Abstract
Dengue viruses (DENV) are transmitted to humans by the bite of Aedes aegypti or Aedes albopictus mosquitoes, with millions of infections annually in over 100 countries. The diseases they produce, which occur exclusively in humans, are dengue fever (DF) and dengue hemorrhagic fever (DHF). We previously developed a humanized mouse model of DF in which mice transplanted with human hematopoietic stem cells produced signs of DENV disease after injection with low-passage, wild-type isolates. Using these mice, but now allowing infected A. aegypti to transmit dengue virus during feeding, we observed signs of more severe disease (higher and more sustained viremia, erythema, and thrombocytopenia). Infected mice mounted innate (gamma interferon [IFN-γ] and soluble interleukin 2 receptor alpha [sIL-2Rα]) and adaptive (anti-DENV antibodies) immune responses that failed to clear viremia until day 56, while a mosquito bite alone induced strong immunomodulators (tumor necrosis factor alpha [TNF-α], IL-4, and IL-10) and thrombocytopenia. This is the first animal model that allows an evaluation of human immunity to DENV infection after mosquito inoculation.
INTRODUCTION
Dengue fever (DF) in humans is characterized by fever, myalgia, arthralgia, abdominal pain, rash, low platelet counts (thrombocytopenia), and a viremia that begins 3 to 4 days after infection by mosquito bite. The more severe form of dengue, dengue hemorrhagic fever (DHF), usually presents as a second phase of disease, at the end of the fever stage, but with a sudden onset of plasma leakage that can result in hemoconcentration, pleural effusion, ascites, shock, hepatic failure, and encephalopathy. While this hemodynamic syndrome can resolve in 2 days, complete convalescence can take weeks to months (reviewed in reference 53). Moreover, ∼5% of DHF patients die, usually from hypotensive shock, due to a delay in the recognition and treatment of the plasma leakage. Dengue virus (DENV)-induced disease has increased markedly due to the global spread of the virus and expansion of its mosquito vectors, and it is now the most important viral illness transmitted by insects (61). However, a clear understanding of the mechanisms leading to DF and DHF has been limited by several factors: (i) inadequate animal models of disease, with most knowledge being derived from clinical studies and in vitro experiments; (ii) the genetic diversity of DENV, with four different antigenic groups or serotypes and with humans potentially infected multiple times; and (iii) the relative risk of severe DENV disease, which is enhanced greatly by secondary infection with a heterologous serotype. The last factor has prompted the development of several models of immunopathogenesis (reviewed in reference 47) that have been difficult to evaluate experimentally, given the absence of reliable immunocompetent-animal models of human disease presenting with clinical signs of DHF after serial infection with wild-type viruses.
We sought to produce an animal model of disease that could mimic the natural cycle of mosquito-human transmission with low-passage-number viruses from clinical samples, using human cells as targets of infection within a neutral background of nonsusceptible tissues (mouse) and using the appropriate species of mosquito vector, to evaluate the influence of biting/probing, virus delivery, and saliva proteins on DENV pathogenesis. Using humanized NOD/SCID/interleukin 2 receptor gamma (IL-2Rγ)-null (hu-NSG) mice that had previously defined differences in the virulence of DENV genotypes (38) and established tropism and kinetics of virus replication (37), we performed mosquito transmission and pathogenesis experiments with a virulent DENV serotype 2 (Southeast Asia strain K0049) (3, 19, 38).
Mosquito saliva has been shown to enhance the replication and pathogenesis of numerous arthropod-borne viruses (reviewed in references 22 and 49). Previous studies examining the effect of A. aegypti saliva on DENV replication were performed on human cells in vitro using saliva proteins collected from mosquitoes (1); in these studies, crude mosquito saliva inhibited DENV infection of human dendritic cells. Other studies showed that flavivirus-susceptible, inbred, immunocompetent mice respond to the bites of uninfected A. aegypti mosquitoes by secreting large amounts of proinflammatory cytokines (63). Here, we investigated the effect of mosquito inoculation on DENV pathogenesis in the context of an animal model with human cells that can develop signs of dengue disease. We show that hu-NSG mice infected with DENV by mosquito bite develop some signs of disease that are more severe than when they are injected with virus alone and contain functional human immune cells that respond to infection by secreting cytokines and DENV-specific antibodies and that the mosquito bite and saliva are necessary for these responses. Our results highlight the importance of including virus delivery by the natural vector in evaluations of models of dengue pathogenesis.
MATERIALS AND METHODS
Mouse reconstitution.
Animal manipulation and procedures were described previously (37, 38). We established a colony of NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice from breeding pairs obtained from the Jackson Laboratory (ME), housing them in a pathogen-free facility. For transplantation, human cord blood from anonymous donors was obtained from the South Texas Blood and Tissue Center (San Antonio, TX), and peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density gradient followed by positive selection of CD34+ hematopoietic stem cells using a CD34+ Progenitor Cell Selection System (Invitrogen) according to the manufacturer's instructions. In preparation for transplantation, newborn mice were sublethally irradiated with 100 cGy using a cesium source; the following day, they received an intrahepatic inoculation of 3 × 105 purified cord blood CD34+ cells. To test for engraftment levels, 6 to 8 weeks after transplantation, peripheral blood was collected via retro-orbital bleed and stained with allophycocyanin (APC)-conjugated anti-human CD45 antibody (BD Biosciences). Flow cytometry was performed on a CyAn ADP analyzer, and the data were processed using Summit software (Beckman Coulter).
Mosquitoes.
A. aegypti (Rockefeller strain) mosquitoes were maintained in an insectary at 26 to 28°C and 70 to 80% relative humidity with a 12-h/12-h light-dark cycle. Eggs were hatched and larvae were reared in water pans at a density of 100 to 300 larvae per liter and fed a mixture of ground rabbit chow (Purina)-liver powder (Bio-Serv)-yeast (Bio-Serv) (4:1:1) ad libitum. Pupae were transferred to screened cages, and emergent adults were maintained on an ad libitum diet of 10% sucrose (Sigma). Successive generations were produced by providing female mosquitoes a 37°C defibrinated rabbit blood (Colorado Serum Company) meal using a water-jacketed membrane (hog intestine) feeder. Eggs were collected, kept moist for at least 24 h, and air dried prior to storage.
Mosquito infection and virus in saliva.
Female mosquitoes, 4 to 7 days postemergence, were intrathoracically inoculated (46) with approximately 45,000 genome copies of DENV type 2 (DENV-2) strain K0049 virus (equal to ∼450 50% tissue culture infective doses [TCID50] in C6/36 cells [21] or ∼45 PFU in LLC-MK2 cells [5]), sufficient to produce a disseminated infection rate of 100%, as determined by previous studies (3). This DENV-2 strain had been isolated from a DHF patient in Kamphaeng Phet, Thailand, in 1995 (45); all mouse and mosquito infections were performed with virus from C6/36 cell culture passage 3 and had been titrated previously (21). Disseminated infection was confirmed by immunofluorescence assay (IFAT) in mosquito head squashes (21) after the mosquitoes had bitten mice. To determine the time point at which the maximum amount of virus was secreted postinoculation, five mosquitoes, taken from the inoculated group every other day, were salivated following methods described previously (20) by inserting the proboscis into mineral oil in capillary pipettes. The salivary secretions and mosquito bodies were analyzed by quantitative reverse transcription (qRT)-PCR (as described below) to determine the quantity of virus contained in whole bodies and eventually secreted by individual mosquitoes. To quantify tissue in bodies and to normalize samples, we performed qRT-PCR in parallel for an A. aegypti housekeeping gene, RpS17, as described previously (3).
DENV infection of reconstituted mice via mosquito bite.
Mosquitoes were starved for 24 h before feeding on mice. DENV-infected mosquitoes were removed from cages and placed individually in 5-dram vials. The vials were covered with a fine white polyester net (BioQuip) held in place by a rubber band. Mice were anesthetized by a 50-μl intraperitoneal injection of ketamine and xylazine (100 mg/kg and 10 mg/kg of body weight). Individual mosquitoes were allowed to feed on mouse footpads and/or ears, with most mosquitoes engorging within 15 min (maximum placement time, 60 min). Since not all mosquitoes bite animals under experimental conditions, we used visual engorgement with blood as a sign of biting and injection of DENV; the mosquitoes were tested for virus in head squashes by IFAT.
Infection via footpad inoculation with virus and saliva or by virus and uninfected-mosquito bite.
Some mice were treated as follows: (i) 4 or 5 uninfected mosquitoes were allowed to bite the footpads of each hu-NSG mouse, and 30 to 60 min later, each mouse received an intradermal inoculation of DENV on its back (as was done previously [37]) with 9 log10 genome equivalents of strain K0049 or (ii) rear footpads were inoculated with a mixture of virus and mosquito salivary secretions from uninfected mosquitoes, collected as described above. Saliva for inoculation was prepared by adding 25 μl sterile 0.9% saline solution, mixing the contents, and centrifuging it at 8,000 × g for 5 min. The aqueous phase was removed and mixed with 9 log10 genome equivalents of strain K0049 in a 40-μl final volume of 0.9% saline and inoculated in the footpads of isofluorane-anesthetized (2% isoflurane plus 2 liter/min O2) mice.
Quantitative RT-PCR analysis of dengue virus RNA.
To measure viremia, blood was collected (25 μl) on even-numbered days, starting at day 2 postinfection (p.i.), by the retro-orbital route using calibrated capillary micropipettes (Drummond Scientific), alternating right and left eyes. For some experimental groups, two different cohorts were infected and bled in the same manner, but every 4 days (due to IACUC limitations on bleeding mice), so those data would be available every 2 days for the mosquito infections. The sera were separated by centrifugation at 10,000 × g for 5 min, and 10 μl of serum was used for RNA extraction. For both mouse sera and mosquito bodies and saliva, a DENV RNA template was amplified in duplicate using the RNA Ultrasense One-step Quantitative RT-PCR System (Invitrogen), as described previously (38). This amplifies a 94-bp fragment of a highly conserved region of the capsid gene of dengue virus and uses a standard curve (in vitro-transcribed RNA standards from the same virus strain) to estimate RNA copies. The sensitivity of the assay is 240 RNA copies per ml or 1.2 copies per reaction tube.
Clinical signs of dengue infection.
Male and female NSG adult mice (6 to 8 weeks old) with engraftment levels of 11 to 88% (total, 56 mice) were used to generate groups of 3 to 6 individuals for infection experiments via mosquito bite and injection, with appropriate controls (Table 1). Mouse temperature and erythema measurements were taken every other day under isoflurane anesthesia. Temperature was measured using a RET-3 rectal probe coupled to a BAT-12 Microprobe Thermometer (Physitemp Instruments), and erythema was obtained using a DSMII ColorMeter (Cortex Technology, Denmark) by measuring the same area of skin (armpit). The erythema index was obtained by measuring the narrow-band simple reflectance of erythema/melanin (520 to 580 nm and 660 to 690 nm, expressed as optical density [OD]) (18). For platelet count determinations, 10 μl of blood was collected retro-orbitally on days 4 to 30 from 6 mice and diluted 1:100 in 1% buffered oxalate for cell lysis, using a Thrombo-tic test capillary pipette (Bioanalytic). The diluted sample was used to charge a Neubauer hemacytometer, and platelets were counted under ×400 magnification using bright-light microscopy. Once it was determined that mosquito-infected mice consistently show their lowest platelet levels on day 14, all platelet measurement samples were taken on that day only for the remainder of the mice.
Table 1.
Descriptions of the 56 mice used in the study
| Mouse ID | Donor ID | Engraftment (%) | Expt | No. of platelets (day 14)a |
|---|---|---|---|---|
| 638 | 472 | 62 | Platelet titration, 5 mosquito bites | 995 |
| 699 | 737 | 51 | Platelet titration, 4 mosquito bites | 1,483 |
| 701 | 737 | 58 | Platelet titration, 4 mosquito bites | 370 |
| 713 | 789 | 20 | Platelet titration, 5 mosquito bites | 1,355 |
| 714 | 789 | 77 | Platelet titration, 5 mosquito bites | 1,525 |
| 715 | 789 | 20 | Platelet titration, 4 mosquito bites | 1,165 |
| 664 | 491 | 59 | Tropism, day 4, 5 mosquito bites | ND |
| 665 | 491 | 47 | Tropism, day 4, 5 mosquito bites | ND |
| 666 | 491 | 30 | Tropism, day 4, 4 mosquito bites | ND |
| 702 | 737 | 49 | Tropism, day 12, 4 mosquito bites | ND |
| 703 | 737 | 24 | Tropism, day 12, 5 mosquito bites | ND |
| 704 | 737 | 80 | Tropism, day 12, 4 mosquito bites | ND |
| 705 | 788 | 49 | Tropism, day 32, 5 mosquito bites | ND |
| 706 | 788 | 82 | Tropism, day 32, 5 mosquito bites | ND |
| 707 | 788 | 73 | Tropism, day 32, 4 mosquito bites | ND |
| 614 | 261 | 68 | Infected, 2 mosquito bites | 600 |
| 631 | 471 | 79 | Infected, 2 mosquito bites | 1,190 |
| 524 | 455 | 41 | Infected, 3 mosquito bites | 1,170 (day 10) |
| 615 | 261 | 78 | Infected, 4 mosquito bites | 1,060 |
| 520 | 455 | 37 | Infected, 4 mosquito bites | 770 (day 10) |
| 521 | 455 | 75 | Infected, 4 mosquito bites | 1,115 (day 10) |
| 517 | 455 | 72 | Infected, 4 mosquito bites | 1,090 (day 11) |
| 643 | 472 | 11 | Infected, 4 mosquito bites | 1,520 |
| 682 | 534 | 88 | Infected, 4 mosquito bites | 1,460 |
| 716 | 789 | 18 | Infected, 4 mosquito bites | 1,810 |
| 723 | 218 | 54 | Infected, 4 mosquito bites | 1,800 |
| 724 | 218 | 40 | Infected, 4 mosquito bites | 1,485 |
| 725 | 218 | 58 | Infected, 4 mosquito bites | 1,093 |
| 729 | 218 | 66 | Infected, 4 mosquito bites | 1,030 |
| 516 | 455 | 66 | Infected, 5 mosquito bites | 1,005 (day 11) |
| 518 | 455 | 76 | Infected, 5 mosquito bites | 920 (day 10) |
| 508 | 441 | 29 | Infected, 5 mosquito bites | 920 |
| 519 | 455 | 56 | Infected, 5 mosquito bites | 865 |
| 712 | 788 | 73 | Infected, 5 mosquito bites | ND |
| 717 | 789 | 22 | Infected, 5 mosquito bites | 1,718 |
| 722 | 218 | 41 | Infected, 5 mosquito bites | 1,450 |
| 591 | 353 | 41 | Infected by inoculation | 1,502 |
| 611 | 261 | 80 | Infected by inoculation | 1,830 |
| 592 | 353 | 40 | Infected by inoculation | 1,070 |
| Second infection 210 days p.i., 4 mosquito bites | 1,445 | |||
| 613 | 261 | 45 | Infected by inoculation | 1,675 |
| Second infection 210 days p.i., 4 mosquito bites | 1,275 | |||
| 593 | 353 | 30 | Infected, inoculation + 4 mosquito bites | 915 |
| 590 | 353 | 49 | Infected, inoculation + 5 mosquito bites | 1,510 |
| 616 | 261 | 32 | Infected, inoculation + 5 mosquito bites | 1,020 |
| 633 | 471 | 81 | Infected, inoculation + 5× saliva | 605 |
| Second infection 182 days p.i., 4 mosquito bites | 1,210 | |||
| 634 | 471 | 71 | Infected, inoculation + 5× saliva | 1,122 |
| Second infection 182 days p.i., 4 mosquito bites | 675 | |||
| 661 | 291 | 48 | Infected, inoculation + 5× saliva | 1,220 |
| Second infection 178 days p.i., 4 mosquito bites | 1,175 | |||
| 662 | 291 | 59 | Infected, inoculation + 5× saliva | 983 |
| Second infection 178 days p.i., 4 mosquito bites | 1,060 | |||
| 663 | 291 | 43 | Infected, inoculation + 5× saliva | 1,208 |
| Second infection 178 days p.i., 4 mosquito bites | 960 | |||
| 589 | 353 | 56 | Uninfected, 5 mosquito bites, day 32 | 1,105 |
| 626 | 471 | 64 | Uninfected, 5 mosquito bites, day 32 | 910 |
| 740 | 316 | 46 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 355 |
| 741 | 316 | 51 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 805 |
| 742 | 316 | 54 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 1,370 |
| 743 | 316 | 42 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 1,235 |
| 744 | 316 | 43 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 1,100 |
| 745 | 316 | 44 | Uninfected, 4 mosquito bites, days 4, 14, 32 | 830 |
ND, not done.
Human cytokine analysis in mouse blood.
Ten human cytokine and chemokine levels were measured in the sera of infected and control humanized mice by multiplex analysis (Luminex), as described previously (37). Levels of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), IL-2, soluble IL-2R (sIL-2R), IL-4, IL-6, IL-8, IL-10, MCP-1, and VEGF were tested using the Miliplex MPXHCYTO-60K-10 kit (Millipore), according to the manufacturer's instructions. At different time points, serum samples from infected and control mice were obtained via retro-orbital bleeding or exsanguination and tested in duplicate, using the Luminex 100 system and software. The results were expressed in picograms per milliliter, and the averages from uninfected mice; infected, mosquito-bitten mice; and mice infected by injection were determined, the last using data from a previous publication (37).
Characterization of human antibody production.
Most mice were euthanized by exsanguination (via retro-orbital bleeding) under deep anesthesia (2 liters/min O2 plus 3% isoflurane) 30 to 56 days postinfection to obtain sufficient blood (0.3 to 0.5 ml per mouse) to determine antibody titers to DENV and the total types and classes of human immunoglobulins made under various experimental conditions. The amount of anti-dengue activity (titer) in mouse sera was determined by an indirect enzyme-linked immunosorbent assay (ELISA) that detects IgM, IgG, and IgA specific for DENV, as described previously (38). Mice having antibody titers to dengue virus of 1:20 or greater (total, 9) were then tested for total human IgM and IgG content in a quantitative ELISA that is specific for human immunoglobulins (Bethyl Laboratories). We developed another capture ELISA with the same IgM and IgG quantitation sets to determine which of the human IgM and IgG antibodies were specific for DENV. As described previously (38), 96-well plates were coated with sucrose-gradient-purified DENV-2 and blocked with milk diluent solution (KPL), and mouse serum samples (1:100 dilution) were added; human antibodies were detected with specific anti-human IgM and IgG, and standard curves were used for quantification (Bethyl). An infected human serum sample was used as a positive control in all ELISAs and for comparison to humanized mouse antibody levels.
A total of 9 mouse sera were tested for DENV neutralizing antibodies by focus-forming assay. One hundred focus-forming units (FFU) of mosquito cell-derived DENV-1 (strain Western Pacific-74), DENV-2 (strain 16681), DENV-3 (strain UNC3043), and DENV-4 (strain 1036) was incubated with medium or serial dilutions of sera for 1 h at 37°C; samples were added to a monolayer of Vero cells and incubated for 1 h at 37°C. The cells were washed and overlaid with 1% methylcellulose mixed with Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS) and incubated for 48 (DENV-1, -2, and -4) or 60 (DENV-3) hours. Monolayers were washed three times with phosphate-buffered saline (PBS) to remove the methylcellulose, fixed with 1% paraformaldehyde in PBS for 10 min at room temperature, rinsed, and permeabilized in Perm Wash (PBS, 0.1% saponin, and 0.1% bovine serum albumin [BSA]). Infected-cell foci were stained by incubating the cells with the flavivirus-cross-reactive, fusion loop-specific monoclonal antibody (MAb) WNV-E18 (1 μg/ml) (40) and quantified as described previously (25). Endpoint titers were given as 50% neutralization.
Statistical analysis.
Data were collected and graphed using Microsoft Excel; statistical analysis was performed and graphics were generated using GraphPad Prism v5.0. For comparison of viremia, erythema indices, and temperature in mice infected by four different modes, t tests were done (Wilcoxon matched-pairs rank tests). For platelet level comparisons, a Mann-Whitney t test was used to compare values of mosquito bites versus injections, and a one-way analysis of variance (ANOVA) with Dunnett's multiple-comparison was used for comparing humanized, uninfected mice to those infected by mosquito bite and injection, all at day 14. For cytokine comparisons on days 4, 12, and 32, infected mice were compared to uninfected humanized mice via t test; we used data published previously (38) from 6 mice infected by injection (day 4, numbers 469, 484, and 489; day 18, numbers 501, 502, and 504) for comparison. We tested for the effects of engraftment levels or cord blood donor in these studies but did not find any correlation with signs of disease (data not shown). For comparisons with mosquito-infected mice, previous data from three mice injected with the same virus (identifiers [IDs] 176 to 178; days 4 to 16) and four uninfected, unbitten humanized mouse controls (IDs 278 to 281) (38) were used.
RESULTS
Inoculated mosquitoes contain DENV in saliva.
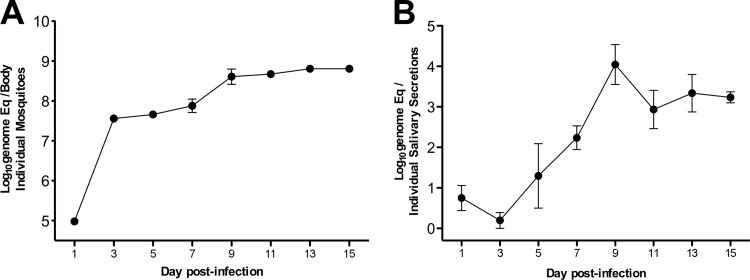
Preliminary tests were performed to determine the peak of DENV infection of inoculated mosquitoes in bodies and saliva. By day 9 postinfection, DENV RNA had reached its peak in mosquito bodies (∼9 log10 genome equivalents/body) (Fig. 1A) and in saliva (∼4 log10 genome equivalents per saliva sample) (Fig. 1B) in five different mosquitoes. Therefore, in subsequent experiments, female mosquitoes were inoculated with 45,000 genome copies of virus strain K0049 and allowed to feed on hu-NSG mice 9 days postinfection. IFA tests of dengue virus-inoculated mosquito heads demonstrated a uniform infection rate (100%) and confirmed that mosquitoes used to bite mice had disseminated infections and were competent to transmit virus (data not shown).
Fig 1.
DENV genome copies in the bodies and saliva of injected A. aegypti mosquitoes over time. (A) Amounts of DENV RNA in mosquito bodies after intrathoracic inoculation with ∼45 PFU of DENV-2 strain K0049 normalized for tissue content with a housekeeping gene (the error bars represent SEM; n = 5 per time point). Eq, equivalents. (B) Amounts of DENV RNA in saliva collected from each of 5 mosquitoes over time.
Multiple mosquito bites are required for mouse infection.
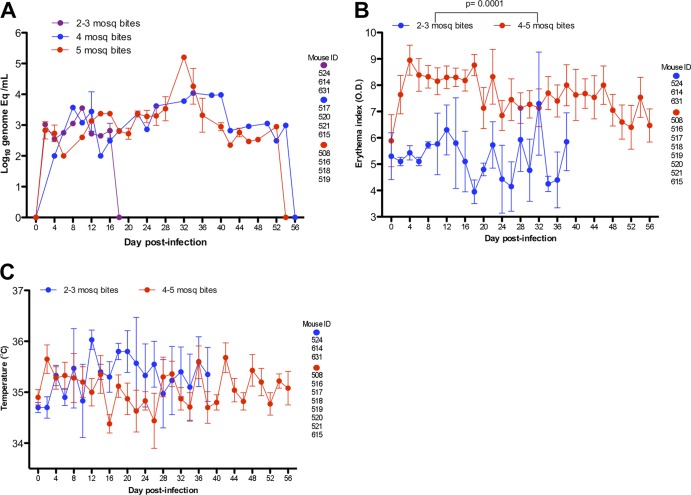
To determine how many DENV-infected-mosquito bites were necessary to infect and produce clinical signs in 100% of hu-NSG mice, we compared viremia, erythema, and temperature values after bites by 2 or 3, 4, and 5 individuals. Viremia levels (Fig. 2A) were clearly different between mice bitten by 2 or 3 mosquitoes versus those bitten by 4 or 5 mosquitoes, with the former showing similar viral RNA blood levels and for a shorter duration (undetectable by day 18), and very similar to those produced in mice with injected virus (Fig. 3A). Humanized mice bitten by 4 or 5 mosquitoes showed equivalent and higher levels of viremia and longer durations, with these curves showing no significant difference between them, and viral RNA reached undetectable levels by day 56. However, we did not evaluate mouse sera for infectious virus particles, and thus, the actual infectious viremia duration could have been shorter. Erythema indices (Fig. 2B) were significantly lower (P = 0.0001) for mice bitten by 2 or 3 mosquitoes than for 4 or 5 mosquito bites combined. Temperature and fever did not differ significantly between those infected by 2 or 3 or by 4 or 5 mosquito bites (Fig. 2C). Although three mice bitten by 2 or 3 mosquitoes showed consistent viremia (short error bars in Fig. 2A), clinical signs were inconsistent (large error bars in Fig. 2B and C). We proceeded to use 4 or 5 infected mosquitoes to bite and transmit virus in subsequent experiments, since these numbers produced uniform rates of infection and clinical signs in all bitten humanized mice. Extrapolating from the data on virus in mosquito saliva at day 9 p.i. (Fig. 1), it is likely that ∼50,000 genomes (5 × 4 log10), 500 TCID50, or 50 PFU (at 100 genome copies/TCID50 or 1,000 genome copies/PFU from our previous studies of strain K0049 [5, 21]) of virus are delivered via saliva from the bites of 5 mosquitoes. Accordingly, the bites of 2 or 3 mosquitoes (or ∼200 to 300 TCID50, or 20 to 30 PFU) were not sufficient to produce consistent clinical signs in hu-NSG mice.
Fig 2.
Comparison of signs of infection in humanized mice bitten by 2, 3, 4, or 5 mosquitoes. (A) Viremia (dengue virus RNA copies in blood) in mice bitten by 2, 3, 4, or 5 mosquitoes (mosq). The error bars represent the SEM; mouse ID numbers per group are given on the right. (B) Erythema index (rash) in mice bitten by 2 or 3 mosquitoes compared to 4 or 5 mosquito bites combined. The statistical significance of the differences is shown above the brackets with the appropriate comparison groups. (C) Temperatures (fever) in mice bitten by 2 or 3 mosquitoes compared to 4 or 5 mosquito bites combined.
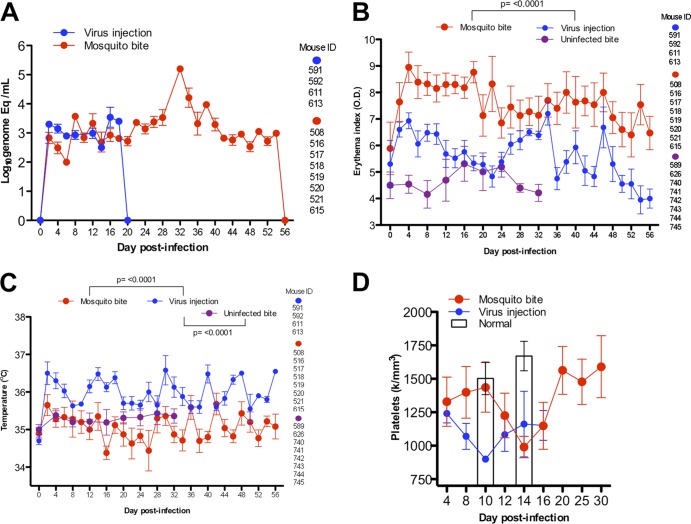
Fig 3.
Comparison of viremia and clinical signs of infection in humanized mice infected by virus intradermal injection (9 log10 genome Eq) or 4 or 5 infected mosquito bites (this group includes the same mice as in Fig. 2) or bitten by 4 or 5 uninfected mosquitoes. The statistical significance of the differences is shown above the brackets with the appropriate comparison groups. The error bars represent the SEM. (A) Viremia in infected mice with injected virus undetectable by day 20, whereas mosquito-bitten mice showed undetectable virus by day 56. The error bars represent the SEM; mouse ID numbers per time point are given on the right. (B) Erythema or rash measurements for humanized mice infected by injection or bite. (C) Temperature or fever in mice infected by injection or bite. (D) Platelet levels or thrombocytopenia peaks in humanized mice infected by injection (peak, day 10) or by bite (peak, day 14), with three additional injected mice (days 4 to 16) and four humanized, healthy mouse controls included for comparison (days 10 and 14) from previous data (38).
Mosquito bite infection produces greater viremia, erythema, and thrombocytopenia.
There were noticeable differences in viremia, erythema, temperature, and platelet levels between hu-NSG mice infected by virus injection and by infected-mosquito bites (Fig. 3). Viremia in hu-NSG mice injected directly with 9 log10 RNA copies (determined previously to produce 100% infection [38]) reached a peak of less than 4 log10 genome copies/ml (<100 TCID50, or <10 PFU per ml of blood) on day 16 and was undetectable by day 20 (Fig. 3A). In comparison, when mice were bitten by 4 or 5 infected mosquitoes, the levels of viral RNA in the blood reached slightly more than 5 log10 copies/ml on day 32 (>1,000 TCID50 or >100 PFU per ml) (Fig. 3A). The latter values are similar to those reached in adult dengue fever patients with clinical signs, according to prospective studies in Vietnam (57), where a similar qRT-PCR assay (with a cutoff of 1 RNA copy per reaction tube versus 1.2 in our study) was used for viral-RNA detection and quantification (29). Erythema and rash indices (Fig. 3B) were also significantly higher (P < 0.0001) for humanized mice infected by mosquito bite throughout the course of infection (days 4 to 56). However, temperatures were significantly lower (P < 0.0001) in mice infected by mosquito bite than in those infected by injection (Fig. 3C) or in those mice bitten by uninfected mosquitoes (P < 0.0001). Platelet values (Fig. 3D) were significantly different between mice infected by injection and mice infected by mosquito bite on day 10 postinfection (P < 0.0001), and both were lower than those in control uninfected humanized mice on day 14 (P = 0.0016). However, hu-NSG mice infected by mosquito bite showed severe thrombocytopenia on day 14 postinfection in a delayed manner, similar to the pattern of delayed viremia, compared to experimental virus injection (Fig. 3D). Humanized mice bitten by 4 or 5 uninfected or 4 or 5 infected mosquitoes developed thrombocytopenia compared to uninfected, unbitten hu-NSG mice (Fig. 4D) on day 14 (P = 0.0034).
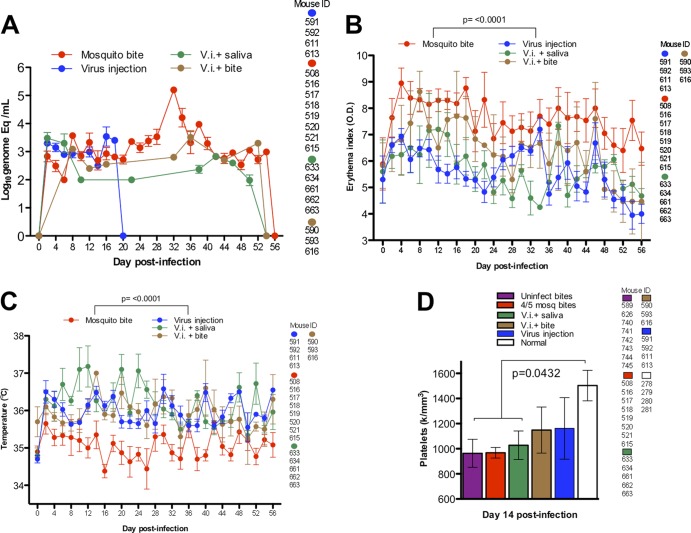
Fig 4.
Comparison of viremia and clinical signs for humanized mice after four different treatments: (i) 4 or 5 infected-mosquito bites (this group includes the same mice as in Fig. 2), (ii) intradermal injection (virus injection [V.i.]) on the back (these are the same mice shown in Fig. 3), (iii) injection of virus plus mosquito saliva on the back, and (iv) injection on the back plus uninfected mosquito bite on the footpad. The statistical significance of the differences is shown above the brackets with the appropriate comparison groups. The error bars represent the SEM. (A) DENV RNA in blood of humanized mice after four different infection models. (B) Erythema indices after four different modes of infection. (C) Temperature after four different modes of infection. (D) Platelet levels after four different modes of infection compared to mice bitten by uninfected mosquitoes and uninfected, unbitten humanized mice, the last from previous data (38).
Infection by inoculation produces fever, and mosquito saliva enhances thrombocytopenia.
To test the effects of salivary secretions alone, without delivery of virus via mosquito bite, we infected hu-NSG mice with 9 log10 genome copies of virus suspended in mosquito saliva collected from 5 adult female mosquitoes. Viremia trended lower but was not statistically different from infection by virus injection alone or by infected mosquito bites (Fig. 4A). Erythema values were significantly lower (P < 0.0001) than for infection by mosquito bites (Fig. 4B), and temperatures were significantly higher (P < 0.0001) by inoculation, whether or not mosquito saliva was included in the intradermal injection (Fig. 4C). Platelet values on day 14 p.i. were lower (P < 0.04) in animals receiving virus plus saliva than in control, uninfected hu-NSG mice but were not significantly different from values in hu-NSG mice bitten by uninfected mosquitoes (Fig. 4D). Thus, mosquito saliva delivered by syringe does not increase viremia or rash in infected, humanized mice but does increase the body temperature of infected mice, as does inoculation of virus alone. In addition, saliva itself (and not the biting mechanism) seems to promote thrombocytopenia on day 14 postinfection when delivered at the same site as the virus. However, we did not measure this directly by including a group inoculated with saliva alone.
A mosquito bite at a site remote from infection can impact viremia.
Previous investigators had reported that tick saliva enhances the transmission and pathogenesis of several other viruses, including tick-borne encephalitis (TBE) virus (another Flavivirus) (39). Tick salivary gland extracts were shown to enhance TBE virus infection in animals, and the effects also occurred if these compounds were injected distally from the site of virus inoculation. This led us to test the hypothesis that a mosquito bite and/or saliva could enhance DENV replication in humanized mice at sites distant from the inoculation site. We inoculated hu-NSG mice with the same virus dose as before (9 log10 genome copies) intradermally on the back but allowed (4 or 5) uninfected mosquitoes to bite their footpads immediately before injection. Virus injection plus mosquito bites produced viremia that was almost as high and sustained as infection via mosquito bite, with only one point, at day 32 p.i., noticeably lower for this route of infection (Fig. 4A). However, erythema indices were significantly (P < 0.0001) lower for these mice than for those infected by mosquito bite alone (Fig. 4B), and temperatures were higher than in humanized mice infected by mosquito bite (Fig. 4C). Platelet counts in these mice at day 14 were not significantly different from uninfected controls, nor were they different from other modes of infection (Fig. 4D). Thus, virus injection alone induces a fever response in hu-NSG mice, whereas mosquito saliva can enhance virus replication distally, resulting in prolonged viremia, but does not affect rash or platelet counts.
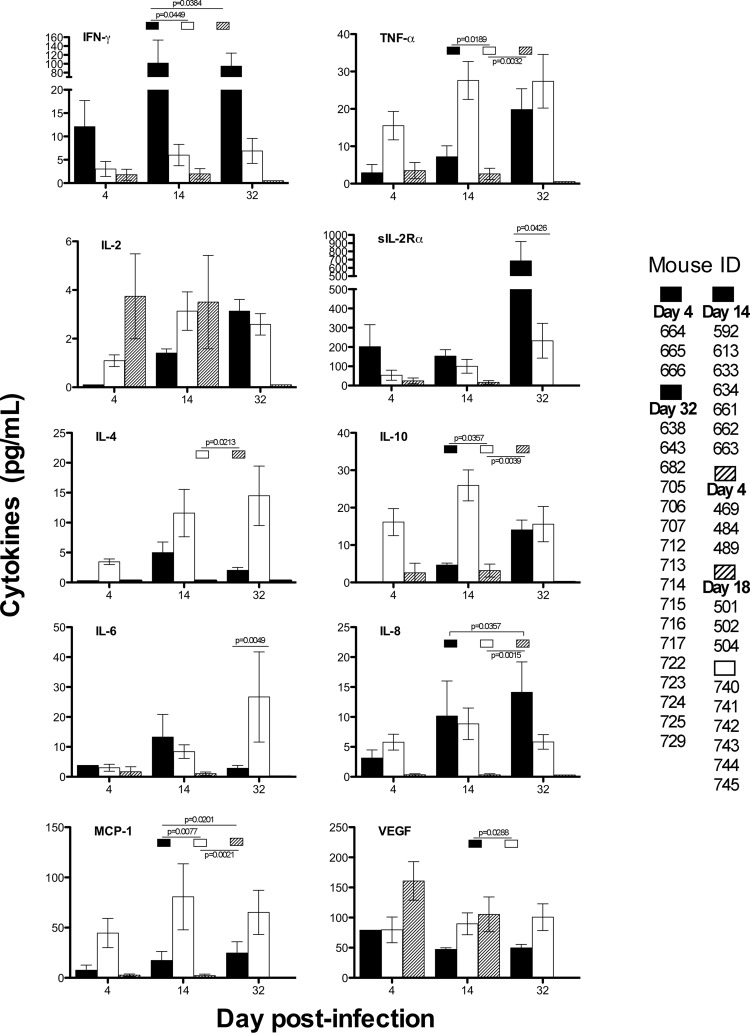
Humanized mice bitten by mosquitoes produce human cytokines.
We tested for evidence of immune system function in hu-NSG mice by measuring 10 different human cytokines in mouse blood at different time points (days 4, 14, and 32) postinfection after mosquito bites (4 or 5 mosquitoes; n = 3 to 17) and direct virus injection (n = 6) and in comparison with hu-NSG mice bitten by 4 or 5 uninfected mosquitoes (n = 6) (Fig. 5). Hu-NSG mice produced substantial amounts of the chemokine MCP-1 and the growth factor VEGF (products of monocyte and mast cell activation), the Th2 cytokine, IL-4, the inhibitory cytokine IL-10, and the proinflammatory cytokine TNF-α after mosquito bite without virus (Fig. 5, white bars). Thus, hu-NSG mice react to mosquito bites/saliva by initially producing some Th2 cytokines, results that agree with studies analyzing the effects of hematophagous arthropods that bite humans (reviewed in reference 23). In contrast, hu-NSG mice bitten by DENV-infected mosquitoes produced significantly higher levels of IFN-γ and the soluble IL-2 receptor alpha than uninfected mice (P = <0.05) (Fig. 5, black bars); these proteins are secreted by activated T cells in DENV-infected patients and have been correlated with the severity of disease (12, 27, 28, 32). In comparison, hu-NSG mice infected by direct virus injection produced weaker human cytokine responses, with only IL-2 and VEGF showing higher levels 4 to 18 days after infection (37) (Fig. 5; hatched bars); this suggests that syringe delivery of virus does not stimulate the same immune responses as infection by mosquito bite.
Fig 5.
Comparison of 10 human cytokines and chemokines measured in humanized mouse serum that were (i) infected by mosquito bite (black bars), (ii) bitten by uninfected mosquitoes (white bars), and (iii) infected by virus injection (hatched), the last values (n = 6) from a previous publication (available for days 4 and 18 only) (38). The error bars represent the SEM; mouse IDs per time point are given on the right. The statistical significance of the differences is shown above the bars with the appropriate comparison groups.
Humanized mice make antibodies to DENV after mosquito bites.
We tested the effect of mosquito bite delivery on the induction of adaptive immunity. In our previous studies of DENV infection in humanized mice, we used only intradermal (37, 38) or subcutaneous (8) injection, and very few of these mice developed anti-DENV antibodies (3/45 total mice infected with K0049 at titers of <1:20). Initially, we used a “screening” ELISA to detect human IgA, IgG, and IgM antibodies specific for DENV-2 in all mouse serum samples. Of the 32 hu-NSG mice that were infected by mosquito bite, 14 developed anti-dengue virus antibodies (Table 2). The screening ELISA titers ranged from 1/20 to 1/2,560 (human serum was >1/40,000), but three mice with higher titers received cord blood cells from only one human donor (Table 2, numbers 664 and 666).
Table 2.
Antibody titers in humanized mice
| Mouse ID | Engraftment (%) | Donor ID | Treatment | Day collected | Anti-DENV titera | Total IgM (ng/ml) | DENV IgM (ng/ml) | Total IgG (ng/ml)b | DENV IgG (ng/ml) | Homologous neut50c titer |
|---|---|---|---|---|---|---|---|---|---|---|
| 638 | 62 | 472 | 5 mosq.e bites | 30 | Neg | 26,169 | 653 | |||
| 699 | 51 | 737 | 4 mosq. bites | 30 | 1:160 | 15,370 | 6,436 | Neg | Neg | |
| 701 | 58 | 737 | 4 mosq. bites | 30 | 1:20 | 73,840 | 6,233 | 13,478 | Neg | <10 |
| 713 | 20 | 789 | 5 mosq. bites | 30 | Neg | 5,031 | 1,864 | |||
| 714 | 77 | 789 | 5 mosq. bites | 30 | Neg | 1,171 | 191 | |||
| 715 | 20 | 789 | 4 mosq. bites | 30 | 1:640 | 118,200 | 66,811 | 16,529 | Neg | <10 |
| 664 | 59 | 491 | 5 mosq. bites | 4 | 1:2560 | 119,363 | 134,218 | 57,500 | 135 | >10 |
| 665 | 47 | 491 | 5 mosq. bites | 4 | 1:640 | 25,230 | 30,161 | 2,686 | Neg | >10 |
| 666 | 30 | 491 | 4 mosq. bites | 4 | 1:80 | 46,941 | NDd | Neg | ND | |
| 702 | 49 | 737 | 4 mosq. bites | 12 | Neg | 9,459 | 3,386 | |||
| 703 | 24 | 737 | 5 mosq. bites | 12 | Neg | 10,360 | Neg | Neg | ||
| 704 | 80 | 737 | 4 mosq. bites | 12 | 1:20 | 49,097 | 2,068 | Neg | ||
| 705 | 49 | 788 | 5 mosq. bites | 32 | Neg | 41,417 | 1,387 | |||
| 706 | 82 | 788 | 5 mosq. bites | 32 | 1:40 | 84,090 | 6,605 | Neg | Neg | |
| 707 | 73 | 788 | 4 mosq. bites | 32 | 1:20 | 69,840 | 3,232 | Neg | Neg | |
| 643 | 11 | 472 | 4 mosq. bites | 32 | Neg | 18,400 | Neg | |||
| 712 | 73 | 788 | 5 mosq. bites | 32 | Neg | 52,600 | 3,125 | |||
| 682 | 88 | 534 | 4 mosq. bites | 32 | 1:160 | 161,000 | 18,267 | 95,650 | Neg | <10 |
| 716 | 18 | 789 | 4 mosq. bites | 32 | Neg | 145,000 | 23,950 | |||
| 723 | 54 | 218 | 4 mosq. bites | 32 | Neg | 8,600 | 9,650 | |||
| 725 | 58 | 218 | 4 mosq. bites | 32 | Neg | 71,200 | Neg | |||
| 724 | 40 | 218 | 4 mosq. bites | 32 | Neg | Neg | Neg | |||
| 729 | 66 | 218 | 4 mosq. bites | 32 | 1:20 | 51,200 | 4,148 | 2,450 | Neg | <10 |
| 722 | 41 | 218 | 5 mosq. bites | 32 | Neg | 23,900 | 11,750 | |||
| 717 | 22 | 789 | 5 mosq. bites | 32 | Neg | 26,500 | 4,531 | |||
| 592 | 40 | 353 | Inoculation | 30 | Neg | |||||
| 4 mosq. bites 210 days p.i. | 14 | 1:2560 | 167,000 | 141,498 | 1,360 | Neg | <10 | |||
| 613 | 45 | 261 | Inoculation | 30 | Neg | |||||
| 4 mosq. bites 210 days p.i. | 14 | Neg | 28,300 | 1,090 | ||||||
| 633 | 81 | 471 | Inoculation + saliva | 30 | Neg | |||||
| 4 mosq. bites 182 days p.i. | 14 | Neg | 9,500 | 1,950 | ||||||
| 634 | 71 | 471 | Inoculation + saliva | 30 | Neg | |||||
| 4 mosq. bites 182 days p.i. | 14 | Neg | 32,500 | Neg | ||||||
| 661 | 48 | 291 | Inoculation + saliva | 30 | Neg | |||||
| 4 mosq. bites 182 days p.i. | 14 | 1:80 | 150,800 | 6,822 | 93,000 | Neg | <10 | |||
| 662 | 59 | 291 | Inoculation + saliva | 30 | Neg | |||||
| 4 mosq. bites 182 days p.i. | 14 | Neg | 59,700 | 9,750 | ||||||
| 663 | 43 | 291 | Inoculation + saliva | 30 | Neg | |||||
| 4 mosq. bites 178 days p.i. | 14 | 1:320 | 175,000 | 21,685 | 93,300 | Neg | <10 | |||
| 740 | 46 | 316 | Uninfected, 4 bites | 4 | Neg | 164,480 | Neg | |||
| 12 | Neg | 152,380 | Neg | |||||||
| 32 | Neg | 211,111 | 232 | |||||||
| 741 | 51 | 316 | Uninfected, 4 bites | 4 | Neg | 113,760 | 128 | |||
| 12 | Neg | 111,520 | 143 | |||||||
| 32 | Neg | 145,160 | 304 | |||||||
| 742 | 54 | 316 | Uninfected, 4 bites | 4 | Neg | 153,360 | 61,605 | |||
| 12 | Neg | 147,040 | 67,536 | |||||||
| 32 | Neg | 201,834 | 116,666 | |||||||
| 743 | 42 | 316 | Uninfected, 4 bites | 4 | Neg | 137,240 | 293 | |||
| 12 | Neg | 131,160 | 270 | |||||||
| 32 | Neg | 150,420 | 548 | |||||||
| 744 | 43 | 316 | Uninfected, 4 bites | 4 | Neg | 110,320 | 7,644 | |||
| 12 | Neg | 93,720 | 12,725 | |||||||
| 32 | Neg | 88,046 | 45,523 | |||||||
| 745 | 44 | 316 | Uninfected, 4 bites | 4 | Neg | 147,480 | Neg | |||
| 12 | Neg | 124,000 | Neg | |||||||
| 32 | Neg | 186,070 | Neg | |||||||
| Human serum | Positive control | >1:40,000 | 686,364 | 84,254 | 5.5e6 | 1.5e5 |
Neg, average optical density (OD) of negative controls plus 1 standard deviation (SD).
Neg, <15.6 ng/ml for IgM and <7.8 ng/ml for IgG.
neut50, 50% neutralization.
ND, not done.
mosq., mosquito.
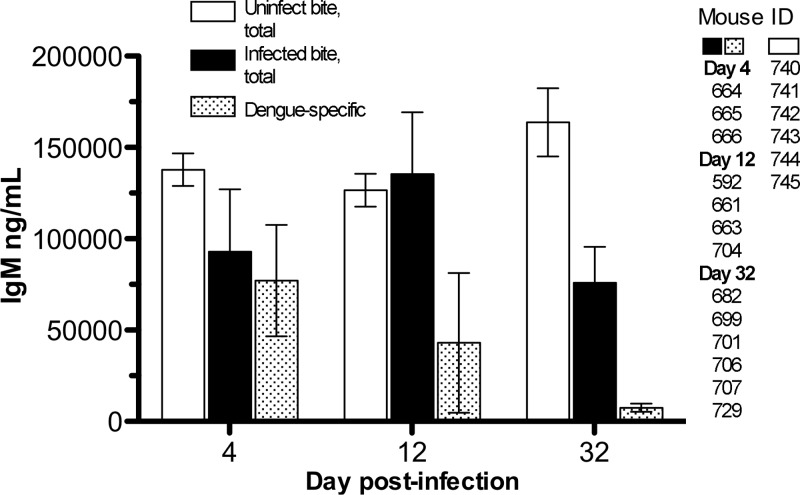
To determine the quantities and types of antibodies made by humanized mice after infection, we used a commercially available kit for quantification of total human IgM and IgG antibodies and a modification of the same assay to measure DENV-specific antibodies, using whole DENV as a capturing antigen. All the mice that had screening ELISA titers of ≥20 (total, 14) had detectable amounts of human IgM and/or IgG (Table 2); the IgM totals ranged from 15,370 to 175,000 ng/ml, and for IgG, the totals ranged from undetectable to 95,650 ng/ml. When testing for specific anti-DENV antibodies, the IgM quantities ranged from 2,068 to 141,498 ng/ml, and the IgG quantities were mostly undetectable. Thus, the humanized mice produced strong DENV-specific IgM responses; nevertheless, some of the antibody response was likely generated against mosquito salivary antigens inserted via bite. Indeed, when humanized mice were tested for quantifiable human IgM, all except one (number 724) had values ranging from 1,171 to 175,000 ng/ml; mice that had been bitten only by uninfected mosquitoes also made human IgM, ranging from 93,720 to 164,480 ng/ml, and some human IgG (2 of 6; range, 7,644 to 67,536 ng/ml), but none of these were specific for DENV (Fig. 6). The results of antibody tests for hu-NSG mice bitten by uninfected mosquitoes compared to those bitten by infected mosquitoes are shown in Fig. 6; the differences (total versus dengue specific) illustrate how some of the IgM levels are likely specific for saliva components. At present, we cannot evaluate the fraction of antibodies made specifically against mosquito saliva components, since it is technically difficult to obtain sufficient salivary secretions to use as antigens in an ELISA (potentially thousands of mosquitoes need to be salivated, at 1 to 2 μg/ml of protein per mosquito) (2). It is also unclear if these antibodies to mosquito saliva impact DENV disease, as few studies have addressed this issue, with inconclusive results (35, 59).
Fig 6.
Comparison of total and dengue-specific human IgM antibodies in uninfected-mosquito-bitten hu-NSG mice and those infected via mosquito bite. The error bars represent the SEM; mouse IDs per time point are given on the right.
To test the biological activity of the serum antibodies, we used samples from humanized mice with the highest ELISA titers in neutralization tests of four viruses, representing all DENV serotypes. All the mice showed some virus-neutralizing activity at the lowest (1:10) dilution for a homologous serotype 2 DENV (Southeast Asia strain 16681) and some for the other, heterologous serotypes (Table 3). Only two mice had neutralizing titers of >1:10, and these samples were taken on day 4 postinfection after 4 or 5 mosquito bites. This correlates with the timing of IgM antibodies made by human patients with DENV (reviewed in reference 41). However, the levels of neutralizing anti-dengue virus antibodies we measured in hu-NSG mice were substantially lower than those produced in humans, likely due to the inability of mouse B cell-activating factor (BAFF) to signal human B cells (48).
Table 3.
Results of antibody neutralization assays
| Serum ID | Virus serotype | % Neutralization at dilutiona: |
|||||
|---|---|---|---|---|---|---|---|
| 1:10 | 1:40 | 1:160 | 1:640 | 1:2,560 | 1:10,240 | ||
| 664 | DV1 | 42 | 22 | 7 | 3 | 0 | 3 |
| 665 | 18 | 7 | 0 | 0 | 0 | 0 | |
| 701 | 13 | 7 | 8 | 13 | 0 | 0 | |
| 715 | 17 | 1 | 0 | 0 | 3 | 1 | |
| 682 | 29 | 6 | 7 | 1 | 0 | 1 | |
| 729 | 14 | 11 | 0 | 0 | 0 | 0 | |
| 592 | 21 | 6 | 0 | 0 | 0 | 0 | |
| 661 | 27 | 2 | 0 | 2 | 0 | 0 | |
| 663 | 20 | 12 | 8 | 0 | 0 | 0 | |
| 664 | DV2 | 77 | 35 | 9 | 1 | 4 | 6 |
| 665 | 57 | 34 | 12 | 9 | 7 | 10 | |
| 701 | 49 | 15 | 3 | 0 | 3 | 0 | |
| 715 | 32 | 19 | 12 | 9 | 5 | 0 | |
| 682 | 33 | 12 | 11 | 0 | 0 | 0 | |
| 729 | 11 | 11 | 11 | 2 | 0 | 0 | |
| 592 | 16 | 0 | 5 | 0 | 2 | 0 | |
| 661 | 37 | 21 | 6 | 0 | 0 | 0 | |
| 663 | 27 | 6 | 8 | 0 | 0 | 0 | |
| 664 | DV3 | 47 | 15 | 0 | 0 | 0 | 4 |
| 665 | 34 | 19 | 3 | 0 | 0 | 0 | |
| 701 | 32 | 5 | 0 | 0 | 0 | 0 | |
| 715 | 28 | 12 | 0 | 0 | 0 | 3 | |
| 682 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 729 | 51 | 41 | 19 | 4 | 15 | 6 | |
| 592 | 64 | 28 | 26 | 6 | 4 | 15 | |
| 661 | 68 | 49 | 13 | 19 | 0 | 0 | |
| 663 | 62 | 33 | 11 | 4 | 0 | 0 | |
| 664 | DV4 | 58 | 43 | 33 | 17 | 0 | 4 |
| 665 | 60 | 40 | 20 | 7 | 0 | 0 | |
| 701 | 59 | 41 | 31 | 13 | 7 | 0 | |
| 715 | 63 | 43 | 22 | 9 | 1 | 5 | |
| 682 | 59 | 37 | 11 | 13 | 2 | 0 | |
| 729 | 41 | 38 | 17 | 16 | 1 | 5 | |
| 592 | 53 | 30 | 11 | 6 | 0 | 0 | |
| 661 | 64 | 45 | 16 | 18 | 0 | 0 | |
| 663 | 64 | 36 | 21 | 18 | 0 | 0 | |
The values indicate the percent neutralization (performed in duplicate) after 1 h preincubation at 37°C with each of four different serotype viruses.
First infection produces protective immunity to homologous virus.
Using hu-NSG mice (n = 7) that had been infected by inoculation of virus or by simultaneous inoculation of mosquito saliva and virus, we performed secondary infections with the same virus via mosquito bite to determine if this would boost antibody titers and functional memory. Mice that were inoculated with 9 log10 genomes of DENV-2 and bitten by 4 infected mosquitoes 210 days after the inoculation did not have detectable viremia on day 14 after a second infection and had platelet levels similar to those of healthy, uninfected hu-NSG mice (mean = 1,360; standard error of the mean [SEM] = 85)) (Table 1). Five mice that were infected by inoculation of virus plus mosquito saliva and bitten 178 or 182 days after inoculation also did not develop viremia on day 14 but did have lower (P < 0.02) platelet values (mean = 1,016; SEM = 96) than other mice bitten by mosquitoes on day 14 p.i. Antibody production (IgM) was detectable after the second infection in 3 of 7 mice at a rate similar to those after primary infection (11 of 25). Cytokine values for these secondary infections on day 14 after infected-mosquito bites showed that IFN-γ and sIL-2Rα were elevated, as they were during primary infection by mosquito bite (Fig. 5, days 4 and 32). Thus, these hu-NSG mice were protected from reinfection by the homologous virus strain, with cytokines and dengue-specific IgM antibodies that were made up to 6 months after the first infection in the absence of viremia. This implies that hu-NSG mice have functional memory against DENV up to 8 months after transplantation with human stem cells. Studies of long-term engraftment have shown that the numbers of human lymphocytes actually increase after transplantation (60) and that hu-NSG mice can produce IgM to pneumococcus vaccine >6 months after transplantation (4); in addition, functional T and B cells with specific immune responses to viruses (e.g., HIV and Epstein-Barr virus [EBV]) have been demonstrated in these mice (reviewed in reference 52).
DISCUSSION
Since the 1930s, studies of DENV pathogenesis have been hampered by a lack of suitable animal models of disease (reviewed in reference 9). Even higher primates, including chimpanzees, fail to develop dengue disease, and currently, monkeys are used primarily to establish the safety and immunogenicity of potential dengue vaccines. The advent of mouse-human chimeras, especially those with transplanted human hematopoietic stem cells, allowed the generation of the first animal model of dengue fever (8). The hu-NSG mice that we have worked with more recently (37, 38) and reported here have functional human T, B, and NK cells, along with other cells of the immune system (monocytes/macrophages, neutrophils, and mast and dendritic cells) (14, 30, 56, 58) that reside in human epithelium and capillaries, the targets of mosquito bites (24) and DENV infection (11, 15, 36, 62). However, hu-NSG mice lack other possible human target cells, such as hepatocytes and endothelial cells, and do not secrete human complement factors that could modify infection or disease (6). Therefore, the studies we report here are the result of infection of an animal model that contains many, but not all, of the components of the human immune system and targets of DENV infection. While they are not replacements for human pathogenesis studies, hu-NSG mice can provide new insights into salient aspects of DENV infection, including the impact of the mosquito vector.
To obtain a blood meal, blood-feeding arthropods must cope with a variety of challenges posed by the host: hemostasis, pain and itch responses, and local immune responses (43, 44). Host hemostatic defenses include coagulation pathways, platelet aggregation, and vasoconstriction; rapidly engorging arthropods, such as mosquitoes, have developed countermeasures to each of these responses in the form of molecules in saliva that modulate these systems (22, 49, 51). Although salivary gland extracts from female A. aegypti mosquitoes inhibited release of TNF-α from rat mast cells (10), there is evidence that these cells are important in the human innate immune response to DENV infection. Mast cells are first activators of the inflammatory response (via release of preformed TNF-α) and activate human endothelial cells after DENV infection, a first step in producing plasma leakage (15). They are situated perivascularly in the dermis, and they also contribute to the rash typical of DF and the increased histamine in dengue disease patient serum and urine (15). Mast cells also contribute to NK and T cell recruitment and DENV clearance in vivo (54). Although hu-NSG mice contain functional human mast cells (56) that clinically produce a rash after DENV infection, these mice appear not to contain enough cells to recruit functional NK and T cells for virus clearance.
With regard to the adaptive immune response, in general, hematophagous arthropod saliva downregulates the expression and secretion of Th1 cytokines, thus skewing the response to an antibody-mediated Th2 response (22). Systemic cytokine responses of mice to A. aegypti mosquito bites were downregulated 7 days after feeding (63), and injection with sialokinin I, a neuropeptide from salivary glands, downregulated Th1 cytokines and upregulated Th2 cytokines 4 days after injection. Our results show hu-NSG mice produce cytokines mimicking this response when bitten by uninfected mosquitoes, and most of these cytokines were still detectable at day 32 postbite. At least 4 different mosquito salivary proteins have been shown to affect platelet, thrombin, or other clotting factor function (23). Increased cytokine production by splenocytes of mice bitten by A. aegypti and Culex pipiens mosquitoes has been reported even after 7 to 10 days, suggesting possible delayed or persistent immunomodulatory effects on platelet or T cell functions (63). Thus, similar to our findings, others have reported extended effects on the immune response after mosquito bites. We speculate that the late effects of mosquito saliva observed in our study (low platelet levels and prolonged cytokine production) could be due to persistent alterations in cytokines. Exactly how this occurs from a mechanistic standpoint remains unknown and is an area of active study by several groups.
However, once DENV is introduced, along with these immune function effects of mosquito saliva, most of the hu-NSG mice secreted proinflammatory cytokines typically seen in human infections (28). In addition, hu-NSG mice produced DENV-specific IgM only after infection by mosquito bite, and not by virus injection. Some mice, receiving cord blood cells from the same donor, produced higher tiers of neutralizing antibodies than all others. Rather than the donor cells determining why these mice produced more anti-dengue virus antibodies, we believe that early sampling (day 4) explains the difference in levels because of the type and class made early after infection (IgM); most other mice were sampled for antibodies after days 12 to 32 p.i. Other investigators have recently described rates of repopulation and specific T and B cell functions in hu-NSG mice, with colocalization of human T and dendritic cells in mouse liver and development of B cells in the bone marrow up to 5 months postengraftment (17). T cells from these mice (splenocytes) also produced human cytokines (IFN-γ, IL-2, IL-4, and IL-5) ex vivo after stimulation with human major histocompatibility complex (MHC) molecules (via coculture with peripheral blood mononuclear cells [PBMCs]). Here, we have shown evidence for functional T cells that secrete human cytokines after DENV infection and for memory up to 6 months postinfection. While others have reported on the sporadic production of anti-dengue virus IgM antibodies in humanized mice, it was after injection with a combination of 3 or 4 DENV strains, in another strain of mouse (hu-Rag) (33), or in hu-NSG mice injected with a laboratory-adapted strain of DENV-2 (New Guinea C) (31). However, neither of these models showed consistent evidence of dengue disease after infection with single or multiple dengue viruses; the rates and levels of antibody production in the mice were also low.
Our data provide evidence that immune system function is modulated, induced by the host responses to mosquito saliva proteins, and that this enhances DENV replication. This finding was also observed recently after West Nile virus infection of immunocompetent mice via mosquito bite (50, 55). However, the investigators did not measure fever or blood counts, as their endpoints of disease were ataxia, paralysis, and death. In addition, those studies did not include the effects of mosquito bites on the induction of human cytokines and antibodies, as wild-type and not chimeric mice were studied. Thus, our results showing enhanced DENV replication and disease after mosquito bites are analogous to those seen with other members of the genus Flavivirus; however, none of the other virus-vector pairs tested monitored changes in hemostasis, as it is more DENV specific in humans.
Increased T cell activation and cytokine production have been reported in patients with DHF during primary or secondary infections (16, 26). However, it has been shown that the timing of Th1 cytokine (IFN-γ, IL-10, sIL-2R, and TNF-β) responses varies by stage of disease in humans and whether the patient presents with DF or DHF (34). A more recent study of cytokines secreted by patients with primary DENV infections in Gabon showed that TNF-α levels are not significantly altered but IFN-γ levels are much higher in adults and children (7). Humanized mice infected by mosquito bite reacted in a similar manner during primary infection, with very high levels of IFN-γ (approximately 1/10 of that seen in humans) and sIL-2Rα (approximately 1/2 of that seen in humans) (27, 28), with both cytokines produced during the viremic phase. Thus, DENV in mosquito saliva induces a Th1 response, although hu-NSG mice cannot mount strong enough innate or adaptive immune responses to clear virus early. There is a consensus that mild and severe dengue syndromes represent a disease continuum that is differentiated by the degree of vascular leakage in the patient. However, vascular leakage begins during the febrile phase, when the virus load is declining, so it seems more likely to be a consequence of the response to the infection and not a direct effect of viral replication. It is widely believed that there is an immunological basis for the pathogenesis of endothelial permeability and vascular leakage, but the mechanisms leading to it and the hemostatic abnormalities seen in both DF and DHF are not known. However, by including the effects of mosquito saliva in the hu-NSG mouse model of infection, we can begin to address whether salivary components contain factors that contribute to more severe dengue disease in humans.
Our data clarify that DENV inoculation alone produces higher temperature or fever in humanized mice, and this should be compared to other animal models of disease to determine if syringe inoculation iatrogenically causes disease signs. This fever response might be expected, since injection involves inoculation with cell culture medium components (antigens that might serve as irritants), along with virus, with administration by puncture of the skin with a needle, which is much more traumatic than a mosquito proboscis. Thrombocytopenia did not occur in humanized mice infected by inoculation and with distal mosquito bites, and this suggests that mosquito delivery of virus at the same site in a suspension of saliva components results in lower platelet counts without producing rash. Thus, a mosquito bite or saliva alone can cause thrombocytopenia on its own, most probably by injecting soluble factors that act directly on platelets, causing activation and aggregation (22), and by inhibiting the coagulation cascade only locally at the bite site. It has been suggested that patients suffering from other, underlying diseases or conditions (e.g., asthma, diabetes, or pregnancy) that affect immunity or hemostasis have a tendency to develop DHF (13, 42). These patients could experience a more excessive reaction to mosquito saliva, which could modulate immunopathogenesis (47). Although we have no direct evidence for this, we propose to include the effects of mosquito bites and saliva in future evaluations of dengue virus pathogenesis, especially after serial infection with heterologous serotypes.
ACKNOWLEDGMENTS
We thank John F. Anderson for providing A. aegypti strain Rockefeller eggs for mosquito breeding.
This work was supported in part by U.S. National Institutes of Health grants R01-AI050123 (R.R.-H.) and R01-AI077955 and U01-AI 061373 (M.S.D.), a Cowles Postdoctoral Fellowship (J.C.), and a TBRI Forum pilot study grant (J.C. and R.R.-H.).
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Ader DB, et al. 2004. Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 17:252–265 [DOI] [PubMed] [Google Scholar]
- 2. Almeras L, et al. 2010. Salivary gland protein repertoire from Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis. 10:391–402 [DOI] [PubMed] [Google Scholar]
- 3. Anderson JR, Rico-Hesse R. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am. J. Trop. Med. Hyg. 75:886–892 [PMC free article] [PubMed] [Google Scholar]
- 4. Andre MC, et al. 2010. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J. Immunol. 185:2710–2720 [DOI] [PubMed] [Google Scholar]
- 5. Armstrong PM, Rico-Hesse R. 2003. Efficiency of dengue serotype 2 virus strains to infect and disseminate in Aedes aegypti. Am. J. Trop. Med. Hyg. 68:539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avirutnan P, et al. 2011. Complement-mediated neutralization of dengue virus requires mannose-binding lectin. MBio 2:e00276-11 doi:10.1128/mBio.00276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becquart P, et al. 2010. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon alpha production. BMC Infect. Dis. 10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. 2005. Dengue fever in humanized NOD/SCID mice. J. Virol. 79:13797–13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bente DA, Rico-Hesse R. 2006. Models of dengue virus infection. Drug Discov. Today Dis. Models 3:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bissonnette EY, Rossignol PA, Befus AD. 1993. Extracts of mosquito salivary gland inhibit tumour necrosis factor alpha release from mast cells. Parasite Immunol. 15:27–33 [DOI] [PubMed] [Google Scholar]
- 11. Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. 2011. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J. Virol. 85:1671–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bozza FA, et al. 2008. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bravo JR, Guzman MG, Kouri GP. 1987. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans. R. Soc. Trop. Med. Hyg. 81:816–820 [DOI] [PubMed] [Google Scholar]
- 14. Brehm MA, et al. 2010. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin. Immunol. 135:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown MG, et al. 2011. Dengue virus infection of mast cells triggers endothelial cell activation. J. Virol. 85:1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chau TN, et al. 2008. Dengue in Vietnamese infants—results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J. Infect. Dis. 198:516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi B, et al. 2011. Human T cell development in the liver of humanized NOD/SCID/IL-2Rgamma(null)(NSG) mice generated by intrahepatic injection of CD34(+) human (h) cord blood (CB) cells. Clin. Immunol. 139:321–335 [DOI] [PubMed] [Google Scholar]
- 18. Clarys P, Alewaeters K, Lambrecht R, Barel AO. 2000. Skin color measurements: comparison between three instruments: the Chromameter the DermaSpectrometer and the Mexameter. Skin Res. Technol. 6:230–238 [DOI] [PubMed] [Google Scholar]
- 19. Cologna R, Armstrong PM, Rico-Hesse R. 2005. Selection for virulent dengue viruses occurs in humans and mosquitoes. J. Virol. 79:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colton L, Biggerstaff BJ, Johnson A, Nasci RS. 2005. Quantification of West Nile virus in vector mosquito saliva. J. Am. Mosq. Control Assoc. 21:49–53 [DOI] [PubMed] [Google Scholar]
- 21. Cox J, Brown HE, Rico-Hesse R. 2011. Variation in vector competence for dengue viruses does not depend on mosquito midgut binding affinity. PLoS Negl. Trop. Dis. 5:e1172 doi:10.1371/journal.pntd.0001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fontaine A, et al. 2011. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit. Vectors 4:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fontaine A, et al. 2011. Relationship between exposure to vector bites and antibody responses to mosquito salivary gland extracts. PLoS One 6:e29107 doi:10.1371/journal.pone.0029107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frischknecht F. 2007. The skin as interface in the transmission of arthropod-borne pathogens. Cell Microbiol. 9:1630–1640 [DOI] [PubMed] [Google Scholar]
- 25. Fuchs A, Pinto AK, Schwaeble WJ, Diamond MS. 2011. The lectin pathway of complement activation contributes to protection from West Nile virus infection. Virology 412:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green S, et al. 1999. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J. Infect. Dis. 180:1429–1435 [DOI] [PubMed] [Google Scholar]
- 27. Green S, et al. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755–762 [DOI] [PubMed] [Google Scholar]
- 28. Gunther VJ, et al. 2011. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 29:3895–3904 [DOI] [PubMed] [Google Scholar]
- 29. Hue KD, et al. 2011. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J. Virol. Methods 177:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishikawa F, et al. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaiswal S, et al. 2009. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS One 4:e7251 doi:10.1371/journal.pone.0007251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurane I, et al. 1991. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Invest. 88:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuruvilla JG, Troyer RM, Devi S, Akkina R. 2007. Dengue virus infection and immune response in humanized RAG2(-/-)gamma (c) (-/-) (RAG-hu) mice. Virology 369:143–152 [DOI] [PubMed] [Google Scholar]
- 34. Libraty DH, et al. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185:1213–1221 [DOI] [PubMed] [Google Scholar]
- 35. Machain-Williams C, et al. 2012. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol. 34:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marovich M, et al. 2001. Human dendritic cells as targets of dengue virus infection. J. Investig. Dermatol. Symp. Proc. 6:219–224 [DOI] [PubMed] [Google Scholar]
- 37. Mota J, Rico-Hesse R. 2011. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One 6:e20762 doi:10.1371/journal.pone.0020762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mota J, Rico-Hesse R. 2009. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J. Virol. 83:8638–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nuttall PA, Labuda M. 2004. Tick-host interactions: saliva-activated transmission. Parasitology 129(Suppl.):S177–S189 [DOI] [PubMed] [Google Scholar]
- 40. Oliphant T, et al. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J. Virol. 80:12149–12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peeling RW, et al. 2010. Evaluation of diagnostic tests: dengue. Nat. Rev. Microbiol. 8:S30–S38 [DOI] [PubMed] [Google Scholar]
- 42. Ribeiro CF, Silami VG, Brasil P, Nogueira RM. 2012. Sickle-cell erythrocytes in the placentas of dengue-infected women. Int. J. Infect. Dis. 16:e72 doi.org/10.1016/j.ijid.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 43. Ribeiro JM. 2000. Blood-feeding in mosquitoes: probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med. Vet. Entomol. 14:142–148 [DOI] [PubMed] [Google Scholar]
- 44. Ribeiro JM, Francischetti IM. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73–88 [DOI] [PubMed] [Google Scholar]
- 45. Rico-Hesse R, et al. 1998. Molecular evolution of dengue type 2 virus in Thailand. Am. J. Trop. Med. Hyg. 58:96–101 [DOI] [PubMed] [Google Scholar]
- 46. Rosen L, Gubler D. 1974. The use of mosquitoes to detect and propagate dengue viruses. Am. J. Trop. Med. Hyg. 23:1153–1160 [DOI] [PubMed] [Google Scholar]
- 47. Rothman AL. 2011. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 11:532–543 [DOI] [PubMed] [Google Scholar]
- 48. Schmidt MR, et al. 2008. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One 3:e3192 doi:10.1371/journal.pone.0003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneider BS, Higgs S. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 102:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider BS, et al. 2006. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 19:74–82 [DOI] [PubMed] [Google Scholar]
- 51. Schoeler GB, Wikel SK. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95:755–771 [DOI] [PubMed] [Google Scholar]
- 52. Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7:118–130 [DOI] [PubMed] [Google Scholar]
- 53. Srikiatkhachorn A, Green S. 2010. Markers of dengue disease severity. Curr. Top. Microbiol. Immunol. 338:67–82 [DOI] [PubMed] [Google Scholar]
- 54. St John AL, et al. 2011. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. U. S. A. 108:9190–9195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Styer LM, et al. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 85:1517–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takagi S, et al. 2012. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood 119:2768–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl. Trop. Dis. 5:e1309 doi:10.1371/journal.pntd.0001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. 2009. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J. Leukoc. Biol. 86:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wasinpiyamongkol L, et al. 2010. Blood-feeding and immunogenic Aedes aegypti saliva proteins. Proteomics 10:1906–1916 [DOI] [PubMed] [Google Scholar]
- 60. Watanabe S, et al. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212–218 [DOI] [PubMed] [Google Scholar]
- 61. WHO 2011, posting date Working to overcome the global impact of neglected tropical diseases. World Health Organization, Geneva, Switzerland: http://www.who.int/neglected_diseases/2010report/en/index.html [Google Scholar]
- 62. Wu SJ, et al. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816–820 [DOI] [PubMed] [Google Scholar]
- 63. Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. 1999. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 21:35–44 [DOI] [PubMed] [Google Scholar]