Abstract
A study was undertaken to compare the host immune responses to herpes simplex virus 1 (HSV-1) and HSV-2 infection by the ocular or genital route in mice. Titers of HSV-2 from tissue samples were elevated regardless of the route of infection. The elevation in titers of HSV-2, including cell infiltration and cytokine/chemokine levels in the central nervous system relative to those found following HSV-1 infection, was correlative with inflammation. These results underscore a dichotomy between the host immune responses to closely related alphaherpesviruses.
TEXT
Herpes simplex virus 1 (HSV-1) is a significant human pathogen, and between 1 and 2 billion adults have been exposed to the pathogen and develop an immune response by the age of 59 (38). Components of the innate and adaptive immune responses, including type I interferons (IFN) and T and B lymphocytes, are thought to be responsible for managing the infection and maintaining surveillance during latency (27). Similar to HSV-1, HSV-2 is a highly successful human pathogen with a worldwide prevalence of more than 500 million infections and an annual acquisition rate of close to 25 million/year (21). As with HSV-1, a robust and protective innate and adaptive immune response to genital HSV-2 infection has been noted (1, 12, 23, 24, 25, 28).
HSV-1 and HSV-2 encode at least 84 different polypeptides that show enough similarity to allow intertypic recombinants to be readily generated (38). Whereas it was previously suggested that oral and genital infections were primarily associated with HSV-1 and HSV-2, respectively, an increase in the incidence of genital HSV-1 infection has been seen over the past 20 years (4, 9, 16, 18, 29). As a result of the clinical significance of the pathogens in terms of prevalence and morbidity, a number of investigative teams have developed vaccines to HSV-1 or HSV-2, some of which have been evaluated against HSV-1 and/or HSV-2 (2, 5, 11, 15, 20, 22, 26, 32, 33, 41). While sequence conservation is widely appreciated for HSV-1- and HSV-2-related genes, side-by-side comparisons of the immune responses to these pathogens in the eye or genital tract have not been extensively studied.
One study comparing HSV-1 to HSV-2 and the immune responses at specific anatomical sites reported that ocular challenge of mice with HSV-1 (strain F) elicited a robust expression of several cytokines, including interleukin-1 alpha (IL-1α), gamma IFN (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-6, in the brain, whereas ocular infection with an unknown strain of HSV-2 resulted in a reduction in the cytokine signature (19). However, a caveat to this observation was that there was a 10-fold reduction in the HSV-2 challenge employed to infect mice compared to the HSV-1 challenge applied to the eye. In another study, an intertypic recombinant HSV-1 strain expressing the HSV-2 (strain 333) virion host shutoff (vhs) protein was found to elicit less blepharitis associated with increased clearance of the recombinant virus in comparison to the parental or marker-rescued virus following ocular infection (31). Since the HSV-2-encoded vhs protein displayed significantly greater activity than its HSV-1 counterpart in vitro, those authors concluded that vhs expression alters the host immune response to the pathogen, including the activation of T cells and the IFN pathway (31).
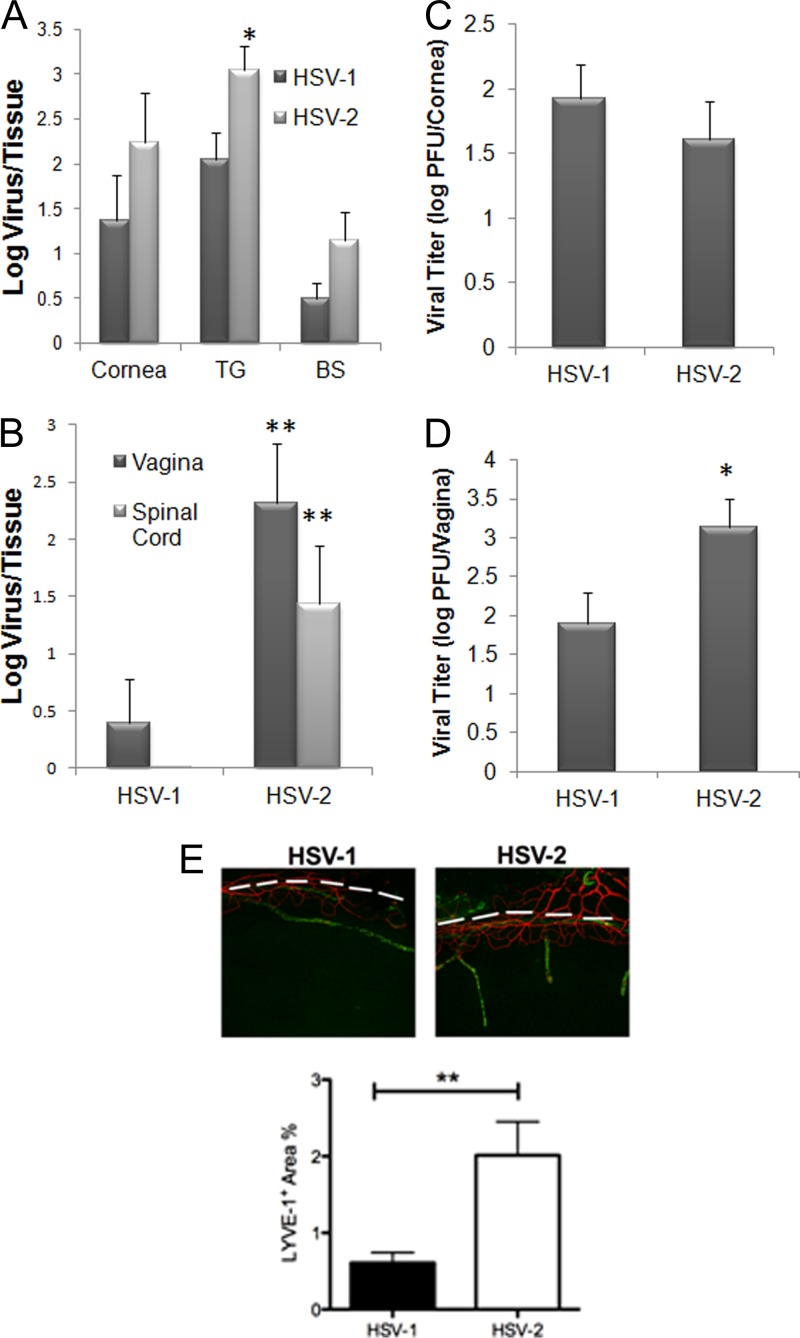
The present study was undertaken to more fully compare the immune responses to HSV-1 versus HSV-2 at two distinct mucosal sites, namely, the eye and the genital tract. Comparing the highly neurovirulent HSV-1 McKrae strain (10) to a clinical HSV-2 isolate (Charity Hospital, New Orleans, LA, 1998) (13) following corneal infection, the results show a trend toward an increased amount of HSV-2 recovered in the cornea and brain stem (BS) at day 7 postinfection (p.i.) compared to HSV-1 following administration of equivalent infectious inocula of 1,000 PFU/cornea, but such results did not reach significance (P ≥ 0.06) (Fig. 1A). HSV-2 levels were significantly elevated in the trigeminal ganglion (TG) at the same time point (Fig. 1A). At an earlier time point (day 3 p.i.), HSV-1 and HSV-2 titers in the cornea were similar (Fig. 1C). By comparison, following genital infection, HSV-2 levels were significantly elevated in the vaginal tract at day 3 p.i. (Fig. 1D) and in the vaginal tract and spinal cord day 7 p.i. (Fig. 1B) compared to the levels seen with mice infected with HSV-1. Even though the levels of virus recovered from the cornea were not significantly different in the comparisons of HSV-1- to HSV-2-infected animals, there was a pronounced increase in the genesis of lymphatic but not blood vessels in the cornea from HSV-2-infected samples, indicating a greater degree of inflammation (Fig. 1E). Production of natural killer (NK) cells and T lymphocytes is attributed to host resistance to HSV-1 and HSV-2 infection (6, 7). Since changes in viral titer were observed in comparisons of HSV-1- to HSV-2-infected mice, the causality of such findings may be reflected by the recruitment of effector innate and/or adaptive immune cells that are typically associated with viral surveillance. As such, we investigated leukocyte infiltration and cytokine/chemokine production in the cornea, TG, BS, vagina, spinal cord, and draining lymph nodes in HSV-1- and HSV-2-infected mice. Relative to leukocyte infiltration, flow cytometry was performed on processed tissue and gated on the CD45hi-expressing cells to determine the number of NK (NK1.1+ CD3−), CD4 T (CD3+ CD4+), CD8 T (CD3+ CD8+), and HSV-specific CD8 T (CD8+ gB495-505 tetramer+) cells as well as macrophages (F4/80+ Gr-1−), inflammatory monocytes (F4/80+ Gr-1+), neutrophils (F4/80− Gr-1+), plasmacytoid dendritic cells (B220+ CD11c+), and conventional dendritic cells (B220− CD11c+) (36, 39). There were no significant changes in the leukocyte populations residing in the cornea, TG, or draining (mandibular) lymph node following ocular viral infection in comparisons of HSV-1- to HSV-2-infected mice (data not shown). However, within the BS, the total number of CD8+ T cells and HSV-specific CD8+ T cells from HSV-2-infected mice was significantly lower than in HSV-1-infected animals (Fig. 2A). This might have been due to reduced recruitment to the BS or a loss of T cells via infection and subsequent fratricide (30). There was also a noticeable reduction in the total count of leukocyte (CD45hi) cells that resided in the BS of mice infected ocularly with HSV-2 (Fig. 2B). These results are consistent with findings showing that the presence of HSV-specific CD8+ T cells corresponds to resistance following ocular HSV infection in this tissue (39).
Fig 1.
HSV-2 yields greater numbers of infectious progeny than HSV-1 regardless of route of infection at 7 days postinfection. Depo-Provera-treated (genital infection) and non-Depo-Provera-treated (ocular infection) female C57BL/6 mice were infected with HSV-1 (McKrae strain) or HSV-2 (clinical isolate) by the (A and C) corneal (1,000 PFU/eye) or (B and D) vaginal (2,000 PFU/vagina) route. At 7 days (A and B) or 3 days (C and D) postinfection, the mice were euthanized and the indicated tissue was removed, processed, and assayed for viral content by plaque assay. The results are expressed as mean log ± standard errors of the means (SEM) (n = 6 to 10/group). (E) C57BL/6 mice (n = 4) were infected as described above and euthanized at day 7 p.i. The corneas were removed, processed, and imaged to visualize lymphatic (green) and blood (red) vessels as described previously (40). Vessels were imaged and quantified to compare the areas occupied by lymphatic vessels (LYVE-1+) within the cornea proper (below to the dashed white line) for HSV-1 versus HSV-2 samples by the use of Metavue software. **, P < 0.01; *, P < 0.05 (comparing HSV-1- to HSV-2-infected groups for each designated tissue as determined by Student's t test [two sample, pooled variances, and Bonferonni adjusted for alpha]).
Fig 2.
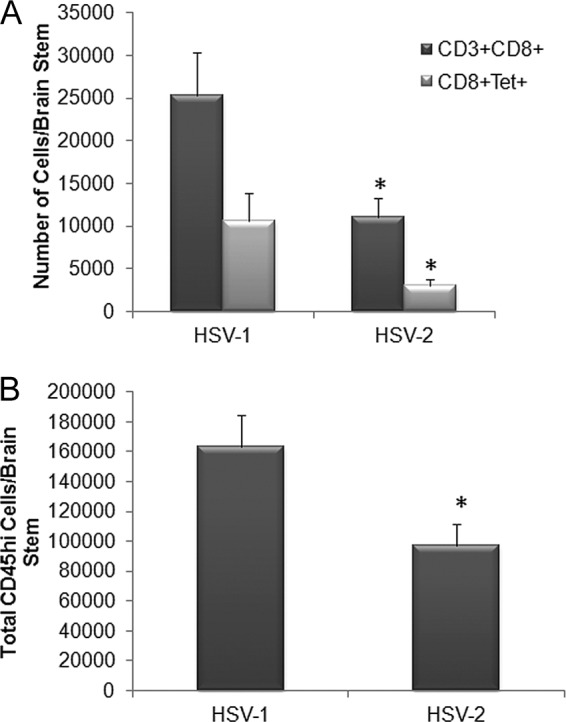
HSV-2-infected mice show a deficiency in the number of HSV-specific CD8+ T cells resident in the brain stem following ocular infection. Female C57BL/6 mice (n = 7 to 9/group) were infected with 1,000 PFU of HSV-1 or HSV-2/cornea. Seven days postinfection, the mice were exsanguinated and the brain stems were processed for (A) total CD8+ and HSV gB-specific CD8+ T cell numbers and (B) total leukocyte (CD45hi) cell numbers by flow cytometry. The results are expressed as mean numbers of cells ± SEM; *, P < 0.05 (comparing HSV-1 to HSV-2 groups for each phenotypic set of markers as determined by Student's t test [two sample, pooled variances, and Bonferonni adjusted for alpha]).
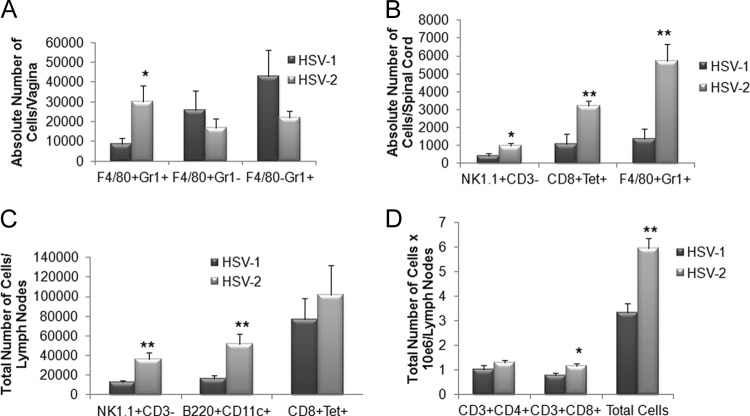
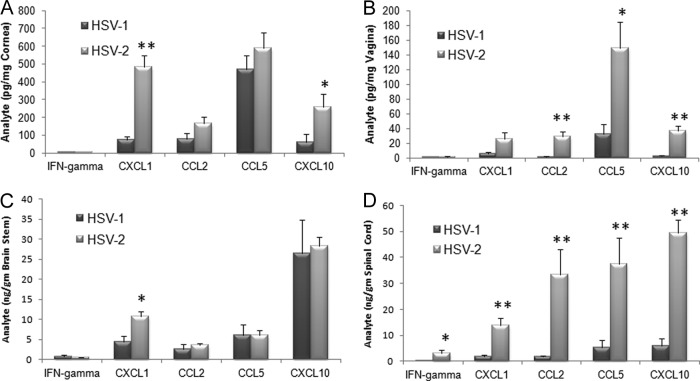
Genital infection with HSV pathogens led to dramatically different host immune responses, as measured by leukocyte infiltration, depending on the tissue evaluated. In the vaginal tissue, inflammatory monocyte levels were significantly elevated in HSV-2-infected mice in comparison to HSV-1-infected animals (Fig. 3A). However, other leukocyte populations, including those of macrophages and neutrophils (Fig. 3A) as well as CD4+ and CD8+ T cells, dendritic cells, and HSV-specific CD8+ T cells (data not shown), were not significantly changed. Within the spinal cord, levels of NK cells, HSV-specific cytotoxic T cells, and inflammatory monocytes were all elevated in HSV-2-infected mice (Fig. 3B) whereas other leukocyte populations were similar to those seen in HSV-1-infected mice (data not shown). Within the draining (inguinal/iliac) lymph nodes, a greater response was observed in HSV-2-infected mice compared to their HSV-1-infected counterparts as measured by total leukocyte counts (Fig. 3D) as well as by individual cell populations, including those of NK cells, plasmacytoid dendritic cells, and total CD8+ T cells (Fig. 3C and D). Such results are correlative with antigenic load found in the tissue and would predict an increase in the expression of chemokines that drive recruitment of the leukocyte populations. Indeed, of the four chemokines analyzed, including CCL2, CCL5, CXCL1, and CXCL10 along with IFN-γ, levels of all analytes were elevated in the spinal cord of mice genitally infected with HSV-2 compared to the levels seen with those infected with HSV-1 (Fig. 4D). Likewise, CCL2, CCL5, and CXCL10 levels were elevated in the vaginal tissue of HSV-2-infected mice in comparison to levels seen with those infected with HSV-1 (Fig. 4B). By comparison, the CXCL1 levels were elevated in the cornea and BS of mice infected ocularly with HSV-2 (Fig. 4A and C) whereas only CXCL10 was upregulated in the cornea of animals infected ocularly with HSV-2 (Fig. 4A). Since HSV-specific CD8+ T cell numbers were significantly reduced in the BS of HSV-2-infected mice following ocular challenge, a lack of change of expression of chemokines, including CCL5 and CXCL10, that are associated with the recruitment of these effector cells suggests that other mediators are likely involved in this event.
Fig 3.
An increase in effector immune cell recruitment in the spinal cord of HSV-2-infected mice correlates with viral titers. Depo-Provera-treated female C57BL/6 mice (n = 9 to 10) were infected with 2,000 PFU of HSV-1 or HSV-2/vagina. Seven days postinfection, the mice were exsanguinated and the vaginal tissue (A), spinal cord (B), and iliac-inguinal lymph node (C and D) samples were removed and processed for flow cytometry. The results are expressed as mean numbers of cells ± SEM; **, P < 0.01; *, P < 0.05 (comparing the HSV-1- to HSV-2-infected tissue for each phenotypic set of markers as determined by Student's t test [two sample, pooled variances, and Bonferonni adjusted for alpha]).
Fig 4.
Chemokine expression levels in mice genitally infected with HSV-2 correlate with viral titer. Depo-Provera-treated (genital infection) and non-Depo-Provera-treated (ocular infection) female C57BL/6 mice (n = 5 to 8/tissue/analyte) were infected with HSV-1 (McKrae strain) or HSV-2 (clinical isolate) by (A and C) the corneal (1,000 PFU/eye) or (B and D) vaginal (2,000 PFU/vagina) route. Seven days postinfection, the mice were exsanguinated and the cornea (A), vaginal tissue (B), brain stem (C), and spinal cord (D) samples were obtained and processed for analyte content by suspension array. The results are expressed as mean picograms or nanograms per milligram of tissue ± SEM (n = 7/group). **, P < 0.01; *, P < 0.05 (comparing the HSV-1- to HSV-2-infected tissue for each analyte per tissue as determined by Student's t test [two sample, pooled variances, and Bonferonni adjusted for alpha]).
This study was undertaken to directly compare HSV-1 infection to HSV-2 infection using a mouse model that can be controlled genetically according to the background haplotype and virus inoculum. Despite the elevated chemokine and cellular responses to HSV-2 in the cornea, vagina, BS, spinal cord, and lymph nodes, HSV-2 still replicates at a greater rate than HSV-1 in the genital mucosa and presents higher viral titers in the TG as well. Interestingly, a significant difference between the two strains in IFN-γ production was seen only in the spinal cord in response to HSV-2. While it is not currently known why such closely related viruses yield different host responses following challenge at the same mucosal site, newly described intracellular pattern recognition receptors (e.g., IF116, DHX9, and DHX36) and their cellular distribution may have important consequences with respect to the innate responses to HSV-1 and HSV-2 that are currently poorly understood (17, 37). Additionally, differences in the immune responses during neuronal infection and reactivation may be explained, in part, by cell tropism. Specifically, A5(+) neurons support HSV-2 replication whereas KH10(+) neurons support HSV-1 productive infection (3). Since A5(+) neurons are noradrenergic (34) and KH10(+) neurons are sensory in nature (8) and both adrenergic and sensory neuronal pathways influence the host immune response or sparing of neurons following HSV infection (14, 35), it is not surprising that disparate immune responses to these two closely related but distinct viral pathogens ensue within the nervous system. A randomized, double-blind HSV vaccine trial utilizing glycoprotein D from HSV-2 (gD-2, with alum and 3-O-deacylated monophosphoryl lipid A as an adjuvant) was recently performed to test for efficacy against HSV-1 and HSV-2 genital disease and infection. Surprisingly, the vaccine was more efficacious against HSV-1 (35%) than HSV-2 (20%) genital disease and provided 35% efficacy against HSV-1 infection (with or without the presence of disease) but did not provide any protection against infection by HSV-2 (2). These results suggest divergent immune responses to a conserved protein that shares 89% homology with gD-2 from HSV-1. It is also likely the relatively low passage number (approximately 25 passages) of the HSV-2 isolate drives a more robust host response in comparison to the high-passage-number (unknown passage number) HSV-1 McKrae strain. A comparison of HSV-1 and HSV-2 pathogens (clinical isolate or laboratory strains) with nearly equivalent passage numbers may yield a better model. Ultimately, the results may suggest that, apart from the use of live attenuated vaccine or vaccine vectors (2), dual protection may require a unique design directed against each of the alphaherpesviruses to elicit protective immunity.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI053108 to D.J.J.C. Additional support includes a RPB Senior Investigator Award to D.J.J.C. and P20 RR017703. D.J.J.C. is an OUHSC Presbyterian Health Foundation Presidential Professor.
Footnotes
Published ahead of print 24 April 2012
REFERENCES
- 1. Ashkar AA, Rosenthal KL. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168–10171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belshe RB, et al. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertke AS, et al. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J. Virol. 85:6669–6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattarakosol P, Visaprom S, Sangdara A, Mungmee V. 2005. Increase of genital HSV-1 and mixed HSV-1 and HSV-2 infection in Bangkok, Thailand. J. Med. Assoc. Thai. 88(Suppl. 4):S300–S304 [PubMed] [Google Scholar]
- 5. Brans R, Yao F. 2010. Immunization with a dominant-negative recombinant herpes simplex virus (HSV) type 1 protects against HSV-2 genital disease in guinea pigs. BMC Microbiol. 10:163 doi:10.1186/1471-2180-10-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr DJJ, Wuest T, Ash J. 2008. An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3. J. Interferon Cytokine Res. 28:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan T, Barra NG, Lee AJ, Ashkar AA. 2011. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J. Reprod. Immunol. 88:210–218 [DOI] [PubMed] [Google Scholar]
- 8. Dodd J, Jessell TM. 1985. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 5:3278–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert M, et al. 2011. Using centralized laboratory data to monitor trends in herpes simplex virus type 1 and 2 infection in British Columbia and the changing etiology of genital herpes. Can. J. Public Health 102:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halford WP, Gebhardt BM, Carr DJJ. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542–3549 [PubMed] [Google Scholar]
- 11. Halford WP, et al. 2011. A live-attenuated HSV-2 ICP0− virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 6:e17748 doi:10.1371/journal.pone.0017748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845–853 [DOI] [PubMed] [Google Scholar]
- 13. Härle P, Noisakran S, Carr DJJ. 2001. The application of a plasmid DNA encoding IFN-α1 postinfection enhances cumulative survival of herpes simplex virus type 2 vaginally infected mice. J. Immunol. 166:1803–1812 [DOI] [PubMed] [Google Scholar]
- 14. Henken DB, Martin JR. 1992. The proportion of galanin-immunoreactive neurons in mouse trigeminal ganglia is transiently increased following corneal inoculation of herpes simplex virus type-1. Neurosci. Lett. 140:177–180 [DOI] [PubMed] [Google Scholar]
- 15. Hu K, et al. 2011. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine 29:1455–1462 [DOI] [PubMed] [Google Scholar]
- 16. Kaneko H, et al. 2008. Evaluation of mixed infection cases with both herpes simplex virus types 1 and 2. J. Med. Virol. 80:883–887 [DOI] [PubMed] [Google Scholar]
- 17. Kim T, et al. 2010. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 107:15181–15186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kortekangas-Savolainen O, Vuorinen T. 2007. Trends in herpes simplex virus type 1 and 2 infections among patients diagnosed with genital herpes in a Finnish sexually transmitted disease clinic, 1994–2002. Sex. Transm. Dis. 34:37–40 [DOI] [PubMed] [Google Scholar]
- 19. Lewandowski G, Hobbs MV, Bloom FE. 1994. Alteration of intracerebral cytokine production in mice infected with herpes simplex virus types 1 and 2. J. Neuroimmunol. 55:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindqvist M, Persson J, Thorn K, Harandi AM. 2009. The mucosal adjuvant effect of α-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J. Immunol. 182:6435–6443 [DOI] [PubMed] [Google Scholar]
- 21. Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Z, et al. 2009. High-level expression of glycoprotein D by a dominant-negative HSV-1 virus augments its efficacy as a vaccine against HSV-1 infection. J. Invest. Dermatol. 129:1174–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milligan GN. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milligan GN, Bernstein DI. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259–268 [DOI] [PubMed] [Google Scholar]
- 26. Morello CS, Levinson MS, Kraynyak KA, Spector DH. 2011. Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease. J. Virol. 85:3461–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. 2011. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parr MB, Parr EL. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peña KC, Adelson ME, Mordechai E, Blaho JA. 2010. Genital herpes simplex virus type 1 in women: detection in cervicovaginal specimens from gynecological practices in the United States. J. Clin. Microbiol. 48:150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raftery MJ, et al. 1999. Herpes simplex virus type-1 infection of activated cytotoxic T cells: induction of fratricide as a mechanism of viral immune evasion. J. Exp. Med. 190:1103–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith TJ, Ackland-Berglund CE, Leib DA. 2000. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J. Virol. 74:3598–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanberry LR, et al. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 33. Straus SE, et al. 1993. Induction and enhancement of immune responses to herpes simplex virus type 2 in humans by use of a recombinant glycoprotein D vaccine. J. Infect. Dis. 167:1045–1052 [DOI] [PubMed] [Google Scholar]
- 34. Taxini CL, Takakura AC, Gargaglioni LH, Moreira TS. 2011. Control of the central chemoreflex by A5 noradrenergic neurons in rats. Neuroscience 199:177–186 [DOI] [PubMed] [Google Scholar]
- 35. Templeton A, Nguyen G, Ash JD, Straub RH, Carr DJJ. 2008. Chemical sympathectomy increases susceptibility to ocular herpes simplex virus type 1 infection. J. Neuroimmunol. 197:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thapa M, Kuziel WA, Carr DJJ. 2007. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 81:3704–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unterholzner L, et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitley RJ, Roizman B. 2009. Herpes simplex viruses, p 409–449 In Richman DD, Whitley RJ, Hayden FG. (ed), Clinical virology, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 39. Wuest TR, Carr DJJ. 2008. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J. Immunol. 181:7985–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wuest TR, Carr DJJ. 2010. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 207:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, et al. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2:129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]