Abstract
The influenza virus H1N1 pandemic of 1918 was one of the worst medical catastrophes in human history. Recent studies have demonstrated that the hemagglutinin (HA) protein of the 1918 virus and 2009 H1N1 pandemic virus [A(H1N1)pdm09], the latter now a component of the seasonal trivalent inactivated influenza vaccine (TIV), share cross-reactive antigenic determinants. In this study, we demonstrate that immunization with the 2010-2011 seasonal TIV induces neutralizing antibodies that cross-react with the reconstructed 1918 pandemic virus in ferrets. TIV-immunized ferrets subsequently challenged with the 1918 virus displayed significant reductions in fever, weight loss, and virus shedding compared to these parameters in nonimmune control ferrets. Seasonal TIV was also effective in protecting against the lung infection and severe lung pathology associated with 1918 virus infection. Our data demonstrate that prior immunization with contemporary TIV provides cross-protection against the 1918 virus in ferrets. These findings suggest that exposure to A(H1N1)pdm09 through immunization may provide protection against the reconstructed 1918 virus which, as a select agent, is considered to pose both biosafety and biosecurity threats.
INTRODUCTION
The Spanish influenza pandemic of 1918 resulted in an estimated 20 to 50 million deaths worldwide and a 10-year reduction in the average life expectancy (13). During that year in the United States, outbreaks of respiratory disease occurred simultaneously in humans and swine; whether the H1N1 virus jumped species from humans to pigs or vice versa remains unclear. Recently, it has been suggested that the 1918 pandemic virus was derived from an influenza virus of swine origin and that the precursor of this virus was a descendant of a distinct avian H1N1 virus (1). The H1N1 virus became established in domestic pigs after 1918, forming the classical swine H1N1 lineage, and continued to circulate as the dominant influenza virus in North American swine populations until 1998 (7, 29). Although the human 1918 and swine H1N1 viruses evolved separately in different hosts and thus diverged genetically (32, 33), the hemagglutinin (HA) of the H1N1 viruses maintained significant antigenic similarities (40, 42).
In April 2009, a novel influenza A H1N1 virus [A(H1N1)pdm09] was first detected in humans and spread throughout the world to cause the first influenza pandemic of the 21st century. This pandemic virus of apparent swine origin was derived from a reassortment in nature of avian, human, and swine influenza A viruses and contains an HA gene belonging to the classical swine H1N1 lineage (12). During the subsequent 2009-2010 and 2010-2011 winter seasons, the 2009 H1N1 subtype virus continued to circulate throughout the world and appears to have replaced the seasonal H1N1 virus (30). In September 2009, the Food and Drug Administration (FDA) licensed the first A(H1N1)pdm09 vaccine, available as a monovalent vaccine used during the 2009-2010 season. Subsequently, the WHO recommended that vaccine strains for the 2010-2011 trivalent influenza vaccine contain a pandemic 2009 H1N1 (A/California/7/2009-like) component (8). For the inactivated trivalent influenza vaccine (hereinafter referred to as TIV), each 0.5-ml dose of TIV contains 15 μg of influenza HA of each strain (45 μg total) from three viral strains: two influenza A virus subtypes (H1N1 and H3N2) and one influenza B virus strain. The seasonal inactivated TIV is the most commonly administered influenza vaccine, and each year hundreds of millions of individuals are vaccinated worldwide (20, 21).
Several serological studies of the A(H1N1)pdm09 virus have provided evidence for the presence of preexisting cross-reactive antibodies to a 1918-like H1N1 virus from previous vaccinations or infections (15, 18, 26). Using stored serum samples from trials of seasonal TIV predating the 2009 pandemic, Hancock et al. showed that vaccination with contemporary seasonal influenza vaccines containing former seasonal H1N1 viruses provided little cross-reactive immunity to the A(H1N1)pdm09 virus; however, subjects who were born before 1930 (and therefore were probably exposed to a 1918-like H1N1 virus) possessed cross-reactive antibodies to the A(H1N1)pdm09 virus (15). Thus, despite more than 90 years of separation between the pandemic viruses, the 1918 and A(H1N1)pdm09 viruses induced antibodies that demonstrated cross-neutralization. Moreover, immunization of mice with a 1918 virus vaccine conferred protection against the A(H1N1)pdm09 virus, documenting cross-protection in vivo (25, 42). Conversely, using the mouse model, a recent study addressed the impact of A(H1N1)pdm09 influenza monovalent vaccination on 1918 virus protection (26). However, this study did not test the efficacy of contemporary seasonal TIV against 1918 virus challenge in ferrets.
Ferrets are considered to be the most suitable animal model for influenza vaccine efficacy studies (5, 17, 36), and this animal model has been used to demonstrate the inherent virulence of the 1918 influenza viruses in comparison to that of other influenza viruses (41). Here, we demonstrate that vaccination with the seasonal 2010-2011 TIV induced neutralizing antibodies to the 1918 virus and that immune ferrets displayed a significant reduction in viral shedding and fever following 1918 virus challenge. Influenza vaccination with TIV also prevented significant virus replication in the lung and trachea tissues of ferrets on subsequent challenge, further demonstrating that the current influenza vaccine would provide protection against the 1918 pandemic virus. These findings have implications for biosafety and biosecurity precautions that are designed to protect laboratorians and the public from possible exposure to this virus.
MATERIALS AND METHODS
Viruses.
Stocks for the H1N1 influenza viruses used in the study, A/Mexico/4482/2009 (Mex/4482) and the rescued 1918 virus, were propagated in Madin-Darby canine kidney (MDCK) cells as previously described (39, 43). Mex/4482 has been found to be antigenically and genetically similar to the prototype A(H1N1)pdm09 vaccine virus, A/California/7/2009 (12). The seasonal human H3N2 virus, A/Perth/16/2009 (Perth/16), and the influenza B virus, B/Brisbane/60/2008 (B/Brisbane/60), were grown in the allantoic cavities of 10-day-old embryonating hens' eggs at 34°C for 48 to 72 h. Pooled cell supernatant or allantoic fluid was clarified by centrifugation and stored at −70°C. All stocks were titrated in a standard plaque assay and expressed as PFU (43). All experiments were performed in biosafety level 3 laboratories with enhancements (BSL3+) as outlined in Biosafety in Microbiological and Biomedical Laboratories (9). Animal research was conducted under the guidance of the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility.
Vaccination and challenge.
Adult male Fitch ferrets, 5 to 12 months of age (Triple F Farms, Sayre, PA), serologically negative by hemagglutination inhibition (HI) assay for currently circulating influenza viruses, were used in this study. For vaccinations, ferrets were each injected 3 times (4 to 5 weeks between injections) intramuscularly (i.m.) with an adult-human dose (0.5 ml) of the 2010-2011 seasonal inactivated split-product TIV or phosphate-buffered saline (PBS) (controls). To determine viral replication in nasal washes, as well as clinical signs of infection, ferrets were randomly assigned to be challenged with 1918 virus (5 TIV inoculated and 5 PBS controls), Perth/16 virus (3 TIV inoculated and 4 PBS controls), or Mex/4482 (3 TIV inoculated and 4 PBS controls). In a second experiment (experiment 2) to study the effect of vaccination on virus replication in respiratory tissues and lung histopathology, ferrets were TIV inoculated (n = 6) or given PBS (n = 6) on the schedule detailed above.
Prior to initial vaccination, vaccine boosts, and viral challenge, all ferrets were bled for collection of serum. Following anesthesia with an i.m. injection of a ketamine-xylazine-atropine cocktail, ferrets were challenged intranasally with 106 PFU of virus in a total volume of 1 ml (500 μl per nostril) diluted in PBS (31). Challenge with 1918, Mex/4482, or Perth/16 virus occurred 5 to 8 weeks after final vaccine boost. Following challenge, ferrets were monitored daily for changes in body weight and temperature, as well as clinical signs of illness (24). The statistical significance of differences in weight loss and virus titers (nasal washes and tissues) between vaccinated and control animals was determined by Student's t test.
Histopathology.
Necropsies were performed on three ferrets from each group at three and 5 days postinoculation (d.p.i.). Three 5-mm lung sections of each left lung lobe were collected for histopathology. Lung sections were fixed in 10% buffered formalin, processed routinely, and embedded in paraffin. Duplicate 5-μm sections were immunohistochemically (IHC) stained to demonstrate influenza A nucleoprotein (NP) by first microwaving the sections in Citra antigen retrieval solution (Biogenex, San Ramon, CA) for antigen exposure. A monoclonal antibody (P13C11) specific for type A influenza virus NP was used as the primary antibody for a streptavidin-biotin-alkaline phosphatase complex-based IHC method as previously described (35, 38). Only cells with red-staining nuclei were considered positive with the IHC assay.
Antibody assays.
All sera were initially diluted 1:10 in receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan) and tested for neutralizing antibody titers against 1918, Mex/4482, Perth/16, or B/Brisbane/60 virus as described previously (27). Titers were calculated as the reciprocal of the highest dilution of serum that neutralized 100 PFU of virus in MDCK cell cultures. The neutralization titers are also presented as the geometric mean titers (GMT) from vaccinated or control ferrets.
RESULTS
Immunogenicity of 2010-2011 seasonal inactivated TIV in ferrets.
We first assessed the level of cross-reactive antibody to 1918 (H1N1) influenza virus following vaccination with the 2010-2011 seasonal TIV. Prior to vaccine boost and viral challenge, ferret sera were collected to assess neutralization antibody responses against the 1918 virus and against the three homologous viruses in the 2010-2011 TIV. As shown in Table 1 (experiment 1), a single dose of vaccine did not induce detectable neutralizing antibody titers to 1918, seasonal H3N2 (Perth/16), or H1N1 (Mex/4482) virus. Neutralizing antibody titers to influenza B (B/Brisbane/60) virus were detected that ranged from 20 to 80 (geometric mean titer [GMT] = 40). Following vaccine boost, TIV elicited low neutralizing antibody (<10 to 40) titers to 1918, Perth/16, and Mex/4482 virus, whereas a single vaccine boost led to substantial increases in antibody titers (160 to 320) to B/Brisbane/60 virus (GMT = 211). A second vaccine boost was required to obtain detectable homologous antibody levels in all ferrets, measured 3 days before challenge. The prechallenge neutralizing titers were highest against B/Brisbane/60 virus (GMT = 485), followed by Mex/4482 virus (GMT = 121) and then Perth/16 (GMT = 35) virus. Neutralizing activity to 1918 virus was also detected, at titers that ranged from 10 to 40 (GMT = 23). As expected, all sera from PBS control or prevaccinated ferrets failed to neutralize any of the viruses in the test.
Table 1.
Serum antibody neutralizing responses following seasonal TIV administration in ferrets
| Group | Neutralizing antibody titer (GMT)a |
|||
|---|---|---|---|---|
| A/Perth/16/2009 | A/Mexico/4482/2009 | B/Brisbane/60/2008 | 1918 | |
| Experiment 1 | ||||
| Prevaccination | <10 | <10 | <10 | <10 |
| Pre-1st booster vaccination | <10 | <10 | 20–80 (40) | <10 |
| Pre-2nd booster vaccination | 10–20 (11) | 10–40 (17) | 160–320 (211) | <10–10 (8) |
| Prechallenge | 20–80 (35) | 40–320 (121) | 160–640 (485) | 10–40 (23) |
| Experiment 2 | ||||
| Prevaccination | <10 | <10 | <10 | <10 |
| Pre-1st booster vaccination | <10 | <10–20 (5.9) | 20–80 (38) | <10 |
| Pre-2nd booster vaccination | ND | ND | ND | ND |
| Prechallenge | 20–160 (47) | 40–320 (136) | 40–320 (143) | 20–80 (28) |
Serum neutralizing antibody responses following primary and booster immunizations of 2010-2011 seasonal TIV are shown. ND, not done.
In a second vaccine experiment aiming to evaluate TIV-induced protection against viral replication in respiratory tract tissues, preboost and prechallenge ferret neutralizing titers against 1918 virus were determined (Table 1, experiment 2). Similar to the antibody responses in the first experiment, a single immunization with 2010-2011 seasonal TIV failed to elicit a significant antibody response to 1918, H3N2 (Perth/16), or Mex/4482 (H1N1) virus. Again, a second vaccine boost elicited measurable homologous antibody levels in all ferrets. The prechallenge neutralizing antibody titers ranged from 20 to 160 (GMT = 47) against Perth/16 virus and from 40 to 320 (GMT = 136) to Mex/4482 virus. The prechallenge virus neutralizing antibody titers to B/Brisbane/60 ranged from 40 to 320 (GMT = 143). Importantly, neutralizing activity to 1918 virus that ranged from 20 to 80 (GMT = 28) was detected in all ferrets (Table 1, experiment 2). Overall, these data indicate that the licensed vaccine for seasonal 2010-2011 influenza elicits neutralizing antibodies to the 1918 pandemic virus.
Protective efficacy of seasonal TIV to homologous virus challenge.
We first determined the level of protection against homologous virus challenge induced by seasonal 2010-2011 TIV. Vaccine protection was measured by reductions in fever, weight loss, and viral shedding in ferrets after challenge with Perth/16 or Mex/4482 virus. Following either virus challenge, no significant differences in body temperatures were detected between TIV-immunized and unimmunized control ferrets observed for 14 days postchallenge (p.c.). All TIV-immunized ferrets displayed a peak in body temperature on days 1 and 2 p.c., with mean maximum fever of 1.2 or 1.3°C above the baseline (Table 2). Similarly, Perth/16 or Mex/4482 virus challenge induced an increase in temperature among all corresponding control ferrets, with a mean maximum fever of 1.5 or 1.1°C above the baseline, respectively.
Table 2.
Clinical symptoms observed in TIV-immunized ferrets challenged with homologous virus or heterologous 1918 virus
| Group | No. of animals with indicated result following challenge/total no. of animalsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A/Perth/16/2009 (homologous) |
A/Mexico/4482/2009 (homologous) |
1918 (heterologous) |
|||||||
| Wt loss (%) | Fever (°C) | Virus detected (peak titer) | Wt loss (%) | Fever (°C) | Virus detected (peak titer) | Wt loss (%) | Fever (°C) | Virus detected (peak titer) | |
| TIV immunized | 3/3 (4.6) | 3/3 (1.3) | 3/3 (5.2) | 3/3 (6.5) | 3/3 (1.2) | 3/3 (5.2) | 5/5 (2.8) | 5/5 (1.1) | 5/5 (3.7) |
| Mock vaccinated | 4/4 (5.7) | 4/4 (1.5) | 4/4 (5.3) | 4/4 (16.8) | 4/4 (1.1) | 4/4 (4.9) | 5/5 (13.2) | 5/5 (1.4) | 5/5 (5.6) |
Ferrets were vaccinated i.m. three times with the 2010-2011 seasonal trivalent influenza vaccine (TIV) or with PBS. Data show either the number of animals in which weight loss was observed, with mean maximum weight loss percentage in parentheses, the number of animals in which fever was observed, with mean maximum increase over baseline in parentheses, or the number of animals in which virus was detected in nasal wash samples, with mean peak titer (log10 PFU/ml) in parentheses.
Viral challenge with the seasonal Perth/16 virus resulted in minimal morbidity for vaccinated and control ferrets, causing 4.6% and 5.7% maximum weight loss, respectively (Fig. 1A). In contrast, the more pathogenic Mex/4482 virus caused greater morbidity among unvaccinated control ferrets (16.8% mean maximum weight loss) and TIV provided partial protection against weight loss; none of the vaccinated animals lost more than 9.7% of their initial body weight (6.5% mean maximum weight loss) during any point in the study (Fig. 1B and Table 2).
Fig 1.
Protective efficacy of seasonal TIV (VAX) in ferrets following homologous virus challenge. The morbidity of ferrets was determined for 14 days following challenge with seasonal human H3N2 virus A/Perth/16/2009 (Perth/16) (A) or H1N1 virus A/Mexico/4482/2009 (Mex/4482) (B). For each virus challenge, three TIV-inoculated ferrets (open circle) and four PBS control ferrets (filled circle) were challenged with 106 PFU of virus. Differences in weight loss between groups were analyzed by Student's t test (*, P ≤ 0.005). Error bars show standard deviations.
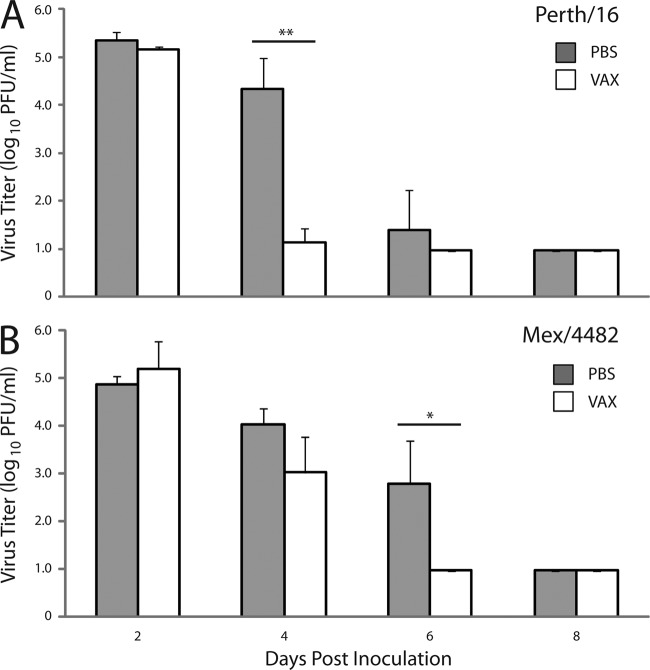
The extent of virus replication in the upper respiratory tract was determined by titrating nasal wash samples collected from immune and control ferrets following challenge with Perth/16 or Mex/4482 virus. In comparison to unimmunized control animals, TIV-immunized ferrets displayed a significant reduction in viral shedding among Perth/16 virus-challenged ferrets on day 4 p.c. (P ≤ 0.0005) and Mex/4482 virus-challenged ferrets on day 6 p.c. (P ≤ 0.020) (Fig. 2). Moreover, TIV-immunized ferrets cleared Perth/16 and Mex/4482 virus 2 days earlier than control ferrets. These data indicate that although TIV does not provide sterilizing immunity against homologous virus challenge, the seasonal influenza vaccine offers some level of protection against viral shedding and against A(H1N1)pdm09-induced severe morbidity. Such data can be compared to the level of cross-protection against heterologous 1918 H1N1 virus challenge.
Fig 2.
Seasonal TIV efficacy against virus replication of A/Perth/16/2009 (Perth/16) or A/Mexico/4482/2009 (Mex/4482) virus. The nasal cavities of ferrets were washed with 1 ml of PBS every other day starting 2 days p.c., and virus titers were determined in a standard plaque assay. Gray-shaded bars represent the results for unimmunized (PBS) control ferrets, and open bars represent the results for TIV-immunized ferrets. The data shown are mean values plus standard deviations (error bars) for 3 or 4 ferrets per group (A and B). Differences in viral titers of nasal washes between groups were analyzed by Student's t test (*, P ≤ 0.020; **, P ≤ 0.0005).
Cross-protective efficacy of 2010-2011 seasonal TIV to 1918 pandemic H1N1 virus.
We next assessed the degree of cross-protection against the 1918 H1N1 virus conferred by seasonal TIV. TIV-immunized and unimmunized control ferrets received a similar high-titer (106 PFU) challenge dose of the reconstructed 1918 virus. On day 1 p.c., all unimmunized control ferrets exhibited an early spike in body temperature, ranging from 1.3 to 1.6°C above the baseline (mean maximum = 1.4°C) (Table 2), and elevated temperatures (>0.8°C above the baseline) were maintained for 7 days p.c. (not shown). TIV-immunized animals also displayed an early spike in body temperature (mean maximum = 1.1°C) but had significantly lower (P ≤ 0.05) body temperatures than controls on days 4 and 6 p.c. Significant differences in weight loss were also observed between the two groups on day 1 p.c. onwards (P ≤ 0.04). Control animals exhibited 13.2% mean maximum weight loss compared with 2.8% weight loss for vaccinated ferrets (Fig. 3A and Table 2).
Fig 3.

Clinical symptoms and viral shedding in TIV-immunized and control ferrets following 1918 virus challenge. (A) Mean weight loss observed among vaccinated (open circles) and PBS control (filled circles) ferrets following challenge with 106 PFU of the 1918 virus. Nasal washes (B) and tissues (C) at 3 (left) and 5 (right) days p.c. were collected and viruses titrated in a standard plaque assay. Gray-shaded bars represent results for unimmunized (PBS) control ferrets, and open bars represent results for TIV-immunized ferrets. The data shown are mean values plus standard deviations (error bars) for 5 ferrets per group (A and B) and for 3 ferrets per group for each time period (C). Differences in viral titers between groups were analyzed by Student's t test (*, P ≤ 0.04; **, P ≤ 0.005; ***, P ≤ 3E-06).
Prior seasonal-TIV administration also reduced shedding of 1918 virus in challenged ferrets. The viral titers measured in nasal washes were significantly reduced (P ≤ 0.04) on days 2, 4, and 6, and vaccinated ferrets exhibited higher rates of virus clearance than controls (Fig. 3B). Taken together, these data suggest that TIV immunization offered significant protection against substantial weight loss and viral shedding following 1918 virus challenge.
The effect of 2010-2011 seasonal-TIV vaccination on virus replication in the respiratory tract and histopathology in lung tissue.
In another experiment, the extent of TIV protection against the 1918 virus was measured in terms of (i) viral titers in the upper (nasal turbinate and trachea) and lower (lung) respiratory tract tissues of individual ferrets on days 3 and 5 p.c. and (ii) lesion formation in lung tissues. Vaccinated and control ferrets were similarly challenged with 106 PFU of the 1918 virus 5 weeks after the second vaccine boost (Table 1, experiment 2). On days 3 and 5 p.c., unimmunized control animals showed high titers (≥4.7 log10 PFU/ml) of replicating virus in each of the three respiratory tract tissues (Fig. 3C). In contrast, the mean tissue titers among vaccinated animals were lower and significant reductions were noted in the tracheal (day 5 p.c., P < 0.005) and lung tissues (days 3 and 5 p.c., P < 0.005). The mean viral titers for tracheal samples on day 5 were 17,000-fold less than those in unimmunized controls, while the mean viral titers in lung tissue on days 3 and 5 p.c. were at least 4,600-fold less than those in unimmunized controls. On day 3 p.c., only trace amounts of infectious virus were found in lung tissue of one of three vaccinated ferrets, and on day 5 p.c., no virus was measured above the limit of detection (1.0 log10 PFU/g).
Lung tissue sections from TIV-immunized and unimmunized control ferrets were also examined for viral antigen and microscopic lesion formation. Following 1918 virus challenge, lung lesions from unimmunized controls resembled lesions previously reported in 1918 virus-infected ferrets (41). The damage affected multiple lung lobes and consisted of moderate to severe necrotizing bronchiolitis and alveolitis with focal areas of acute alveolar necrosis, edema, and hemorrhage. (Fig. 4A to D). At both 3 and 5 days p.c., control lungs displayed mild epithelial necrosis in bronchus submucosal serous glands and moderate to severe bronchitis and bronchiolitis accompanied by mild to moderate peribronchiolar accumulation of mixed inflammatory cells. The lumen of the bronchi and bronchioles contained cellular debris and various amounts of neutrophils and mononuclear cells. Fibrin and edema were also observed within the airways of unimmunized control ferrets. Additionally, mild to severe, focal to diffuse alveolitis and alveolar epithelial necrosis with hemosiderosis, intra-alveolar edema, and hemorrhage were observed, especially at 5 days p.c. Virus antigen was present in epithelial cells and in detached cells in the lumen of bronchi and bronchioles, in submucosal serous glands, in alveolar epithelium, and in alveolar macrophages (Fig. 4A to D).
Fig 4.
Immunohistochemistry of 1918 TIV-immunized and control ferrets following 1918 virus challenge. Photomicrographs of lung tissue sections from control (A to D) and TIV-immunized (E and F) ferrets stained (in red) for the presence of viral antigen 3 and 5 days p.c. Viral antigen was found in bronchiolar epithelial cells and in desquamated cells in the lumen of bronchioles on day 3 p.c. (A and B), in cells of submucosal serous glands of the bronchus on day 5 p.c. (C), and in alveolar cells and macrophages on day 5 p.c. (D). TIV-immunized ferrets challenged with the 1918 virus examined at day 3 p.c. displayed milder lung lesions than controls, with no specific viral antigen staining detected on day 3 p.c. (E) and day 5 p.c. (F).
Two of three TIV-immunized ferrets challenged with the 1918 influenza virus examined at day 3 p.c. displayed milder lung lesions than control lungs, characterized by mild peribronchial and perivascular interstitial pneumonia with focal areas of congestion and edema (Fig. 4E). Thickening of the alveolar epithelium and some hemosiderosis were also observed; however, no specific viral antigen staining was detected in these lung samples (Fig. 4E). The third ferret examined at this same time point displayed bronchopneumonia, with macrophages, neutrophils, and cellular debris present in the airways and multifocal edema and hemorrhage. Viral staining was infrequent in bronchiole and bronchus respiratory epithelium but common in alveolar epithelium and macrophages (not shown). In general, the three vaccinated ferrets examined at day 5 p.c. displayed milder lung disease; some sections showed mild to moderate bronchointerstitial pneumonia with edema and congestion (Fig. 4F). No viral antigen was detected in lung sections in any of the vaccinated animals at this time point (Fig. 4F).
DISCUSSION
Following multiple reassortment events, the A(H1N1)pdm09 virus emerged from North American swine to infect humans and possessed an HA gene belonging to the classical swine lineage (12). The ancestry of the classical swine influenza HA can be traced back to H1N1 viruses that circulated in pigs since 1918. The presence of a swine influenza-like HA in the genome of the A(H1N1)pdm09 virus which has largely remained antigenically intact suggested that seasonal influenza vaccination would provide some level of protection against the 1918 pandemic virus. Using the ferret model, regarded as the most relevant model for influenza vaccine efficacy assessments, we showed that the 2010-2011 seasonal TIV was able to induce neutralizing antibodies that reacted with 1918 virus and provided a significant reduction in morbidity and virus shedding compared to these parameters in 1918-challenged control ferrets. Seasonal TIV was also effective at reducing virus replication and pathology in the lung associated with 1918 virus infection.
Although the prechallenge cross-neutralizing titers against the 1918 virus were generally low (GMT of 23 and 28) relative to homologous titers to seasonal B/Brisbane/60 and Mex/4482 H1N1 viruses (GMT of 121 to 485), the antibody titers to one homologous virus, Perth/16 (H3N2) were also low (GMT of 35 and 47) and in the same titer range as the neutralizing titers generated to the 1918 virus. In general, inactivated influenza vaccines are poorly immunogenic in immunologically naïve animals and appreciable antibody responses are induced only at high vaccine doses or when vaccines are adjuvanted (6, 28). In particular, purified surface antigen preparations of TIV (also referred to as “split” vaccines) are weakly immunogenic in naïve ferrets, often necessitating multiple rounds of vaccine boosting when adjuvants are not used (2, 3, 11). For the current study, ferrets were vaccinated with TIV manufacturer-filled syringes designed for single-dose administration. We chose not to use an adjuvant to vaccinate animals since the standard TIVs against influenza are mostly nonadjuvanted vaccines (21). It is worth noting that adjuvanted vaccines have demonstrated the ability, particularly in immunologically naïve animals, to significantly augment the cross-reactivity of antibodies induced by influenza virus vaccines (23, 37). Thus, it would be of interest to evaluate the potential for adjuvants in TIV formulations to induce greater cross-neutralizing antibody titers against the 1918 virus than were shown in this study for the group that received TIV alone. Although the precise immunological mechanism conferring cross-protection was not addressed in the current study, the generation of TIV-induced neutralizing antibodies against the 1918 virus most certainly played a prominent role in decreasing viral shedding and replication in trachea and lung tissues. TIV generally does not effectively induce mucosal immune responses or influenza-specific CD8+ T cells, two immune effectors capable of protecting the host (10, 14, 16, 19).
The antigenic similarities between the 1918 and A(H1N1)pdm09 viruses are supported by the results of passive transfer experiments in which 1918 HA-specific monoclonal antibodies administered to mice protected them against the A(H1N1)pdm09 virus (25). Taking an opposite experimental approach, Medina and colleagues showed that human A(H1N1)pdm09-positive sera was sufficient to protect mice from 1918 lethal challenge (26). The same study demonstrated that a partially purified whole-virus vaccine preparation from a recombinant influenza virus carrying the HA gene from A/California/04/2009 virus provided protection against 1918 virus challenge in mice. Conversely, immunization with an inactivated 1918 virus vaccine but not a former seasonal H1N1 influenza vaccine conferred protection against the A(H1N1)pdm09 virus in mice, further demonstrating the antigenic similarity between these viruses (34, 42).
The A(H1N1)pdm09 virus is now part of seasonal influenza virus circulation and is a component of the seasonal trivalent vaccine. The 2009 H1N1 antigen became one of three components in the 2010-2011 seasonal TIV and is the same vaccine virus as was used in the 2009 H1N1 monovalent vaccine. Moreover, there was no change in vaccine components for the 2011-2012 Northern Hemisphere TIV. The current worldwide production capacity for trivalent influenza vaccine is approximately 300 million doses per year (20, 21). Among persons 6 months of age or older, an estimated 130.9 million people, or 43% of the U.S. population, received a seasonal influenza vaccine during August 2010 through May 2011 (http://www.cdc.gov/flu/professionals/vaccination/vaccinecoverage.htm). The results of our study suggest that individuals who receive the contemporary seasonal influenza vaccine, which contains the A(H1N1)pdm09 virus component, may develop neutralizing antibodies that cross-react against the 1918 virus. Indeed, it was demonstrated that the 2009 H1N1 monovalent vaccine induced cross-reactive antibodies to the 1918 virus among adults ≥18 years enrolled in an A(H1N1)pdm09 influenza vaccine trial (26). Furthermore, human serology studies showed a high prevalence of A(H1N1)pdm09 cross-reactive antibodies in people born before 1930 due to probable exposure to 1918 or 1918-like H1N1 virus (15, 25). This correlated with a reduced frequency of hospitalization during the 2009 pandemic among individuals aged ≥65 years when compared with the risks for complications and hospitalizations from seasonal influenza virus infection (4, 22). A recent study by Reed et al. (C. Reed, J. M. Katz, K. Hancock, A. Balish, J. Singleton, and A. M. Fry for the H1N1 Serosurvey Working Group, unpublished data) estimated that by December 2009, approximately 20% of the U.S. population, including over half of school-aged children, were infected with A(H1N1)pdm09 virus. The extent to which human infection with A(H1N1)pdm09 virus elicits neutralizing antibody against the 1918 virus warrants further study.
Improving biosafety and assessing the dual-use potential and risks of working with highly pathogenic influenza viruses are integral components of public health research. This is the first report to demonstrate that the seasonal trivalent influenza vaccine for 2010-2011 offers protection against the reconstructed 1918 virus. Although more human serology data are needed to determine the frequency of cross-reactive antibodies to the 1918 H1N1 virus, these data (along with other data cited in the text) suggest that there is significant preexisting immunity to potential 1918 virus infection in the human population, which is likely to increase as more individuals acquire immunity through influenza vaccination or infection.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
We thank Eddie Jackson and his team for exceptional care of animals kept under biosafety level 3 containment (with appropriate enhancements).
Footnotes
Published ahead of print 2 May 2012
REFERENCES
- 1. Anhlan D, Grundmann N, Makalowski W, Ludwig S, Scholtissek C. 2011. Origin of the 1918 pandemic H1N1 influenza A virus as studied by codon usage patterns and phylogenetic analysis. RNA 17:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baras B, et al. 2011. Pandemic H1N1 vaccine requires the use of an adjuvant to protect against challenge in naive ferrets. Vaccine 29:2120–2126 [DOI] [PubMed] [Google Scholar]
- 3. Baras B, et al. 2011. Longevity of the protective immune response induced after vaccination with one or two doses of AS03A-adjuvanted split H5N1 vaccine in ferrets. Vaccine 29:2092–2099 [DOI] [PubMed] [Google Scholar]
- 4. Barker WH, Mullooly JP. 1980. Impact of epidemic type A influenza in a defined adult population. Am. J. Epidemiol. 112:798–811 [DOI] [PubMed] [Google Scholar]
- 5. Belser JA, Szretter KJ, Katz JM, Tumpey TM. 2009. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv. Virus Res. 73:55–97 [DOI] [PubMed] [Google Scholar]
- 6. Bresson JL, et al. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657–1664 [DOI] [PubMed] [Google Scholar]
- 7. Brockwell-Staats C, Webster RG, Webby RJ. 2009. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influenza Other Respi. Viruses 3:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention 2010. Update: influenza activity—United States, August 30, 2009–March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 59:423–430 [PubMed] [Google Scholar]
- 9. Chosewood LC, Wilson DE. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, Washington, DC [Google Scholar]
- 10. Forrest BD, et al. 2008. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin. Vaccine Immunol. 15:1042–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galarza JM, Latham T, Cupo A. 2005. Virus-like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:365–372 [DOI] [PubMed] [Google Scholar]
- 12. Garten RJ, et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glezen WP. 1996. Emerging infections: pandemic influenza. Epidemiol. Rev. 18:64–76 [DOI] [PubMed] [Google Scholar]
- 14. Gorse GJ, et al. 1996. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J. Infect. Dis. 173:285–290 [DOI] [PubMed] [Google Scholar]
- 15. Hancock K, et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 16. He XS, et al. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756–11766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herlocher ML, et al. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J. Infect. Dis. 184:542–546 [DOI] [PubMed] [Google Scholar]
- 18. Itoh Y, et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. 1986. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J. Infect. Dis. 154:121–127 [DOI] [PubMed] [Google Scholar]
- 20. Kieny MP, et al. 2006. A global pandemic influenza vaccine action plan. Vaccine 24:6367–6370 [DOI] [PubMed] [Google Scholar]
- 21. Lang PO, Govind S, Mitchell WA, Siegrist CA, Aspinall R. 2011. Vaccine effectiveness in older individuals: what has been learned from the influenza-vaccine experience. Ageing Res. Rev. 10:389–395 [DOI] [PubMed] [Google Scholar]
- 22. Louie JK, et al. 2009. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302:1896–1902 [DOI] [PubMed] [Google Scholar]
- 23. Lu X, et al. 2006. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 24:6588–6593 [DOI] [PubMed] [Google Scholar]
- 24. Maines TR, et al. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manicassamy B, et al. 2010. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog. 6:e1000745 doi:10.1371/journal.ppat.1000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medina RA, et al. 2010. Pandemic 2009 H1N1 vaccine protects against 1918 Spanish influenza virus. Nat. Commun. 1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. 1997. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J. Virol. 71:4347–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicholson KG, et al. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937–1943 [DOI] [PubMed] [Google Scholar]
- 29. Olsen CW. 2002. The emergence of novel swine influenza viruses in North America. Virus Res. 85:199–210 [DOI] [PubMed] [Google Scholar]
- 30. Palese P, Wang TT. 2011. Why do influenza virus subtypes die out? A hypothesis. mBio 2:e00150–11 doi:10.1128/mBio.00150-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearce MB, Belser JA, Houser KV, Katz JM, Tumpey TM. 2011. Efficacy of seasonal live attenuated influenza vaccine against virus replication and transmission of a pandemic 2009 H1N1 virus in ferrets. Vaccine 29:2887–2894 [DOI] [PubMed] [Google Scholar]
- 32. Reid AH, et al. 2003. 1918 influenza pandemic caused by highly conserved viruses with two receptor-binding variants. Emerg. Infect. Dis. 9:1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reid AH, Taubenberger JK. 2003. The origin of the 1918 pandemic influenza virus: a continuing enigma. J. Gen. Virol. 84:2285–2292 [DOI] [PubMed] [Google Scholar]
- 34. Skountzou I, et al. 2010. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J. Immunol. 185:1642–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slemons RD, Swayne DE. 1990. Replication of a waterfowl-origin influenza virus in the kidney and intestine of chickens. Avian Dis. 34:277–284 [PubMed] [Google Scholar]
- 36. Smith H, Sweet C. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10:56–75 [DOI] [PubMed] [Google Scholar]
- 37. Stephenson I, et al. 2005. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 191:1210–1215 [DOI] [PubMed] [Google Scholar]
- 38. Swayne DE, Beck JR, Perdue ML, Brugh M, Slemons RD. 1996. Assessment of the ability of ratite-origin influenza viruses to infect and produce disease in rheas and chickens. Avian Dis. 40:438–447 [PubMed] [Google Scholar]
- 39. Tumpey TM, et al. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77–80 [DOI] [PubMed] [Google Scholar]
- 40. Tumpey TM, et al. 2004. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U. S. A. 101:3166–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tumpey TM, et al. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315:655–659 [DOI] [PubMed] [Google Scholar]
- 42. Wei CJ, et al. 2010. Cross-neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci. Transl. Med. 2:24ra21 doi:10.1126/scitranslmed.3000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeng H, et al. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 81:12439–12449 [DOI] [PMC free article] [PubMed] [Google Scholar]