Abstract
We have previously shown that the porcine alphaherpesvirus pseudorabies virus (PRV) efficiently interferes with phosphorylation of the eukaryotic translation initiation factor eIF2α. Inhibition of phosphorylation of eIF2α has been reported earlier for the closely related alphaherpesvirus herpes simplex virus 1 (HSV-1) through its ICP34.5 and US11 proteins. PRV, however, does not encode an ICP34.5 or US11 orthologue. Assays using cycloheximide, UV-inactivated PRV, or phosphonoacetic acid (PAA) showed that de novo expression of one or more (immediate) early viral protein(s) is required for interference with eIF2α phosphorylation. In line with this, a time course assay showed that eIF2α phosphorylation was abolished within 2 h after PRV inoculation. PRV encodes only one immediate-early protein, IE180, the orthologue of HSV-1 ICP4. As reported earlier, a combinational treatment of cells with cycloheximide and actinomycin D allowed expression of IE180 without detectable expression of the US3 early protein in PRV-infected cells. This led to a substantial reduction in eIF2α phosphorylation levels, indicative for an involvement of IE180. In support of this, transfection of IE180 also potently reduced eIF2α phosphorylation. IE180-mediated interference with eIF2α phosphorylation was not cell type dependent, as it occurred both in rat neuronal 50B11 cells and in swine testicle cells. Inhibition of the cellular phosphatase PP1 impaired PRV-mediated interference with eIF2α phosphorylation, indicating that PP1 is involved in this process. In conclusion, the immediate-early IE180 protein of PRV has the previously uncharacterized ability to suppress phosphorylation levels of the eukaryotic translation initiation factor eIF2α.
INTRODUCTION
The translation initiation factor eIF2α plays a critical role in the onset of translation of mRNA, including viral mRNA. Phosphorylation of eIF2α prevents recycling of GDP-bound eIF2α into its active GTP-bound form, thereby globally inhibiting protein synthesis (23). Phosphorylation of eIF2α represents one of the potent antiviral consequences of the interferon (IFN)-mediated immune response (14). IFN leads to the production of protein kinase PKR, but viral double-stranded RNA (dsRNA) is necessary to mediate PKR dimerization and activation. Activated PKR then phosphorylates eIF2α, shutting down translation and viral protein production.
Because of its central importance in ensuring translation of mRNA, different viruses have evolved mechanisms to counteract phosphorylation of eIF2α. We have recently shown that the porcine alphaherpesvirus pseudorabies virus (PRV) very efficiently counteracts phosphorylation of eIF2α (24). Inhibition of eIF2α phosphorylation has been reported earlier for the human alphaherpesvirus herpes simplex virus 1 (HSV-1) through its US11 and ICP34.5 proteins (6, 8, 16, 20). PRV, however, does not encode an ICP34.5 or US11 orthologue. The aim of the current study was therefore to investigate the mechanism of PRV-mediated dephosphorylation of eIF2α.
In this study, we report that both in rat 50B11 neuronal cells and in swine testicle (ST) cells, de novo synthesis of the immediate-early protein IE180 of PRV is able to interfere with eIF2α phosphorylation. We also show that the cellular protein phosphatase 1 (PP1) is involved in this process.
MATERIALS AND METHODS
Cells and virus.
Sensory neuronal cells originating from rat dorsal root ganglion neurons (50B11) cells were a kind gift from A. Höke (Department of Neurology, Johns Hopkins University). The cells were grown in neurobasal medium supplemented with 1.1% glucose (20%), 0.27% l-glutamine, 10% fetal calf serum (FCS), 2% B-27, and 0.1% blasticidin (7). Before use in experiments, cells were differentiated as described before (7, 24), by treatment with forskolin (50 μM) (Sigma) for 24 h. ST cells were cultivated in Eagle's minimal essential medium (MEM) supplemented with 10% FCS, glutamine (0.3 mg/ml), and antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin). The PRV strain Becker (4) was grown and titrated on ST cells and stored at −80°C.
Antibodies and chemicals.
The rabbit polyclonal anti-IE180 antibody has been described before (13). Mouse monoclonal anti-US3 antibody was kindly provided by L. A. Olsen and L. Enquist (Princeton University, Princeton, NJ, USA). Mouse anti-eIF2α (L57A5), rabbit anti-phospho-eIF2α (D9G8), rabbit anti-phospho-PERK (16F8), and rabbit anti-PP2A (52F8) antibodies were purchased from Cell Signaling. Mouse anti-PP1 (sc-7482) and rabbit anti-GADD34 (sc-8327) antibodies were purchased from Santa Cruz. Rabbit anti-green fluorescent protein (anti-GFP) (G10362) antibody was purchased from Invitrogen, and rabbit anti-alpha tubulin (ab15246) antibody was purchased from Abcam. Horseradish peroxidase (HRP)-conjugated secondary goat anti-rabbit antibodies were purchased from Cell Signaling, and goat anti-mouse antibodies were purchased from Dako Cytomation.
To induce phosphorylation of eIF2α, cells were treated with 1 μM thapsigargin (Invitrogen) for 1 h. Thapsigargin induces eIF2α phosphorylation by activating the pancreatic endoplasmic reticulum PERK protein kinase (26). Cycloheximide (CHX) (Sigma-Aldrich) treatment (10 μg/μl) was used to inhibit protein translation, and phosphonoacetic acid (PAA) (Sigma-Aldrich) treatment (250 μg/ml) was used to inhibit viral replication. When UV-inactivated PRV was used, inactivation was performed by UV irradiating the inoculum in a petri dish on ice with 1,000 mJ/cm2 using the CL-1000 UV Cross-linker (UVP, Inc.), all as described before (11). Sequential treatment with cycloheximide and actinomycin D (ActD) (Invitrogen) was used to allow expression of the immediate-early protein IE180, as described before (2). Briefly, cells were treated with CHX (10 μg/μl) for 30 min prior to inoculation. At 2 hours postinoculation (hpi), CHX was washed away and medium with ActD (5 μg/ml) was added. PP1 and PP2 inhibitors inhibitor 2 (I2) and okadaic acid (OA) were obtained from Millipore and Sigma-Aldrich, respectively, and used at concentrations recommended by the manufacturer (I2) or found in the literature (25).
Infection and transfection.
For infection assays, monolayers of cells, pretreated with thapsigargin (1 μM for 1 h), were inoculated with PRV strain Becker at a multiplicity of infection (MOI) of 1. Cells were lysed for immunoblotting at 4 hpi. For transfection assays, subconfluent 50B11 cells were transfected with an IE180-expressing plasmid (21) or an eGFP-expressing plasmid (pTrip, obtained from B. Verhasselt, Ghent University) using the jetPRIME reagent (Polyplus) according to the manufacturer's protocol. Medium was renewed 4 h posttransfection. Cells were treated with thapsigargin (1 μM for 1 h) prior to lysis 24 h posttransfection.
Western blotting.
SDS-PAGE and Western blotting were performed as described previously (10). Protein concentrations of 30 to 40 μg were used for all experiments. Protein concentration was determined using the BCA protein assay reagent and spectrophotometry according to the manufacturer's instructions (Pierce Biotechnology). Blots were blocked in 5% nonfat dry milk in phosphate-buffered saline (PBS)–Tween 20 for 1 h at room temperature. The blots were incubated for 1 h or overnight (according to the manufacturer's instructions) with primary antibodies and washed 3 times in 0.1% Tris-buffered saline (TBS)–Tween 20 (TBS-T). Blots were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature and, after several washing steps, developed using enhanced chemiluminescence (ECL Plus; GE Healthcare). Protein band intensities were measured using Image J software. Statistical analysis on three independent replicates was performed using GraphPad Prism software using a t test.
RESULTS
PRV interferes with eIF2α phosphorylation.
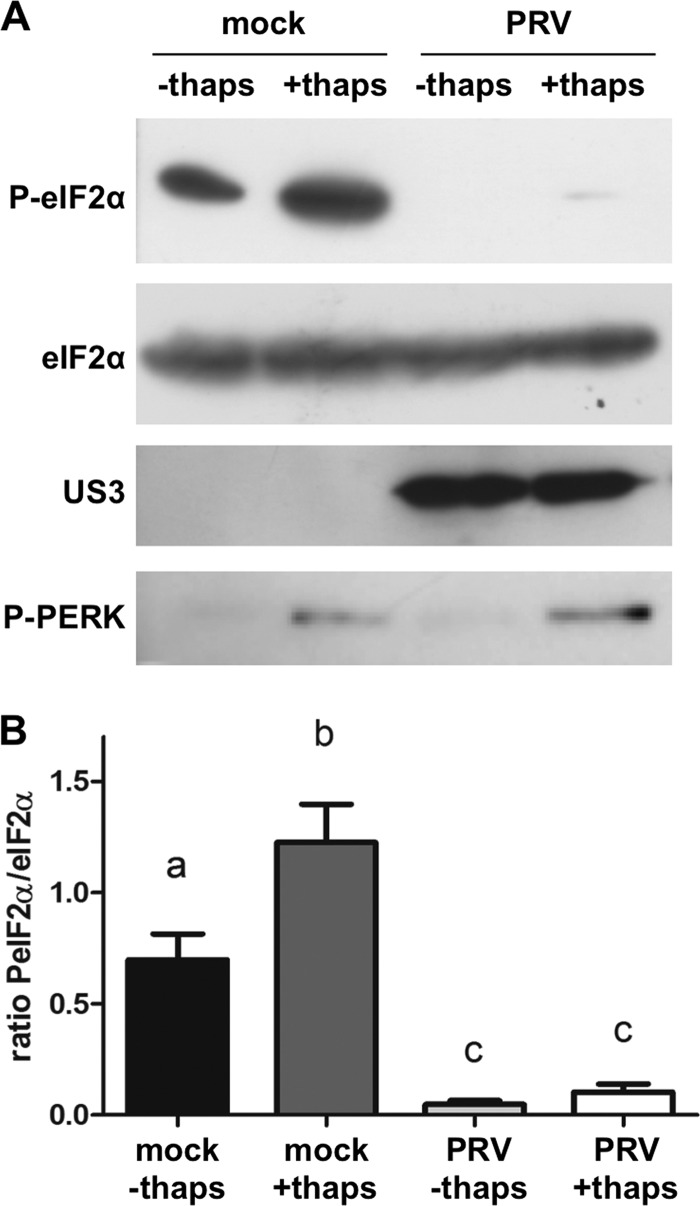
We previously described that PRV is able to counteract IFN-mediated phosphorylation of eIF2α in 50B11 neuronal cells (24). Here, we used thapsigargin to induce phosphorylation of eIF2α. Thapsigargin induces eIF2α phosphorylation by activating the pancreatic endoplasmic reticulum PERK protein kinase (26). Differentiated 50B11 cells were pretreated or not with thapsigargin (1 μM) for 1 h prior to inoculation with PRV. Lysates were collected at 4 hpi, and Western blotting was performed for total and phosphorylated eIF2α. Thapsigargin treatment effectively induced phosphorylation of eIF2α (Fig. 1A, lanes 1 and 2, and B). PRV efficiently counteracted phosphorylation of eIF2α in thapsigargin-stimulated cells and also reduced basal levels of eIF2α phosphorylation in nonstimulated cells (Fig. 1A, lanes 3 and 4, and B). This indicates that PRV is able to interfere with phosphorylation of eIF2α induced by different stimuli. Since PRV is able to counteract both preinduced (by thapsigargin) and basal levels of phosphorylation of eIF2α, this suggests that PRV leads to dephosphorylation of eIF2α rather than preventing phosphorylation of eIF2α. In support of the latter, PRV did not influence thapsigargin-induced phosphorylation of PERK, the kinase that phosphorylates eIF2α upon thapsigargin treatment (Fig. 1A).
Fig 1.
PRV suppresses phosphorylation of eIF2α. (A) Differentiated 50B11 cells were pretreated or not with thapsigargin (1 μM) for 1 h prior to mock inoculation or inoculation with PRV (MOI, 1). Lysates were collected at 4 hpi, and Western blot detection was performed for total and phosphorylated eIF2α (P-eIF2α) and phosphorylated PERK (P-PERK). US3 detection was included as a control for infection. (B) Ratios of PeIF2α/total eIF2α from three independent replicates. Different letters indicate statistically significant differences (P < 0.05).
An immediate-early or early protein is involved in PRV-mediated interference with eIF2α phosphorylation.
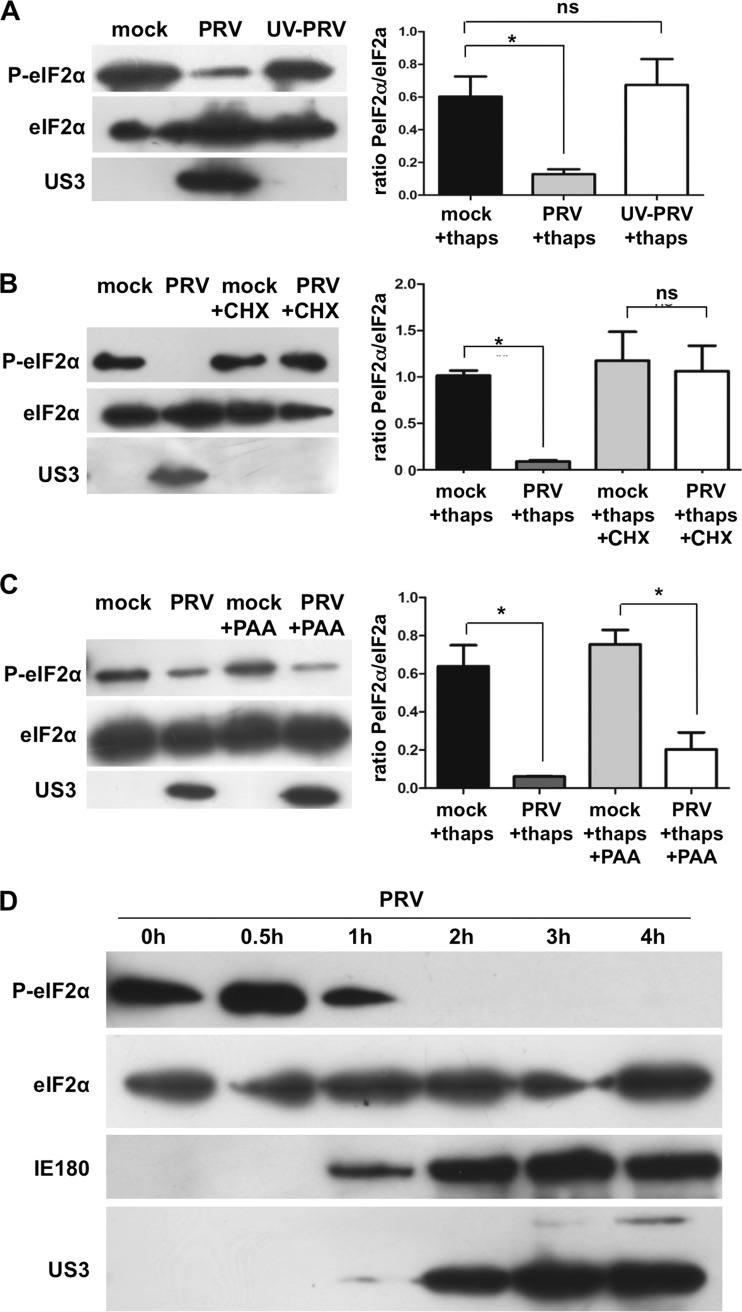
To elucidate which viral protein(s) is responsible for interference with eIF2α phosphorylation in PRV-infected cells, we first determined at which stage of infection interference with eIF2α phosphorylation occurs. To investigate whether de novo viral protein synthesis is required, thapsigargin-stimulated cells either were treated with cycloheximide (CHX), a translation inhibitor, before and during virus inoculation or were inoculated with UV-inactivated PRV. Both in PRV-infected cells treated with CHX and in cells inoculated with UV-inactivated PRV, the level of phosphorylation of eIF2α was comparable to that of mock-infected cells, indicating that viral protein synthesis is required for interference with eIF2α phosphorylation (Fig. 2A and B). Phosphonoacetic acid (PAA) inhibits viral replication and thus late viral protein expression. PAA did not affect PRV-mediated interference with eIF2α phosphorylation (Fig. 2C), implicating that one or more (immediate) early viral proteins are involved.
Fig 2.
An immediate-early or early protein is involved in PRV-mediated suppression of eIF2α phosphorylation. (A, B, C) Differentiated 50B11 cells were pretreated with thapsigargin (1 μM for 1 h). Subsequently, cells were mock inoculated or inoculated with UV-inactivated PRV (theoretical MOI, 1) (A) or treated with cycloheximide (10 μg/μl) (B) or PAA (250 μg/ml) (C) prior to inoculation with PRV (MOI, 1). Lysates were collected at 4 hpi, and Western blot detection was performed for total and phosphorylated eIF2α. US3 detection was included as a control for treatment and infection. Graphs show ratios of PeIF2α/total eIF2α from three independent replicates. Asterisks indicate significant differences (P < 0.05). (D) Differentiated 50B11 cells were pretreated with thapsigargin (1 μM for 1 h). Subsequently, cells were inoculated with PRV (MOI, 1). Lysates were collected at different time points postinoculation, and Western blot detection was performed for total and phosphorylated eIF2α, the immediate-early protein IE180, and the early protein US3.
In order to confirm these findings, a time course experiment of PRV infection was performed to determine the time point of suppression of eIF2α phosphorylation. Figure 2D shows that a reduction in phosphorylation of eIF2α is already evident at 1 hpi. From 2 hpi onwards, phosphorylation levels were very strongly reduced. Expression of the PRV immediate-early protein IE180 was first detected at 1 hpi, while substantial expression of the early viral protein US3 was detected from 2 hpi onwards. These data indicate that PRV interference with eIF2α phosphorylation occurs very early in infection and involves the expression of one or more immediate-early or early viral proteins.
Expression of IE180 interferes with eIF2α phosphorylation.
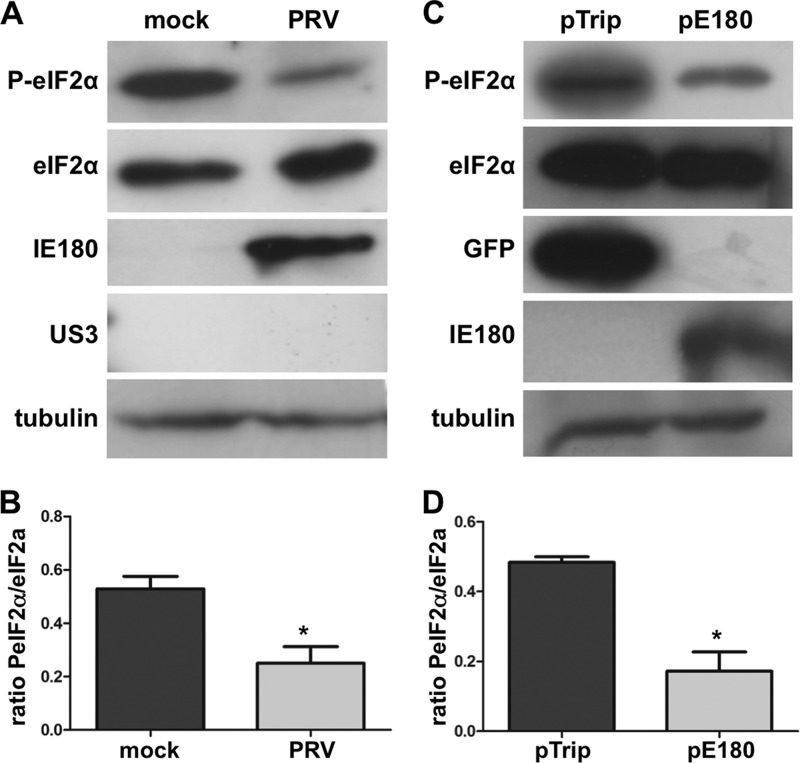
PRV encodes only one protein with immediate-early kinetics, immediate-early protein 180 (IE180), an orthologue of HSV-1 ICP4. To investigate the possible involvement of IE180, first, a sequential treatment of CHX and actinomycin D (ActD) was used. This treatment results in protein expression of viral genes with immediate-early characteristics (transcribed without de novo viral protein synthesis) (2, 9, 15). In the case of PRV, this results in expression of IE180, the only viral gene product with immediate-early characteristics (2). As shown in Fig. 3A and B, phosphorylation of eIF2α is significantly reduced under these circumstances, indicating that the expression of IE180 interferes with eIF2α phosphorylation. A time course analysis of PRV-infected cells treated with CHX alone or sequentially with CHX and ActD confirmed these findings (data not shown).
Fig 3.
Expression of IE180 causes suppression of eIF2α phosphorylation. (A, B) Differentiated 50B11 cells were pretreated with thapsigargin (1 μM for 1 h). Subsequently, cells were treated with cycloheximide (10 μg/μl) prior to mock inoculation or inoculation with PRV (MOI, 1). At 2 hpi, CHX was washed away, and actinomycin D (5 μg/ml) was added to the cells for the remainder of the experiment. At 4 hpi, lysates were collected and Western blot detection was performed for total and phosphorylated eIF2α. IE180 and US3 were included as controls for treatment, and tubulin detection was included as loading control. (C, D) Differentiated 50B11 cells were transfected with a eukaryotic expression vector encoding IE180 (pIE180) or eGFP (pTrip) for 24 h. Upon stimulation with thapsigargin (1 μM for 1 h), lysates were collected and Western blot detection was performed for total and phosphorylated eIF2α. GFP and IE180 were included as transfection controls, and tubulin detection was included as loading control. Panels B and D show ratios of P-eIF2α/total eIF2α from three independent replicates. Asterisks indicate statistically significant differences (P < 0.05).
To confirm a potential involvement of IE180, phosphorylation of eIF2α was assessed in 50B11 cells transfected with a eukaryotic expression vector encoding either IE180 or (as a control) eGFP. Fig. 3C and D show that in samples containing IE180-transfected cells phosphorylation of eIF2α was significantly diminished. The degree of reduction corresponded well with the transfection efficiency of ∼40%. In conclusion, expression of IE180 protein is sufficient to interfere with phosphorylation of eIF2α.
PRV IE180-mediated suppression of eIF2α phosphorylation also occurs in ST cells.
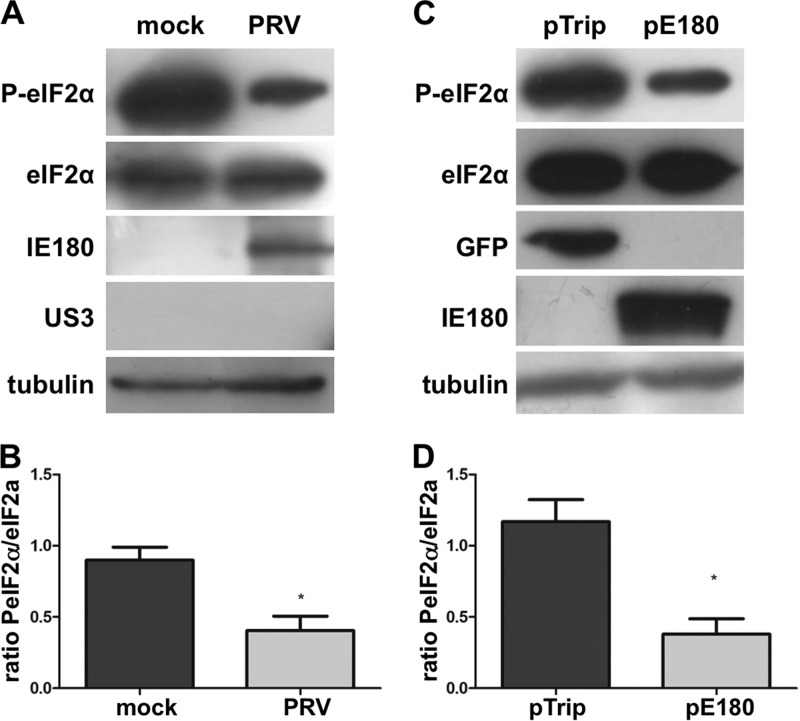
To determine whether our observations hold true in other cells, particularly swine cells, key experiments were repeated in ST cells. A time course infection experiment showed rapid suppression of eIF2α phosphorylation in ST cells, within 2 hpi, similar to what was observed in 50B11 cells (data not shown). Also, like in 50B11 cells (Fig. 3A), subjection of ST cells to a sequential treatment with CHX and ActD that leads to IE180 protein expression during PRV infection again resulted in a substantial reduction in eIF2α phosphorylation (Fig. 4A). In addition, like in 50B11 cells (Fig. 3B), transfection of IE180 in ST cells resulted in substantial interference with eIF2α phosphorylation that corresponded well with the ∼30% transfection efficiency (Fig. 4B).
Fig 4.
PRV IE180-mediated suppression of eIF2α phosphorylation in ST cells. (A, B) ST cells were pretreated with thapsigargin (1 μM for 1 h). Subsequently, cells were treated with cycloheximide (10 μg/μl) prior to mock inoculation or inoculation with PRV (MOI, 1). At 2 hpi CHX was washed away, and actinomycin D (5 μg/ml) was added to the cells for the remainder of the experiment. At 4 hpi, lysates were collected and Western blot detection was performed for total and phosphorylated eIF2α. IE180 and US3 were included as controls for treatment, and tubulin detection was included as a loading control. (C, D) ST cells were transfected with a eukaryotic expression vector encoding IE180 (pIE180) or eGFP (pTrip) for 24 h. Upon stimulation with thapsigargin (1 μM for 1 h), lysates were collected and Western blot detection was performed for total and phosphorylated eIF2α. GFP and IE180 were included as transfection controls, and tubulin detection was used as loading control. Panels B and D show ratios of P-eIF2α/total eIF2α from three independent replicates. Asterisks indicate statistically significant differences (P < 0.05).
Protein phosphatase 1 but not protein phosphatase 2 is involved in PRV-mediated interference with eIF2α phosphorylation.
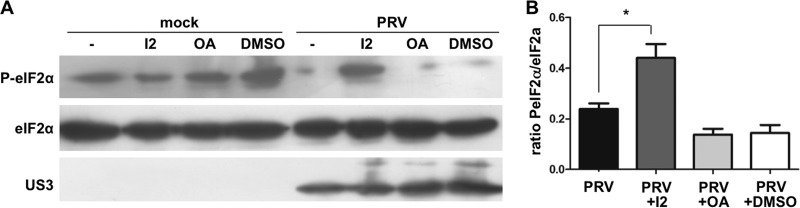
As described above, the observations that PRV suppresses both basal and thapsigargin-preinduced levels of eIF2α phosphorylation and does not affect thapsigargin-induced phosphorylation of PERK (Fig. 1) suggest that PRV leads to dephosphorylation of eIF2α rather than preventing phosphorylation of eIF2α. This would imply the involvement of a phosphatase. PRV does not encode a viral phosphatase, suggesting that a cellular phosphatase may be recruited or induced by PRV to induce dephosphorylation of eIF2α. Both protein phosphatase 1 (PP1) and protein phosphatase 2 (PP2A) have been reported to dephosphorylate eIF2α (12). To investigate whether PP1 and/or PP2A are involved in PRV-mediated interference with eIF2α phosphorylation, assays were done in the presence of 0.2 μM I2 to inhibit PP1 activity or 20 nM OA to inhibit PP2 activity. Treatment with I2 significantly counteracted PRV-mediated interference with eIF2α phosphorylation, while treatment with OA had no effect (Fig. 5A and B). These results indicate that PP1 is involved in PRV-mediated dephosphorylation of eIF2α.
Fig 5.
Protein phosphatase PP1 but not PP2 is involved in PRV-mediated suppression of eIF2α phosphorylation. (A) 50B11 cells were pretreated with thapsigargin (1 μM for 1 h). Prior to and during inoculation with PRV (MOI, 1), cells were treated with PP1-specific inhibitor I2 (200 nM), PP2-inhibiting okadaic acid (OA, 20 nM), or a dimethyl sulfoxide (DMSO) control. At 4 hpi, lysates were collected and Western blotting was performed for total and phosphorylated eIF2α. US3 detection was included as a control for infection. Panel B shows ratios of P-eIF2α/total eIF2α from three independent replicates. The asterisk indicates a statistically significant difference (P < 0.05).
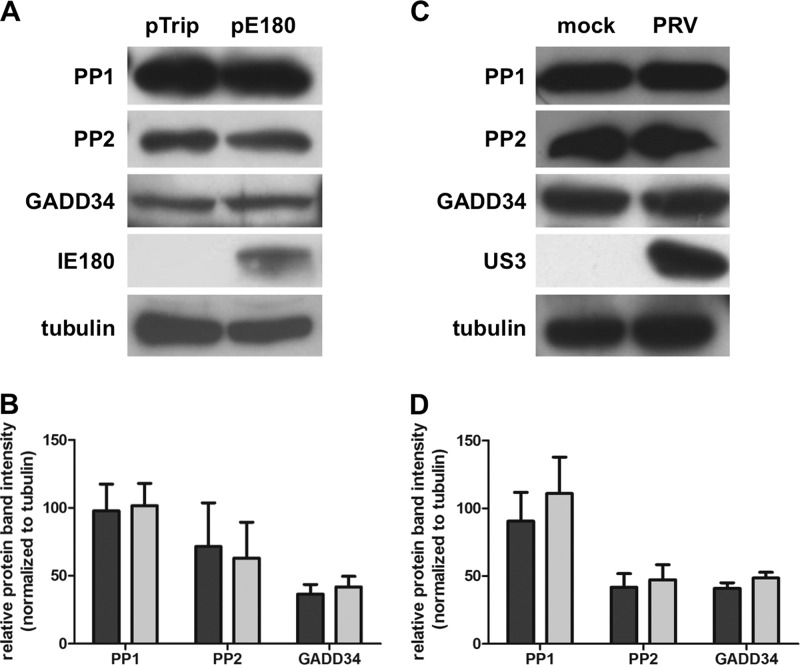
PRV infection or IE180 transfection does not affect PP1 or GADD34 protein expression levels.
Since IE180 is a transcriptional transactivator, one possibility could be that IE180 increases expression levels of PP1 or its regulatory complex partner GADD34, thereby promoting dephosphorylation of eIF2α. However, as shown in Fig. 6, neither PRV infection nor IE180 transfection affected PP1, PP2A, or GADD34 protein levels.
Fig 6.
PRV infection or IE180 transfection does not affect protein expression levels of PP1, PP2, or GADD34. (A, B) Differentiated 50B11 cells were transfected with pIE180 or the control plasmid pTrip for 24 h and subsequently pretreated with thapsigargin (1 μM for 1 h). Lysates were collected and subjected to Western blot detection of PP1, PP2, and GADD34. IE180 and tubulin were included as controls. (C, D) Differentiated 50B11 cells were pretreated with thapsigargin (1 μM for 1 h) and subsequently mock inoculated or subjected to inoculation with PRV (MOI, 1). Lysates were collected at 4 hpi, and Western blot detection was performed for PP1, PP2, and GADD34. US3 and tubulin were included as controls. Graphs show relative protein band intensities normalized to tubulin from three independent replicates. Dark gray bars represent data from control plasmid-transfected (B) or mock-infected (D) cells, and light gray bars represent data from IE180-transfected (B) or PRV-infected (D) cells.
In addition, we found that an N-terminally His-tagged version of IE180 that retains its transcriptional transactivating activity lost its ability to suppress phosphorylation of eIF2α (data not shown).
These findings indicate that IE180 does not alter the protein expression levels of PP1 or GADD34 to initiate dephosphorylation of eIF2α and that the transcriptional transactivating activity of IE180 is not sufficient to suppress phosphorylation of eIF2α.
DISCUSSION
Here, we show that the PRV immediate-early protein IE180 interferes with phosphorylation of eIF2α. We also show that the cellular phosphatase PP1 is involved in PRV-mediated interference with eIF2α phosphorylation.
PRV IE180 is the orthologue of HSV-1 ICP4 and varicella-zoster virus (VZV) IE62. Before the current report, three alphaherpesvirus proteins had been reported to interfere with phosphorylation of eIF2α: ICP34.5 and US11 of HSV-1 (neither of which have orthologues in PRV) and IE63 of VZV (the putative homolog/orthologue of HSV-1 ICP22 and PRV RSp40/ICP22). ICP34.5 and US11 of HSV-1 counteract phosphorylation of eIF2α via different mechanisms. US11 binds to PKR, preventing its activation and thus phosphorylation, while ICP34.5 recruits PP1 to eIF2α to mediate its dephosphorylation (5, 16, 18). The mechanism underlying VZV immediate-early protein IE63-mediated dephosphorylation of eIF2α is unknown (1).
Our finding that the only immediate-early protein encoded by PRV, IE180, interferes with phosphorylation of eIF2α ensures that phosphorylation of eIF2α is rapidly suppressed upon infection. VZV also interferes with phosphorylation through a protein with immediate-early kinetics, IE63 (the putative homolog/orthologue of HSV-1 ICP22 and PRV RSp40/ICP22) (1). Perhaps surprisingly, both HSV-1 proteins that interfere with eIF2α phosphorylation, ICP34.5 and US11, are reported to be late proteins (3, 5, 16). However, at least for ICP34.5, this late expression is leaky, and small amounts of ICP34.5 protein can already be detected 3 h postinoculation (19, 22). These small amounts of ICP34.5 early in infection are sufficient to generate an effect on eIF2α phosphorylation (22).
The overall amino acid homology between IE180 orthologues is relatively low, ranging from 32% to 40% in HSV ICP4, VZV IE62, and bovine and equine ICP4. However, ClustalW analysis showed that amino acid homology is condensed in 4 highly conserved regions over the different alphaherpesviruses. Hence, it will be interesting to investigate whether other IE180 orthologues affect eIF2α phosphorylation and, if so, to analyze the involvement of these four conserved domains.
How does IE180 affect phosphorylation levels of eIF2α? IE180 acts as a transcriptional transactivator to control the expression of different genes. From this viewpoint, one potential explanation could be that IE180 affects gene expression of cellular factors that influence the phosphorylation status of eIF2α. Although we provide evidence that the cellular phosphatase PP1 is involved in IE180-mediated dephosphorylation of eIF2α, IE180 did not affect protein expression levels of either PP1 or its regulatory complex partner GADD34. In addition, we found that an N-terminally His-tagged version of IE180 that retains transcriptional transactivating activity lost its ability to suppress phosphorylation of eIF2α. Together with our observation that the decrease of eIF2α phosphorylation is already evident at 1 hpi, this may point toward an interaction of IE180 with components that mediate PP1-dependent dephosphorylation of eIF2α. Such putative interaction of IE180 with cellular components affecting eIF2α phosphorylation would be in line with mechanisms employed by various other viruses. For example, ICP34.5 of HSV-1 displays sequence homology to both PP1 and eIF2α. This allows ICP34.5 to serve as a molecular bridge, thereby mediating eIF2α dephosphorylation (16–18).
In conclusion, we report that the PRV immediate-early protein IE180 has the previously uncharacterized ability to suppress phosphorylation of eIF2α, which may have important consequences for the virus during early stages of virus replication, in the face of an active innate immune response.
ACKNOWLEDGMENTS
N.V.O. is supported by a Ph.D. grant from the Agency for Innovation by Science and Technology (I.W.T.-Vlaanderen), C.V.D.B. is supported by a post-doc grant of the F.W.O.-Vlaanderen, and research described in the manuscript was supported by a grant of the F.W.O.-Vlaanderen (G.0835.09) to H.W.F.
We thank C. Van Waesberghe for technical assistance. We thank L. Enquist for the mouse monoclonal anti-US3 antibodies and the PRV strain Becker.
Footnotes
Published ahead of print 24 April 2012
REFERENCES
- 1. Ambagala AP, Cohen JI. 2007. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J. Virol. 81:7844–7851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambagala AP, Hinkley S, Srikumaran S. 2000. An early pseudorabies virus protein down-regulates porcine MHC class I expression by inhibition of transporter associated with antigen processing (TAP). J. Immunol. 164:93–99 [DOI] [PubMed] [Google Scholar]
- 3. Bryant KF, et al. 2008. ICP34.5-dependent and -independent activities of salubrinal in herpes simplex virus-1 infected cells. Virology 379:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Card JP, et al. 1990. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J. Neurosci. 10:1974–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassady KA, Gross M. 2002. The herpes simplex virus type 1 U(S)11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cassady KA, Gross M, Roizman B. 1998. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W, Mi R, Haughey N, Oz M, Hoke A. 2007. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Peripher. Nerv. Syst. 12:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou J, Chen JJ, Gross M, Roizman B. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5-mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 92:10516–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. 1995. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J. Virol. 69:3240–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. 2007. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res. 128:65–70 [DOI] [PubMed] [Google Scholar]
- 11. Deruelle MJ, Van den Broeke C, Nauwynck HJ, Mettenleiter TC, Favoreel HW. 2009. Pseudorabies virus US3- and UL49.5-dependent and -independent downregulation of MHC I cell surface expression in different cell types. Virology 395:172–181 [DOI] [PubMed] [Google Scholar]
- 12. Ernst V, Levin DH, Foulkes JG, London IM. 1982. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc. Natl. Acad. Sci. U. S. A. 79:7092–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez-Sebastian S, Tabares E. 2004. Negative regulation of herpes simplex virus type 1 ICP4 promoter by IE180 protein of pseudorabies virus. J. Gen. Virol. 85:2125–2130 [DOI] [PubMed] [Google Scholar]
- 14. Goodbourn S, Didcock L, Randall RE. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341–2364 [DOI] [PubMed] [Google Scholar]
- 15. Hayes MK, Rock DL. 1990. Identification of a novel bovine herpesvirus type 1 immediate-early infected cell protein. Arch. Virol. 112:291–300 [DOI] [PubMed] [Google Scholar]
- 16. He B, Gross M, Roizman B. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 94:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He B, Gross M, Roizman B. 1998. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737–20743 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, et al. 2011. ICP34.5 Protein of herpes simplex virus facilitates the initiation of protein translation by bridging eukaryotic initiation factor 2{alpha} (eIF2{alpha}) and protein phosphatase 1. J. Biol. Chem. 286:24785–24792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKay EM, McVey B, Marsden HS, Brown SM, MacLean AR. 1993. The herpes simplex virus type 1 strain 17 open reading frame RL1 encodes a polypeptide of apparent Mr 37K equivalent to ICP34.5 of herpes simplex virus type 1 strain F. J. Gen. Virol. 74:2493–2497 [DOI] [PubMed] [Google Scholar]
- 20. Mulvey M, Poppers J, Ladd A, Mohr I. 1999. A herpesvirus ribosome-associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73:3375–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munoz AL, Torres M, Martin B, Lerma L, Tabares E. 2010. Regulation of pseudorabies virus gG glycoprotein gene promoter independently of pseudorabies immediate early IE180 protein. Arch. Virol. 155:515–523 [DOI] [PubMed] [Google Scholar]
- 22. Pasieka TJ, et al. 2006. Functional genomic analysis of herpes simplex virus type 1 counteraction of the host innate response. J. Virol. 80:7600–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider RJ, Mohr I. 2003. Translation initiation and viral tricks. Trends Biochem. Sci. 28:130–136 [DOI] [PubMed] [Google Scholar]
- 24. Van Opdenbosch N, De Regge N, Van Poucke M, Peelman L, Favoreel HW. 2011. Effects of interferon on immediate-early mRNA and protein levels in sensory neuronal cells infected with herpes simplex virus type 1 or pseudorabies virus. Vet. Microbiol. 152:401–406 [DOI] [PubMed] [Google Scholar]
- 25. Wang X, et al. 2009. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J. Virol. 83:12462–12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong WL, Brostrom MA, Kuznetsov G, Gmitter-Yellen D, Brostrom CO. 1993. Inhibition of protein synthesis and early protein processing by thapsigargin in cultured cells. Biochem. J. 289:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]