Abstract
Two-component systems (TCSs) consisting of a membrane-anchored histidine kinase (HK) and a response regulator (RR) with DNA-binding activity. are major players in signal transduction in prokaryotes. Whereas most TCSs in Escherichia coli are well characterized, almost nothing is known about the LytS-like HK YehU and the corresponding LytTR-like RR YehT. To identify YehT-regulated genes, we compared the transcriptomes of E. coli cells overproducing either YehT or the RR KdpE (control). The expression levels of 32 genes differed more than 8-fold between the two strains. A comprehensive evaluation of these genes identified yjiY as a target of YehT. Electrophoretic mobility shift assays with purified YehT confirmed that YehT interacts directly with the yjiY promoter. Specifically, YehT binds to two direct repeats of the motif ACC(G/A)CT(C/T)A separated by a 13-bp spacer in the yjiY promoter. The target gene yjiY encodes an inner membrane protein belonging to the CstA superfamily of transporters. In E. coli cells growing in media containing peptides or amino acids as a carbon source, yjiY is strongly induced at the onset of the stationary-growth phase. Moreover, expression was found to be dependent on cyclic AMP (cAMP)/cAMP receptor protein (CRP). It is suggested that YehU/YehT participates in the stationary-phase control network.
INTRODUCTION
The prototypic two-component system (TCS) comprises a membrane-anchored, stimulus-sensing histidine kinase (HK) and a response regulator (RR) with DNA-binding activity. Escherichia coli encodes 32 RRs and 30 HKs. Although many of these are well characterized (25), almost nothing is known about the YehU/YehT system (see Fig. 1).
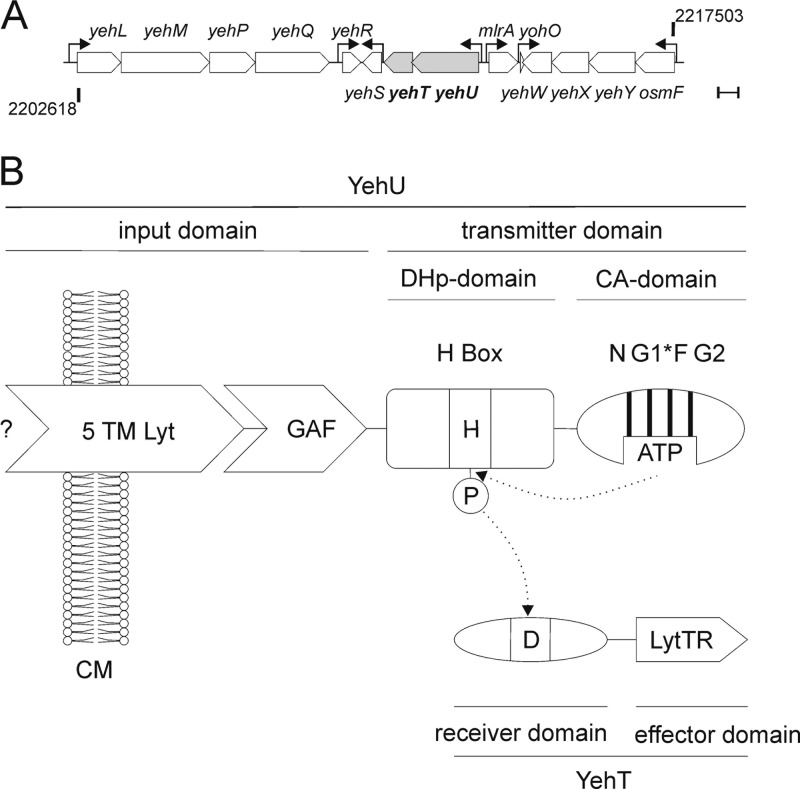
Fig 1.
The YehU/YehT system of E. coli. (A) The region between 47.48 and 47.77 centisomes (bp 2202618 to 2217503) around the yehUT locus on the E. coli MG1655 chromosome is shown. See the text for a description of the neighboring genes. Arrows mark transcription start sites as indicated by EcoCyc (www.ecocyc.org) (32). Bar, 500 bp. (B) Domain structures of YehU and YehT. YehU is a class I HK in which DHp and CA domains are connected (30). The input domain of YehU consists of the 5TM Lyt (LytS-YhcK) domain (2) and a GAF domain. The G1 box of YehU is incomplete (G1*). YehT is made up of a CheY-like receiver domain and a LytTR-type DNA-binding domain (44). The phosphorylation sites are indicated (H, His; D, Asp). N, G1, F, and G2 are conserved boxes in HKs; CM, cytoplasmic membrane.
The YehU/YehT system is one of the most widespread TCSs in bacteria and is found in several human and plant pathogens. Various systematic studies have failed to identify either the stimulus or the target gene(s) for this TCS (45). Under all conditions tested, autophosphorylation of YehU and phosphotransfer to YehT were barely detectable (65). A yehUT deletion mutant exhibited no phenotypic alterations when subjected to phenotypic microarray analysis (67). However, overproduction of YehT was associated with moderate resistance to agents such as crystal violet or deoxycholate (28).
The yehU and yehT genes form an operon with a 4-bp overlap, which is localized at 47.638 centisomes in the E. coli MG1655 genome (Fig. 1A). The neighboring genes are mlrA (221 bp upstream of yehU) and yehS (46 bp downstream of yehT) (Fig. 1A). mlrA encodes a regulator of curli production in pathogenic E. coli (9). The function of yehS is unknown. Adjacent to these genes are the genes/operons yohO, osmF-yehYXW, yehR, and yehLMPQ. yehL encodes a putative ATP-binding subunit of the ABC transporter family (56); yohO encodes a small membrane protein (27); and the product of the osmF-yehYXW operon is a putative ABC transporter (13). Thus far, no functions are predicted for the products of yehM, yehP, yehQ, or yehR.
Based on sequence and homology analysis, YehU is a LytS-like HK and YehT a LytTR-like RR (2, 48). LytS HKs are putative sensors for peptidoglycan subunits in Gram-positive bacteria (11). The input domain of YehU consists of the 5TM Lyt (LytS-YhcK) domain (2) and a GAF domain (Fig. 1B). Bioinformatic analysis suggests that YehU is anchored in the membrane by at least five transmembrane (TM) helices (according to the TMHMM, MEMSAT3, and OCTOPUS programs [29, 35, 62]). The GAF domain is commonly found in cyclic GMP (cGMP)-specific phosphodiesterases, adenylyl cyclases, and the FhlA protein (hence GAF) and is capable, e.g., of binding cGMP and ions (12), but its function in most proteins is still unknown (43). YehT is composed of a CheY-like receiver and a LytTR-homologous DNA-binding domain (44). In general, transcriptional regulators with a LytTR domain are involved in the biosynthesis of extracellular polysaccharides, in fimbriation, and in quorum sensing in several Gram-positive and Gram-negative species (44). The LytTR binding motif was originally described as (T/A)(A/C)(C/A)GTTN(A/G)(T/G) (44). Subsequently, the structure of the LytTR domain of AgrA from Staphylococcus aureus was resolved, revealing a 10-stranded beta fold with an unusual mode of binding to the DNA (54). This and other studies have indicated that the recognition motif of LytTR-containing RRs is more variable than previously supposed (19, 22, 54).
Here we have undertaken a comprehensive characterization of the YehU/YehT system of Escherichia coli K-12. We identified and characterized yjiY as a direct target of YehT. Furthermore, our data suggest a role of the YehU/YehT system in the stationary-phase control network.
MATERIALS AND METHODS
Strains, plasmids, and oligonucleotides.
E. coli strains and their genotypes are listed in Table 1. Mutants were constructed by using the E. coli Quick and Easy gene deletion kit and the Bac Modification kit (both from Gene Bridges) as reported previously (26). Both kits rely on the Red/ET recombination technique. The plasmids used in this work are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or descriptiona | Reference or source |
|---|---|---|
| E. coli strains | ||
| MG1655 | F− λ− ilvG rfb50 rph-1 | 8 |
| MG1655 rspL150 | F− λ− ilvG rfb50 rph-1 rpsL150; Strr | 26 |
| MG2 | MG1655 ΔyehUT | This work |
| MG3 | MG1655 rpsL150 ΔyehT∷rpsL-neo; Kanr Strs | This work |
| MG6 | MG1655 rpsL150 ΔyehU∷rpsL-neo; Kanr Strs | This work |
| MG10 | MG1655 ΔyjiY∷FRT-PGK-gb2-neo-FRT; Kanr | This work |
| MG11 | MG1655 ΔcyaA∷FRT-PGK-gb2-neo-FRT; Kanr | This work |
| MG12 | MG1655 Δcrp∷FRT-PGK-gb2-neo-FRT; Kanr | This work |
| MG1655-ΔlacZ | MG1655 ΔlacZ∷Tetr | K. Jahreis (personal gift) |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) | 58 |
| Plasmids | ||
| pRed/ET | λ RED recombinase in pBAD24; Ampr | Gene Bridges |
| pCP20 | FLP recombinase, λcI 857+, λpR Repts; Ampr Cmr | 15 |
| pBAD18-Kan | Arabinose-inducible PBAD promoter, pBR322 ori; Kanr | 24 |
| pBAD18-yehT | 6His-yehT and Shine-Dalgarno sequence cloned into the NheI and SphI sites of pBAD18-Kan; Kanr | This work |
| pBAD24 | Arabinose-inducible PBAD promoter, pBR322 ori; Ampr | 24 |
| pBAD24-yehT | 6His-yehT cloned into the EcoRI and XbaI sites of pBAD24; Ampr | This work |
| pBAD24-yehT D54E | yehT D54E cloned into the EcoI and XbaI sites of pBAD24-yehT; Ampr | This work |
| pBAD24-yehT D54N | yehT D54N cloned into the NdeI and XbaI sites of pBAD24-yehT; Ampr | This work |
| pBAD24-kdpE | kdpE cloned into the NdeI and XbaI sites of pBAD24-kdpE; Ampr | This work |
| pBAD24-yjiY | yjiY-6His cloned into the EcoRI and XbaI sites of pBAD24; Ampr | This work |
| pBAD24-yehU | yehU-6His cloned into the EcoRI and XbaI sites of pBAD24; Ampr | This work |
| pBAD24-yehU H382Q | yehU H382Q cloned into the EcoRI and NdeI sites of pBAD24-yehU; Ampr | This work |
| pBAD24-yehUT | yehUT cloned into the EcoRI and XbaI sites of pBAD24; Ampr | This work |
| pUC19 | IPTG-inducible Plac promoter, pMB1 ori; Ampr | 66 |
| pUC19 PyjiY−212/+88 | PyjiY−212/+88 cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY−12/+88 | PyjiY−12/+88 cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY−112/−13 | PyjiY −112/−13 cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY−212/−113 | PyjiY−212/−113 cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY MM3 | PyjiY MM3 (substituted M3 site) cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY MM23 | PyjiY MM23 (substituted M2 or M3 site) cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY MM123 | PyjiY MM123 (substituted M1 to M3 sites) cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pUC19 PyjiY SC14 | PyjiY SC14 (substituted spacer motif, 14 nucleotides replaced) cloned into the EcoRI and BamHI sites of pUC19; Ampr | This work |
| pRS415 | Operon fusion vector | 55 |
| pRS415 PyjiY−212/+88 | PyjiY−212/+88 cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY MM3 | PyjiY MM3 (substituted M3 site) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY MM23 | PyjiY MM23 (substituted M2 and M3 sites) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY MM123 | PyjiY MM123 (substituted M1 to M3 sites) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY SC14 | PyjiY SC14 (substituted spacer motif, 14 nucleotides replaced) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY SC1 | PyjiY SC1 (central nucleotide within the 13-bp spacer replaced) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY SC3 | PyjiY SC3 (three central nucleotides within the 13-bp spacer replaced) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY SC7 | PyjiY SC7 (seven central nucleotides within the 13-bp spacer replaced) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pRS415 PyjiY SC13 | PyjiY SC13 (13 nucleotides within the 13-bp spacer replaced) cloned into the EcoRI and BamHI sites of pRS415; Ampr | This work |
| pBBR1-MCS5-TT-RBS-lux | luxCDABE and terminators lambda T0 rrnB1 T1 cloned into pBBR1-MCS5 for plasmid-based transcriptional fusions; Gmr | 23 |
| pBBR yjiY-lux | PyjiY −212/+88 cloned into the BamHI and EcoRI sites of pBBR1-MCS5-TT-RBS-lux; Gmr | This work |
| pBBR yjiY-sub-CRP-BS-lux | PyjiY sub-CRP-BS (substituted CRP-binding site in PyjiY−212/+88) cloned into the BamHI and EcoRI sites of pBBR1-MCS5-TT-RBS-lux; Gmr | This work |
IPTG, isopropyl-β-d-thiogalactopyranoside.
yehT was amplified by PCR from genomic DNA using primers yehT N-6His EcoRI sense and yehT XbaI anti (see Table S1 in the supplemental material), which introduce codons for an N-terminal hexahistidine tag, a factor Xa site, and an additional NdeI site. The yehT variants yehT D54E and yehT D54N were constructed by two-step PCR using mismatched primers D54E sense, D54E anti, D54N sense, and D54N anti (see Table S1) and the primers mentioned above. yehU was amplified by PCR from genomic DNA using primers yehU EcoRI sense and yehU C6-his XbaI anti (see Table S1), which introduce codons for a C-terminal hexahistidine tag, a factor Xa site, and an additional NdeI site. The yehU variant yehU H382Q was constructed by two-step PCR using mismatched primers H382Q sense and H382Q anti (see Table S1), as well as the primers mentioned above. yjiY-6His and yehUT were amplified by PCR from genomic DNA using primer pairs yjiY EcoRI sense/yjiY C-6His XbaI anti and yehU EcoRI sense/yehT XbaI anti, respectively (see Table S1). Amplified fragments were cloned into the EcoRI and XbaI sites in pBAD24. kdpE was amplified by PCR from genomic DNA using primers kdpE sense and kdpE antisense (see Table S1) and was cloned into the NdeI and XbaI sites in pBAD24-yehT, replacing yehT. Based on pBAD24-yehT, pBAD18-yehT was constructed by inserting the Shine-Dalgarno sequence from pBAD24 together with 6His-yehT into the NheI and SphI sites in pBAD18.
In the case of PyjiY−12/+88, PyjiY−112/−13, PyjiY−212/−113, and PyjiY−212/+88, promoter fragments were amplified by PCR from genomic DNA and were cloned into pUC19 via the EcoRI and BamHI sites. All mutated promoter constructs used for electrophoretic mobility shift assays (EMSAs) were produced by annealing complementary oligonucleotides and were cloned into pUC19.
Mutated yjiY promoter fragments used for in vivo studies were generated by two-step PCR using primers yjiY-5P-3 sense and yjiY-5P-1 anti and appropriate combinations of 2STPCR_ms, 2STPCR_m123, 2STPCR_m23, 2STPCR_m3, yjiY spacer 1, yjiY spacer 3, yjiY spacer 7, and yjiY spacer 13 (see Table S1 in the supplemental material), digested with EcoRI and BamHI, and cloned into vector pRS415. To analyze the expression of yjiY in more detail, a transcriptional fusion of the yjiY promoter (PyjiY−212/+88) and the luciferase operon luxCDABE was generated by amplifying a DNA fragment of the yjiY promoter region using primers up yjiY 300bp BamHI sense and up yjiY EcoRI anti (see Table S1). The fragment was cloned into vector pBBR1-MCS5-TT-RBS-lux (23), resulting in plasmid pBBR yjiY-lux. pBBR1-MCS plasmids are low- to medium-copy-number plasmids (30 copies/cell), with ColE1- and P15a-based replicons (34). No incompatibility group has been defined for them (3), but they are compatible with IncP, IncQ, and IncW group plasmids.
Mutations in the cyclic AMP (cAMP) receptor protein (CRP)-binding site were introduced by amplifying the DNA fragment of the yjiY promoter region by two-step PCR using mismatched primers up yjiY Del CRP BS sense and up yjiY Del CRP BS anti (see Table S1 in the supplemental material) and the primers mentioned above. The resulting fragment was cloned into plasmid pBBR yjiY-lux, replacing the yjiY promoter region.
Molecular biological techniques.
Plasmid DNA and genomic DNA were isolated using the HiYield Plasmid Mini kit (Süd-Laborbedarf GmbH, Gauting, Germany) and the DNeasy Blood and Tissue Kit (Qiagen), respectively. DNA fragments were purified from agarose gels using the Hi-Yield PCR cleanup and gel extraction kit (Süd-Laborbedarf GmbH). Phusion High-Fidelity DNA polymerase or Phire Hot Start DNA polymerase (Finnzymes) was used according to the supplier's instructions. Restriction enzymes and other DNA-modifying enzymes were purchased from New England BioLabs (NEB) and were used according to the manufacturer's directions.
Growth conditions and RNA isolation.
E. coli MG1655 and the mutants MG2 and MG3 were transformed with plasmid pBAD24-yehT, pBAD24-yehT D54E, pBAD24-yehT D54N, or pBAD24-kdpE. Strains were grown overnight in lysogeny broth (LB) medium.
After inoculation of 100 ml of LB medium, cells were grown aerobically at 37°C to the early-exponential phase (optical density at 600 nm [OD600], 0.5). At this point, overexpression of genes encoding the corresponding RRs was induced by adding 0.2% (wt/vol) l-arabinose, and growth was allowed to continue for 45 min. Cells were harvested, and total RNA was isolated essentially as described previously (1) and was treated with DNase I for 30 min to remove residual chromosomal DNA. Subsequently, RNA was purified using the RNA Pure kit (Süd-Laborbedarf GmbH).
Preparation of fluorescence-labeled cDNA, hybridization, and microarray analysis.
Fluorescence-labeled cDNA was prepared, hybridization was performed, and microarray analysis was conducted by the Kompetenzzentrum für Fluoreszente Bioanalytik (KFB; Regensburg, Germany) using Affymetrix E. coli 2.0 chips with Affymetrix chemistry. RNA derived from three biological replicates was prepared and hybridized. Statistical analysis was also performed by KFB (Regensburg, Germany) using MAS5 software (Affymetrix).
Northern blot analysis.
mRNA transcripts of potential target genes were quantitatively analyzed by Northern blot analysis. This technique reveals only relative induction levels by using a radioactively labeled probe that is specific for the mRNA of interest. The protocol used for Northern blot analysis has been described elsewhere (31). Briefly, 20-μg samples of RNA were fractionated by electrophoresis on 1.2% (wt/vol) agarose gels containing 1.1% (vol/vol) formaldehyde in MOPS (4-morpholinepropanesulfonic acid) buffer. RNA was transferred to a Hybond nylon membrane (GE Healthcare) by capillary blotting. Hybridization was performed by following a standard protocol (51) using an [α-32P]dCTP-labeled PCR fragment specific for the first 99 to 1,000 bp of the target mRNA. The radioactive probes were synthesized using the Rediprime II DNA labeling system (GE Healthcare) and were purified with a PCR cleanup and gel extraction kit. Radioactive labeling was quantified with a phosphorimager. As a control, the expression of rpoD, a housekeeping gene of E. coli encoding the sigma 70 subunit of RNA polymerase (32), was analyzed.
5′ RACE.
Rapid amplification of 5′ cDNA ends (5′ RACE) was performed according to protocols described previously (4, 7) with minor modifications. 5′ triphosphates were converted to monophosphates by treatment of 5 μg total RNA with RNA 5′-polyphosphatase (Epicentre Technologies) according to the manufacturer's instructions. Control RNA was incubated under the same conditions in the absence of the enzyme. Reactions were stopped by phenol-chloroform extraction, followed by ethanol-sodium acetate precipitation. Precipitated RNAs were dissolved in water. Five hundred picomoles of a 5′ RNA adapter (see Table S2 in the supplemental material) was added, and the mixture was first heated at 95°C for 5 min and then quick-chilled on ice. The adapter was ligated to the purified total RNA with 50 U of T4 RNA ligase (New England BioLabs) at 17°C overnight. After extraction with phenol-chloroform, the ethanol-precipitated RNA (5 μg) was reverse transcribed with gene-specific primers (2 pmol each) and ThermoScript reverse transcriptase (RT) (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed in three consecutive 20-min steps at 55°C, 60°C, and 65°C. Subsequently, the samples were treated with RNase H. The products of reverse transcription were amplified by PCR. Products were separated on 1.8% (wt/vol) Tris-acetate-EDTA (TAE) agarose gels, and bands of interest were excised, gel-eluted, cloned into pUC19 (linearized by SmaI), and treated with alkaline phosphatase (NEB). After transformation, colonies were screened for the presence of inserts of appropriate sizes by colony PCR with primers uni24 and rev24. Plasmids were purified, and the inserts were sequenced on an ABI 3730 automatic DNA sequencer (Applied Biosystems).
Purification of 6His-YehT.
E. coli BL21(DE3) cells harboring plasmid pBAD24-yehT were cultivated aerobically in LB medium supplemented with ampicillin (100 μg ml−1) at 37°C to an OD600 of 0.8, and overproduction was induced by adding 0.2% (wt/vol) l-arabinose. After 3 h of induction, cells were harvested, washed with buffer (10 mM Tris-HCl [pH 7.5], 5% [vol/vol] glycerol) at 4°C to remove residual medium, and stored at −80°C until use. The cell pellet was resuspended in 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 10% (vol/vol) glycerol, 30 mM imidazole, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 50 μg ml−1 DNase I, and cells were disrupted using a cell disruptor (Constant Systems Ltd.) at 135 MPa and 4°C. Cell debris and unbroken cells were removed by low-speed centrifugation (12,000 × g, 4°C, 20 min), and membranes were removed in a subsequent high-speed centrifugation (144,000 × g, 4°C, 60 min). The Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen) was equilibrated in buffer W1 (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 10% [vol/vol] glycerol, 30 mM imidazole, 2 mM β-mercaptoethanol). The soluble fraction was mixed with preequilibrated Ni2+-NTA resin and was incubated at 4°C for 30 min. The protein-resin complex was loaded onto a column and was washed with buffer W1 (100-fold of the resin volume). Subsequently, YehT was eluted using elution buffer (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 10% [vol/vol] glycerol, 300 mM imidazole, 2 mM β-mercaptoethanol). Eluted YehT was dialyzed against buffer (50 mM Tris-HCl [pH 7.5], 10% [vol/vol] glycerol, 2 mM β-mercaptoethanol, with stepwise reduction of NaCl 500, 300, 150, 100, 75, and 50 mM). All steps were monitored via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (36) and Western blotting by using an anti-His antibody (Qiagen) and an alkaline phosphatase-conjugated anti-mouse antibody as the secondary antibody. The protein concentration was determined by the method of Lowry et al.(38). The protein was about 95% pure as judged by SDS-PAGE and Western blot analysis using an anti-His-Tag antibody.
Acetyl[32P]phosphate was synthesized using a protocol modified from that in reference 57 and was resuspended in a buffer (50 mM Tris-HCl [pH 7.5], 5% [vol/vol] glycerol, 0.1 mM EDTA, 1 mM DTT). The concentration was determined as described previously (37). Phosphorylation of 0.6 mg/ml 6His-YehT was performed at 37°C with 20 mM MgCl2 and 20 mM acetyl[32P]phosphate for a maximum of 60 min. The reaction was stopped by adding SDS sample buffer (36). Samples were the loaded onto an SDS-polyacrylamide (PA) gel. Phosphorylated YehT was detected by Western blotting and was visualized with the Typhoon Trio variable-mode imager (GE Healthcare) after exposure to a phosphor screen.
EMSA.
The upstream regulatory regions of yjiY, cspI, yhjX, and ypjB were amplified using a 5′ primer labeled with the 6-isomer of carboxyfluorescein (6-FAM) (6-FAM uni24) and a unlabeled primer (rev24) (see Table S1 in the supplemental material) with the corresponding pUC19 plasmids as the template. Alternatively, short DNA fragments comprising parts of the yjiY upstream regulatory sequence were obtained by annealing of appropriate oligonucleotide pairs (pairs of yjiY YehT bs, yjiY YehT bs mut 1, yjiY YehT bs mut 2, yjiY YehT bs mut 3, yjiY YehT bs spacer mut), cloned into vector pUC19, and amplified by PCR as described above. After PCR amplification, the DNA fragments were purified by electrophoresis on 7% (wt/vol) polyacrylamide gels according to the protocol provided with the GenElute gel extraction kit (Sigma).
YehT-DNA binding assays were carried out in a total volume of 27.5 μl containing 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), 6% (vol/vol) glycerol, 5.45 ng μl−1 salmon sperm DNA (as a nonspecific competitor), 1.1 nM 6-FAM-labeled DNA, 135 μg ml−1 bovine serum albumin (BSA), and increasing concentrations of 6His-YehT. DNA-protein binding assay mixtures were incubated at room temperature for 15 min. Complexes were resolved by electrophoresis in native TAE polyacrylamide gels (5% [wt/vol]), which were prerun at 70 V for 1 h. After 3 h of electrophoresis at a constant voltage (10 V cm−1) in 0.5× TAE buffer, gels were scanned in a Typhoon Trio variable-mode imager equipped with 488-nm (excitation) and 526-nm (emission) filters. Free DNA and protein-bound DNA were quantified using ImageQuant (version 5.0) analysis software (Molecular Dynamics).
DNase I footprinting.
The DNase I footprinting protocol using 6-FAM-labeled DNA and an ABI 3730 DNA sequencer was adapted from the work of Zianni et al. (2006) (68). Briefly, a 407-bp DNA fragment, including the upstream region of yjiY (bases −212 to +88), was amplified from the corresponding pUC19 derivative and was purified as described above. Aliquots (550 fmol) of this 6-FAM-labeled DNA fragment were incubated with 300 nM, 600 nM, 1,200 nM, or 2,400 nM purified 6His-YehT or, as a control, with BSA in a total volume of 27.5 μl (other components were 50 mM NaCl, 10 mM Tris-HCl [pH 7.5], 6% [vol/vol] glycerol, 135 μg ml−1 BSA, 0.05 mM CaCl2, and 0.25 mM MgCl2) at 25°C for 15 min.
DNase I (0.01 U) was added to the reaction mixture, which was incubated at 25°C for 2 min. The reaction was stopped by adding 137.5 μl of DF buffer (Hi Yield PCR cleanup and gel extraction kit; Süd-Laborbedarf GmbH). After purification, the DNA fragments were analyzed with an ABI 3730 DNA sequencer. The sequences were analyzed with PeakScanner software, version 1.0 (Applied Biosystems). Positions were mapped according to a GeneScan 500 LIZ size standard (Applied Biosystems), which was corun with each sample.
β-Galactosidase activity assay.
Cells from overnight cultures of E. coli MG1655 ΔlacZ transformed with pBAD18-yehT and pRS415 encoding various yjiY promoter∷lacZ fusions were inoculated into LB medium (OD600, 0.05). Cultures were grown aerobically in Erlenmeyer flasks at 37°C to the mid-exponential-growth phase. YehT overproduction was induced by the addition of 0.2% (wt/vol) l-arabinose for 45 min. Cells were harvested, and β-galactosidase activities were measured as described previously (61). Values are given in Miller units, calculated according to the method of Miller (42).
Production and detection of YjiY-6His in the membrane fraction.
E. coli BL21(DE3) cells transformed with pBAD24-yjiY were grown to an OD600 of 0.5 in LB medium, and gene expression was induced by addition of 0.2% (wt/vol) arabinose. After growth for a further 3 h, cells were harvested by centrifugation, disrupted, and fractionated as described above. At each step, pellets were resuspended in equal volumes of TG buffer (50 mM Tris-HCl [pH 7.5], 10% [vol/vol] glycerol). An equal volume of each fraction was fractionated by SDS-PAGE (36) and was analyzed by Western blotting using a primary anti-His antibody as described above.
In vivo yjiY expression studies.
In vivo yjiY expression was probed by a luciferase-based reporter gene assay using the pBBR yjiY-lux plasmid in E. coli MG1655 and its corresponding mutants (Table 1).
For complementation studies, the yehUT operon was expressed under the control of the PBAD promoter in the absence of l-arabinose. Cells of an overnight culture grown in M9 minimal medium with 0.5% (wt/vol) glucose as a C source were inoculated into LB medium or M9 minimal medium (supplemented with different C sources and additives [20 mM amino acids, 0.5% {wt/vol} all other C sources, peptidoglycan fragments at 1/20, 1/200, 1/2,000, and 1/20,000 dilutions of peptidoglycan fragment solutions]), resulting in an OD600 of 0.05. Cells were grown under aerobic growth conditions at 37°C, and the OD600 and luminescence were measured continuously. The optical density of cultures was determined in a microplate reader (Tecan Sunrise) at 600 nm. Luminescence levels were determined in a Centro LB960 luminometer (Berthold Technology) for 0.1 s and are reported as relative light units (counts s−1) (RLU). Peptidoglycan fragment solutions of Bacillus subtilis, Lactobacillus casei, and E. coli were isolated as described by Shah et al. (2008) (53).
Microarray data accession number.
The complete microarray data set is listed in the ArrayExpress database (46) under accession no. E-MTAB-984.
RESULTS AND DISCUSSION
Phenotypic characterization of a yehUT deletion mutant.
The E. coli strains MG1655 and MG2 (ΔyehUT) were phenotypically analyzed with respect to motility, biofilm formation, cell surface hydrophobicity, curli formation, and cell morphology, but no major differences were observed. Furthermore, neither different growth temperatures nor oxygen tensions nor carbon or iron starvation resulted in differences between the two E. coli strains (data not shown). In addition, the survival of the mutant under salt, osmotic, temperature, and heavy-metal stresses was tested and found to be comparable to that of the parental strain. A previous phenotypic microarray analysis that included about 2,000 different growth conditions also failed to uncover any significant difference between the wild-type strain and an isogenic yehUT mutant (67). Thus, under the conditions tested so far, the deletion of yehUT has no obvious phenotypic effect.
Identification of YehT target genes.
To identify genes targeted by YehT, we used various strategies. The first approach was based on the assumption that target genes might lie in the neighborhood of the yehUT operon. We therefore measured levels of the transcripts of the yehR, yehS, mlrA, and yohO genes and the osmF-yehYXW and yehLMPQ operons (Fig. 1A) in E. coli MG1655 and the isogenic yehUT deletion mutant by Northern blot analysis, but we detected no differences between the two strains (data not shown). Each promoter was also tested by the electrophoretic mobility shift assay for the ability to interact in vitro with 6His-YehT. The calculated KD (equilibrium dissociation constant) values were in the range of 300 nM, indicating, at most, only low-affinity binding. These promoters did not contain the YehT-binding site that was subsequently characterized (see below). The discrepancy between the earlier transcriptome results and our study could be explained by indirect effects due to the yehUT deletion or strain-specific effects of E. coli BW25113.
For the second approach, we focused on the expression of genes that had previously been found to be differentially expressed as a consequence of yehUT deletion in strain BW25113 (yafT, cspB, rfc, yehR, rfaS, yjhC, and yjhA were downregulated, and mlc, hemH, focA, yccY, narG, narK, napD, nirB, feoA, malT, nikA, ilvC, and yjfO were upregulated) (45). We compared levels of the transcripts of these genes in E. coli MG1655 and an isogenic yehUT deletion strain after growth to exponential or stationary phase under microaerobic conditions at 30°C, but again, no differences were noted (data not shown). In vitro data from EMSAs performed with 6His-YehT and DNA fragments encompassing the promoters of these genes yielded KD values in the range of 0.8 to 1.2 μM, indicating extremely low affinities (see below).
The final approach was based on overproduction of YehT and subsequent transcriptome analysis. Such an artificial microarray-based strategy has already been used successfully, e.g., for identification of the targets of Rap proteins in Bacillus subtilis (5) or of EvgA in E. coli (39). yehT was overexpressed from the arabinose-inducible promoter of plasmid pBAD24 in E. coli strain MG3, which is deficient in yehT. To avoid any side effects due to protein overproduction, cells with the same genetic background but overproducing KdpE instead of YehT were used as a negative control. KdpE is an RR required under conditions of K+ limitation with a distinct DNA-binding sequence that is found only once in the E. coli genome (59, 63). The two strains grew at comparable rates under overproducing conditions (data not shown). Overexpression of yehT and kdpE was limited to 45 min; then total RNA was prepared. Production of YehT and KdpE was monitored by Western blot analysis and was found to be indistinguishable (data not shown). The global expression patterns of the two strains were analyzed using RNA obtained from three independent biological experiments using Affymetrix E. coli (version 2.0) chips. In the evaluation of differentially expressed genes, we excluded intergenic regions and considered only those genes that were well expressed (fluorescence, ≥100 arbitrary units) and showed a highly significant (P, ≤0.01 by the t test) difference in expression (at least 8-fold) between strains overexpressing YehT versus KdpE. A total of 32 genes met these criteria, with expression differences ranging from a maximum of 200-fold induction to 120-fold repression. As anticipated, yehT expression was increased about 5-fold, and kdpE expression rose 18-fold, in the respective strains. Overproduction of KdpE resulted in significant induction of the genes of the kdpFABC operon (see Table S3 in the supplemental material) in the absence of the natural stimulus, providing an internal control for the reliability of this approach. The complete microarray data set is listed in the ArrayExpress database (46) under accession no. E-MTAB-984. Nevertheless, because overproduction of RRs is an artificial procedure, the microarray data had to be carefully validated. For this reason, we focused on those genes that showed the greatest differences in expression between the two test strains (see Table S2 in the supplemental material).
Validation of YehT-dependent target gene expression by Northern blot analysis.
To validate the data obtained by the transcriptome analysis, we probed the transcription levels of all genes identified in strains MG1655 and MG2 following overproduction of YehT-D54E or KdpE (negative control) by Northern blotting (see Table S2 in the supplemental material). The YehT-D54E variant, in which the putative phosphorylation site was replaced by glutamate, was chosen to mimic the phosphorylated state of YehT (see below). The expression of all genes tested was affected either by overproduction of YehT-D54E or by overproduction of KdpE (see Table S2). According to this analysis, only 4 of the 32 genes identified (yjiY, cspI, ypjB, and yhjX) (Table 2; Fig. 2A) were directly dependent on YehT, while overproduction of KdpE directly or indirectly (e.g., due to increased K+ accumulation) influenced the expression of 28 genes (see Table S2). Among the YehT-dependent genes, expression of yjiY, which encodes a putative carbon starvation protein, was strongly induced (Table 2; Fig. 2A). Expression of yhjX (coding for a putative resistance protein), cspI (a cold shock-like protein), and ypjB (a hypothetical protein) was found to be repressed upon overproduction of YehT (Table 2; Fig. 2A). Since the stimulus perceived by the YehU/YehT system is unknown, we assayed transcript levels for all four genes in E. coli MG1655 and MG2 (ΔyehUT) following transient (45-min) overproduction of YehT, the putative phosphorylation-independent variant YehT-D54E, or the putative phosphorylation-resistant variant YehT-D54N (Fig. 2A). Wild-type YehT and both variants significantly induced yjiY expression, while the expression of cspI, yhjX, and ypjB was repressed (Fig. 2A). yjiY was induced about 40-fold when YehT or either of its variants was overproduced in E. coli MG1655 or the yehUT mutant. yjiY expression was absent in the yehUT mutant (Fig. 2A). Increased expression of cspI (about 2-fold), yhjX (about 50-fold), and ypjB (about 1.5-fold) was observed in the yehUT deletion mutant, while overproduction of YehT or either variant caused repression of these genes (Fig. 2A). Thus, Northern blot analysis indicated a strong effect of YehT overproduction on the regulation of yjiY and yhjX and a much weaker effect on cspI and ypjB. At this time, we did not observe any differences between wild-type YehT and the putative phosphorylation-independent YehT-D54E variant or the phosphorylation-resistant, inactive YehT-D54N variant. As described below, an increase in the copy number of YehT overrides the necessity of phosphorylation.
Table 2.
Genes most affected by the overexpression of yehTa
| Gene | b-no. | rFb |
Log2 ratioc | Pd | Function | Transcriptional regulation by YehTe | |
|---|---|---|---|---|---|---|---|
| YehT | KdpE | ||||||
| yjiY | b4354 | 9,170 | 50 | 7.6 | ≤10−3 | Predicted inner membrane protein | ↑ |
| cspI | b1552 | 300 | 2,480 | −3.0 | 0.01 | Cold shock-like protein CspI | ↓ |
| yhjX | b3547 | 80 | 3,120 | −5.3 | ≤10−3 | Uncharacterized member of the major facilitator superfamily of transporters | ↓ |
| ypjB | b2649 | 20 | 1,790 | −8.3 | ≤10−3 | Hypothetical protein | ↓ |
Gene names, b-numbers, and gene product function are adopted from http://www.ecocyc.org (32) and the Affymetrix Expression Analysis Sequence Information Database (14).
rF, relative fluorescence (arbitrary units).
Log2 ratios of transcript levels for the yehT and kdpE overexpression strains, calculated from the ratio of the mean fluorescence intensity of the respective transcript in the yehT-overexpressing strain to that in the kdpE overexpression strain. A negative value indicates a decrease, and a positive value indicates an increase, in the transcription level upon YehT overproduction over that seen with KdpE overproduction.
Significance (by the t test) of single rF values.
Effect of YehT overproduction on the transcript levels of the respective genes in E. coli MG2/pBAD24-yehT compared to control cells (E. coli MG2/pBAD24), as determined by Northern blot analysis. YehT-dependent induction (↑) or repression (↓) of the gene is indicated (see Fig. 2A).
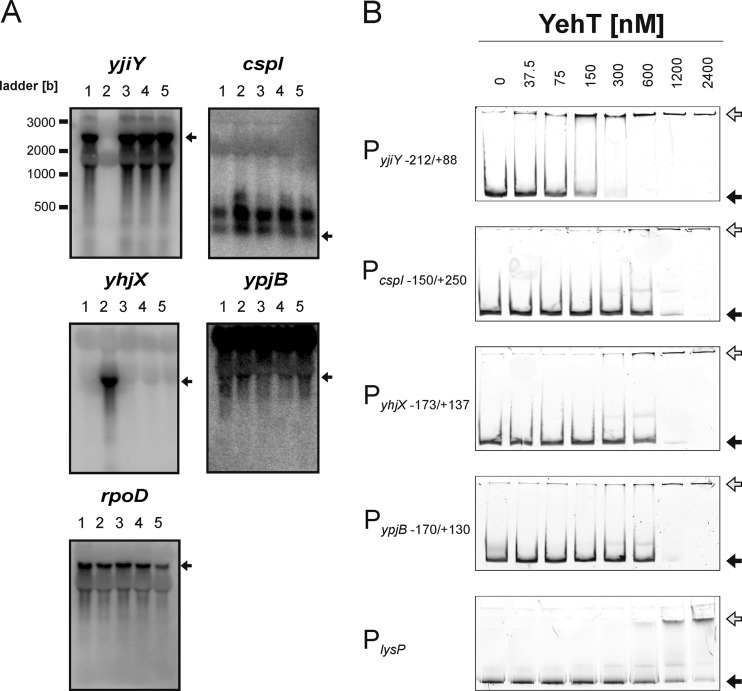
Fig 2.
Evaluation of potential YehT target genes. (A) Northern blot analysis was used to measure the effect of overproduction of YehT-D54E on the expression of the yjiY, cspI, yhjX, ypjB, and rpoD (control) genes in E. coli MG1655 (lanes 1). The expression levels of these genes were also assessed in the E. coli strain MG2 (ΔyehUT) (lanes 2 to 5) in the absence of YehT (i.e., MG2 transformed with the empty pBAD24 vector) (lanes 2) or upon overproduction of YehT (lanes 3), YehT-D54E (lanes 4), or YehT-D54N (lanes 5). Twenty micrograms of total RNA was loaded per lane, and the transcripts were detected with the corresponding gene-specific DNA probes. Transcripts of the corresponding genes are marked by arrows. (B) EMSA of fluorescence-labeled DNA fragments comprising the indicated regions of the 5′-regulatory sequences for interaction with increasing concentrations of purified 6His-YehT. The numbers indicate nucleotide positions relative to the transcriptional start site of +1. The positions of free DNA (filled arrows) and YehT-DNA complexes (shaded arrows) are marked. A DNA fragment encompassing the lysP promoter (PlysP) (50) was used as a control for unspecific binding.
Identification of transcription initiation points in putative YehT target genes.
Since the sequences upstream of yjiY, cspI, yhjX, and ypjB lacked any LytTR-like sequence motifs (44) and shared no obvious sequence similarity overall (data not shown), we suspected that not all were directly dependent on YehT. To localize possible YehT-binding sites, we first mapped the 5′ untranslated regions (5′ UTRs) of these genes using rapid amplification of 5′ cDNA ends (5′ RACE) with PCR. The same RNA used for the microarray analysis was analyzed by 5′ RACE. The transcription initiation points were determined to lie at −88 bp for yjiY, −45/−145 bp for cspI, −37 bp for yhjX, and −389 bp for ypjB (data not shown). Numbers correspond to positions upstream of the translation start point at +1.
YehT–promoter DNA interaction studies.
To quantitatively analyze the interaction between YehT and its potential target promoters, we used EMSAs. For this purpose, we cloned DNA fragments comprising the transcriptional start point, the putative −35 and −10 boxes, and sufficiently large upstream and downstream regions into vector pUC19. These fragments were amplified and were simultaneously labeled by using a primer pair that included one 6-FAM-labeled primer.
YehT with an N-terminal hexahistidine tag (6His-YehT) was purified by Ni2+-NTA affinity chromatography. The purified protein could be phosphorylated with acetyl[32P]phosphate, indicating its native state.
The fluorescence-labeled DNA fragments were incubated with increasing concentrations of 6His-YehT (0 to 2,400 nM). 6His-YehT bound tightly to the yjiY promoter region (KD, 75 nM) (Fig. 2B). The corresponding KD values for all other fragments were determined to be in the range of 275 to 800 nM (275 nM for cspI, 600 nM for yhjX, 800 nM for ypjB) (Fig. 2B). A 276-bp fragment amplified from the lysP promoter of E. coli (PlysP [50]) was used as a negative control. A KD value of ≥900 nM was determined for this control fragment. Since nonspecific protein-DNA interactions are also influenced by the lengths of the DNA fragments, we defined a KD value of 275 nM or higher as nonspecific binding.
Specific binding affinities for LytTR RRs are generally in the nanomolar range (54). Thus, the affinity of the monomeric DNA-binding domain of AgrA for its target sequence was determined by a KD of 80 nM (54), a value similar to that for YehT. However, the affinity of the full-length AgrA for its target is about 10-fold higher and is further increased in the phosphorylated state (33). Phosphorylation of 6His-YehT did not affect the affinity of the RR for the yjiY promoter region (data not shown). It is worth mentioning that 6His-YehT has a high tendency to aggregate. The active protein fraction was determined to be 10 to 20% by measurement of binding in the presence of an excess of the labeled yjiY promoter DNA fragment (data not shown). This finding might explain the differences between AgrA and YehT. Nonetheless, our results indicated specific and high-affinity binding of 6His-YehT to the yjiY promoter region. Taken together, the data indicate that YehT binds with the highest affinity to the yjiY promoter, and induction of this gene was clearly dependent on YehU/YehT. Hence, subsequent detailed analysis focused on the yjiY promoter.
Characterization of the yjiY promoter and definition of the YehT-binding site.
Although PSI-BLAST analysis and secondary-structure prediction clearly identified YehT as a protein with a LytTR-binding domain, the promoter region of yjiY shows hardly any similarity to the consensus LytTR-binding sequence (44). Therefore, we directly localized the YehT-binding site within the yjiY promoter by DNase I footprinting. A DNA fragment comprising the −212-to-+88 segment of the yjiY promoter/control region was incubated with various concentrations (0 to 2,400 nM) of 6His-YehT. YehT specifically protected a 58-bp stretch between positions −101 and −44 (Fig. 3A and C). The degree of protection increased with the concentration of 6His-YehT (data not shown). Within the protected stretch, three repeats of the motif CC(G/A)CT(C/T)A (Fig. 3C), designated M1 to M3, were identified using MEME (http://meme.sdsc.edu/meme/intro.html). The distances determined were typical for protein binding sites in general (40).
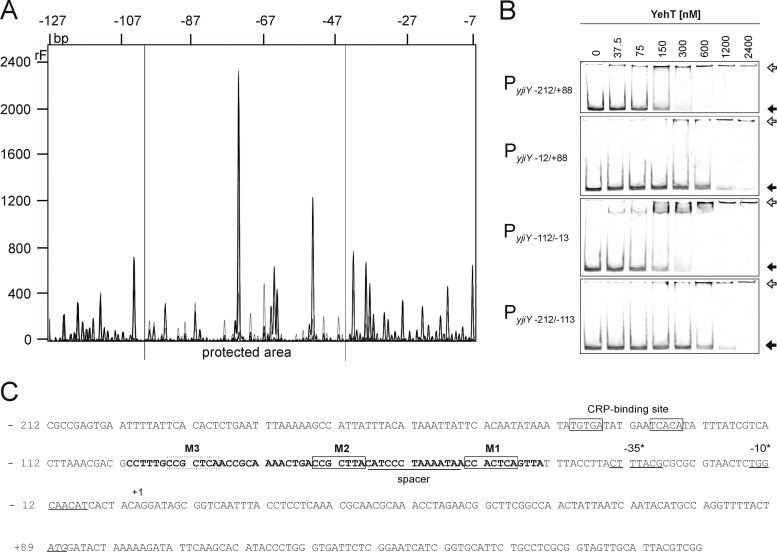
Fig 3.
Characterization of the YehT-binding site within the yjiY promoter. (A) DNase I digestion patterns were determined for DNA fragments comprising bp −212 to +88 of the yjiY promoter that had been incubated in the presence of 2.4 μM 6His-YehT (black) or BSA (gray). The DNA was labeled with fluorescein at the 5′ end of the top strand. The protected region lies within the gray horizontal lines (−101 to −44 bp). (B) EMSA of fluorescence-labeled DNA fragments comprising different segments of the yjiY promoter region following incubation with the indicated concentrations of purified 6His-YehT. The largest fragment (PyjiY−212/+88) includes the +1 transcriptional start site and the −35/−10 boxes. The positions of free DNA (filled arrows) and YehT-DNA complexes (shaded arrows) are marked. (C) Nucleotide sequence of the yjiY upstream region (positions −212 to +189). Predicted −35 and −10 promoter motifs (BProm, SoftBerry) and the transcription start site (+1) identified by 5′ RACE are indicated. The start codon is italicized and underlined, and the CRP-binding site (6) is boxed. The region protected from digestion by DNase I is marked in boldface. This 58-bp stretch includes a triple repeat of the motif CC(G/A)CT(C/T)A (M1, M2, M3) (boxed), which was identified using the MEME server (http://meme.sdsc.edu/meme/intro.html).
In parallel, the 300-bp yjiY fragment was divided into three 100-bp fragments (Fig. 3B), each of which was tested in EMSAs. 6His-YehT bound very tightly to the PyjiY−112/−13 fragment (KD 75 nM) (Fig. 3B), while its affinity for the other two fragments was about 5-fold lower (KD, 300 to 500 nM) (Fig. 3B). Thus, these data reveal that 6His-YehT binds within the region between positions −112 and −13 upstream of the yjiY transcriptional start site (Fig. 3C).
Tight binding of YehT at sites within the sequence from bp −101 to bp −44 was confirmed by EMSAs testing PyjiY P (Fig. 4A). 6His-YehT bound very tightly to this DNA fragment, with an affinity (KD 75 nM) in the same range as that for the PyjiY−112/−13 fragment (Fig. 3A). We then probed the roles of the three repeat motifs in the YehT-binding site separately, using mutated fragments in EMSAs (Fig. 4A). In these variants, one or all of the three motifs were partially or completely altered by substituting purines for pyrimidines and vice versa. Complete replacement of motif M3 (PMM3) did not significantly alter the affinity for 6His-YehT (KD 120 nM). However, replacement of M2 in addition (PMM23) or removal of all three motifs (PMM123) significantly reduced the affinity of 6His-YehT for the corresponding DNA fragments (KD 280 nM in each case). Substitution of the spacer sequence between M1 and M2 (PyjiY SC14) also decreased the affinity for 6His-YehT (KD 300 nM).
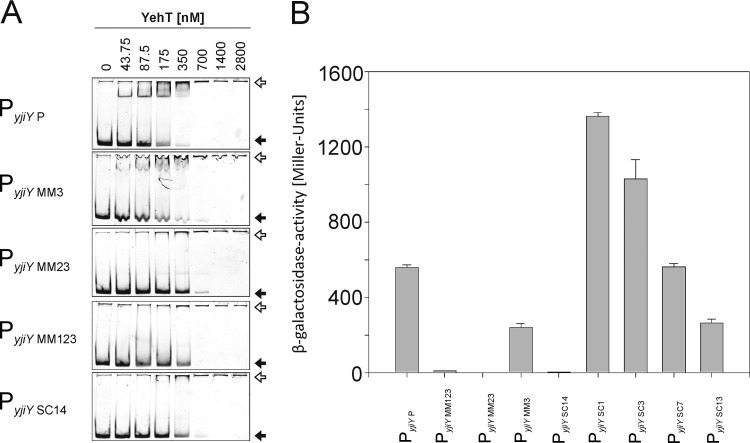
Fig 4.
Effects of substitution mutations in the yjiY promoter on in vitro YehT binding and YehT-dependent promoter activity in vivo. (A) The wild-type core region PyjiY P (bp −101 to −44) (Fig. 3C) of the yjiY promoter, comprising motifs M1, M2, and M3, and several mutated variants of the same region were analyzed by EMSA following incubation with the indicated concentrations of 6His-YehT. In the mutant fragments, motifs M1 to M3 were systematically removed by substituting purines in the sequence for pyrimidines and vice versa. The resulting fragments were designated PyjiY MM3 (M3 site replaced), PyjiY MM23 (M2 and M3 replaced), and PyjiY MM123 (all three M sites replaced). In addition, the 14-bp spacer between M1 and M2 was mutated in the same way, resulting in fragment PyjiY SC14. All fragments were analyzed by EMSAs. The positions of free DNA (filled arrows) and YehT-DNA complexes (open arrows) are marked. (B) Effects of the various substitutions within the yjiY promoter on YehT-dependent transcription in vivo. E. coli MG1655-ΔlacZ/pBAD18-yehT was transformed with plasmid pRS415 encoding various yjiY promoter∷lacZ fusions (see the text for detailed descriptions). Bacteria were cultivated under aerobic growth conditions in LB medium at 37°C until the exponential-growth phase. Then yehT overexpression was induced by adding 0.2% (wt/vol) l-arabinose, and cells were harvested after 45 min. β-Galactosidase (LacZ) activity was then determined as a measure of yjiY promoter activity. Experiments were performed at least three times, and error bars indicate the standard deviations of the means.
In addition, the effect of alterations within the yjiY promoter was tested in vivo. Reporter strains carrying various yjiY promoter∷lacZ fusions were tested for β-galactosidase activity. E. coli MG1655 ΔlacZ was transformed with plasmid-encoded yehT and various pRS415 PyjiY∷lacZ fusions representing the same variants of the YehT-binding motif (Fig. 4B). Overproduction of YehT resulted in maximal yjiY promoter activity in the case of the wild-type YehT-binding motif (PyjiY P) (Fig. 4B). Elimination of all three motifs (PyjiY MM123) or motifs M2 and M3 (PyjiY MM23) by mutagenesis prevented the induction of yjiY. In contrast, cells with a mutated motif M3 (PyjiY MM3) were able to induce the yjiY promoter, albeit to a significantly lesser degree than the wild type (Fig. 4B). Also, the spacer sequence between motifs M1 and M2 (PyjiY SC14) was found to be important for yjiY induction (Fig. 4B). These data are in complete accordance with the results of the EMSAs (Fig. 4A).
In addition, we tested the importance of the individual motifs M1 and M2, as well as the spacer between M2 and M3. Substitutions in either M1 (PyjiY MM1) or M2 (PyjiY MM2) abolished yjiY promoter activity, whereas yjiY promoter activity was detectable, albeit significantly reduced, when the spacer between motifs M2 and M3 was mutated (data not shown).
A more-detailed mutagenesis study of the 14-bp spacer between motifs M1 and M2 revealed that replacement (substitution of purines for pyrimidines and vice versa) of only 13 nucleotides (PyjiY SC13) instead of all 14 nucleotides (PyjiY SC14) partially restored promoter activity (Fig. 4B). These data suggest that the adenine nucleotide in front of M1 and hence M2 seems to be part of the DNA-binding motif. The substitution of one (PyjiY SC1), three (PyjiY SC3), or seven (PyjiY SC7) central nucleotides within the 13-bp spacer enhanced promoter activity (Fig. 4B), indicating an influence of surrounding DNA structure on yjiY expression.
In summary, these data suggest a core YehT-binding site within the yjiY promoter consisting of two direct repeats of the sequence motif ACC(G/A)CT(C/T)A, which are linked by a 13-bp spacer. The third motif (M3), as well as the intervening spacer, probably stabilizes the YehT-DNA complex.
The LytTR-like transcriptional activator BrsR of Streptococcus mutans binds to a similar DNA motif, which is composed of a direct repeat of 9 bp (ACCGTTTAG) with a 12-bp spacer (41, 64). These data are in accordance with the general description of LytTR-binding sites, which are often made up of two direct repeats of 9 to 10 bp separated by 12- or 13-bp spacers (16, 20, 33, 54).
Furthermore, we suggest that binding of YehT to the yjiY promoter is modulated by the sequence-dependent structure of the surrounding DNA, as has been described for FsrA, a LytR-like RR in Enterococcus faecalis (19). Similarly, binding of PlnD and PlnC to target promoter DNA was also strongly influenced by variations within the spacer sequence (49). LytTR-like proteins might induce bending of DNA, a process that may play a hitherto underappreciated role in transcriptional regulation (17).
Strikingly, a search of the E. coli genome revealed that the YehT-binding motif is found only within the yjiY promoter. In addition, we checked all differentially expressed genes identified by our microarray analysis (fold change, >1.5) as well as those described in the earlier transcriptome study (45), for the presence of the YehT-binding motif. However, this motif was not found in the regulatory regions of these genes. Therefore, yjiY seems to be the only target regulated by the YehU/YehT system.
Characterization of YjiY.
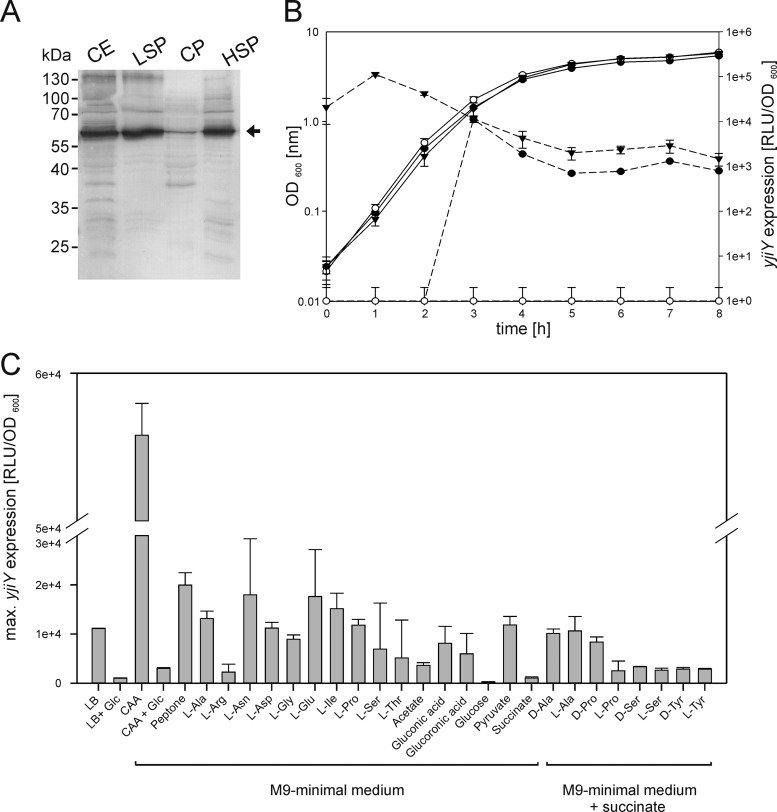
yjiY is located at 98.87 centisomes on the E. coli chromosome, disconnected from the yehUT operon. In the genomes of other bacteria, e.g., Aeromonas hydrophila, Aeromonas salmonicida, Photorhabdus asymbiotica, and several species of Shewanella, Vibrio, and Clostridium, the yehUT operon and yjiY, or a gene encoding a similar sequence, are colocalized (60). This finding provides another hint for a functional relationship between YehU/YehT and YjiY. yjiY, which codes for an inner membrane protein with 16 to 18 predicted transmembrane helices (18), is homologous to CstA, a carbon starvation protein and putative peptide transporter (52). YjiY and CstA share high degrees of sequence similarity (75.4%) and identity (61.1%). To obtain experimental proof for YjiY production and membrane integration, yjiY-6His with six codons encoding a C-terminal histidine tag under the control of the arabinose promoter was overexpressed in E. coli BL21(DE3). Cells were then disrupted and fractionated. Immunological analysis with an anti-His antibody indicated that YjiY-6His was indeed produced as a membrane-integrated protein (Fig. 5A).
Fig 5.
Localization of YjiY-6His and characterization of yjiY expression during growth in various culture media. (A) yjiY-6His was overexpressed in E. coli BL21(DE3). Subsequently, cells were disrupted and fractionated, and the cellular localization of YjiY was analyzed by SDS-PAGE and Western blotting. An equal volume of each fraction was loaded in each lane, and a monoclonal mouse antibody against the 6-His tag was used for detection. YjiY-6His was detected in the crude cell extract (CE), the low-speed pellet (LSP), and the high-speed pellet (HSP), but not in the cytoplasmic fraction (CP). (B) A luciferase-based reporter assay was used to determine the pattern of yjiY expression. E. coli MG1655 (●) and E. coli MG2 (ΔyehUT) (○) were transformed with pBBR yjiY-lux and were grown in LB medium. In addition, these reporter strains contained plasmid pBAD24 or pBAD24-yehUT. The latter was used for complementation of strain E. coli MG2 (▼). Bacteria were cultivated under aerobic conditions, and growth (solid lines) and the activity of the reporter enzyme luciferase (dashed lines) were determined. The luciferase activity (expressed as relative light units divided by the optical density of the culture [RLU/OD600]) served as a measure of yjiY expression. (C) Influence of medium composition and supplements on yjiY expression, as measured with the reporter strain E. coli MG1655/pBBR yjiY-lux. Bacteria were cultivated under aerobic conditions in complex medium (LB) or M9 minimal medium with the indicated carbon sources (20 mM amino acids, 0.5% [wt/vol] all other carbon sources; CAA, Casamino Acids). Glucose (Glc) (0.5%, wt/vol) was added where indicated. Bacteria were also grown in M9 minimal medium with succinate as a carbon source, supplemented with the indicated amino acids (20 mM). Growth and luciferase activity were monitored continuously. The maximal luciferase activity normalized to an optical density of 1 (RLU/OD600) was used as a measure of the degree of induction of yjiY. All experiments were performed at least three times, and the error bars indicate the standard deviations of the means.
Stimulus-response analysis of the YehU/YehT system.
To gain insight into the in vivo expression pattern of yjiY, a transcriptional fusion of the yjiY promoter (PyjiY−212/+88) and the luciferase luxCDABE operon was constructed (plasmid pBBR yjiY-lux). E. coli MG1655 and E. coli MG2 were transformed with this plasmid and either pBAD24 or pBAD24-yehUT. Growth and luminescence (as a measure of yjiY expression) under aerobic conditions were monitored in these cultures over time. All strains tested showed comparable growth patterns (Fig. 5B). yjiY was expressed in E. coli MG1655 after approximately 3 h of growth. At this time, the population was undergoing the transition into the stationary-growth phase. No induction of yjiY was observed in the yehUT deletion mutant MG2 (Fig. 5B). Complementation of this mutant with plasmid-encoded yehUT restored yjiY expression, but growth-phase-dependent induction was no longer observed (Fig. 5B). Furthermore, we tested the effect of substitution mutations in the predicted phosphorylation sites of YehU and YehT on yjiY promoter activity. Replacement of the conserved histidine (H382) in YehU with a glutamine prevented yjiY induction (Table 3). Mutation of the conserved aspartate in YehT to glutamate (resulting in the putative phosphorylation-independent variant YehT-D54E) or to glutamine (resulting in the putative phosphorylation-resistant, inactive variant YehT-D54N) caused about a 2-fold increase or a 10-fold decrease in yjiY induction, respectively (Table 3). This yjiY induction pattern clearly indicated the importance of phosphorylation for the YehU/YehT-system. However, when the copy number of YehT was increased (by the addition of arabinose as an inducer), yjiY induction became independent of the phosphorylation state of YehT (Table 3), which explained the results of the Northern blot analysis (see above).
Table 3.
yjiY induction is affected by mutations in the proposed phosphorylation sites of YehU/YehT and underlies cAMP/CRP regulation
| Type of regulation and E. coli strain/reporter plasmid | Additional plasmid | Description of deletion or substitution | Maximal induction of yjiY (RLU/OD600)a | ||
|---|---|---|---|---|---|
| LB | LB + 0.00006% Ara | LB + 0.2% Ara | |||
| YehU/YehT regulation | |||||
| E. coli MG1655 rpsL150/pBBR yjiY-lux | pBAD24 | Wild type | 22,000 | 19,000 | 5,000 |
| E. coli MG3/pBBR yjiY-lux | pBAD24 | ΔyehT | 1 | 1 | 1 |
| E. coli MG3/pBBR yjiY-lux | pBAD24-yehT | yehT | 23,000 | 20,000 | 170,000 |
| E. coli MG3/pBBR yjiY-lux | pBAD24-yehT-D54E | yehT-D54E | 39,000 | 87,000 | 200,000 |
| E. coli MG3/pBBR yjiY-lux | pBAD24-yehT-D54N | yehT-D54N | 2,200 | 17,000 | 170,000 |
| E. coli MG6/pBBR yjiY-lux | pBAD24 | ΔyehU | 1 | 1 | 1 |
| E. coli MG6/pBBR yjiY-lux | pBAD24-yehU | yehU | 42,000 | 44,000 | 300 |
| E. coli MG6/pBBR yjiY-lux | pBAD24-yehU-H382Q | yehU-H382Q | 1 | 1 | 1 |
| cAMP/CRP regulation | |||||
| E. coli MG1655/pBBR yjiY-lux | None | Wild type | 12,000 | ND | ND |
| E. coli MG10/pBBR yjiY-lux | None | ΔyjiY | 42,000 | ND | ND |
| E. coli MG11/pBBR yjiY-lux | None | ΔcyaA | 2,700 | ND | ND |
| E. coli MG12/pBBR yjiY-lux | None | Δcrp | 1 | ND | ND |
| E. coli MG1655/pBBR yjiY-sub-CRP-BS-lux | None | Substitution of the CRP-binding site in PyjiY | 2,300 | ND | ND |
Strains were grown aerobically in a complex medium (LB) in the absence or presence of l-arabinose (Ara). Growth and luciferase activity were monitored continuously. The maximal luciferase activity normalized to an optical density of 1 (RLU/OD600) was used as a measure of the degree of induction of yjiY. Data were obtained from at least three independent experiments, and average values (the standard deviation was less than 15%) were used for calculations. ND, not determined.
In an effort to identify the stimulus perceived by the YehU/YehT system, E. coli strains MG1655 and MG2 were transformed with plasmid pBBR yjiY-lux. Cells were grown in aerobic culture in the presence of different carbon sources, and luminescence and growth were continuously monitored. No yjiY expression was recorded in the yehUT mutant under any of the conditions tested (data not shown). In strain MG1655/pBBR yjiY-lux, the maximal luciferase activity was determined and was used as an indicator for the degree of induction of yjiY (Fig. 5C). We found strong induction of yjiY when this reporter strain was grown in media containing mixtures of peptides and amino acids, such as LB, Casamino Acids (CAA), or peptone. Induction was significantly reduced, or even absent altogether, when glucose was available concurrently. Induction of yjiY was detectable when cells were grown in minimal medium with amino acids (Ala, Asn, Asp, Gly, Glu, Ile, Pro, Ser, Thr) or acetate, gluconic acid, glucuronic acid, or pyruvate as the sole carbon source. Growth on typical phosphotransferase system (PTS) sugars, e.g., glucose, galactose, mannose, mannitol, and fructose, as sole carbon sources did not activate yjiY expression (data not shown). However, the same phenomenon was also detected for non-PTS sugars, such as lactose, maltose, and glycerol (data not shown). Induction of yjiY was also detectable when cells were grown with succinate as the C source in the presence of different amino acids as supplements (Fig. 5C). Remarkably, both the l- and d-isomers of the amino acids were efficient inducers.
Murein subunits have been postulated as potential stimuli for the homologous HK LytS in S. aureus (10). Therefore, we isolated peptidoglycan fragments from B. subtilis, Lactobacillus casei, and E. coli and tested them as additives in our reporter strain assay. None of these fragments caused significant induction of yjiY (data not shown).
The induction profile raised the possibility that the yjiY gene might also be regulated by cAMP and the cAMP receptor protein (CRP). Indeed, yjiY expression was significantly reduced or prevented in a cyaA or a crp mutant (Table 3). Moreover, a CRP consensus sequence was identified in the upstream regulatory region (Fig. 3C). Mutation of this motif reduced yjiY induction to the same degree as in the cyaA mutant (Table 3).
Finally, we tested whether YjiY, a putative transporter, had an effect on yjiY induction. Induction of the PyjiY-luxCDABE reporter was found to be 4-fold higher in the yjiY mutant than in the parental strain (Table 3). Even so, the growth-phase-dependent induction pattern of the mutant was indistinguishable from that of the parental strain (data not shown). Therefore, it seems unlikely that YjiY exerts a feedback regulatory effect on its own expression.
In summary, expression of yjiY is induced at the transition of the E. coli culture into the stationary-growth phase, and induction requires cAMP and CRP. This profile is similar to that of the induction of cstA (52). Synthesis of CstA is subject to an additional regulation by CsrA, a protein that blocks the ribosome binding site in cstA mRNA (21, 52). yjiY is expressed primarily in E. coli grown on amino acids or peptides as carbon sources. The concurrent availability of glucose, which is accompanied by low intracellular cAMP levels (47), almost abolishes the expression of yjiY. These results suggest that YjiY, like CstA, takes part in amino acid/peptide utilization. However, the function of YjiY is unknown thus far. The participation of YjiY in peptide/amino acid uptake or the export of some still- unknown quorum-sensing molecule can only be speculated on. Further studies are required to identify the function of YjiY, its mode of transport (importer or exporter), its specific substrate, and the mode of energization.
In conclusion, the YehU/YehT-system was identified as another player within the complex carbon starvation control network. Subsequent experiments will focus on the elucidation of the stimulus perceived by YehU and the function of YjiY.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (Exc114/1).
We thank Ingrid Weitl and Sonja Kroll for excellent technical assistance and Björn Schwalb, Thomas Engleitner, and Achim Tresch for bioinformatics advice.
Footnotes
Published ahead of print 8 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Aiba H, Adhya S, de Crombrugghe B. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256: 11905–11910 [PubMed] [Google Scholar]
- 2. Anantharaman V, Aravind L. 2003. Application of comparative genomics in the identification and analysis of novel families of membrane-associated receptors in bacteria. BMC Genomics 4: 34 doi:10.1186/1471-2164-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antoine R, Locht C. 1992. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol. Microbiol. 6: 1785–1799 [DOI] [PubMed] [Google Scholar]
- 4. Argaman L, et al. 2001. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 11: 941–950 [DOI] [PubMed] [Google Scholar]
- 5. Auchtung JM, Lee CA, Grossman AD. 2006. Modulation of the ComA-dependent quorum response in Bacillus subtilis by multiple Rap proteins and Phr peptides. J. Bacteriol. 188: 5273–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benoff B, et al. 2002. Structural basis of transcription activation: the CAP-α CTD-DNA complex. Science 297: 1562–1566 [DOI] [PubMed] [Google Scholar]
- 7. Bensing BA, Meyer BJ, Dunny GM. 1996. Sensitive detection of bacterial transcription initiation sites and differentiation from RNA processing sites in the pheromone-induced plasmid transfer system of Enterococcus faecalis. Proc. Natl. Acad. Sci. U. S. A. 93: 7794–7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blattner FR, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1462 [DOI] [PubMed] [Google Scholar]
- 9. Brown PK, et al. 2001. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 41: 349–363 [DOI] [PubMed] [Google Scholar]
- 10. Brunskill E, Bayles K. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 178: 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunskill E, Bayles K. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178: 5810–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cann M. 2007. Sodium regulation of GAF domain function. Biochem. Soc. Trans. 35: 1032–1034 [DOI] [PubMed] [Google Scholar]
- 13. Checroun C, Gutierrez C. 2004. σs-dependent regulation of yehZYXW, which encodes a putative osmoprotectant ABC transporter of Escherichia coli. FEMS Microbiol. Lett. 236: 221–226 [DOI] [PubMed] [Google Scholar]
- 14. Cheng J, et al. 2004. NetAffx Gene Ontology Mining Tool: a visual approach for microarray data analysis. Bioinformatics 20: 1462–1463 [DOI] [PubMed] [Google Scholar]
- 15. Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158: 9–14 [DOI] [PubMed] [Google Scholar]
- 16. Cheung JK, Rood JI. 2000. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J. Bacteriol. 182: 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coulombe B. 1999. DNA wrapping in transcription initiation by RNA polymerase II. Biochem. Cell Biol. 77: 257–264 [PMC free article] [PubMed] [Google Scholar]
- 18. Daley DO, et al. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308: 1321–1323 [DOI] [PubMed] [Google Scholar]
- 19. Del Papa MF, Perego M. 2011. Enterococcus faecalis virulence regulator FsrA binding to target promoters. J. Bacteriol. 193: 1527–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diep DB, Havarstein LS, Nes IF. 1996. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J. Bacteriol. 178: 4472–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubey AK, et al. 2003. CsrA regulates translation of the Escherichia coli carbon starvation gene, cstA, by blocking ribosome access to the cstA transcript. J. Bacteriol. 185: 4450–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galperin MY. 2008. Telling bacteria: do not LytTR. Structure 16: 657–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Godeke J, Heun M, Bubendorfer S, Paul K, Thormann KM. 2011. Roles of two Shewanella oneidensis MR-1 extracellular endonucleases. Appl. Environ. Microbiol. 77: 5342–5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzman L, Belin D, Carson M, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177: 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heermann R, Jung K. 2010. Stimulus perception and signaling in histidine kinases, p 135–161 In Krämer R, Jung K. (ed), Bacterial signaling, vol 1 Wiley-VCH, Weinheim, Germany [Google Scholar]
- 26. Heermann R, Zeppenfeld T, Jung K. 2008. Simple generation of site-directed point mutations in the Escherichia coli chromosome using Red/ET recombination. Microb. Cell Fact. 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemm MR, Paul BJ, Schneider TD, Storz G, Rudd KE. 2008. Small membrane proteins found by comparative genomics and ribosome binding site models. Mol. Microbiol. 70: 1487–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirakawa H, Nishino K, Hirata T, Yamaguchi A. 2003. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 185: 1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones DT. 2007. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23: 538–544 [DOI] [PubMed] [Google Scholar]
- 30. Jung K, Fried L, Behr S, Heermann R. 2012. Histidine kinases and response regulators in networks. Curr. Opin. Microbiol. 15: 118–124 [DOI] [PubMed] [Google Scholar]
- 31. Jung K, Krabusch M, Altendorf K. 2001. Cs+ induces the kdp operon of Escherichia coli by lowering the intracellular K+ concentration. J. Bacteriol. 183: 3800–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keseler IM, et al. 2009. EcoCyc: a comprehensive view of Escherichia coli biology. Nucleic Acids Res. 37: D464–D470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. 2004. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 186: 7549–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kovach ME, et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166: 175–176 [DOI] [PubMed] [Google Scholar]
- 35. Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305: 567–580 [DOI] [PubMed] [Google Scholar]
- 36. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- 37. Lipmann F, Tuttle LC. 1945. A specific micromethod for the determination of acyl phosphates. J. Biol. Chem. 159: 21–28 [Google Scholar]
- 38. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275 [PubMed] [Google Scholar]
- 39. Masuda N, Church GM. 2002. Escherichia coli gene expression responsive to levels of the response regulator EvgA. J. Bacteriol. 184: 6225–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mendoza-Vargas A, et al. 2009. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS One 4: e7526 doi:10.1371/journal.pone.0007526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merritt J, Qi F. 2012. The mutacins of Streptococcus mutans: regulation and ecology. Mol. Oral Microbiol. 27: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, 4th ed Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 43. Möglich A, Ayers RA, Moffat K. 2009. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17: 1282–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolskaya AN, Galperin MY. 2002. A novel type of conserved DNA-binding domain in the transcriptional regulators of the AlgR/AgrA/LytR family. Nucleic Acids Res. 30: 2453–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oshima T, et al. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46: 281–291 [DOI] [PubMed] [Google Scholar]
- 46. Parkinson H, et al. 2011. ArrayExpress update—an archive of microarray and high-throughput sequencing-based functional genomics experiments. Nucleic Acids Res. 39: D1002–D1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peterkofsky A, Gazdar C. 1974. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc. Natl. Acad. Sci. U. S. A. 71: 2324–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riley M, et al. 2006. Escherichia coli K-12: a cooperatively developed annotation snapshot—2005. Nucleic Acids Res. 34: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Risøen P, et al. 2001. Regulation of bacteriocin production in Lactobacillus plantarum depends on a conserved promoter arrangement with consensus binding sequence. Mol. Genet. Genomics 265: 198–206 [DOI] [PubMed] [Google Scholar]
- 50. Ruiz J, Haneburger I, Jung K. 2011. Identification of ArgP and Lrp as transcriptional regulators of lysP, the gene encoding the specific lysine permease of Escherichia coli. J. Bacteriol. 193: 2536–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 52. Schultz JE, Matin A. 1991. Molecular and functional characterization of a carbon starvation gene of Escherichia coli. J. Mol. Biol. 218: 129–140 [DOI] [PubMed] [Google Scholar]
- 53. Shah IM, Laaberki MH, Popham DL, Dworkin J. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135: 486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sidote DJ, Barbieri CM, Wu T, Stock AM. 2008. Structure of the Staphylococcus aureus AgrA LytTR domain bound to DNA reveals a beta fold with an unusual mode of binding. Structure 16: 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simons R, Houman F, Kleckner N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53: 85–96 [DOI] [PubMed] [Google Scholar]
- 56. Snider J, et al. 2006. Formation of a distinctive complex between the inducible bacterial lysine decarboxylase and a novel AAA+ ATPase. J. Biol. Chem. 281: 1532–1546 [DOI] [PubMed] [Google Scholar]
- 57. Stadtman ER. 1957. Preparation and assay of acetyl phosphate. Methods Enzymol. 3: 228–231 [Google Scholar]
- 58. Studier FW, Moffatt BA. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189: 113–130 [DOI] [PubMed] [Google Scholar]
- 59. Sugiura A, Nakashima K, Tanaka K, Mizuno T. 1992. Clarification of the structural and functional features of the osmoregulated kdp operon of Escherichia coli. Mol. Microbiol. 6: 1769–1776 [DOI] [PubMed] [Google Scholar]
- 60. Szklarczyk D, et al. 2011. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39: D561–D568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tetsch L, Koller C, Haneburger I, Jung K. 2008. The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP. Mol. Microbiol. 67: 570–583 [DOI] [PubMed] [Google Scholar]
- 62. Viklund H, Elofsson A. 2008. OCTOPUS: improving topology prediction by two-track ANN-based preference scores and an extended topological grammar. Bioinformatics 24: 1662–1668 [DOI] [PubMed] [Google Scholar]
- 63. Weber A, Jung K. 2002. Profiling early osmostress-dependent gene expression in Escherichia coli using DNA macroarrays. J. Bacteriol. 184: 5502–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie Z, Okinaga T, Niu G, Qi F, Merritt J. 2010. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol. Microbiol. 78: 1431–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto K, et al. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J. Biol. Chem. 280: 1448–1456 [DOI] [PubMed] [Google Scholar]
- 66. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119 [DOI] [PubMed] [Google Scholar]
- 67. Zhou L, Lei XH, Bochner BR, Wanner BL. 2003. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. J. Bacteriol. 185: 4956–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zianni M, Tessanne K, Merighi M, Laguna R, Tabita FR. 2006. Identification of the DNA bases of a DNase I footprint by the use of dye primer sequencing on an automated capillary DNA analysis instrument. J. Biomol. Tech. 17: 103–113 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.