubMed] [Google Scholar]
- 55. Simonsson T. 2001. G-quadruplex DNA structures—variations on a theme. Biol. Chem. 382: 621–628 [DOI] [PubMed] [Google Scholar]
- 56. Smajs D, et al. 2011. Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One 6: e20415 doi:10.1371/journal.pone.0020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Small JD, Newman B. 1972. Venereal spirochetosis of rabbits (rabbit syphilis) due to Treponema cuniculi: a clinical, serological, and histopathological study. Lab. Anim. Sci. 22: 77–89 [PubMed] [Google Scholar]
- 58. Smith JL, et al. 1971. Neuro-ophthalmological study of late yaws and pinta. II. The Caracas Project. Br. J. Vener. Dis. 47: 226–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith JL, Pesetsky BR. 1967. The current status of Treponema cuniculi. Review of the literature. Br. J. Vener. Dis. 43: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thornburg RW, Baseman JB. 1983. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect. Immun. 42: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Turner TB, Hollander DH. 1957. Biology of the treponematoses. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 62. van Belkum A, Scherer S, van Alphen L, Verbrugh H. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62: 275–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van der Ende A, Hopman CT, Dankert J. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68: 6685–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yonaha M, Proudfoot NJ. 1999. Specific transcriptional pausing activates polyadenylation in a coupled in vitro system. Mol. Cell 3: 593–600 [DOI] [PubMed] [Google Scholar]
- 65. Zhou W, Brand NJ, Ying L. 2011. G-quadruplexes-novel mediators of gene function. J. Cardiovasc. Transl. Res. 4: 256–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.