Abstract
Yersinia entomophaga MH96, which was originally isolated from the New Zealand grass grub, Costelytra zealandica, produces an orally active proteinaceous toxin complex (Yen-Tc), and this toxin is responsible for mortality in a range of insect species, mainly within the Coleoptera and Lepidoptera. The genes encoding Yen-Tc are members of the toxin complex (Tc) family, with orthologs identified in several other bacterial species. As the mechanism of Yen-Tc activity remains unknown, a histopathological examination of C. zealandica larvae was undertaken in conjunction with cultured cells to identify the effects of Yen-Tc and to distinguish the contributions that its individual subunit components make upon intoxication. A progressive series of events that led to the deterioration of the midgut epithelium was observed. Additionally, experiments using a cell culture assay system were carried out to determine the cellular effects of intoxication on cells after topical application and the transient expression of Yen-Tc and its individual components. While observations were broadly consistent with those previously reported for other Tc family members, some differences were noted. In particular, the distinct stepwise disintegration of the midgut shared features associated with both apoptosis and necrotic programmed cell death pathways. Second, we observed, for the first time, a contribution of toxicity from two chitinases associated with the Yen-Tc complex. Our findings were suggestive of the activities encoded within the subunit components of Yen-Tc targeting different sites along putative programmed cell death pathways. Given the observed broad host range for Yen-Tc, these targeted loci are likely to be widely shared among insects.
INTRODUCTION
The toxin complex (Tc) family is a bacterially produced family of multisubunit supramolecular “ABC” protein complexes, with the individual Tc protein subunits defined as subunits A, B, and C; all subunit classes are required for full insecticidal activity (10). Since their original discovery, orthologs of the Tc components have since been identified in several bacterial species from a wide variety of Gram-negative bacteria (e.g., Serratia entomophila Sep; Photorhabdus luminescens Tca, Tcb, Tcc, and Tcd; Xenorhabdus nematophilus Xpt; and Yersinia pseudotuberculosis Tca-like) (5, 15, 18, 31) and at least two Gram-positive bacteria (e.g., Paenibacillus nematophila Tca-like and Bacillus thuringiensis Tca-like) (4, 11).
Currently, the mode of action of Tc is not well described. The histopathological effects of Tc complexes from P. luminescens against Manduca sexta (hawk moth) and Leptinotarsa decemlineata (Colorado potato beetle) demonstrated that exposure to the Tca Tc complex triggered the destruction of the midgut through the lysis of midgut epithelia (2, 3). In contrast to this, no discernible effect of the S. entomophila Sep Tc complex on larvae of the New Zealand grass grub, Costelytra zealandica, was observed after treatment; the amber disease caused by the Sep proteins is chronic, with the larvae taking 3 to 4 months to die after the ingestion of S. entomophila (21).
Work to date on the understanding of the role of individual Tc subunits in the observed toxicity has provided evidence that the A subunit component contributes to host range specificity, with the Xenorhabdus A protein subunit (XptA1) being shown to bind to brush border membranes of Pieris brassicae (cabbage butterfly) (28). Second, host toxicity can be changed by interchanging the A subunit component from different Tc loci within or between bacterial species with that of the BC subcomplex (32, 34). Subsequently, Lang et al. (27) showed that the C subunits function as the major toxin components, with the C3 and C5 subunits from P. luminescens Tca encoding ADP-ribosylate activities against actin and RhoA, respectively. To date, no specific function has been demonstrated for the B subunit component.
The bacterium Yersinia entomophaga MH96 was originally isolated from a diseased C. zealandica larva (16). Subsequent host range testing revealed that the bacterium is able to cause mortality across a broad range of insect species, including those of the orders Coleoptera (e.g., C. zealandica) and Lepidoptera (e.g., Plutella xylostella, the diamondback moth) (19). The major pathogenicity determinant for Y. entomophaga has since been identified as a 2.46-MDa multisubunit protein Tc and is referred to as Yen-Tc (10). The genes that encode the individual A, B, and C subunits of the Yen-Tc complex are located on a 31.8-kb pathogenicity island, with the Tc operon containing two tcA-like genes (yenA1 and yenA2), a tcB-like gene (yenB), and two tcC-like genes (yenC1 and yenC2) (19). Interestingly, a point of difference with Yen-Tc compared to other members of the Tc family is the required coexpression of two chitinase genes (chi1 and chi2 [both protein products are enzymatically active]), whose positions flank the yenA1 and yenA2 genes (6, 19, 25). The structure of Yen-Tc has recently been resolved by negative-stain reconstruction to a resolution of 17 Å (25). The A1 and A2 components were found to form a pentameric pyramidal cage-like structure with the chitinase protein subunits decorating the outer shell. The B and C components remained unresolved but were found through density mapping to be positioned at the pyramidal point of the pentamer.
Larvae of the New Zealand grass grub, C. zealandica, are the original hosts of two different Tc-bearing bacteria, with distinct Tc variants (Sep and Yen-Tc) producing different pathologies. This places C. zealandica as a model to delineate potential differences or commonalities between these Tc clusters. The chronic (but relatively benign) effects of Tcs from pADAP-bearing strains of S. entomophila on C. zealandica have been well studied (17, 18, 20, 21); however, the effects of the highly potent Yen-Tc toxin have yet to be fully elucidated (19). We present here a detailed histological study of the effects of the ingestion of Yen-Tc on C. zealandica larvae to determine the histopathology of this toxin. Additionally, we have extended our study to cultured cell lines to investigate the effects that individual Yen-Tc subunit components have on the observed histopathology and cytology.
MATERIALS AND METHODS
Source of bacteria and production of toxin complex proteins.
Stock cultures of Y. entomophaga (strain MH96T [ATCC BAA-1678T]) are maintained at AgResearch, Lincoln, New Zealand (AgResearch Bacteria Culture Collection) (16). Methods for the production and purification of Yen-Tc were described previously by Landsberg et al. (25). Briefly, 50-ml cultures of Y. entomophaga were grown overnight at 25°C in LB broth and spun down to collect the Yen-Tc-containing supernatant. This supernatant was subjected to ammonium sulfate precipitation and size-exclusion chromatography. After fractions containing pure Yen-Tc were identified, pooled fractions were spun through an Amicon Ultracel-30K device to concentrate purified Yen-Tc in Tris-buffered saline (TBS) (25 mM Tris, 130 mM NaCl [pH 7.5]). The protein concentration of purified Yen-Tc was determined by using a Bradford assay (Bio-Rad).
Collection, handling, and treatment of C. zealandica larvae.
Live third-instar C. zealandica larvae were collected from pastures in the Canterbury region of South Island, New Zealand, and held in soil at 4°C. Prior to the experimental treatments being set up, larvae were removed from soil, fed carrot (3-mm cube), and incubated at 15°C. After 3 days of assessment for feeding activity and the absence of disease symptoms, healthy larvae were selected, randomly separated into groups, and starved of food for 24 h prior to experimental treatment. Bioassays were carried out in triplicate, with each replicate consisting of 4 larvae per treatment over a time course (0-, 16-, 24-, 48-, 72-, and 96-h intervals). The starved, healthy larvae were placed into 24-well culture trays (1 larva per well) and incubated at 15°C. Experimental treatments were administered as a single 5-μl dose of purified Yen-Tc (250 ng) or 5 μl of TBS (control treatment) via direct oral injection into the larval foregut. After treatment, larvae were fed carrot (3-mm cube), which was replenished daily, and symptom phenotypes were recorded for each time interval.
Histopathology studies.
Larvae were removed from trays at appropriate time intervals and prepared for histological processing. For the sample from each time point, the larval cuticle was opened to expose the digestive tract, and the grub was fixed for 48 h in a 10% neutral buffered formalin solution. Standard paraffin embedding, serial sectioning, and hematoxylin and eosin (H&E) staining methods (23) were carried out on samples by Gribbles Veterinary Services (Christchurch, New Zealand). Stained slides of the alimentary tract were examined under bright-field and differential interference contrast (DIC) optics by using an Olympus BX50 upright microscope and photographed with an Olympus DP-12 digital camera.
Electron microscopy.
Transmission electron microscopy (TEM) was carried out on midgut samples collected from third-instar C. zealandica larvae 24 h after treatment with either TBS or Yen-Tc. Tissue was dissected in ice-cold primary fixative (2% formaldehyde, 2.5% glutaraldehyde, and 2 mM CaCl2 in 0.1 M cacodylate buffer [pH 7.2] with hydrogen peroxide drops added just prior to dissection). The dissected midgut was then transferred into fresh fixative for 1 to 1.5 h at room temperature (∼22°C). Following three 15-min washes in buffer (0.1 M cacodylate, 2 mM CaCl2 [pH 7.2]), samples were stored overnight at 4°C and then placed in 4°C secondary fixative {1% OsO4, 1.5% K3[Fe(CN)6]} for 1 h, followed by three washes (5 min each) with ultrapure water at room temperature. Samples were soaked in mordant (1% tannic acid in phosphate buffer [pH 7.0]) for 2 h and washed for 15 min in a solution of 1% Na2SO4 in phosphate buffer (pH 7.0), followed by three 5-min water washes, before being placed into a tertiary fixation solution (1% uranyl acetate) at room temperature for 1 h in the dark. Samples were washed three times (5 min each) in water and stored overnight at 4°C. Dehydration was done via a graded ethanol series (70, 80, and 90% ethanol for 15 min each at room temperature), twice in 100% ethanol (20 min each), and then in dry acetone (20 min). Samples were embedded in Procure 812 (ProSciTech Pty., Australia) epoxy resin (50% resin–50% acetone for 1 h, three changes of 100% resin for 1 h, and 100% resin overnight with rotation). Resin blocks were polymerized at 60°C for 22 h. Sections (100 nm thick) were cut by using an Ultracut UCT ultramicrotome (Leica, Germany) fitted with a 45° diamond knife (Diatome, Switzerland). Sections were cut onto ultrapure water, collected onto 100-mesh copper Formvar-coated grids, and examined by using an FEI Morgagni 268D transmission electron microscope. Micrographs were captured by using an SIS/Olympus Megapixel III digital camera mounted above the phosphor screen.
Programmed cell death assays.
Sectioned larval samples for terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining were prepared from paraffin blocks as described above but without H&E staining. TUNEL reactions were conducted according to the manufacturer's instructions, using an in situ cell death detection kit (tetramethylrhodamine [TMR] red; Roche). Sections were counterstained to visualize nuclei by incubating slides at room temperature for 5 min in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, and 10 mM phosphate buffer solution [pH 7.4]) containing 100 ng ml−1 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI; Roche), followed by three 5-min rinses in PBS. Prior to coverslipping, the stained sections were mounted with a drop of antifade mountant consisting of 1% (wt/vol) Mowiol 4-88 (Calbiochem) and 2.5% (wt/vol) 1,4-diazabicyclo[2.2.2]octane (DABCO; Sigma) in Tris-buffered glycerol (pH 8.5) (1). Fluorescence staining was observed by using Olympus filter sets U-MWIG2 (for TMR) (520- to 550-excitation wavelength [ex]/588-nm emission wavelength [em]) and U-MWU2 (for DAPI) (330- to 385-nm ex/420-nm em).
Samples for the monitoring of caspase-3/7 activity were collected by dissecting midguts from TBS- and Yen-Tc-treated grubs at various time points, with three replications of six larvae per treatment. Following dissection, midguts were rinsed in 1 to 2 ml PBS and ground with micropestles in 1.7-ml tubes containing 100 μl of ice-cold extraction buffer (20 mM Tris-HCl, 5 mM EDTA, 5 mM EGTA, 1 mM dithiothreitol [DTT], 150 mM NaCl [pH 7.5]). Samples were centrifuged for 10 min at 13,000 × g, and supernatants were used for enzyme assays. Caspase-3/7 activity was measured by the addition of 5 μl of midgut extract to 100 μl caspase reaction buffer (25 mM HEPES-KOH, 10% glycerol, 0.25 mM EDTA, 2.5 mM DTT [pH 7.5]), with the reaction initiated by the addition of N-acetyl–Asp–Glu–Val–Asp–p-nitroanilide (DEVDpNA) (caspase-3/7 substrate) to a final concentration of 0.1 mM. For inhibitor experiments, prior to the addition of DEVDpNA, the midgut sample was incubated for 30 min in caspase reaction buffer supplemented with either 0.1 mM N-acetyl–Asp–Glu–Val–Asp–Val (DEVD-CHO) or 0.1 mM (each) leupeptin and aprotinin. The reactions were monitored at 410 nm by using a BMG Fluorostar Optima microplate reader, and initial velocities were determined.
Topical application of the native Yen-Tc complex.
Clonal Spodoptera frugiperda IPLB-Sf-21-AE (Sf9) cells (Invitrogen) were seeded into 6-well plates containing borosilicate glass coverslips (BDH Merck Ltd.) and grown for 24 h in Grace's medium, supplemented with 3.3 g liter−1 lactalbumin hydrolysate hydrosolate (Invitrogen), 3.3 g liter−1 yeastolate l-glutamine (Invitrogen), and gentamicin (25 μg ml−1) (Sigma) and incubated in air at 28°C until cells were 80% confluent. Purified Yen-Tc in a solution containing 20 mM Tris-HCl (pH 7.5) and 130 mM NaCl was added to 1 ml of fresh medium to a final concentration of 5 ng ml−1 and incubated for 24 h at 28°C. After 24 h, cells were washed with PBS and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature before being permeabilized with 0.02% Tween 20 in PBS for 20 min. Cells were washed three times in PBS before being incubated with 50 μg ml−1 fluorescein isothiocyanate (FITC) phalloidin (Sigma) for 1 h at room temperature in the dark, followed by a 10-min incubation with 0.12 mg ml−1 Hoechst 33258 DAPI stain (Sigma). Cells were subsequently washed twice with PBS, followed by two washes with distilled water. Coverslips were mounted with Mowiol mounting medium (0.12 M Tris-HCl [pH 6.8], 30% glycerol, 12% Mowiol, 2.5% DABCO). Slides were examined and photographed with an Olympus BX61 microscope. Each experiment was conducted in triplicate and repeated twice.
The human Caucasian colon adenocarcinoma cell line (Caco-2) (12) was treated as described above, except that cells were incubated with Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal calf serum, 1% nonessential amino acids (Sigma), and 1% penicillin and streptomycin at 37°C with 5% CO2.
Construction of Y. entomophaga Tc Myc fusion expression vectors.
Full-length Yen-Tc-associated coding sequences were PCR amplified by using primers Chi1, A1 (yenA1), A2 (yenA2), Chi2, B (yenB), C1 (yenC1), and C2 (yenC2), which contain appropriate Myc (forward) and Myc-STOP (reverse) primer combinations (Table 1), to allow the introduction of an N-terminal Myc fusion tag. PCRs were performed by using Accuprime Pfx DNA polymerase (Invitrogen) according to the manufacturer's instructions, with amplified products subsequently being cloned into the BamHI and XbaI restriction sites of the cytomegalovirus (CMV) promoter-based eukaryotic expression vector pRK5myc (Clontech). PCR products and plasmid templates were purified by using the High Pure PCR product purification kit and the High Pure plasmid isolation kit (Roche Diagnostics GmbH), respectively. All pRK5myc-Tc subunit-derived clones were sequenced to confirm the integrity of the expression constructs.
Table 1.
Primer sequences and their resultant pRK5myc clone designations
| Primer | Primer sequence (5′→3′)a | Plasmid |
|---|---|---|
| Chi1-Myc | AAAGGATCCATGGAAAAAGAAGAAAAAAGCAATCTCATCTACG | pRK5myc-Chi1 |
| Chi1-Myc-STOP | AAATCTAGATTATTTGATATTACATGCTTCGCACTGTGGC | |
| A1-Myc | AAAGGATCCATGGATAAATATAATAATTATTCTAATGTAA | pRK5myc-A1 |
| A1-Myc-STOP | AAATCTAGATTAGACATTATCGCTATCTCCTTGTGCCC | |
| A2-Myc | AAAGGATCCATGTCTAATTCTATTGAAGCGAAACTACAGG | pRK5myc-A2 |
| A2-Myc-STOP | AAATCTAGATTATTCATCGTTGTTTATATTGGCCAATATTTTCC | |
| Chi2-Myc | AAAGGATCCATGGTCAATAAATATACTTACACCTCATC | pRK5myc-Chi2 |
| Chi2-Myc-STOP | AAATCTAGATTTACTTGCTCTTAATTTCAGAAATTGATTTTAAG | |
| B-Myc | AAAGGATCCATGCAAAATTCACAGGAAATGGCTATCACG | pRK5myc-B |
| B-Myc-STOP | AAATCTAGATCTAAATCGCCAGATTTGCTGCCGTATCG | |
| C1-Myc | AAAGGATCCATGAACCAGTTTGATTCTGCGCTCCATC | pRK5myc-C1 |
| C1-Myc-STOP | AAATCTAGATCAGCGTAATTTAACGACGGTATTCTTTC | |
| C2-Myc | AAAGGATCCATGGACATACAGCTGTTTAGTAAAACCCC | pRK5myc-C2 |
| C2-Myc-STOP | AAATCTAGACTAAAATGACTTTCGACGCTTTAGTCTTACATC |
Underlining indicates the restriction enzyme site used for cloning; boldface type denotes the Tc-derived termination codon (STOP).
Transient expression of individual Yen-Tc components in cell culture.
Caco-2 cells were seeded into 6-well plates containing borosilicate glass coverslips (BDH) and grown for 24 h in 2 ml DMEM (Invitrogen) supplemented with 10% fetal calf serum, 1% nonessential amino acids (Sigma), and 1% penicillin and streptomycin at 37°C in 95% air–5% CO2 (vol/vol) until cells were 80% confluent. One microgram of each RK5myc-Tc subunit clone was cotransfected with 0.5 μg pEGFP-actin by using Nanojuice (Invitrogen). Each experiment was performed in triplicate and repeated independently three times. After 24 h, the cells were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 in PBS, and blocked with Image-iT FX signal enhancer (Invitrogen) according to the manufacturer's instructions. Cells were labeled with monoclonal mouse anti-Myc primary antibody (Invitrogen) and visualized with Alexa Fluor 594 goat anti-mouse dye-labeled secondary antibody (Molecular Probes) to detect the Myc-labeled protein. Nuclei were visualized by incubation with Hoechst 33258 DAPI stain (Sigma) at a final concentration of 0.12 mg ml−1 in PBS for 10 min, followed by two washes each with PBS and double-distilled water. Coverslips were mounted in Mowiol mounting medium and subsequently examined and photographed by using an Olympus BX61 microscope.
RESULTS
Gross external phenotypic effects of Yen-Tc exposure on C. zealandica larvae.
C. zealandica larvae exposed to 250 ng of purified Yen-Tc toxin displayed the same progression of symptoms that was observed previously for larvae that ingested live Y. entomophaga cells (19). Prior to treatment, healthy larvae exhibited active movement, a darkened gut filled with ingested food/soil matter, and an expulsion of discrete frass pellets. Within 1 to 2 days following the ingestion of Yen-Tc, C. zealandica larvae displayed an amber coloration and clearance of gut contents (see Fig. S1 in the supplemental material), which was similar to the phenotype described previously by Hurst et al. (19). This was accompanied by notable fluid loss through vomiting (larvae also became increasingly flaccid), an excessive expulsion of discrete frass material, and the progressive development of lethargy and browning coloration. The average time to death was 5.7 ± 0.4 days posttreatment, which was estimated by using the Kaplan-Meier method (22), with the observed mortality rates being 41.7% by day 4 and 80.6% by day 9 posttreatment (see Table S1 in the supplemental material).
Histopathological examination of Yen-Tc treatment of C. zealandica larvae.
All third-instar C. zealandica tissues were initially examined for damage caused by Yen-Tc exposure. However, prior to larvae reaching a moribund stage, only the midgut displayed major alterations in morphology. Therefore, we focused on the changes occurring in the larval midgut as a result of Yen-Tc intoxication. In conjunction with an examination of the histopathological effects of purified Yen-Tc on the C. zealandica midgut, we conducted control oral injections with TBS buffer (the carrier buffer for purified Yen-Tc) to ensure that the detection of any effects was not caused by the delivery procedure itself. No damage or alteration to the midgut was observed for larvae injected with TBS.
In healthy third-instar C. zealandica larvae, the anterior midgut is comprised of densely packed elongated columnar cells neatly arranged in a typical invaginated configuration, with large ovoid nuclei lined up in a chain-like fashion, while the posterior midgut is similarly arranged but lacks the invaginations prominent in the anterior midgut (Fig. 1A and B). The tissue folds in the anterior midgut are thought to be caused by the high abundance of underlying regenerative nidi (RN) and presumptive endocrine cells; note that goblet cells, which are typical of other holometabolous insects, are absent in the C. zealandica larval midgut. Histological observations also revealed well-defined microvilli lining the midgut lumen (Fig. 1A and B). The gut lumens from TBS-treated larvae were filled with ingested food/soil material enclosed by a peritrophic membrane to form a discrete food bolus (Fig. 1A and B). Other features observed included the occasional presence of nucleus-free vesicles, which are thought to be either putative aprocrine secretory vesicles or “old/damaged” columnar cells sloughed off from the midgut epithelium (data not shown).
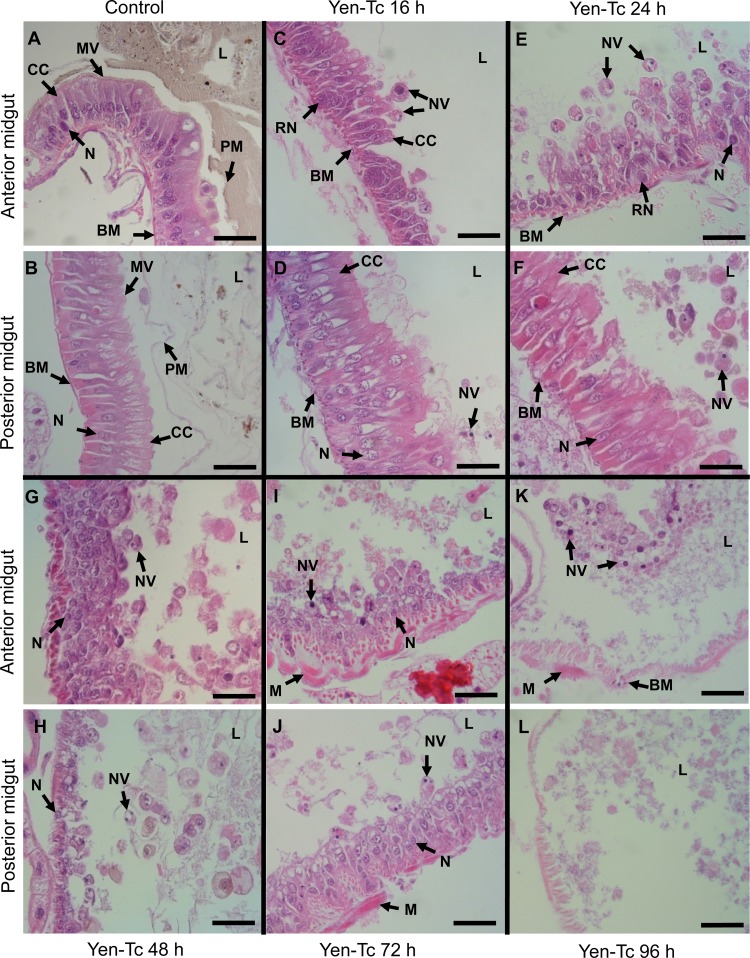
Fig 1.
Longitudinal midgut sections from third-instar C. zealandica larvae stained with hematoxylin and eosin following oral ingestion of either a TBS buffer-only (control) treatment (24 h posttreatment) (A and B) or the purified Y. entomophaga Tc protein complex at the indicated time points (C to L). All images are representative of midgut tissue located either anteriorly or posteriorly (as indicated and in relation to the first and second cecal rows of the C. zealandica digestive tract). (A and B) For the control treatment, note the well-formed columnar cells, an intact microvillus surface lining the luminal surface of the midgut, and the presence of ingested soil matter enclosed by a peritrophic membrane. (C and D) At 16 h post-Yen-Tc treatment (C), food matter had generally disappeared, the anterior columnar cells had shrunk, and a sloughing of nucleus-containing vesicles into the gut lumen was observed; during this time, the columnar cells in the posterior gut (D) were only just beginning to show signs of disorganization and shrinkage. (E and F) By 24 h, both the anterior (E) and posterior (F) regions of the gut epithelium showed obvious signs of intoxication, with nucleus-containing vesicles being easily observed within the luminal space. (G to L) From 48 h onwards, the entire gut was in the process of disintegration, and by 96 h (K and L), remnants of the midgut were only occasionally detected. Arrows point to highlighted structures labeled as follows: BM, basement membrane; CC, columnar cell; L, gut lumen; M, muscle; MV, microvilli; N, nucleus; NV, nucleus-containing vesicles; PM, peritrophic membrane; RN, regenerative nidi; V, vesicle. Scale bars represent 50 μm.
For the purposes of comparison against both control-treated (TBS, as described above) and Yen-Tc-treated (see below) larvae, histology sections from midgut tissues of larvae orally treated with the Serratia entomophila Sep toxin (another member of the Tc family which also induces a gut-clearing, amber coloration phenotype in C. zealandica larvae) were also examined (see Fig. S2 in the supplemental material). Although the Sep complex itself elicited the clearance of the gut contents (resulting in an amber gut coloration), no other obvious changes in the midgut epithelial structure were observed after the administration of the Sep protein complex. Although the reasons for this remain unknown, the lack of an obvious effect was not unexpected, since the ingestion of the amber disease-causing bacterium, S. entomophila, was previously demonstrated to have limited toxicity to C. zealandica larvae (20, 21).
During the early stages following the oral injection of purified Yen-Tc (prior to approximately 16 h posttreatment), any observed change or damage was inconsistent and difficult to detect, with only a few affected cells seen among a mass of relatively healthy-looking midgut cells. Beginning approximately 16 h after the oral administration of the Yen-Tc dose, an anterior-to-posterior sequence of events was consistently observed for the C. zealandica larval midgut (Fig. 1C and D). Due to the anterior-posterior progression of the observed effects, we have arbitrarily divided the midgut into two portions. The anterior section begins at the cardiac valve and ends just ahead of the first cecal row, while the posterior section of the midgut extends from the two rows of cecae and continues to the pyloric sphincter.
From 16 to 48 h after treatment (Fig. 1C to H), the most conspicuous histological changes were the clearing of gut contents accompanied by the progressive deterioration and eventual loss of the peritrophic membrane. Concurrently, patches of microvilli became less distinct and eventually disappeared throughout the midgut by 48 h.
Between approximately 16 and 24 h post-Yen-Tc treatment (Fig. 1C to F), the anterior midgut columnar cells displayed prominent vacuolization and began to lose their normal neatly ordered rectangular form, with the cells taking on an oval and slightly shrunken and ragged appearance and with the arrangement of the nuclei now appearing disarrayed. The loss of columnar cell integrity coincided with the appearance in the gut lumen of rounded vesicles containing putative condensed pyknotic nuclei (“nuclear vesicles”). The posterior midgut columnar cells displayed a similar progressive disorganization and extrusion of these cell-like bodies; however, the timing of events was shifted approximately 4 to 8 h after the events of the anterior midgut (Fig. 1D and F).
By 48 h, a large number of vesicles had already been sloughed off into the midgut lumen; this was accompanied by the large-scale deterioration of the gut epithelium (Fig. 1G and H). At 72 h post-Yen-Tc treatment, the epithelial cells of the midgut had rapidly degenerated to a point where it was difficult to discern any specific pattern of events (Fig. 1I and J). By 96 h, the midgut epithelium had completely broken down, with remnants of the putative midgut being observed in only a few sections from the specimens examined (Fig. 1K and L).
Ultrastructural changes observed for C. zealandica midgut cells exposed to Yen-Tc.
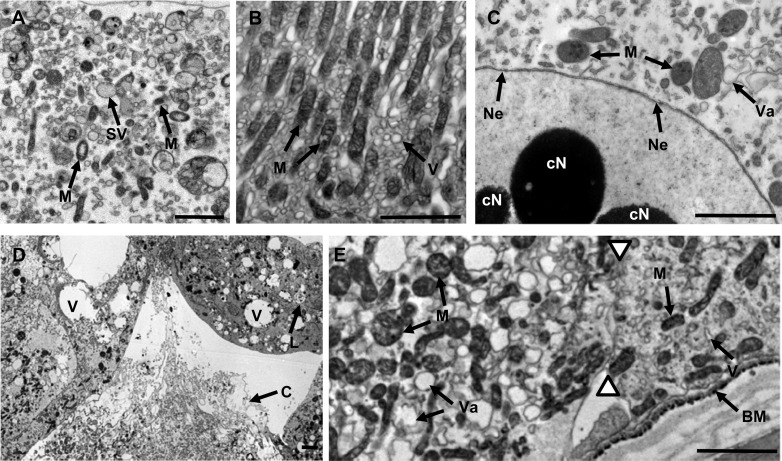
To examine more closely the subcellular effects of Yen-Tc intoxication, ultrathin sections of midgut tissue were examined 24 h after treatment (Fig. 2). For larvae treated with TBS as a control treatment (Fig. 2A and B), a wide array of structures (presumptive secretory vesicles, mitochondria, and other unidentified structures) were observed, and the nucleus appeared to be intact, with chromatin material being well dispersed. Toward the basal end of the cell, the mitochondria were packed in an anisotropic configuration, being oriented perpendicular to the nearby basal membrane (Fig. 2B). However, 24 h after Yen-Tc treatment (Fig. 2C to E), many changes were observed. Large, roughly circular patches of condensed chromatin became apparent within the nuclei of several cells (although this was not observed for all cells), and breakages in one layer of the nuclear envelope double layers were also detected (Fig. 2C). The cells' basal mitochondria had a more isotropic appearance than those of the control cells, and throughout the cells, most mitochondria appeared slightly swollen, with dark spots now being easily observed within them (Fig. 2C and E). As shown in Fig. 2D, cells that displayed a cytoplasm containing numerous vacuoles and dark electron-dense staining material were also observed, and there was also evidence for the cytoplasm being released from cells with broken membranes. Finally, as indicated by histology sections (Fig. 1), not all cells responded uniformly to toxin treatment (as shown in Fig. 2E).
Fig 2.
Transmission electron micrographs of ultrathin sections (stained with 1% uranyl acetate) of midgut tissue from third-instar C. zealandica larvae 24 h following oral ingestion of either control treatment (TBS buffer only) (A and B) or purified Yen-Tc toxin (C to E). (A) The apical portion of a healthy active midgut cell showing structures involved in secretion and absorption. (B) The basal portion of a healthy midgut cell showing elongated mitochondria oriented along a basal-apical axis, surrounded by small smooth vesicles. (C) At 24 h post-toxin treatment, the region around the nucleus had variable-sized, irregular-shaped vesicles and swollen mitochondria with prominent dark spots, while the nucleus displayed a globular electron-dense condensation of nuclear material and evidence of breaks in the double membrane of the nuclear envelope. (D) Presence of both apoptotic-like cells (upper cell showing a condensed cytoplasm with evidence of organelles being broken down in lysosomes) and necrotic-like cells (lower cell showing cytoplasm escaping from broken membranes). (E) The two white triangles highlight the junction of two cells, revealing cell-to-cell variation, with the left-hand cell displaying variable-sized vesicles with irregular profiles and less-well-oriented mitochondria (both swollen and elongated mitochondria are present) and the right-hand cell showing less dramatic changes. Arrows point to highlighted structures labeled with as follows: B, basement membrane; cN, nucleus with condensed chromatin; M, mitochondria; Ne, nuclear envelope disrupted; SV, secretory vesicle; Va, vacuole. Paired white triangles indicate the boundary of two adjoining cells. Scale bars represent 2 μm.
Programmed cell death assays on C. zealandica larvae.
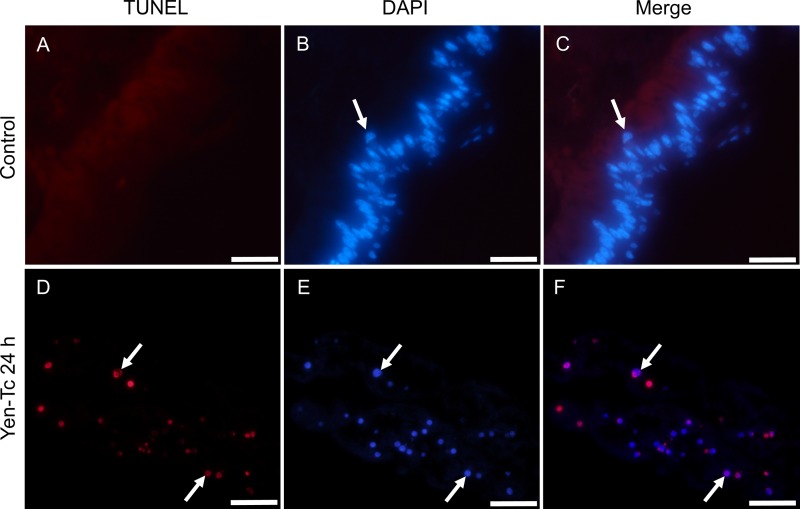
From the histopathological observations made subsequent to Yen-Tc exposure, it appeared that an ordered series of cellular events led to the eventual breakdown of the midgut tissue. In particular, the appearance of cell body-like vesicles containing condensed nuclear material displayed a striking similarity to one of the hallmark features described previously for cells undergoing an apoptosis-like processes (29). To determine if the condensed chromatin material was fragmenting (another characteristic of apoptotic nuclei), in situ TUNEL assays were carried out on midgut sections collected 24 h after either Yen-Tc or TBS buffer treatment (Fig. 3). TUNEL-positive cells were observed only in Yen-Tc treated larvae (Fig. 3D to F), indicative of nicked or broken DNA being present in those cells. Although positively stained cells were not as numerous as expected, nor were they as brightly labeled (compared to the positive-control DNase I-treated sections [data not shown]), the TUNEL negative-control sections (data not shown) and healthy midgut sections (Fig. 2A to C) were almost completely devoid of any staining in the TMR channel. Associated with the sloughed cell-like bodies within the gut lumen, the DAPI-counterstained sections revealed a pattern of densely staining DNA material, which corroborated the concentrated purple eosin pigmentation observed for the H&E-stained histology sections and the condensed chromatin in the TEM observations.
Fig 3.
TUNEL staining of longitudinal sections from third-instar C. zealandica larvae 24 h post-oral treatment with either the control (TBS buffer only) (A to C) or purified Yen-Tc (D to F). Arrows indicate examples of either TUNEL-positive nuclei (TMR label appears as red fluorescence) (A and D), nuclei stained with DAPI (blue fluorescence) (B and E), or nuclei displaying both TUNEL-positive and DAPI staining (purple appearance in merged images) (C and F). Scale bars represent 50 μm.
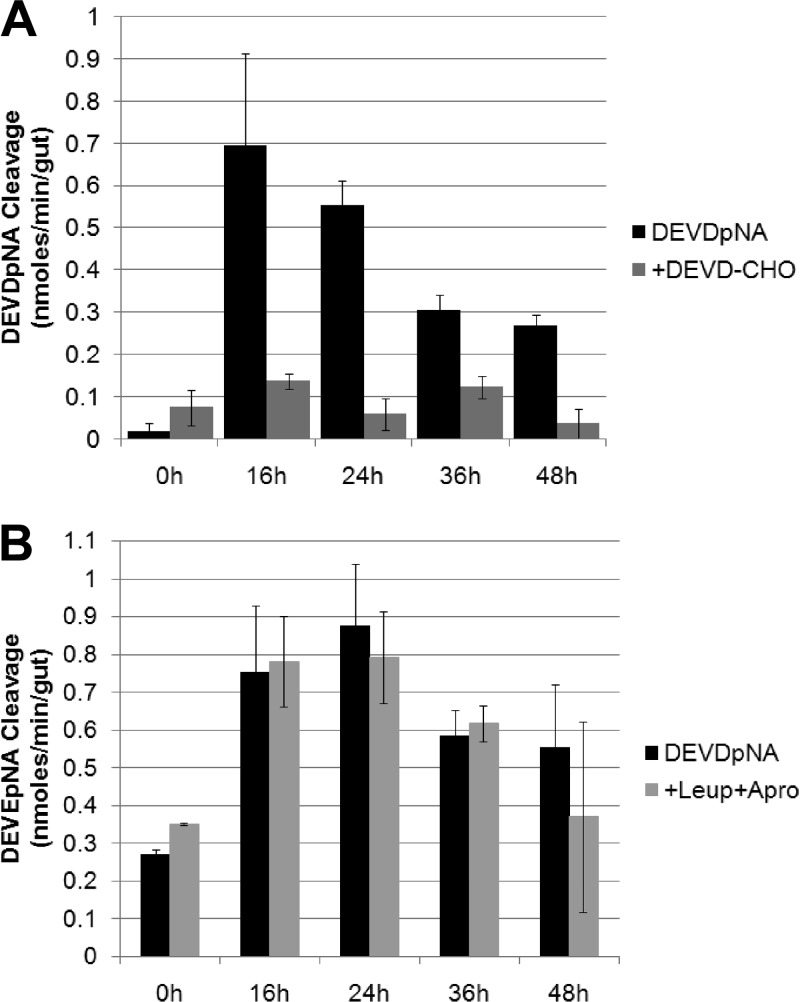
DNA damage can be caused by other processes, including other cell death pathways, such as programmed necrosis (24). Generally speaking, two principal mechanisms of apoptotic programmed cell death have been identified: the intrinsic pathway, where agents interact with the mitochondria, and the extrinsic pathway, where agents directly activate the family of cell death receptors. This results in the activation of a defined cascade of proteins known as the caspases, which initiate (caspase-2, -8, -9, and -10) and induce (caspase-3, -6, and -7) a progressive series of events which ultimately leads to cell death (24). To investigate if another component part of an apoptosis-like response was being activated, the appearance of cysteine aspartate-specific proteinase (caspase) activity in C. zealandica larval midguts exposed to the purified Yen-Tc protein complex was monitored (Fig. 4). The hydrolysis of the substrate DEVDpNA is generally accepted as a measure of caspase-3/7 activity being present. As shown in Fig. 4A, a negligible cleavage of DEVDpNA was observed for the mock-treated larvae (0 h), but by 16 h post-Yen-Tc treatment, a 43-fold increase in DEVDpNA hydrolysis was measured (0.694 versus 0.016 nmol min−1 gut−1); significant levels of caspase activity were also observed over time points from 24 to 48 h posttreatment. While a decline in the level of caspase activity over this time was observed, the levels were still manyfold higher than those observed for healthy larvae (with 34-, 19-, and 16-fold increases over the mock-treated larvae at 24, 36, and 48 h, respectively). The specific inhibition of this activity was demonstrated by the addition of the reversible inhibitor DEVD-CHO prior to the measurement of DEVDpNA cleavage (Fig. 4A). As C. zealandica midguts are known to contain a high level of serine protease activity, a second experiment was conducted, whereby extracts were preincubated with the serine protease inhibitors leupeptin and aprotinin (Fig. 4B) prior to the measurement of DEVDpNA hydrolysis, to ensure that promiscuous substrate utilization was not contributing to the activity levels observed. No significant inhibition was recorded when extracts were preincubated with the serine protease inhibitors leupeptin and aprotinin (Fig. 4B).
Fig 4.
Caspase activity present in gut extracts collected from third-instar C. zealandica larvae at 0, 16, 24, 36, or 48 h after control or Yen-Tc treatment (as indicated). (A) Hydrolysis of the caspase-3/7 substrate (DEVDpNA at a 0.1 mM final concentration) measured after incubation in either the absence or the presence of the caspase-3/7 inhibitor (DEVD-CHO at a 0.1 mM final concentration). (B) Hydrolysis of the caspase-3/7 substrate (DEVDpNA) measured after incubation in either the absence or the presence of the serine protease inhibitors leupeptin and aprotinin (0.1 mM each) (+Leu+Apro). The cleavage of DEVDpNA was measured as the release of p-nitroaniline (pNA).
Effects of Yen-Tc and individual Yen-Tc components on cultured cell lines.
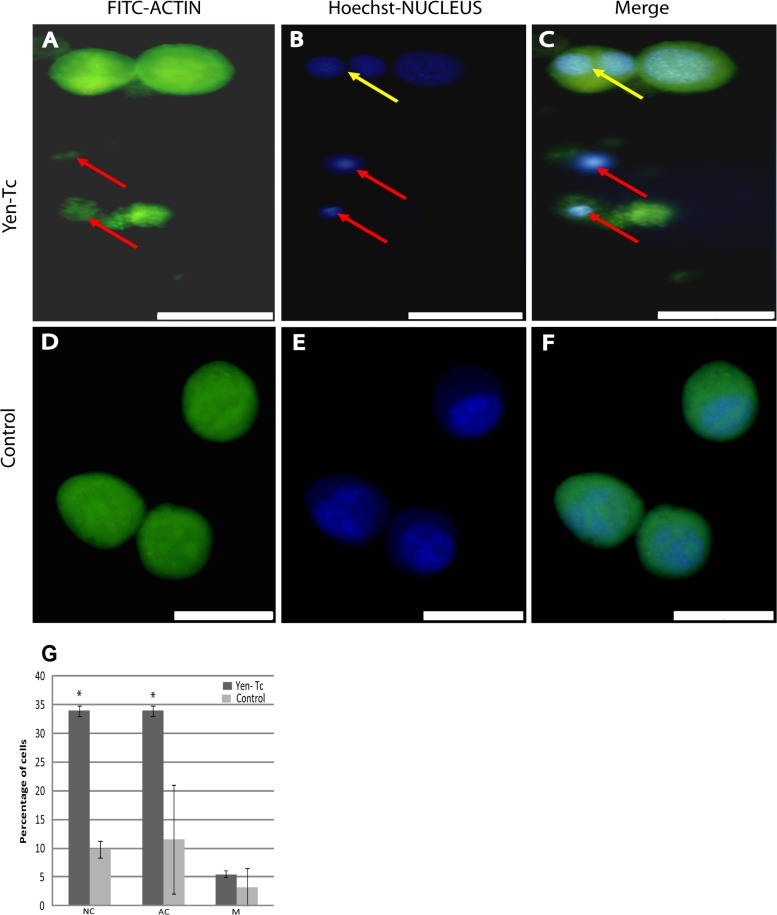
To delineate the effects observed with the histological examinations described above, Yen-Tc was topically applied to the Spodoptera frugiperda clonal insect cell line Sf9. Strikingly, after 24 h, 34% (standard error [SE] = 0.9) of cells showed apoptosis, as demonstrated by pyknotic condensed nuclei (NC) (Fig. 5B and C, red arrows), compared to 10% (SE = 1.5) of TBS-treated control cells (Fig. 5E and F), and was associated with actin condensation (AC) (Fig. 5A, C, and G). Yen-Tc-treated Sf9 cells either were observed to be normal compared to the control cells or showed nuclear and actin condensation. Few cells showed any intermediate stages, such as actin vacuoles (14%), and these effects were comparable to those on the control cells (13.5%). Interestingly, a small but not significant effect, an increase in the numbers of multinucleated cells (M) (5% of cells [SE = 0.9]) (Fig. 5B, C, and G), compared to 3% (SE = 3.25) of TBS-treated control cells, was also observed.
Fig 5.
(A to F) Fluorescence micrographs of Sf9 cells topically treated with the native Y. entomophaga toxin complex (A to C) or the Tris buffer control (D to F). Cells were stained for F-actin with FITC-phalloidin (A and D) and for nuclei with Hoechst 33258 DAPI stain (B and E); merged images are also presented (C and F). Red arrows indicate actin and nuclear condensation (A to C), and yellow arrows indicate multinucleated cells (B and C). (G) Graph presenting percentages of cells (minimum of 110 cells counted) scored that displayed actin condensation (AC), nuclear condensation (NC), and multinucleation (M). Asterisks indicate high numbers of cells showing actin and nuclear condensation compared to the control. Error bars represent standard errors. Scale bars represent 20 μm.
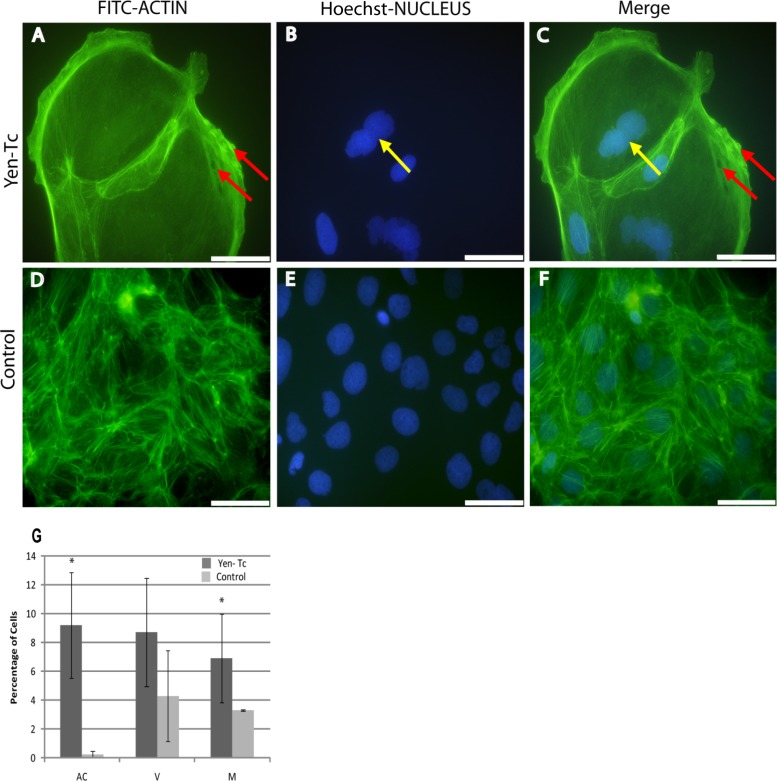
When Yen-Tc was applied to Caco-2 cells, only 0.8% of cells showed pyknotic condensed nuclei, which is considerably lower than that observed for the Sf9 cells topically treated with Yen-Tc (34%), while 9% (SE = 3.15) of Caco-2 cells showed AC (control, 0.25% [SE = 0.25]) (Fig. 6A, C, and G). However, increases in numbers of intermediate stages such as actin vacuoles (V) (9% [SE = 3.57]) (Fig. 6A and C) compared to the control (4.3% [SE = 3.15]) (Fig. 6D and F) as well as enlarged cells (7% [SE = 3.4]) compared to the control (2.4% [SE = 2.4]) were observed. An increase in numbers of multinucleated cells (7% [SE = 3.0]) (Fig. 6B and C), compared to 3% (SE = 0.4) of TBS-treated control cells (Fig. 6E and F), was also observed.
Fig 6.
(A to F) Fluorescence micrographs of Caco-2 cells topically treated with the native Y. entomophaga toxin complex (A to C) or the Tris buffer control (D to F). Cells were stained for F-actin with FITC-phalloidin (A and D) and for nuclei with Hoechst 33258 DAPI stain (B and E); merged images are also presented (C and F). Red arrows indicate vacuoles (A and C), and yellow arrows indicate multinucleated cells (B and C). (G) Graph presenting percentages of cells (minimum of 360 cells) with multinucleation (M), actin condensation (AC), or vacuole (V) phenotypes observed. Asterisks indicate high numbers of cells showing multinucleation and actin condensation compared to the control. Error bars represent standard errors. Scale bars represent 50 μm.
To further ascertain the importance of the individual components of Yen-Tc as well as the significance of Chi1 and Chi2, pRK5myc constructs of the components of the Yen-Tc complex (A, B, and C subunits as well as the two chitinases) were transfected into cells. As described above, similar phenotypic effects were observed for Caco-2 and Sf9 cells. However, the observed phenotypic effects of Yen-Tc on Sf9 cells (as shown in Fig. 5) occurred very rapidly (i.e., cell death within 24 h) in comparison to the events observed for Caco-2 cells (Fig. 6). Indeed, an analysis of Caco-2 cell viability by trypan blue staining or 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-hydroxide (XTT) colorimetric analysis showed an increased number of cells at 24 h; only after 48 h did the number of viable cells decrease (data not shown). The slower progression of Yen-Tc toxicity in Caco-2 cells likely reflects differences based on relative cell size, with Sf9 cells being smaller than Caco-2 cells, although a difference in host specificity may also contribute. Based on this slower progression and to allow comparisons with data from previously reported Tc-related studies undertaken by Waterfield et al. (34) and Hares et al. (15), Caco-2 cells were used for transfection studies. Additionally, Caco-2 cells are significantly larger than Sf9 cells and therefore easier to study for phenotypic variation.
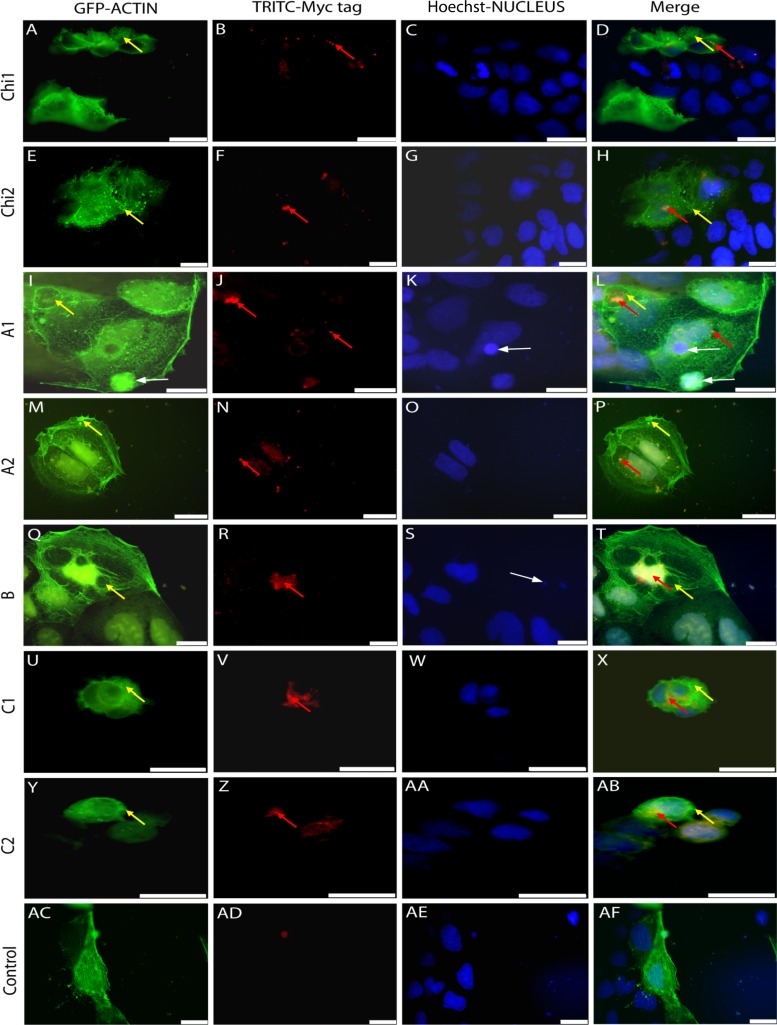
Surprisingly, after 24 h, 20% (SE = 10.2) of Caco-2 cells were observed to have actin blebs when treated with pRK5myc-Chi1 (Fig. 7A and D), and increases in numbers of cells with nuclear condensation (17% [SE = 4.6]) and nuclear blebbing (16% [SE = 3.7]) compared to the control (11% [SE = 1.6] and 10% [SE = 0.7], respectively) were also observed (Fig. 7AC and AF), while pRK5myc-Chi2 was observed to have an increase in the number of cells showing actin condensation (24% [SE = 5]), actin vacuoles (19% [SE = 3.8]), and multinucleation (10% [SE = 7.4]) compared to control cells (12.5% [SE = 2.3], 10% [SE = 6.3], and 6% [SE = 3], respectively) (Fig. 7AC to AF). Both pRK5myc-Chi1 and pRK5myc-Chi2 were observed to colocalize to the cytoplasm (Fig. 7B, D, F, and H). While effects were observed with Chi1 and Chi2, these were not as striking as those observed with the Yen-Tc A, B, or C construct.
Fig 7.
Intracellular transient expression of tc toxin genes from Y. entomophaga. Expressed cMyc fusion proteins were visualized with Alexa Fluor 594 goat anti-mouse dye-labeled secondary antibody (Molecular Probes) (B, F, J, N, R, V, and Z, compared to AD [control]). Actin was detected with an expressed actin-enhanced green fluorescent protein (EGFP) fusion protein (A, E, I, M, Q, U, Y, and AC), while nuclei were highlighted with Hoechst 33258 DAPI stain (C, G, K, O, S, W, AA, and AE). Merged fluorescence signals are presented in panels D, H, L, P, T, X, AB, and AF. (A to D) Cells transfected with pRK5myc-Chi1 and pEGFP-actin. These cells show actin blebs (yellow arrows) (A and D) and are localized to the cytoplasm (B and D) (red arrows). (E to H) Cells transfected with pRK5myc-Chi2 and pEGFP-actin. As can be observed, pRK5myc-Chi2 causes actin vacuoles (E and H) (yellow arrows) although to a lesser degree than those observed for RK5myc-A1 (I and L) and RK5myc-B (Q and T). Again, pRK5myc-Chi2 is localized to the cytoplasm (F and H). (I to L) Cells transfected with pRK5myc-A1 and pEGFP-actin. pEGFP-actin in RK5myc-A1 shows vacuoles (I and L) (yellow arrows) and nuclear (K and L) and actin (I and L) condensation (white arrows). The RK5myc-A1 tag shows localization to the cytoplasm (J and L) (red arrows). (M to P) Cells transfected with pRK5myc-A2 and pEGFP-actin. pEGFP-actin in RK5myc-A2 shows vacuoles (M and P) (yellow arrows). The RK5myc-A2 tag shows localization to the cytoplasm (N and P) (red arrows). (Q to T) Cells transfected with pRK5myc-B and pEGFP-actin. pEGFP-actin in RK5myc-B shows vacuoles (Q and T) (yellow arrows). Here the RK5myc-B tag shows localization to the nucleus (R and T) (red arrows) and causes nuclear blebbing (S and T) (white arrow). (U to X) Cells transfected with pRK5myc-C1 and pEGFP-actin. As can be seen, pRK5myc-C1 shows actin blebs (U and X) (yellow arrows) and is localized to the cytoplasm (V and X) (red arrows). (Y to AB) Cells transfected with pRK5myc-C2 and pEGFP-actin. As observed for pRK5myc-C1, pRK5myc-C2 shows actin blebs (Y and AB) (yellow arrows) and is localized to the cytoplasm (Z and AB) (red arrows). (AC to AF) Control cells transfected with pEGFP alone. The control cells show normal actin and nuclear morphology and no cMyc colocalization. Scale bars represent 20 μm.
Transfections with pRK5myc-A1 and pRK5myc-A2 both showed significant increases in the numbers of cells with actin condensation (pRK5myc-A1, 29% [SE = 6.8]; pRK5myc-A2, 23% [SE = 10.3]) and actin vacuoles (pRK5myc-A1, 18% [SE = 6.3]; pRK5myc-A2, 36% [SE = 8.7]) (Fig. 7I, L, M, and P) compared to the pRK5myc control (12.5% [SE = 2.3] and 10% [SE = 3.8], respectively) (Fig. 7AC and AF), consistent with the phenotype observed with Yen-Tc topically applied to Sf9 (Fig. 5) and Caco-2 (Fig. 6) cells. Interestingly, only pRK5myc-A1 showed increases in nuclear condensation (27.5% [SE = 3.3]) and numbers of multinucleated cells (10% [SE = 8.5]) (Fig. 7K and L), whereas pRK5myc-A2 (Fig. 7O and P) showed 18% (SE = 0.8) of cells with nuclear condensation (controls displayed a nuclear condensation of 11% of cells [SE = 5.0] and multinucleation of 6% of cells [SE = 3.0]) (Fig. 7AE and AF). As can be seen in Fig. 7J, N, L, and O, pRK5myc-A1 and pRK5myc-A2 localized to the cytoplasm.
The transfection of cells with pRK5myc-B also resulted in increases in the numbers of cells with actin condensation (31% [SE = 16.4]) and large actin vacuoles (24% [SE = 2.8]) (Fig. 7Q and T). Nuclear fragmentation and blebbing were also observed for 21.5% (SE = 21.5) of cells (Fig. 7S, white arrow) compared to 0.7% (SE = 0.7) of control cells, as observed by the histological examination of C. zealandica larvae fed Yen-Tc (Fig. 1), and were localized to the nucleus (Fig. 7Q and S).
Both pRK5myc-C1 and pRK5myc-C2 caused increases in numbers of actin aberrations, including actin blebs (32% [SE = 3.7] and 23% [SE = 2.3], respectively), actin vacuoles (27% [SE = 3.3] and 30% [SE = 8.8], respectively), and actin condensation (34% [SE = 9.9] and 24% [SE = 12.2], respectively) (Fig. 7U, X, Y, and AB), compared with the control cells (2% [SE = 1.6], 10% [SE = 6.3], and 12.5% [SE = 2.3], respectively) (Fig. 7AC and AF). As observed for the Sf9 cells with topically applied treatment (Fig. 5), there was also an increase in the number of cells with condensed nuclei (18% [SE = 0.8] and 20% [SE = 11.5]), indicative of apoptosis. pRK5myc-C2 was also observed to have an increase in the number of multinucleated cells (16% [SE = 8.3]) compared to control cells (6% [SE = 3.0]). Both pRK5myc-C1 and pRK5myc-C2 were observed to colocalize to the cytoplasm (Fig. 7V, X, Z, and AB).
DISCUSSION
The main disease determinant of the insecticidal bacterium Y. entomophaga has been identified as Yen-Tc, which is a novel member of the toxin complex family (19). To better understand the lethal effects that Yen-Tc has on insects and to determine its direct contribution to insect mortality from that of the entire Y. entomophaga bacterium, a histopathology study of the C. zealandica larval midgut after exposure to purified Yen-Tc protein was conducted. This study demonstrated that purified Yen-Tc alone was able to cause a series of sequential and predictable events that eventually led to death of the intoxicated insects. Following the oral ingestion of the Yen-Tc toxin, a distinctive clearing of gut contents was coupled with a progressive loss of the peritrophic membrane and the disappearance of microvilli. Prior to the eventual complete deterioration of the gut epithelium, midgut columnar cells became progressively more disorganized in appearance, which was coupled with the extrusion and sloughing of cell-like bodies (containing condensed pyknotic nuclei and fragmented nuclear material) into the gut lumen. The observed ultrastructural changes included the appearance of grainy patches and prominent electron-dense material, a reorganization of basally located mitochondria, and a rippling of the basal membrane.
The destruction of the C. zealandica larval midgut epithelium by Yen-Tc displayed a histopathology similar to those observed previously for other Tc-intoxicated insects, including that for P. xylostella intoxicated by Yen-Tc (19, 25). The oral exposure of M. sexta larvae to the P. luminescens Tca toxin complex was demonstrated previously to cause an accelerated release of vesicles from the midgut epithelium into the gut lumen, followed by the eventual disintegration of the entire gut epithelial wall (2). Further testing of P. luminescens Tca against L. decemlineata showed that the toxin effects were equally as disruptive to the gut epithelium (3). While such similarities seem obvious, given the shared sequence similarity of key regions within each of the A, B, and C subunit classes, there are also regions of amino acid sequence variability between the orthologous subunits encoding the various Tc components (13, 15, 19, 26). These observed differences may explain, at least in part, the spectrum of phenotypes observed, whereby different host effector molecules are being targeted by a range of activities encoded by the variety of Tc subunit combinations.
Interestingly, the destruction of the gut epithelium caused by the Tc toxins described above is in marked contrast to the absence of any observed histopathological effects on C. zealandica larvae inoculated with S. entomophila strains carrying the Sep Tc toxin (the causative agent of amber disease) (21) or semipurified Sep toxin (presented here in the supplemental material). While we do not yet understand the exact significance, it is interesting that the amber coloration and gut clearance phenotypes observed after the early stages of Yen-Tc intoxication share similarity to the observed phenology after the ingestion of S. entomophila (strain AM1O2 is the causative agent of amber disease). In amber disease, the gut color turns from dark brown-black to amber, and this change is accompanied by a dramatic clearance of gut contents through the discharge of frass pellets; however, the gut epithelium remains intact (20, 21). This contrasts with observations of Yen-Tc, whereby the complete dissolution of the gut is preceded by an “amber-like” physical appearance of the gut (prior to the formation of a moribund brown coloration) and is accompanied by vomiting and the discharge of frass material. That the Sep complex has only ever shown activity against C. zealandica points to a specific coevolution, and this “mild” effect could possibly provide an as-yet-unidentified ecological advantage to Serratia species carrying the Sep Tc genes. This concept has some support from the observation that the Tc activity associated with Yersinia pestis has been tailored so that it is highly active against mammalian cells but is not active against its insect host (9). Within the C. zealandica system, a reduced dose of either S. entomophila Sep or Yen-Tc results in a reversion-like effect, whereby intoxicated C. zealandica larvae that began to display disease symptoms would then revert to a healthy phenotype (17, 19). These observations suggest that a continuous dose of Tc is likely to be required throughout the disease process.
The apparent sloughing of vesicle-like structures and the putative condensation of DNA material (pyknotic nuclei) are reminiscent of events often described for cells undergoing apoptosis (24). The presence of TUNEL-positive cells and the activation of caspase-3/7 enzyme activity in Yen-Tc-treated larvae provide evidence that apoptosis-like programmed cell death is activated. Whether this is a direct or indirect effect of the toxin has not yet been determined. The apparent Tc-induced induction of an apoptosis-like event has also been described for other Tc family members (e.g., P. luminescens and other Yersinia species), and the results for Yen-Tc presented here are consistent with this view (15, 34). However, a closer inspection of the data also reveals an apparent shrinkage of columnar cells within the deteriorating gut epithelium and the presence of large amounts of cell debris (putatively from cell lysis) within the gut lumen. Both observations have been associated with programmed necrosis (7, 30). Given the current uncertainty over how to specifically define and characterize apoptotic and necrotic programmed cell death pathways (14, 24), it is interesting to speculate that perhaps a combination of both pathways, or, indeed, a novel pathway, is triggered by Yen-Tc activity. Although the current study was unable to definitively differentiate between the activation of a single apoptotic or necrotic programmed cell death pathway, the predictable sequence of observed events after Yen-Tc intoxication coupled with the above-described evidence supports the idea that Yen-Tc does activate a programmed cell death pathway. As the mode of action of the majority of Tc family members remains largely unknown, an experimental reevaluation of other Tc members may shed light on what programmed cell death mechanisms are involved, as it appears that the interconnectedness of the various death pathways is more common than previously believed.
The ectopic application of Yen-Tc onto Sf9 and Caco-2 cells established that Yen-Tc was able to elicit a cytopathic effect (i.e., vacuole formation, cell actin contraction, and the formation of pyknotic nuclei), and these indicators are consistent with phenotypes observed previously for programmed cell death (8, 24). That Yen-Tc was active against these cell lines allowed their use to further examine the contribution of each individual Yen-Tc subunit component (A, B, and C subunits, plus the two associated chitinases) via transient expression.
Unexpectedly, the transient expression of the Chi components in Caco-2 cells provided observable effects. The intracellular expression of either of the two Chi components was able to cause a 1.5- to 2-fold increase in levels of actin reorganization, vacuole formation, and multinucleation (compared with mock-treated cells). Although reasons for such effects are not immediately obvious, it is known that the presence of both Chi subunits in the Yen-Tc ABC complex is required for insecticidal activity (19), both Chi1 and Chi2 possess endochitinase activity (6, 25), and the presence of chitinase genes in close proximity to Tc loci was noted previously for P. luminescens and Xenorhabdus nematophila Tc loci (although the were not reported to be associated with the Tc complex) (6, 35). Interestingly, specific actin-binding activity has been demonstrated for a 32-kDa chitinase found in STDR-1 potato cells (33), which leaves the potential for such a nonobvious function being present within the Yen-Tc-associated Chi subunits.
The cellular effects caused by the individual Yen-Tc A, B, and C subunits are broadly consistent with observations previously reported for other members of the Tc family, such as other Yersinia species and P. luminescens (27). The transient expressions of the three main Yen-Tc subunit components (A, B, and C subunits) revealed that they all have a role in actin organization (blebbing, condensation, and/or vacuole formation). In addition, Yen-Tc subunits A1 and C1 were both associated with nuclear condensation, and the intracellular expression of subunits A1 and C2 caused multinucleation in some cells, which is consistent with an apoptotic phenotype. Yen-Tc B subunit expression was shown to be responsible for nuclear fragmentation and blebbing, which is consistent with necrosis, with the protein being localized to the nucleus, as opposed to the cytoplasm for the Tc A and Tc C subunits. From this, it would appear that the Yen-Tc A and C subunits have a role initiating an apoptosis-like programmed cell death pathway, while the effects of the Yen-Tc B subunit correlate more with a necrosis-type programmed cell death event.
The finding that all three of the A, B, and C subunits and the two Chi proteins affect the actin cytoskeleton is both interesting and somewhat puzzling. The effects on actin of the topical application of native Yen-Tc toxin onto Sf9 and Caco-2 cells demonstrate a role in cytoskeleton remodeling (as previously shown for other Tc members); however, the specific mechanisms responsible for this remain to be determined. The discovery that P. luminescens Tc-mediated actin clustering was a direct result of the ADP-ribosylation activity contained within two different Photorhabdus Tc complexes provides some direction for future investigations into the mechanisms of Yen-Tc-mediated cytoskeletal changes (26).
The C3 and C5 subunits of the P. luminescens Tc complexes were shown previously to possess ADP-ribosylation activities, which directly modify ADP-ribosylate residue T148 of actin and residues Q-61 and Q-63 of the RhoA GTPase, respectively (26, 27). This activity was predicted from sequence comparisons with known protein domains, but the demonstration of activity has been a breakthrough in the identification of specific molecular targets for Tc toxins. The Yen-Tc C1 and C2 subunits, by sequence comparison, are predicted to have a Rho-activating function similar to that of cytotoxic necrotizing factor and deaminase activity, respectively (19), while the Y. pseudotuberculosis Tc C subunit (from the Tcc complex) displays similarity to tyrosine phosphatase (15). Assuming that these predicted activities are present, this diversity of activity is suggestive of the Tc family members being composed of subunits encoding domains that are able to target different sites along putative programmed cell death pathways and other as-yet-unknown pathways.
Supplementary Material
ACKNOWLEDGMENTS
This research has been funded by the Ministry of Science and Innovation, New Zealand (contract number C10X0804).
We thank Richard Townsend (AgResearch) for field collection of C. zealandica larvae, Richard Walls (AgResearch) for assistance with preparing samples for electron microscopy studies, Chikako van Koten (AgResearch) for statistical assistance, Jennifer Lucas at Gribbles Veterinary Pathology (Christchurch, New Zealand) for providing histology services, and Richard ffrench-Constant (University of Exeter) for use of cell culture facilities.
Footnotes
Published ahead of print 27 April 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allan VJ. 2000. Basic immunofluorescence, p 1–26 In Allan VJ. (ed), Protein localization by fluorescence microscopy: a practical approach. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 2.Blackburn M, Golubeva E, Bowen D, ffrench-Constant RH. 1998. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl. Environ. Microbiol. 64: 3036– 3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn MB, Domek JM, Gelman DB, Hu JS. 2005. The broadly insecticidal Photorhabdus luminescens toxin complex a (Tca): activity against the Colorado potato beetle, Leptinotarsa decemlineata, and sweet potato whitefly, Bemisia tabaci. J. Insect Sci. 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn MB, Martin PAW, Kuhar D, Farrar RR, Jr, Gundersen-Rindal DE. 2011. The occurrence of Photorhabdus-like toxin complexes in Bacillus thuringiensis. PLoS One 6:e18122 doi:10.1371/journal.pone.0018122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen D, et al. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129– 2132 [DOI] [PubMed] [Google Scholar]
- 6.Busby JN, et al. 2012. Structural analysis of Chi1 chitinase from Yen-Tc: the multisubunit insecticidal ABC toxin complex of Yersinia entomophaga. J. Mol. Biol. 415: 359– 371 [DOI] [PubMed] [Google Scholar]
- 7.Christofferson DE, Yuan J. 2010. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22: 263– 268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman ML, Olson MF. 2002. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 9: 493– 504 [DOI] [PubMed] [Google Scholar]
- 9.Erickson DL, et al. 2007. Acute oral toxicity of Yersinia pseudotuberculosis to fleas: implications for the evolution of vector-borne transmission of plague. Cell. Microbiol. 9: 2658– 2666 [DOI] [PubMed] [Google Scholar]
- 10.ffrench-Constant R, Waterfield N. 2005. An ABC guide to the bacterial toxin complexes, p 169–183 In Laskin AI, Bennett JW, Gadd GM, Sariaslani S. (ed), Advances in applied microbiology, vol 58 Elsevier Academic Press Inc, San Diego, CA: [DOI] [PubMed] [Google Scholar]
- 11.ffrench-Constant RH, Dowling A, Waterfield NR. 2007. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 49: 436– 451 [DOI] [PubMed] [Google Scholar]
- 12.Fogh J, Wright WC, Loveless JD. 1977. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J. Natl. Cancer Inst. 58: 209– 214 [DOI] [PubMed] [Google Scholar]
- 13.Fuchs TM, Bresolin G, Marcinowski L, Schachtner J, Scherer S. 2008. Insecticidal genes of Yersinia spp.: taxonomical distribution, contribution to toxicity towards Manduca sexta and Galleria mellonella, and evolution. BMC Microbiol. 8: 214 doi:10.1186/1471-2180-8-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, et al. 2012. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19: 107– 120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hares MC, et al. 2008. The Yersinia pseudotuberculosis and Yersinia pestis toxin complex is active against cultured mammalian cells. Microbiology 154: 3503– 3517 [DOI] [PubMed] [Google Scholar]
- 16.Hurst MR, Becher SA, Young SD, Nelson TL, Glare TR. 2011. Yersinia entomophaga sp. nov. isolated from the New Zealand grass grub Costelytra zealandica. Int. J. Syst. Evol. Microbiol. 61: 844– 849 [DOI] [PubMed] [Google Scholar]
- 17.Hurst MR, Jones SM, Tan B, Jackson TA. 2007. Induced expression of the Serratia entomophila Sep proteins shows activity towards the larvae of the New Zealand grass grub Costelytra zealandica. FEMS Microbiol. Lett. 275: 160– 167 [DOI] [PubMed] [Google Scholar]
- 18.Hurst MRH, Glare TR, Jackson TA, Ronson CW. 2000. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182: 5127– 5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst MRH, et al. 2011. The main virulence determinant of Yersinia entomophaga MH96 is a broad-host-range toxin complex active against insects. J. Bacteriol. 193: 1966– 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson TA, Boucias DG, Thaler JO. 2001. Pathobiology of amber disease, caused by Serratia spp. , in the New Zealand grass grub, Costelytra zealandica. J. Invertebr. Pathol. 78: 232– 243 [DOI] [PubMed] [Google Scholar]
- 21.Jackson TA, Huger AM, Glare TR. 1993. Pathology of amber disease in the New Zealand grass grub Costelytra zealandica (Coleoptera: Scarabaeidae). J. Invertebr. Pathol. 61: 123– 130 [Google Scholar]
- 22.Kaplan EL, Meier P. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53: 457– 481 [Google Scholar]
- 23.Kiernan JA. 1990. Histological and histochemical methods: theory and practice, 2nd ed. Pergamon Press, Toronto, Ontario, Canada [Google Scholar]
- 24.Kroemer G, et al. 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16: 3– 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landsberg MJ, et al. 2011. 3D structure of the Yersinia entomophaga toxin complex and implications for insecticidal activity. Proc. Natl. Acad. Sci. U. S. A. 108: 20544– 20549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang AE, et al. 2010. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 327: 1139– 1142 [DOI] [PubMed] [Google Scholar]
- 27.Lang AE, Schmidt G, Sheets JJ, Aktories K. 2011. Targeting of the actin cytoskeleton by insecticidal toxins from Photorhabdus luminescens. Naunyn Schmiedebergs Arch. Pharmacol. 383: 227– 235 [DOI] [PubMed] [Google Scholar]
- 28.Lee SC, et al. 2007. Structural characterisation of the insecticidal toxin XptA1, reveals a 1.15 MDa tetramer with a cage-like structure. J. Mol. Biol. 366: 1558– 1568 [DOI] [PubMed] [Google Scholar]
- 29.Martinez MM, Reif RD, Pappas D. 2010. Detection of apoptosis: a review of conventional and novel techniques. Anal. Methods 2: 996– 1004 [Google Scholar]
- 30.McCall K. 2010. Genetic control of necrosis—another type of programmed cell death. Curr. Opin. Cell Biol. 22: 882– 888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sergeant M, et al. 2006. Identification, typing, and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci. Appl. Environ. Microbiol. 72: 5895– 5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sergeant M, Jarrett P, Ousley M, Morgan JAW. 2003. Interactions of insecticidal toxin gene products from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 69: 3344– 3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemoto D, Furuse K, Doke N, Kawakita K. 1997. Identification of chitinase and osmotin-like protein as actin-binding proteins in suspension-cultured potato cells. Plant Cell Physiol. 38: 441– 448 [DOI] [PubMed] [Google Scholar]
- 34.Waterfield N, Hares M, Yang G, Dowling A, ffrench-Constant R. 2005. Potentiation and cellular phenotypes of the insecticidal toxin complexes of Photorhabdus bacteria. Cell. Microbiol. 7: 373– 382 [DOI] [PubMed] [Google Scholar]
- 35.Waterfield NR, Bowen DJ, Fetherston JD, Perry RD, ffrench-Constant RH. 2001. The tc genes of Photorhabdus: a growing family. Trends Microbiol. 9: 185– 191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.