Abstract
Persistent viral infections often overburden the immune system and are a major cause of disease in humans. During many persistent infections, antiviral T cells are maintained in a state of immune exhaustion characterized by diminished effector and helper functions. In mammalian systems, an extensive immune regulatory network exists to limit unwanted, potentially fatal immunopathology by inducing T cell exhaustion. However, this regulatory network at times overprotects the host and fosters viral persistence by severely dampening adaptive immune responsiveness. Importantly, recent studies have shown that T cell exhaustion is mediated in part by host immunoregulatory pathways (e.g., programmed death 1 [PD-1], interleukin 10 [IL-10]) and that therapeutic blockade of these pathways either before or during persistent infection can promote viral clearance. Transforming growth factor beta (TGF-β) is another immunosuppressive cytokine known to impede both self- and tumor-specific T cells, but its role in regulating antiviral immunity is not entirely understood. In this study, we inhibited TGF-β with three potent antagonists to determine whether neutralization of this regulatory molecule is a viable approach to control a persistent viral infection. Our results revealed that these inhibitors modestly elevate the number of antiviral T cells following infection with a persistent variant of lymphocytic choriomeningitis virus (LCMV) but have no impact on viral clearance. These data suggest that therapeutic neutralization of TGF-β is not an efficacious means to promote clearance of a persistent viral infection.
INTRODUCTION
Persistent viral infections pose a considerable challenge to the immune system, particularly when immunoregulatory pathways engage to dampen the adaptive immune system (9, 33, 56). During persistence, the responding T cell response can progress through states of functional exhaustion characterized by the loss of effector functions (47, 69, 70, 76). However, recent studies in a murine model have identified molecular determinants (e.g., programmed death 1 [PD-1], and interleukin 10 [IL-10]) that negatively regulate antiviral T cell responses, promote functional exhaustion, and facilitate viral persistence (3, 12, 18). Importantly, these determinants were also identified in humans persistently infected with human immunodeficiency virus type 1 (HIV-1) (17, 50, 62) and hepatitis B and C virus (HBV and HCV) (10, 24, 64) as well as primates infected with simian immunodeficiency virus (SIV) (65). Thus, commonalities exist in how the adaptive immune system from different species dampens antiviral T cell responses. Because therapeutic neutralization of immunoregulatory pathways has the potential to promote clearance of persistent viral infections in humans, it is important to identify and mechanistically understand the entire regulatory network that controls antiviral immunity.
The lymphocytic choriomeningitis virus (LCMV) model has been instrumental for elucidating pathways that foster viral persistence as well as those that promote viral clearance (or control) (11). A case in point stems from the immunotherapeutic treatment of carrier mice persistently infected from birth with LCMV. If mice are infected at birth or in utero with LCMV (referred to as carrier mice), they grow to adulthood with high viral loads persisting in nearly every tissue compartment (21). Importantly, adoptive transfer of memory T lymphocytes or splenocytes from LCMV-immune animals into persistently infected carrier mice results in complete eradication of the pathogen from all tissue compartments (38, 48, 66, 67). This demonstrates that despite a high, systemically distributed viral load (21) and the induction of thymic tolerance (27, 51), it is possible to completely eradicate a persistent virus from its host by using antiviral memory T cells as a therapy. This adoptive transfer paradigm also facilitates the study of memory T cells and the factors that regulate them, because recipient carrier mice are completely purged of high viral loads without the induction of fatal immunopathology, which likely requires strict immune regulation.
Another LCMV model used to study immune regulation involves infection of mice with persistence-prone strains of the virus, such as clone 13 (CL13) (1, 2). CL13 differs from the nonpersistent Armstrong (Arm) strain by only three amino acids (7, 55, 58). Two of these mutations are known to confer a replicative advantage to CL13 in its murine host. Following intravenous inoculation, CL13 persists in most peripheral tissues (e.g., spleen, liver, lymph nodes, blood) until day 60, the brain until day 200, and the kidneys for life (37, 70). In contrast, LCMV Arm is cleared from all murine tissues in 8 to 10 days. Comparison of antiviral immune responses directed against Arm (acute) versus CL13 (persistent) have yielded great insights into mechanisms that facilitate viral persistence. For example, CL13 infection induces a state of antiviral T cell exhaustion characterized by diminished effector and helper functions (47, 70, 71, 76). T cell exhaustion helps prevent the development of severe immunopathology but also promotes viral persistence. Recent studies in the CL13 model have shown that two dominant immune regulators (i.e., PD-1 and IL-10) are largely responsible for the T cell exhaustion that facilitates viral persistence (3, 12, 18). Genetic knockout or therapeutic neutralization of these key regulators restores antiviral T cell function and promotes viral clearance.
In addition to PD-1 and IL-10, other members of the immunoregulatory network include but are not limited to cytotoxic T lymphocyte-associated 4 (CTLA-4) (13, 60, 68), T cell Ig and mucin domain-containing molecule 3 (TIM-3) (45), lymphocyte-activation gene 3 (LAG-3) (63), and transforming growth factor beta (TGF-β) (31, 53). CTLA-4 does not impede antiviral immunity to LCMV CL13 (3), but expression on CD4+ T cells was recently shown to correlate positively with disease progression and T cell dysfunction in HIV-infected patients (30). TIM-3 and LAG-3 also appear to play a minor role in regulating antiviral immunity to LCMV CL13 (8, 28, 52). Neutralization of these regulators has no impact on viral clearance unless done in combination with blockade of a major regulator like PD-1. Thus, there appears to be major (PD-1, IL-10) and minor (TIM-3, LAG-3) regulators that modulate immunity to persistent viral infections like LCMV CL13.
We recently became interested in TGF-β because of its importance in preventing autoimmunity (25, 36, 57) and hypothesized that this potent immunoregulatory cytokine might also act directly on antiviral T cells to control their pathogenicity. TGF-β is a member of a cytokine superfamily that participates in development, cancer, and immune regulation (22, 43, 53). Three isoforms of TGF-β exist in mammals (TGF-β1, -2, -3). TGF-β1 is the predominant isoform expressed by the immune system and is a powerful regulatory cytokine that controls differentiation, proliferation, and apoptosis of immune cells (43, 53). TGF-β1 knockout mice are able to survive only 3 to 4 weeks, at which point spontaneous activation of self-reactive immune cells leads to death (36, 57). TGF-β is also required for the maintenance of peripheral CD4+ regulatory T cells, a cell population that expresses the transcription factor FoxP3 (20, 39, 41, 42) and can reversibly suppress the cytotoxicity of CD8+ T cells through a TGF-β-dependent pathway (15, 44). TGF-β signals by assembling two families of receptor serine/threonine kinases known as the type I and type II receptors. The only known function of the type II receptor is to activate the type I receptors, which propagate their signal by phosphorylating Smads (72).
Given the critical role TGF-β plays in preventing self-reactive immunity, we set out to evaluate the importance of this potent regulator using two different models of LCMV persistence. A recent study suggested that TGF-β is indeed a major immune regulator comparable to PD-1 and IL-10 (59). The conclusions in this study were based entirely on infection of transgenic mice expressing a dominant negative TGF-β receptor (dnTGF-β RII) (25). Study of these mice is confounded by the fact that they develop severe autoimmune disease at 3 to 4 months of age and thus possess a highly activated immune environment (25, 40). We therefore conducted a series of studies in wild-type (wt) mice using three potent antagonists of TGF-β signaling. Our results revealed that neutralization of TGF-β has no impact on clearance of a persistent LCMV CL13 infection, and we propose that the accelerated viral clearance observed in dnTGF-β RII mice is due to a highly activated immune repertoire.
MATERIALS AND METHODS
Mice.
C57BL/6J (B6) Thy1.1+ and Thy1.2+ DbGP33–41 T cell receptor (TCR) transgenic (P14) mice (51) as well as B6 Ly5.1+ I-AbGP61–80 TCR transgenic (SMARTA) mice (49) were bred and maintained in a closed breeding facility. Eight-week-old B6 and B6.Cg-Tg(Cd4-TGFBR2)16Flv/J (dnTGF-β RII) transgenic mice (25) were purchased from The Jackson Laboratories (Bar Harbor, ME). F1 crosses between B6 Thy1.1+DbGP33–41 TCR-tg and dnTGF-β RII mice were conducted in a closed breeding colony. All mice were housed under specific pathogen-free conditions and handled in accordance with the Institutional Animal Care and Use Committee of The National Institutes of Health.
Virus.
Eight-week-old C57BL/6J mice were infected intravenously (i.v.) with 2 × 106 PFU of LCMV Armstrong or clone 13 to generate an acute or persistent viral infection, respectively. Viral titers were determined by plaque assay on Vero cells. A colony of persistently infected LCMV carrier mice was originally established by infecting 1-day-old C57BL/6J mice with 103 PFU of LCMV Armstrong intracerebrally (38). Because LCMV is known to spread through vertical transmission (i.e., mother to offspring), the resultant carrier mice were then interbred to establish and maintain a colony of persistently infected mice for experimentation. To generate a carrier colony of dnTGF-β RII mice, persistently infected female B6 mice were crossed with uninfected male dnTGF-β RII mice. Carrier mice obtained from this F1 cross were used as recipients for adoptive immunotherapy experiments.
CD8 T cell isolations.
CD8+ T cells were purified from Thy1.1+ P14 and Thy1.1+ dnTGF-β RII P14 by negative selection (Stem Cell Technologies). CD4+ T cells were also purified by negative selection from naïve Ly5.1+ SMARTA mice. The purity after enrichment was determined to be greater than 98%. For flow cytometric studies, 5,000 purified P14 T cells and/or 5,000 SMARTA T cells were injected i.v. into naïve mice. Mice were subsequently challenged 1 day later with LCMV.
Surface staining and antibodies.
Mononuclear cell suspensions were incubated for 15 min with a rat anti-CD16/32 antibody to block Fc receptors and then with primary antibodies for 30 min on ice. Cells were stained with the following antibodies: anti-CD4 PerCP-Cy5.5 antibody or Pacific Blue (clone RMA4-5; BD Pharmingen), CD8 Pacific Blue (clone 53.6.7; Caltag), CD122 Pacific Blue (clone TM-b1; eBioscience), CD4 phycoerythrin (PE) (clone RM4-5; BD Pharmingen), Thy1.2 PE (clone 53-2.1; BD Pharmingen), Thy1.1 PerCP (clone OX-7; BD Pharmingen), CD44 allophycocyanin (APC) (clone IM7; eBioscience), CD8 APC-Cy7 (clone 53.6.7; BD Pharmingen), and CD45.1 Alexa 700 (clone A20; Biolegend). For major histocompatibility complex class I (MHC-I) tetramer stains, mononuclear cells were stained for 1 h on ice with either DbGP33–41 PE or DbNP396–404 PE. For MHC-II tetramer stains, mononuclear cells were stained for 1.5 h at room temperature with I-AbGP61–80 PE. MHC-I and -II tetramers were provided by the NIH tetramer core facility at Emory University. All cells were acquired using a digital flow cytometer (Digital LSR II; Becton Dickinson), and data were analyzed with FlowJo software (Tree Star).
Phospho-flow.
For phospho-flow experiments (35), 1 × 106 splenocytes were fixed with 1.5% formaldehyde for 10 min at room temperature (RT). Cells were washed, incubated in ice-cold methanol for an additional 10 min at 4°C, washed, and then stained with surface antibodies (see surface stain section) combined with goat anti-pSmad2/3 antibody (Ser 423/425) or a goat IgG isotype control (Santa Cruz Biotechnology, Inc.). Both the anti-pSmad2/3 and isotype control antibodies were directly conjugated to Alexa Fluor 647 using a conjugation kit from Invitrogen. Following flow cytometric acquisition, the isotype control geometric mean fluorescent intensities (GMFI) were subtracted from the pSMAD2/3 GMFI for all samples.
Intracellular cytokine analyses.
Splenocytes were stimulated for 5 h at 37°C with 2 μg/ml of MHC-I-restricted LCMV peptides (GP33–41 or NP396–404) or 4 μg/ml of an MHC class II-restricted peptide (GP61–80) in the presence of 50 U/ml recombinant murine IL-2 (National Institutes of Health) and 1 mg/ml brefeldin A (Sigma). All peptides were greater than 95% pure. Following the 5-h in vitro stimulation, cells were surface stained with anti-CD8 Pacific Blue (53.6.7; Caltag) or anti-CD4 PerCP-Cy5.5 (RMA4-5; BD Pharmingen). Afterward, cells were fixed, permeabilized, and stained with the following cytokine antibodies: gamma interferon (IFN-γ) PE (XMG1.2; BD Pharmingen), tumor necrosis factor alpha (TNF-α) fluorescein isothiocyanate (FITC) (MP6-XT22; eBioscience), and IL-2 APC (JES6-5H4; BD Pharmingen). All cell populations were acquired using a digital flow cytometer.
TGF-β blockade and quantification.
IN1233 (generously provided by Dae-Kee Kim, Ewha Womans University, Seoul, South Korea), an activin receptor-like kinase 5 (ALK5) inhibitor (32), was resuspended in phosphate-buffered saline (PBS) and injected intraperitoneally (i.p.) (25 mg/kg/day) starting the day before LCMV infection and every day thereafter for 8 days. Anti-TGF-β antibody (1.0 to 1.5 mg; mouse IgG1; clone 1D11.16.8; BioXcell) or mouse IgG isotype control (Jackson ImmunoResearch) was injected i.p. every other day for 8 days, starting at day −1. Recombinant TGF-β RII-Fc (50 μg; R&D Systems) or vehicle was injected i.p. the day of LCMV infection and every other day thereafter for 8 days. The serum concentration of TGF-β was determined using a Quantikine TGF-β1 enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems.
Adoptive immunotherapy.
To generate LCMV memory donors, wild-type and dnTGF-β RII littermate control mice were infected intraperitoneally (i.p.) with 105 PFU of LCMV Armstrong. Memory splenocytes were harvested at day 45 postinfection. For all adoptive immunotherapy experiments, 1.5 × 107 to 4.5 × 107 memory splenocytes were injected i.p. into uninfected or LCMV carrier mice persistently infected from birth. For wild-type memory cells, 1.5 × 107 splenocytes were always injected into wild-type carrier recipients. Because dnTGF-β RII mice infected i.p. with LCMV consistently had reduced numbers of LCMV-specific memory T cells in the spleen at day 45 compared to wild-type controls, we increased the number of dnTGF-β RII memory splenocytes injected into dnTGF-β RII carrier recipients for all adoptive immunotherapy experiments. This was done to ensure that the absolute number of virus-specific T cells administered was similar between the groups receiving wild-type and dnTGF-β RII splenocytes. The correction factor (which ranged from 1.5- to 3.0-fold) was calculated for each experiment by quantifying the frequency of DbGP33–41 MHC-I tetramer+ CD8+ T cells in the pooled splenocytes before transfer. The frequency found in the wild-type mice was divided by the frequency from the dnTGF-β RII mice and used as the correction factor.
Quantitative PCR.
Spleens were homogenized and resuspended in TRIzol before performing a column-based RNA purification using the PureLink RNA minikit (Invitrogen). Total splenic RNA was further treated with amplification-grade DNase I (Invitrogen) prior to reverse transcription. cDNA's were synthesized from 1.0 μg of DNase I-treated RNA using the iScript (Bio-Rad) reverse transcription reagent kit without any gene-specific primers. For determination of relative expression of TGF-β1, -2, and -3, cDNA (250 ng) was used for quantitative real-time PCRs (qPCR) in which beta-actin expression was used as a reference. All qPCRs were performed using SYBR green PCR master mix (Applied Biosystems) in a 96-well optic tray on a CFX96 real-time PCR detection system (Bio-Rad). The reactions were performed in duplicates, and RNA templates without reverse transcriptase were used as a negative control for each gene candidate. qPCRs were run with an initial denaturation temperature of 95°C for 10 min, which was followed by 40 cycles of a three-step amplification reaction. Annealing temperatures for specific primers were optimized individually on positive controls before performing experimental runs. The following primers were used: Actb (NM_007393), Fwd (AGCTCATTGTAGAAGGTGTGG) and Rev (GTGGGAATGGGTCAGAAG); Tgfb1 (NM_011577), Fwd (CTATGCTAAAGAGGTCACCCG) and Rev (ACTGCTTCCCGAATGTCTG); Tgfb2 (NM_009367), Fwd (TGCTAACTTCTGTGCTGGG) and Rev (GCTTCGGGATTTATGGTGTTG); Tgfb3 (NM_009368), Fwd (CAGGATCTAGGCTGGAAATGG) and Rev (GGGTTCAGGGTGTTGTATAGTC).
Data analysis.
Data handling, analyses, and graphical representations were performed using Microsoft Excel and SigmaPlot 11.0. Statistical significance (P < 0.05) was determined using Student's t test (two groups) or a one-way analysis of variance (ANOVA) (more than two groups).
RESULTS
dnTGF-β RII mice acutely clear a persistence-prone strain of LCMV.
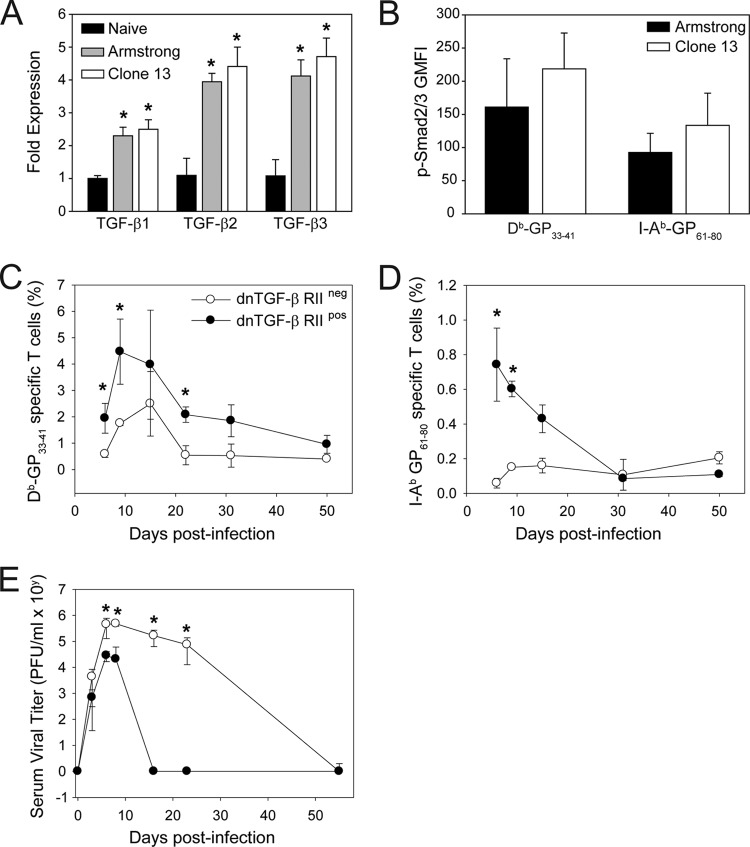
A recent study identified TGF-β as one of the molecules important in preventing antiviral T cells from clearing a persistent LCMV CL13 infection (59). This conclusion was based on the fact that transgenic dnTGF-β RII mice (25) cleared LCMV CL13 acutely compared to wild-type controls. Because this observation could potentially give rise to the development of novel therapeutics for the treatment of persistent viral infections in humans, we set out to confirm and expand upon these studies. To establish whether TGF-β was in fact upregulated following LCMV infection, we inoculated mice intravenously (i.v.) with 2 × 106 PFU of either the acute (Arm) or persistent (CL13) strain of LCMV and then quantified TGF-β1, -2, and -3 transcripts in the spleen at day 7 postinfection (Fig. 1A). This time point was selected because Arm is mostly cleared, whereas CL13 has already established persistence. Interestingly, all TGF-β isoforms were significantly upregulated (P < 0.05) in LCMV-infected mice relative to naïve controls; however, there were no significant differences between Arm- and CL13-infected mice despite a substantial difference in viral loads. To confirm our quantitative PCR data, we also evaluated whether there was evidence of increased TGF-β-mediated signaling in antiviral T cells from LCMV-infected mice (Fig. 1B). For this experiment, mice were seeded with 5,000 naïve DbGP33–41-specific CD8+ T cells (P14 cells) (51) and 5,000 naïve I-AbGP61–80-specific CD4+ T cells (SMARTA cells) (49) and then infected 1 day later with LCMV Arm or CL13. TGF-β induces phosphorylation of Smad2/3; therefore, we quantified pSmad2/3 level flow cytometrically in splenic P14 and SMARTA cells extracted from Arm versus CL13 mice at day 7 postinfection. A slight elevation in pSmad2/3 was noted in CL13 P14 and SMARTA cells (relative to Arm), but this did not reach statistical significance (P > 0.05). Thus, at a time point when Arm- and CL13-infected mice diverge in viral loads, no significant difference in TGF-β mRNA expression or signaling was observed.
Fig 1.
dnTGF-β RII mice rapidly control a persistent LCMV infection. (A) TGF-β1, -2, and -3 expression was quantified by qPCR using splenic RNA extracted from naïve, day 7 Arm-infected, and day 7 CL13-infected mice (n = 4 mice per group). All TGF isoforms were significantly upregulated in LCMV-infected mice. Asterisks denote a statistically significant increase from the naïve control group as determined by one-way ANOVA (P < 0.05). (B) The geometric mean fluorescent intensities of pSmad2/3 (less the isotype control signal) was quantified in splenic DbGP33–41-specific Thy1.1+ CD8+ T cells (P14) and I-AbGP61–80 CD45.1+ CD4+ T cells (SMARTA) 7 days following infection with Arm or CL13 (n = 5 mice per group). No statistically significant differences between the groups were observed. (C, D) Frequencies of Db-GP33–41 tetramer+ CD8+ T cells (B) and I-Ab-GP61–80 tetramer+ CD4+ T cells (C) in the blood were determined in CL13-infected dnTGF-β RII+ and dnTGF-β RII− mice at the denoted time points postinfection (n = 7 mice per group). Tetramer+ T cells were significantly increased (asterisks, P < 0.05) in dnTGF-β RII+ mice relative to in wild-type control mice. (E) At selected time points postinfection, serum viral titers were measured by plaque assay in CL13-infected dnTGF-β RII+ and dnTGF-β RII− mice. dnTGF-β RII+ mice cleared virus significantly faster (asterisks, P < 0.05) than wild-type controls. All data in this figure are plotted as means ± standard deviations (SD) and are representative of at least two independent experiments.
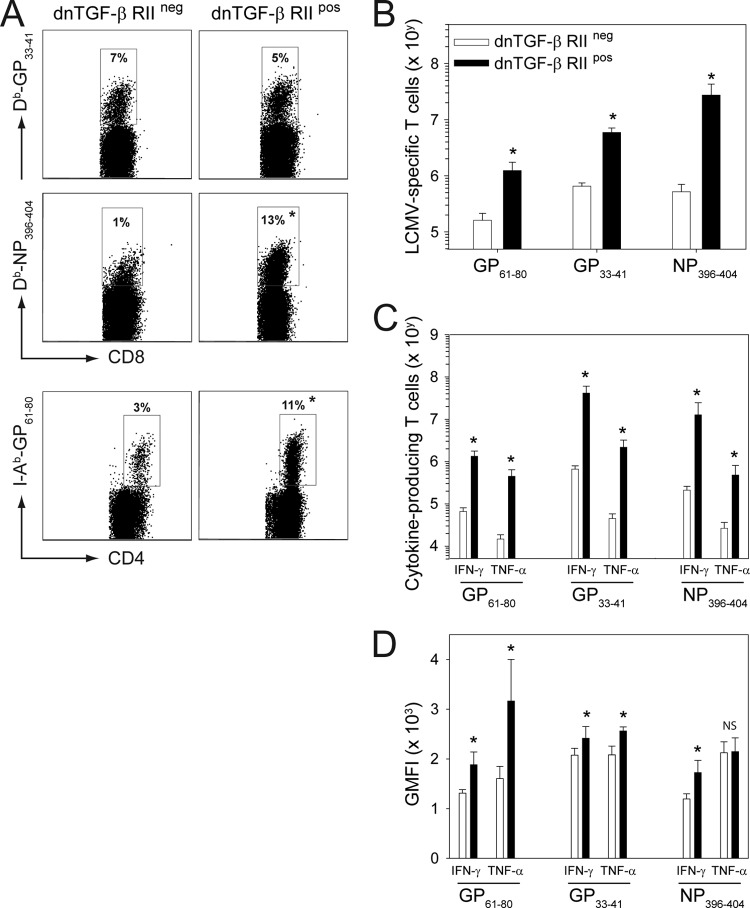
We next set out to determine whether dnTGF-β RII mice do indeed possess the ability to resolve an LCMV CL13 infection acutely. dnTGF-β RII transgenic mice have reduced TGF-β signaling and, consequently, develop spontaneous autoimmune disease at 3 to 4 months of age (25). We therefore selected 8-week-old dnTGF-β RII mice for our LCMV studies to avoid overlap with the period of severe autoimmune disease. Following inoculation with LCMV CL13, dnTGF-β RII mice mounted a significantly increased (P < 0.05) antiviral CD8+ and CD4+ T cell response in the blood relative to wild-type control mice (Fig. 1C and D). This was evidenced by an increased frequency of DbGP33–41 CD8+ (Fig. 1C) and I-AbGP61–80 CD4+ T cells (Fig. 1D). A more thorough assessment of LCMV-specific T cell immunity at a selected time point (day 10) revealed that both the frequency (Fig. 2A) and absolute number (Fig. 2B) of tetramer-positive CD8+ and CD4+ T cells were significantly elevated (P < 0.05) in the spleens of dnTGF-β RII mice compared to in wild-type controls. In addition, the absolute number of cytokine-producing (IFN-γ and TNF-α) CD8+ and CD4+ T cells was increased in dnTGF-β RII mice (Fig. 2C). These cells also produced more cytokine on a per-cell basis when stimulated with MHC-I (GP33–41/NP396–404) and MHC-II (GP61–80) peptides (Fig. 2D). Fitting with the increased antiviral response, and supporting the study by Tinoco and colleagues (59), dnTGF-β RII mice rapidly cleared LCMV CL13 compared to wild-type controls (Fig. 1E). These data suggest that TGF-β is among the potent regulatory molecules that prevent early resolution of LCMV CL13.
Fig 2.
LCMV CL13-infected dnTGF-β RII mice mount an increased antiviral T cell response. (A, B) The frequency (A) and absolute number (B) of MHC-I (DbGP33–41 and Db-NP396–404) and MHC-II (I-AbGP61–80) tetramer+ T cells were calculated in the spleen 10 days following CL13 infection of dnTGF-β RII+ and dnTGF-β RII− mice (n = 5 mice per group). Representative dot plots are gated on CD8+ T cells for MHC-I tetramers and CD4+ T cells for MHC-II tetramers. Boxes denote the mean frequency of tetramer-positive cells in each group. Absolute numbers are plotted as means ± SD. (C) On day 10 postinfection, virus-specific CD8+ and CD4+ T cell responses were assessed by ex vivo peptide stimulation using splenocytes from wild-type and dnTGF-β RII+ mice (n = 5 mice per group). The absolute number of IFN-γ- and TNF-α-producing CD8+ (stimulated with GP33–41 or NP396–404) and CD4+ (stimulated with GP61–80) T cells is plotted as mean ± SD. (D) Geometric mean fluorescent intensities (GMFI) for IFN-γ and TNF-α production from panel C are plotted (n = 5 mice per group). Asterisks on all plots and graphs denote a statistically significant increase (P < 0.05) in dnTGF-β RII+ mice relative to wild-type controls. Data are representative of at least two independent experiments.
TGF-β antagonists do not promote clearance of a persistent LCMV infection.
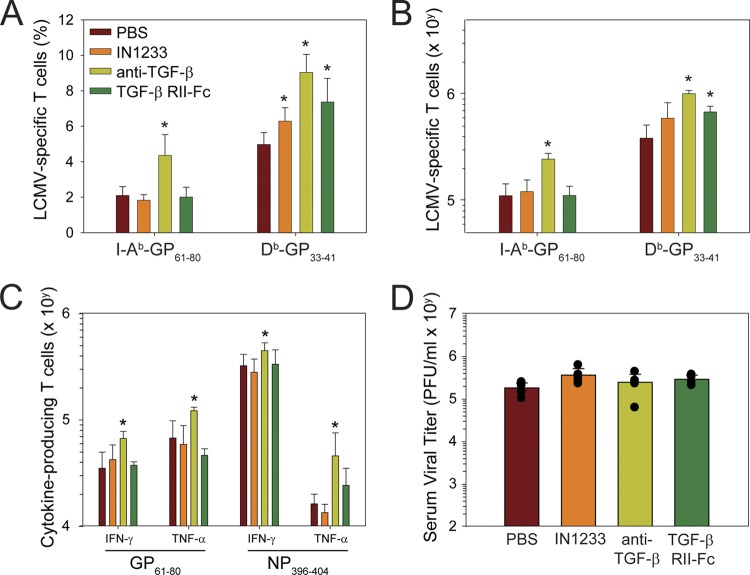
To definitively establish the importance of TGF-β as a dominant regulator of antiviral immunity, we utilized three different approaches to therapeutically block TGF-β signaling. A small-molecule inhibitor (IN-1233) was used to block the type I receptor kinase, ALK5, that TGF-β uses to transduce its cellular signal (32, 74). TGF-β itself was neutralized by administering anti-TGF-β antibodies (4, 16, 19, 75) or TGF-β RII-Fc (a soluble chimeric TGF-β receptor) (34, 73). Importantly, all three antagonistic approaches were shown to be efficacious in other animal models. TGF-β antagonists were administered prior to infection with LCMV CL13 and readministered daily (IN-1233) or every other day (anti-TGF-β antibody and TGF-β RII-Fc) in order to sustain blockade of TGF-β signaling. At day 9 postinfection, we quantified the frequency (Fig. 3A) and absolute number (Fig. 3B) of LCMV-specific tetramer-positive CD8+ and CD4+ T cells in the spleen. A statistically significant increase (P < 0.05) in the frequency of DbGP33–41-specific CD8+ T cells was observed following treatment with all three TGF-β antagonists relative to the control group (Fig. 3A). When absolute numbers were calculated, significant increases (P < 0.05) in DbGP33–41-specific cells were observed in the anti-TGF-β antibody- and TGF-β RII-Fc-treated groups (Fig. 3B). In the CD4 T cell compartment, anti-TGF-β antibody alone induced a statistically significant increase (P < 0.05) in the frequency (Fig. 3A) and absolute number (Fig. 3B) of I-AbGP61–80-specific CD4+ T cells. In fact, anti-TGF-β antibody was the treatment that most consistently elevated the number of virus-specific CD8+ and CD4+ T cells and was the only one that modestly increased (P < 0.05) the absolute number of cytokine-producing CD8+ and CD4+ T cells following ex vivo stimulation with NP396–404 (CD8) or GP61–80 (CD4) peptides (Fig. 3C). Surprisingly, none of the TGF-β antagonists had any impact on viral loads measured in the serum at day 9 postinfection (Fig. 3D). These data indicate that TGF-β neutralization can increase the number of antiviral T cells without accelerating clearance of LCMV CL13.
Fig 3.
Therapeutic blockade of TGF-β increases antiviral T cells but does not impact viral clearance. To block TGF-β signaling, mice were injected with IN1233 (25 mg/kg/day), anti-TGF-β antibody (1 mg every other day), or TGF-β RII-Fc (50 μg every other day) (see Materials and Methods). (A, B) Graphs illustrate the frequency (A) and absolute number (B) of Db-GP33–41 tetramer+ CD8+ T cells and I-Ab-GP61–80 tetramer+ CD4+ T cells in the spleen of treated mice 10 days following infection (mean ± SD) (n = 5 mice per group). (C) The functionality of LCMV-specific CD4+ and CD8+ T cells in the spleen was evaluated by ex vivo peptide stimulation with GP61–80 and NP396–404, respectively. The graph shows the absolute number (mean ± SD) of IFN-γ- and TNF-α-producing T cells that respond to peptide (n = 5 mice per group). (D) Nine days postinfection, serum viral titers were determined by plaque assay (n = 5 mice per group). Data are expressed as PFU/ml of serum sampled (mean ± SD). All data are representative of at least two independent experiments. Asterisks denote a statistically significant increase (P < 0.05) relative to the PBS control group.
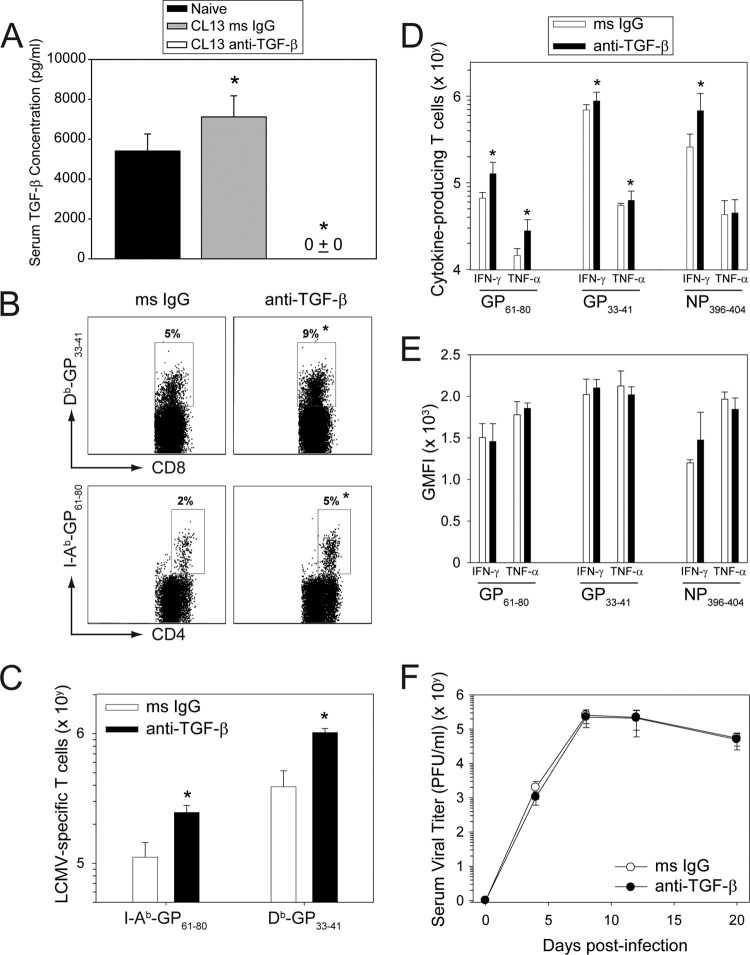
Because these findings were at odds with the accelerated clearance observed in dnTGF-β RII mice, we conducted another study using an increased dose of anti-TGF-β antibody. Beginning 1 day prior to infection, mice were injected every other day i.p. with 1.5 mg of anti-TGF-β or isotype control antibodies. To determine the efficiency of TGF-β neutralization at this dose, we quantified serum cytokine levels by ELISA at day 8 postinfection (Fig. 4A). Relative to the naïve mice, a slight elevation (P < 0.05) in TGF-β was observed in isotype control antibody-treated CL13-infected mice at day 8. Importantly, administration of anti-TGF-β antibody reduced circulating TGF-β to an undetectable level (P < 0.05) in all CL13-infected mice. These data indicate that this dose of antibody was completely effective at neutralizing TGF-β. We then assessed antiviral T cell numbers and function in infected mice treated with the high dose of antibody. Consistent with previous results, anti-TGF-β antibody significantly increased (P < 0.05) the frequency (Fig. 4B) and absolute number (Fig. 4C) of DbGP33–41 CD8+ and I-AbGP61–80 CD4+ T cells in the spleen at day 9 postinfection. An increase in the absolute number of cytokine-producing CD8+ (GP33–41/NP396–404-specific) and CD4+ (GP61–80-specific) T cells was also observed (Fig. 4D); however, no differences in the geometric mean fluorescent intensities of IFN-γ or TNF-α were noted (Fig. 4E). In addition, anti-TGF-β antibody had absolutely no impact (not even a downward trend) on serum viral titers compared to that of the isotype control group (Fig. 4F). These data indicate that anti-TGF-β antibody can increase the number of antiviral T cells but does not improve their functionality or ability to clear a persistent CL13 infection.
Fig 4.
Administration of anti-TGF-β antibodies does not promote clearance of a persistent viral infection. To determine the efficiency of TGF-β neutralization, serum concentrations of the cytokine were quantified by ELISA in day 8 CL13-infected mice treated with anti-TGF-β or isotype control antibodies (1.5 mg every other day). Naïve untreated mice served as a control (n = 5 mice per group). Anti-TGF-β antibodies reduced the cytokine to an undetectable level (mean ± SD = 0 ± 0) in all CL13-infected mice. (B, C) MHC-I (DbGP33–41) and MHC-II (I-AbGP61–80) tetramers were used to calculate frequencies (A) and absolute numbers (B) of virus-specific T cells in the spleens of isotype versus anti-TGF-β antibody (1.5 mg every other day)-treated mice at day 9 postinfection. Representative dot plots (A) are gated on CD8+ T cells for MHC-I tetramers and CD4+ T cells for MHC-II tetramers. Boxes denote the mean frequency of tetramer-positive cells in each group. The absolute number of splenic tetramer-positive T cells was calculated from the frequency data shown in panel A (n = 5 mice per group). (D) On day 10 postinfection, virus-specific CD8+ and CD4+ T cell responses were assessed by ex vivo peptide stimulation of splenocytes from both isotype and anti-TGF-β antibody-treated mice. Boxes denote the absolute number (mean ± SD) of cytokine-producing cells for each group (n = 5 mice per group). (E) Average geometric mean fluorescent intensities (GMFI) from panel C were graphed for all cytokines. (F) At selected time points, serum viral titers were determined by plaque assay and plotted as means ± SD (n = 5 mice per group). Asterisks on all graphs denote a statistically significance increase (P < 0.05) relative to the control group, and data are representative of at least two independent experiments.
Adoptive transfer of dnTGF-β RII memory T cells does not promote accelerated clearance of a persistent viral infection.
Because three different TGF-β neutralization strategies failed to promote clearance of LCMV CL13, we postulated that acute clearance of CL13 in dnTGF-β RII mice might be due to a heightened level of basal immune activity in these transgenic mice prior to infection. Uninfected dnTGF-β RII mice are known to develop spontaneous autoimmune disease within 3 to 4 months and contain a repertoire of activated T cells, owing to the reduction in TGF-β signaling (25). To evaluate the importance of this proinflammatory environment, we conducted several lines of investigation. We decided to first remove dnTGF-β RII antiviral memory T cells from their environment and determine whether they had an enhanced capacity (relative to wild-type control cells) to purge a persistent viral infection in a different model. Mice persistently infected from birth with LCMV become systemic lifelong viral carriers (61). LCMV carrier mice, therefore, provide an excellent model to study adoptive immunotherapies. For example, adoptive transfer of LCMV-specific memory cells into carrier mice is known to completely purge LCMV from most peripheral compartments (serum, spleen, liver, etc.) in 7 to 14 days (48), and clearance is dependent on the coordinated activities of LCMV-specific CD8 and CD4 T cells (5).
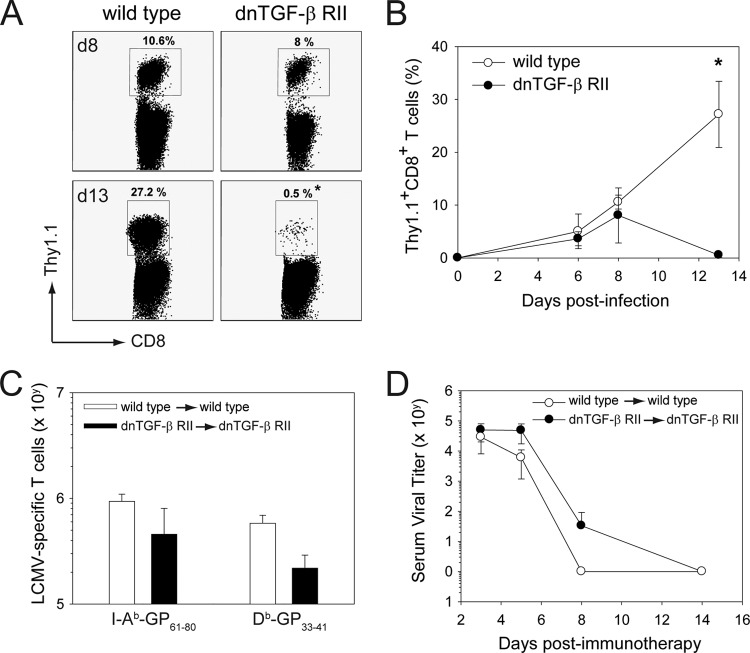
To compare the relative effectiveness of wild-type versus dnTGF-β RII memory cells in purging virus from LCMV carrier mice, we infected 8-week-old wild-type and dnTGF-β RII mice i.p. with 1 × 105 PFU of LCMV Arm. This results in acute viral clearance followed by the generation of LCMV-specific memory T cells in both strains (29, 59). At day 45 postinfection, memory splenocytes from wild-type and dnTGF-β RII “memory” mice were adoptively transferred i.p. into wild-type and dnTGF-β RII mice (respectively) persistently infected from birth with LCMV Arm (Fig. 5). Because the absolute number of MHC-I tetramer-positive cells was reduced in dnTGF-β RII mice relative to wild-type controls at day 45 (data not shown), we introduced a correction factor for each adoptive transfer experiment. This was done to ensure that the memory cells from dnTGF-β RII mice had the same potential to clear LCMV carrier mice as those obtained from wild-type controls. We typically injected 1.5 to 3.0 times more dnTGF-β RII cells, and the exact correction factor was calculated for each experiment based on the frequency of DbGP33–41 CD8+ T cells. In addition, dnTGF-β RII memory cells were always transferred into dnTGF-β RII LCMV carrier recipients, because the dominant negative receptor is of human origin and is immunologically rejected when transferred into wild-type, nontolerant mice (Fig. 5A and B) (25, 54). For example, if B6 mice are seeded with 104 naïve wild-type or dnTGF-β RII P14 CD8+ T cells and infected 1 day later with CL13, then only wild-type P14 cells can be found in the spleen at day 13 postinfection (Fig. 5A and B). This is consistent with published data showing that dnTGF-β RII cells are rejected in B6 recipients (54).
Fig 5.
Adoptively transferred dnTGF-β RII memory T cells do not accelerate clearance of a persistent viral infection. A total of 104 wild-type or dnTGF-β RII Thy1.1+ CD8+ P14 cells was transferred into naïve C57BL/6J mice and then infected intravenously with LCMV CL13 (n = 5 mice per group). (A) Representative dot plots gated on CD8+ T cells show the mean frequency of Thy1.1+ CD8+ T cells (black boxes) in the blood of mice at day 8 and day 13 following LCMV infection. (B) The frequencies of Thy1.1+ CD8+ T cells in the blood are plotted over time (mean ± SD). Note that dnTGF-β RII Thy1.1+ CD8+ P14 cells are rejected from CL13-infected mice by day 13 postinfection. Asterisks denote a statistically significant difference between the groups (P < 0.05). (C) At 45 days following i.p. infection with LCMV Arm, memory splenocytes were extracted from wild-type and dnTGF-β RII mice and then adoptively transferred i.p. into wild-type and dnTGF-β RII LCMV carrier mice, respectively (n = 4 mice per group). The correction factor for dnTGF-β RII splenocytes was calculated by staining pooled memory cells with anti-CD8 antibody and a DbGP33–41 tetramer prior to injection. A correction factor was introduced into each experiment to ensure that comparable numbers of wild-type and dnTGF-β RII memory CD8+ T cells were transferred into LCMV carrier mice (see Materials & Methods). MHC-I (DbGP33–41) and MHC-II (I-AbGP61–80) tetramers were used to calculate the absolute number of transferred virus-specific T cells in the spleens of wild-type and dnTGF-β RII carrier mice at day 11 posttransfer. Data are plotted as means ± SD. (D) At selected time points following adoptive immunotherapy, serum viral titers in recipient carrier mice were determined by plaque assay (n = 4 mice per group). Data are expressed as PFU/ml of serum sampled (mean ± SD) and are representative of three independent experiments.
For all adoptive immunotherapy experiments, donors and recipients were matched to avoid the possibility of immunological rejection. Following adoptive transfer, comparable numbers of donor-derived DbGP33–41 CD8+ and I-AbGP61–80 CD4+ T cells were observed in the spleens of wild-type and dnTGF-β RII carrier recipients at day 11 (Fig. 5C). To determine whether deficiency in TGF-β signaling influenced the clearance kinetics of a persistent viral infection following adoptive immunotherapy, we quantified serum viral titers at different time points postinfection (Fig. 5D). Quantification of serum viral loads is routinely used as a means to monitor the efficacy of adoptive immunotherapeutic regimens in LCMV carrier mice (38). Comparable clearance kinetics were observed in wild-type carrier mice treated with wild-type memory cells and dnTGF-β RII carrier mice treated with dnTGF-β RII memory cells (Fig. 5D), indicating that TGF-β does not negatively regulate antiviral T cells during clearance of a persistent viral infection in this model system. These data were in line with the failure of TGF-β antagonists to accelerate clearance of LCMV CL13 (Fig. 3 and 4).
Uninfected dnTGF-β RII mice contain a highly activated immune repertoire.
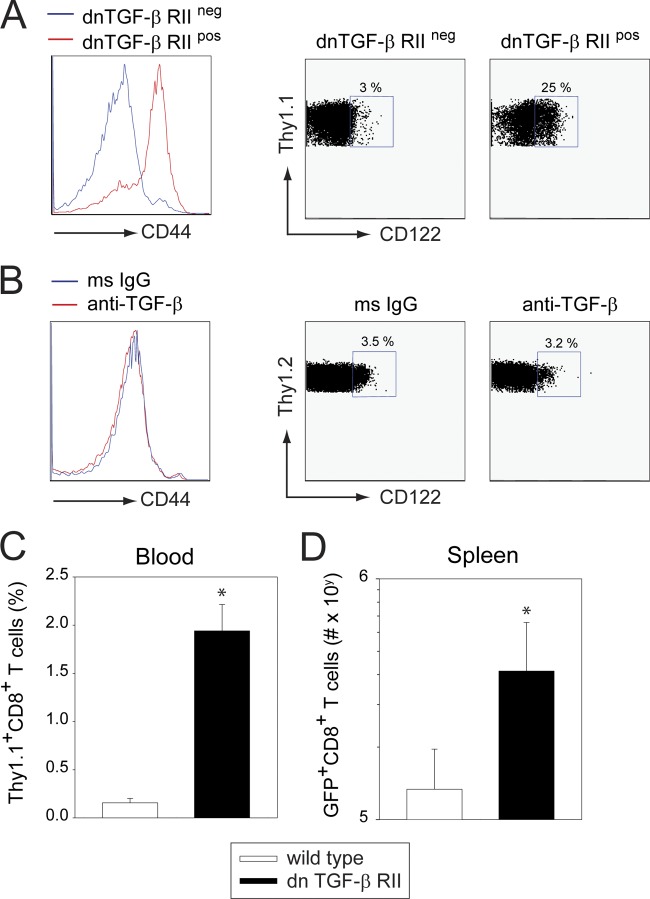
To investigate the activation status of the naïve CD8+ T cell repertoire in dnTGF-β RII mice, we crossed heterozygous dnTGF-β RII mice with homozygous DbGP33–41 T cell receptor (TCR) transgenic mice (P14 mice). In P14 mice, nearly all CD8+ T cells are specific to the LCMV glycoprotein (GP) (amino acids 33 to 41) (51). The resultant F1 mice from this cross all expressed the P14 TCR, but only 50% were positive for dnTGF-β RII. Analysis of individual mice in these litters enabled us to determine the impact of dnTGF-β RII on naïve LCMV-specific CD8+ T cells. At 8 weeks of age, splenic P14 cells from dnTGF-β RII+ and dnTGF-β RII− mice were analyzed for the expression of the activation marker, CD44, and the IL-2 receptor β chain, CD122. P14 cells extracted from dnTGF-β RII+ mice expressed high levels of CD44 (GMFI, 74.2 ± 4.7 for dn versus 3.5 ± 1.7 for wt; P = 0.002) and CD122 (24.7 ± 0.9% positive for dn versus 2.9 ± 0.4% for wt; P = 0.001) relative to wild-type controls (Fig. 6A). We were not able to induce this heightened activation status in naïve, wild-type P14 mice (at 8 weeks of age) by treating them every other day for 3 weeks with anti-TGF-β antibody (Fig. 6B). These data indicate that deficiency in TGF-β signaling results in the generation of highly activated T cells in dnTGF-β RII mice. We postulated that heightened immune activation might give antiviral T cells operating in dnTGF-β RII mice a competitive advantage regardless of whether they expressed the dominant negative receptor or not. To test this possibility, we seeded wild-type and dnTGF-β RII mice with 104 wild-type Thy1.1+P14 cells and then infected i.v. with LCMV CL13. Following infection, the expansion of P14 cells was quantified in the blood and spleen over time. Interestingly, a significant increase (P < 0.05) in the frequency (Fig. 6C) and absolute number (Fig. 6D) of P14 cells was observed in dnTGF-β RII mice relative to wild-type controls. These data indicate that even wild-type P14 cells have an advantage when operating in the dnTGF-β RII environment.
Fig 6.
dnTGF-β RII mice contain a large repertoire of activated T cells prior to infection. (A) Heterozygous dnTGF-β RII mice were crossed with Thy1.1+ P14 mice. Splenic Thy1.1+ CD8+ T cells in the resultant F1 mice were screened at 8 weeks for expression of CD44 and CD122. Histograms and dot plots are gated on Thy1.1+ CD8+ P14 T cells. Note that CD44 and CD122 expressions are elevated on CD8+ T cells from dnTGF-β RII+ mice relative to those of wild-type littermate controls (n = 3 mice per group). Boxes denote the mean frequency of CD122+ Thy1.1+ CD8+ T cells in each group. (B) Naïve 8-week-old Thy1.2+ P14 mice were injected every other day i.p. for 3 weeks with 1 mg of anti-TGF-β or isotype control antibodies. CD44 and CD122 expression was then examined on splenic Thy1.2+ CD8+ T cells as described in panel A. No significant differences were observed between the groups (n = 3 mice per group). (C, D) dnTGF-β RII+ or dnTGF-β RII− mice were seeded with 104 naïve Thy1.1+ CD8+ P14 cells and then infected i.v. with LCMV CL13. The frequency of Thy1.1+CD8+ P14 cells was determined in the blood at day 7 postinfection, and the absolute number of cells was calculated in the spleen at day 10 postinfection (n = 5 mice per group). Asterisks denote a statistically significance increase (P < 0.05) in dnTGF-β RII+ recipients relative to wild-type controls. All data are representative of at least two independent experiments.
DISCUSSION
The immune system is equipped with a broad network of immunoregulatory molecules to prevent autoimmune disease and the development of severe immunopathology following infection (9, 23, 33, 53, 56). While these regulatory pathways have evolved to protect the host, they can also facilitate the establishment of viral persistence and the growth of tumors by hampering the adaptive immune system. It is now well appreciated that potent immune regulators like PD-1, IL-10, and CTLA-4 promote T cell exhaustion in persistently infected mice and humans. TGF-β is a pleiotropic cytokine known for its ability to modulate many different cellular processes (22, 26, 53). From an immunological perspective, TGF-β is required to quench self-reactive T cells, as deficiency in TGF-β signaling results in severe autoimmune disease (25, 36, 57). Given its importance in preventing autoimmunity, it was logical to surmise that TGF-β might also play a major role in controlling antiviral T cell responses during states of persistence. To test this theory, we utilized three different approaches to antagonize TGF-β signaling during the establishment of a persistent LCMV CL13 infection. Each of these approaches was shown previously to block TGF-β-mediated biological effects in other model systems (4, 32, 73). In addition, we demonstrated that anti-TGF-β antibodies reduced circulating TGF-β to an undetectable level in all treated mice (Fig. 4A). Nevertheless, our data with all three antagonists demonstrate convincingly that TGF-β modestly dampens the absolute number of antiviral CD8 and CD4 T cells that expand following viral infection but has no impact on their functionality or ability to clear a persistent viral infection. No significant reductions or downward trends in viral loads were observed in mice treated with TGF-β antagonists. These data indicate that, in contrast to PD-1 and IL-10, TGF-β is not a major immune regulator during a persistent LCMV CL13 infection.
To establish the importance of other major immunoregulatory pathways in the LCMV CL13 system, multiple approaches have been employed. For example, data collected in genetic knockout mice (e.g., PD-L1−/−, IL-10−/−) have been complemented by antibody neutralization studies (e.g., anti-PD-1, anti-PD-L1, anti-IL-10R antibodies) (3, 12, 18). A confounding variable associated with study of genetically deficient mice is that the immune system is often skewed or activated in the absence of immunoregulatory molecules. This is due to the fact that many regulatory pathways are operational under steady-state conditions in order to prevent autoimmune disease and control T cell homeostasis. Thus, genetic deficiency in these pathways can result in immune dysregulation and spontaneous disease. Two well-studied examples include the fatal autoimmune diseases observed in CTLA-4−/− (68) and TGF-β1−/− (36, 57) mice. Severe disease in these knockout mice develops by 1 month of age because of an overly active immune system, making it nearly impossible to assess the importance of CTLA-4 or TGF-β1 on other aspects of immune function (e.g., pathogen-specific immune responses).
Because of the severe autoimmune disease observed in TGF-β1−/− mice, dnTGF-β RII transgenic mice were generated to restrict the TGF-β signaling deficiency to T cells (25). Interestingly, autoimmune disease onset was delayed in dnTGF-β RII mice but nevertheless presented at 3 to 4 months of age and was attributed to the spontaneous development of effector T cells. By 5 months of age, >90% of the T cells obtained from the secondary lymphoid tissues of dnTGF-β RII mice were determined to have an activated effector/memory phenotype (25). We obtained similar results in 8-week-old P14 dnTGF-β RII mice. Despite having a restricted T cell repertoire (i.e., the P14 TCR), nearly all CD8+ T cells in these mice were activated (CD44hi). We postulate that this heightened immune activation status gives these cells a competitive advantage when challenged with a pathogen. Indeed, Tinoco and colleagues demonstrated that dnTGF-β RII mice clear LCMV CL13 acutely (59), and we were able to confirm these findings. However, when LCMV-specific “memory” dnTGF-β RII cells were transferred into dnTGF-β RII mice persistently infected from birth with LCMV, no enhanced ability to clear virus was observed. Thus, dnTGF-β RII T cells appear to have no competitive advantage when placed into an environment with an already established persistent infection. This suggests that TGF-β is not a major regulator of antiviral T cells in the LCMV carrier model. In addition, we propose that the reason LCMV CL13 is cleared acutely in dnTGF-β RII mice is because the “naïve” T cell repertoire is already activated before virus is introduced into the system. CL13 is afforded a competitive advantage over the nonpersistent Arm strain because of higher affinity to its cellular receptor, α-dystroglycan (14, 55). It is conceivable that this replicative advantage is negated by the activated T cells and proinflammatory environment found in dnTGF-β RII mice. In fact, we demonstrated that even wild-type P14 cells performed better in the dnTGF-β RII environment.
In an attempt to validate TGF-β as an important regulator of antiviral immunity, we used three different approaches to block signaling. IN1233 is a small molecule ALK5 inhibitor shown previously to suppress TGF-β-induced fibrosis and hyperplasia (32, 46, 74). We also used anti-TGF-β antibody and TGF-β RII-Fc, which were demonstrated to be effective in multiple inflammatory models (4, 16, 19, 34, 73, 75). Compared side by side, anti-TGF-β antibody was the most effective at increasing the absolute number of virus-specific T cells in the spleen and blood following CL13 infection; however, this treatment did not increase the functionality of the antiviral T cells or their ability to clear virus despite reduction of circulating TGF-β to an undetectable level. Viral titers were identical between anti-TGF-β antibody and isotype control-treated mice at all time points measured. These findings were in stark contrast to what was observed in CL13-infected dnTGF-β RII mice, which showed elevated T cell function and an ability to clear CL13 acutely (59). This discrepancy suggests that TGF-β is not a major immune regulator (comparable to IL-10 or PD-1) in the CL13 model of viral persistence. Given the modest increase in antiviral T cell numbers observed following treatment with all three antagonists, it is possible that anti-TGF-β antibody could offer some benefit in a combinatorial therapy. For example, LAG-3 and TIM-3 are also considered minor immune regulators in the LCMV CL13 system that offer improved antiviral responses only when blocked in combination with a dominant pathway like PD-1 (8, 28).
In conclusion, therapeutic blockade of immune regulators offers great promise for the treatment of persistent viral infections and tumors (33), and clinical trials are already under way (6). Because many regulatory pathways exist, we propose that most infections will have major and minor players. Blockade of major regulators (e.g., PD-1, IL-10) will have a significant impact on viral clearance but may come with the risk of autoimmunity and/or severe immunopathological reactions resulting from rejuvenated T cell activity. Minor regulators (e.g., TGF-β, TIM-3, LAG-3), if blocked, will have only a modest (or no) impact on viral clearance but might be useful to consider in combinatorial therapies designed to improve the efficacy of a dominant regulatory antagonist. After identifying the major and minor regulators that are operational during a persistent infection, the key to designing an efficacious therapy is to next determine the regulator(s) that can be blocked without induction of severe immunopathological reactions.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Intramural Program as well as a grant from the Ray Thomas Edwards Foundation (to D. B. McGavern). L. Garidou was supported by a fellowship from Association pour la Recherche sur la Sclerose en Plaques.
Footnotes
Published ahead of print 2 May 2012
REFERENCES
- 1. Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed R, et al. 1988. Genetic analysis of in vivo-selected viral variants causing chronic infection: importance of mutation in the L RNA segment of lymphocytic choriomeningitis virus. J. Virol. 62:3301–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barber DL, et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 4. Bellavance EC, et al. 2011. Development of tumor-infiltrating CD8+ T cell memory precursor effector cells and antimelanoma memory responses are the result of vaccination and TGF-beta blockade during the perioperative period of tumor resection. J. Immunol. 186:3309–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger DP, Homann D, Oldstone MB. 2000. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology 266:257–263 [DOI] [PubMed] [Google Scholar]
- 6. Berger R, et al. 2008. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14:3044–3051 [DOI] [PubMed] [Google Scholar]
- 7. Bergthaler A, et al. 2010. Viral replicative capacity is the primary determinant of lymphocytic choriomeningitis virus persistence and immunosuppression. Proc. Natl. Acad. Sci. U. S. A. 107:21641–21646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackburn SD, et al. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackburn SD, Wherry EJ. 2007. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 15:143–146 [DOI] [PubMed] [Google Scholar]
- 10. Boni C, et al. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borrow P, Oldstone MBA. 1997. Lymphocytic choriomeningitis virus. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 12. Brooks DG, et al. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunet JF, et al. 1987. A new member of the immunoglobulin superfamily—CTLA-4. Nature 328:267–270 [DOI] [PubMed] [Google Scholar]
- 14. Cao W, et al. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079–2081 [DOI] [PubMed] [Google Scholar]
- 15. Chen ML, et al. 2005. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. 1989. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J. Immunol. 142:1536–1541 [PubMed] [Google Scholar]
- 17. Day CL, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 18. Ejrnaes M, et al. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fahlen L, et al. 2005. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 201:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fantini MC, et al. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153 [DOI] [PubMed] [Google Scholar]
- 21. Fazakerley JK, Southern P, Bloom F, Buchmeier MJ. 1991. High resolution in situ hybridization to determine the cellular distribution of lymphocytic choriomeningitis virus RNA in the tissues of persistently infected mice: relevance to arenavirus disease and mechanisms of viral persistence. J. Gen. Virol. 72(Pt 7):1611–1625 [DOI] [PubMed] [Google Scholar]
- 22. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. 2010. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 10:554–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Francisco LM, Sage PT, Sharpe AH. 2010. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236:219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golden-Mason L, et al. 2007. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 81:9249–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorelik L, Flavell RA. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171–181 [DOI] [PubMed] [Google Scholar]
- 26. Gorelik L, Flavell RA. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46–53 [DOI] [PubMed] [Google Scholar]
- 27. Jamieson BD, Somasundaram T, Ahmed R. 1991. Abrogation of tolerance to a chronic viral infection. J. Immunol. 147:3521–3529 [PubMed] [Google Scholar]
- 28. Jin HT, et al. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 107:14733–14738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaech SM, Hemby S, Kersh E, Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837–851 [DOI] [PubMed] [Google Scholar]
- 30. Kaufmann DE, et al. 2007. Upregulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8:1246–1254 [DOI] [PubMed] [Google Scholar]
- 31. Kehrl JH, et al. 1986. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163:1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JH, et al. 2010. IN-1233, an ALK-5 inhibitor: prevention of granulation tissue formation after bare metallic stent placement in a rat urethral model. Radiology 255:75–82 [DOI] [PubMed] [Google Scholar]
- 33. Kim PS, Ahmed R. 2010. Features of responding T cells in cancer and chronic infection. Curr. Opin. Immunol. 22:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Komesli S, Vivien D, Dutartre P. 1998. Chimeric extracellular domain type II transforming growth factor (TGF)-beta receptor fused to the Fc region of human immunoglobulin as a TGF-beta antagonist. Eur. J. Biochem. 254:505–513 [DOI] [PubMed] [Google Scholar]
- 35. Krutzik PO, Nolan GP. 2003. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55:61–70 [DOI] [PubMed] [Google Scholar]
- 36. Kulkarni AB, et al. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. U. S. A. 90:770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lauterbach H, Truong P, McGavern DB. 2007. Clearance of an immunosuppressive virus from the CNS coincides with immune reanimation and diversification. Virol. J. 4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. 2006. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J. Exp. Med. 203:1963–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li MO, Sanjabi S, Flavell RA. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25:455–471 [DOI] [PubMed] [Google Scholar]
- 40. Lucas PJ, Kim SJ, Melby SJ, Gress RE. 2000. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J. Exp. Med. 191:1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marie JC, Letterio JJ, Gavin M, Rudensky AY. 2005. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marie JC, Liggitt D, Rudensky AY. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25:441–454 [DOI] [PubMed] [Google Scholar]
- 43. Massague J, Blain SW, Lo RS. 2000. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309 [DOI] [PubMed] [Google Scholar]
- 44. Mempel TR, et al. 2006. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25:129–141 [DOI] [PubMed] [Google Scholar]
- 45. Monney L, et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541 [DOI] [PubMed] [Google Scholar]
- 46. Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK. 2006. IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 70:1234–1243 [DOI] [PubMed] [Google Scholar]
- 47. Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758–761 [DOI] [PubMed] [Google Scholar]
- 48. Oldstone MB, Blount P, Southern PJ, Lampert PW. 1986. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321:239–243 [DOI] [PubMed] [Google Scholar]
- 49. Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400 [DOI] [PubMed] [Google Scholar]
- 50. Petrovas C, et al. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559–561 [DOI] [PubMed] [Google Scholar]
- 52. Richter K, Agnellini P, Oxenius A. 2010. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int. Immunol. 22:13–23 [DOI] [PubMed] [Google Scholar]
- 53. Rubtsov YP, Rudensky AY. 2007. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 7:443–453 [DOI] [PubMed] [Google Scholar]
- 54. Sanjabi S, Flavell RA. 2010. Overcoming the hurdles in using mouse genetic models that block TGF-beta signaling. J. Immunol. Methods 353:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sevilla N, et al. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8:239–245 [DOI] [PubMed] [Google Scholar]
- 57. Shull MM, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sullivan BM, et al. 2011. Point mutation in the glycoprotein of lymphocytic choriomeningitis virus is necessary for receptor binding, dendritic cell infection, and long-term persistence. Proc. Natl. Acad. Sci. U. S. A. 108:2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. 2009. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tivol EA, et al. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–547 [DOI] [PubMed] [Google Scholar]
- 61. Traub E. 1938. Factors influencing the persistence of choriomeningitis virus in the blood of mice after clinical recovery. J. Exp. Med. 68:229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trautmann L, et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202 [DOI] [PubMed] [Google Scholar]
- 63. Triebel F, et al. 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171:1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Urbani S, et al. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Velu V, et al. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Volkert M. 1963. Studies on immunological tolerance to Lcm virus. 2. Treatment of virus carrier mice by adoptive immunization. Acta Pathol. Microbiol. Scand. 57:465–487 [PubMed] [Google Scholar]
- 67. Volkert M. 1962. Studies on immunological tolerance to LCM virus. A preliminary report on adoptive immunization of virus carrier mice. Acta Pathol. Microbiol. Scand. 56:305–310 [PubMed] [Google Scholar]
- 68. Waterhouse P, et al. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–988 [DOI] [PubMed] [Google Scholar]
- 69. Wherry EJ. 2011. T cell exhaustion. Nat. Immunol. 12:492–499 [DOI] [PubMed] [Google Scholar]
- 70. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wherry EJ, et al. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684 [DOI] [PubMed] [Google Scholar]
- 72. Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. 1994. Mechanism of activation of the TGF-beta receptor. Nature 370:341–347 [DOI] [PubMed] [Google Scholar]
- 73. Yata Y, Gotwals P, Koteliansky V, Rockey DC. 2002. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology 35:1022–1030 [DOI] [PubMed] [Google Scholar]
- 74. Yoon HJ, et al. 2011. Role of IN-1233 in the prevention of neointimal hyperplasia after stent placement in a rat artery model. J. Vasc. Interv. Radiol. 22:1321–1328 [DOI] [PubMed] [Google Scholar]
- 75. Yu S, Fang Y, Sharp GC, Braley-Mullen H. 2010. Transgenic expression of TGF-beta on thyrocytes inhibits development of spontaneous autoimmune thyroiditis and increases regulatory T cells in thyroids of NOD.H-2h4 mice. J. Immunol. 184:5352–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zajac AJ, et al. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]