Abstract
A rich history of investigation documents various Drosophila-yeast mutualisms, suggesting that Drosophila suzukii similarly has an association with a specific yeast species or community. To discover candidate yeast species, yeasts were isolated from larval frass, adult midguts, and fruit hosts of D. suzukii. Terminal restriction fragment length polymorphism (TRFLP) technology and decimal dilution plating were used to identify and determine the relative abundance of yeast species present in fruit juice samples that were either infested with D. suzukii or not infested. Yeasts were less abundant in uninfested than infested samples. A total of 126 independent yeast isolates were cultivated from frass, midguts, and fruit hosts of D. suzukii, representing 28 species of yeasts, with Hanseniaspora uvarum predominating. This suggests an association between D. suzukii and H. uvarum that could be utilized for pest management of the highly pestiferous D. suzukii.
INTRODUCTION
Yeasts are believed to be an important source of nutrients for and are vectored by Drosophila (27, 32). Drosophila development is affected by the species of yeast available as food for the larvae (33). Also, microbial community composition of the larval substrate affects fitness in terms of susceptibility to parasitism (3). More diverse yeast communities appear to be preferred food sources (26), and bicultures of yeasts are generally more preferred than monocultures (33, 34), though this is not universal (27). Yeasts have been shown to affect Drosophila reproduction, with yeast composition in fly diet affecting egg production by orders of magnitude (8, 9, 29). In some species of Drosophila, yeasts are even offered as nuptial gifts in courtship (35, 36, 37). Yeast-colonized substrates are preferred oviposition sites for most fruit-breeding Drosophila, rather than sites where bacteria or molds predominate (22). Drosophila buzzatii was found to prefer feeding and ovipositing on the same species of yeast, and both Drosophila larvae and adults prefer to feed on particular yeast species when offered a choice of pure yeast cultures (12, 39).
In 2008, a new highly pestiferous Drosophila species, spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), invaded the western United States (7). D. suzukii is unique in that it oviposits on marketable fruit relative to overripe or damaged fruit, and its injury facilitates colonization by other Drosophila species (40). If untreated, it is capable of causing a potential 860 million dollars of revenue loss annually to blackberries, raspberries, and cherries in California, Oregon, and Washington (40). Knowledge of potential yeast associations could be used in lure development. Given that strong associations are common between yeast and Drosophila spp., we hypothesized that D. suzukii has a preferred yeast species or community that both larvae and adults can feed upon.
MATERIALS AND METHODS
Cultivation of yeasts from live D. suzukii larvae.
Infested fruit were collected as available from September 2010 through August 2011 for larval extraction. Individual larvae were removed from infested fruit using Featherweight forceps (BioQuip, Rancho Dominguez, CA). Forceps were dipped into 100% ethanol and flamed twice in succession and allowed to cool for sterilization. The larva was surface sterilized by submergence in 70% ethanol, rinsed in sterile distilled water, and placed in the center of a Rose Bengal chloramphenicol agar (RBCA; prepared according to the manufacturer's instructions; Oxoid, United Kingdom) plate for 30 min to 1 h depending on larval activity. Once a larva began to cross its own trail, it was removed and placed in a Drosophila culture bottle (177-ml stock bottles; Applied Scientific, Schrerville, IN) containing approximately 50 ml of Jazz Mix Drosophila medium (Applied Scientific, Schrerville, IN) with a Kimwipe delicate task wiper (Kimberly-Clark, Fullerton, CA) embedded in the media and closed with BuzzPlugs (Applied Scientific, Schererville, IN). The adult was sexed and identified upon successful eclosion. Yeasts from larvae that did not survive to emergence or were not D. suzukii were not included in this study. One colony of each morphological type was isolated and identified as described below, and at least two colonies per plate were chosen if the morphology appeared identical for the entire plate.
Cultivation of yeasts from live D. suzukii adults.
Adult D. suzukii organisms were collected live from the field during times of peak populations in each crop (July through August 2011) either by sweep netting (cherries) or by vacuum sampling (raspberries). They were immediately returned to the laboratory (within 1 h of collection) and were first submerged in sterile distilled water and then in 70% ethanol for surface sterilization, and then they were rinsed in sterile distilled water. This prevented them from swallowing the ethanol used for surface sterilization and killing the alimentary canal yeasts. The alimentary canal was removed by gently pulling the anterior and posterior parts of the fly simultaneously with sterilized forceps. The alimentary canal was then streaked across an RBCA plate. One colony of each morphological type was isolated and identified, and at least two colonies per plate were chosen if the morphology was identical for the entire plate.
Fruit extractions.
Two to four fruit of each sample (fruit variety and infestation type) were macerated until particles were less than 0.33 mm in diameter inside a Whirl-Pak sterile filter bag (Nasco, Inc., Atlanta, GA). A subsample of the fruit juice taken early in the season (when we would expect the wild Drosophila community to be mostly D. suzukii) was frozen and kept at −80°C for terminal restriction fragment length polymorphism (TRFLP) yeast community analysis. Nine ml of sterile distilled water was added to one ml of the sample juice, and then serial dilutions were performed. These dilutions were plated on RBCA plates by spreading 0.1 ml of the dilution evenly across the plate. The dilution that resulted in 30 to 300 colonies per plate was used to quantify the number of CFU of yeast per gram of sample. Three to five colonies of each morphological type were isolated and identified as described below.
Infested and uninfested raspberry samples (Isabel, Maravilla, and Pacifica varieties) were taken from fields known either to have a fly population or not to have a fly population, as raspberries show no external signs of infestation. Cherries (varieties Rainier, Royal Ann, and unknown) have a characteristic oviposition mark; therefore, cherries were designated infested or uninfested by visual inspection. A subsample of uninfested fruit from each sampling date was also checked for the presence of larvae, and no larvae were found. Fruit were collected from multiple varieties of each fruit type from June to August 2011, as available. Fruit were then inspected when macerated for evidence of larvae within the fruit, and a subsample of infested fruit were used for larval extractions. Ten fruit from each sample were used to measure the Brix sugar content of the sample to account for potential variability in the ripeness of the samples.
Yeast identification.
Approximately 1 μl of yeast from the final potato dextrose agar (PDA) plate was placed in 50 μl of MilliQ water and heated at 95°C for 10 min to lyse the cells. PCR amplification was performed in 50-μl reaction mixtures with 2 μl of the lysed DNA template, 25 μl 2× Promega Go Taq Green Master Mix (Promega, Madison, WI), and 1 mM MgCl2 using the NL1 and NL4 primers at 0.2 μM reaction concentration (23) to amplify the 600-bp D1/D2 domain of the large (26S rRNA) subunit. Samples were sequenced at the UC Davis College of Biological Sciences Sequencing Facility at the University of California, Davis (http://dnaseq.ucdavis.edu/). The sequencing reaction was carried out on a 3730 capillary electrophoresis genetic analyzer with BigDye Terminator v3.1 cycle sequencing chemistry (Applied Biosystems, Foster City, CA). Electropherograms were analyzed using Sequence Scanner (v1.0; Applied Biosystems, Foster City, CA). The rRNA sequences were compared against the NCBI database using the nucleotide Basic Local Alignment Search Tool (BLASTn) program (http://blast.ncbi.nlm.nih.gov) (2) for taxonomic identification of the yeast isolates. A 98% match or higher was used to assign species name; anything less was identified solely to the genus level. Cultures were deposited in the University of California, Davis, Phaff collection (see Table S2 in the supplemental material).
TRFLP.
Yeast communities from infested and uninfested cherries and raspberries were profiled using TRFLP analysis methods that were previously described (6). Briefly, DNA was extracted from the fruit juice samples (1). The internal transcribed spacer (ITS) regions (1 and 2) surrounding the 5.8S rRNA gene were amplified using HEX fluorescent-labeled ITS1 and ITS4 primers (23). Purified PCR products were then digested using the restriction enzymes HaeIII, DdeI, and HinfI. The digested DNA was submitted to the UC Davis College of Biological Science Sequencing Facility at the University of California, Davis, for quantification. Traces were visualized using Peak Scanner Software (v.1.0; Applied Biosystems, Foster City, CA), and peaks with more than 50 fluorescence units were considered true signals. Yeast species assignments were made by referencing the ITS TRFLP database for pure yeast cultures (6). As ITS1 and ITS4 are able to amplify plant 26S rRNA, cherry (Prunus avium) and raspberry (Rubus ideaus) major and minor terminal fragment profiles were predicted using SeqBuilder software (v.7.0; DNAStar) and the publicly accessible ITS rRNA sequences for the fruit. DdeI had the best diversity for our samples in terms of greatest number of operational taxonomic units (OTUs). Therefore, relative abundance for the species in our samples was determined using the height of the fluorescence peak for this restriction cut. HaeIII and HinfI were used for supporting the identification assignment.
RESULTS
D. suzukii larvae were isolated from three varieties of each fruit type for a total of 34 individuals and 46 unique frass-yeast isolations (Table 1). Hanseniaspora uvarum was isolated from 32 of the 34 larvae. The next most frequently isolated species were Pichia kluyveri and Pichia terricola, which were found in 5 and 3 of the larvae, respectively, for a total of 8 isolations of the genus Pichia (see Table S1 in the supplemental material). Yeasts were also isolated from the alimentary canal of both male and female adult D. suzukii flies, for a total of 11 individuals and 14 unique yeast isolations (Table 1). As was the case for the larval extractions, H. uvarum was the most prevalent yeast isolated from D. suzukii adults. The fruit juice dilutions showed some variation in yeast community composition between infested and uninfested cherry samples, with Cryptococcus spp. dominating in uninfested fruit relative to the Metschnikowia spp. in infested fruit (Table 1).
Table 1.
Summary of total yeasts isolated from all locations (n = 126) by species or pooled by genus in cases of multiple species
| Yeast | No. positive for yeast isolation |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cherry |
Raspberry |
Total | |||||||
| Adult (n = 3) | Larva (n = 13) |
Uninfested fruit (n = 4) | Infested fruit (n = 3) | Adult (n = 8) |
Larva (n = 21) |
Uninfested fruit (n = 5) | Infested fruit (n = 5) | ||
| Hanseniaspora spp. | 3 | 13 | 3 | 3 | 8 | 19 | 5 | 8 | 62 |
| Pichia spp. | 1 | 3 | 0 | 2 | 1 | 5 | 1 | 6 | 19 |
| Metschnikowia spp. | 0 | 2 | 0 | 6 | 0 | 0 | 3 | 2 | 13 |
| Cryptococcus spp. | 0 | 0 | 9 | 0 | 0 | 1 | 0 | 1 | 11 |
| Aureobasidium pullulans | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 5 |
| Candida spp. | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 5 |
| Sporobolomyces spp. | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 4 |
| Debaryomyces spp. | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| Udeniomyces pyricola | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Meyerozyma guilliermondii | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Moniliella megachiliensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
The Brix sugar content values and the yeast community profiles were similar for infested and uninfested fruit juices, although fewer yeast colonies were present and fewer were identified in the uninfested samples (Table 2). Infested cherry fruit had >103-fold higher CFU/ml fruit juice than uninfested fruit. Interestingly, in cherries, infested fruit had no mold colonies (Table 2). In raspberries, yeasts and molds were present in infested and uninfested fruit, although yeasts were more prevalent in both. There was no noticeable difference in the quantity of yeast between infested and uninfested raspberries, unlike the cherry samples. However, one sample of infested Pacifica raspberry and one sample of infested Isabel raspberry did exhibit a 103-fold higher CFU than the uninfested samples. Of the yeast colonies isolated, H. uvarum comprised a similar proportion of both uninfested and infested colony isolates (see Table S1 in the supplemental material). Cryptococcus spp. were more prevalent in uninfested samples, with 9 isolations in uninfested and 1 in infested samples (Table 1). Metschnikowia spp. and Pichia spp. showed an opposite trend, with 3 and 1, respectively, in uninfested fruit and 8 each in infested fruit. A small portion of uninfested (3) and infested (2) samples were comprised of the yeast-like fungus Aureobasidium pullulans.
Table 2.
Brix and CFU per ml fruit juice for yeasts and molds from infested and uninfested fruit juice isolations
| Fruit | Date (2011) | Result |
|||||
|---|---|---|---|---|---|---|---|
| Brix sugar content (±SE) |
Yeast CFU |
Mold CFU |
|||||
| Infested | Uninfested | Infested | Uninfested | Infested | Uninfested | ||
| Cherries | |||||||
| Unknown | 14-Jun | 16.5 ± 0.4 | 3.3 × 103 | 1.0 × 102 | |||
| 14-Jun | 15.0 ± 0.4 | 19.6 ± 1.0 | 3.2 × 107 | 1.9 × 103 | 0 | 1.0 × 103 | |
| Royal Ann | 23-Jun | 20.0 ± 0.6 | 20.0 ± 0.6 | 1.1 × 107 | 3.2 × 103 | 0 | 1.0 × 103 |
| Rainier | 12-Aug | 24.0 ± 1.0 | 24.0 ± 1.0 | 4.0 × 106 | <1.0 × 102 | 0 | 0 |
| Raspberries | |||||||
| Isabel | 23-Jun | 10.7 ± 0.3 | 9.8 ± 0.1 | 8.0 × 106 | 4.6 × 102 | 1.0 × 103 | 1.0 × 103 |
| Maravilla | 23-Jun | 11.5 ± 0.4 | 11.3 ± 0.3 | 6.0 × 102 | 3.0 × 102 | 1.0 × 103 | 1.0 × 103 |
| 21-Jul | 10.2 ± 0.2 | 8.8 ± 0.4 | 5.0 × 104 | 2.6 × 103 | 1.0 × 103 | 1.0 × 103 | |
| Pacifica | 23-Jun | 9.0 ± 0.3 | 10.5 ± 0.2 | 1.0 × 107 | >1.0 × 103 | 0 | 1.0 × 102 |
| 21-Jul | 9.8 ± 0.4 | 9.1 ± 0.1 | 4.0 × 105 | 2.8 × 105 | 1.0 × 104 | 1.0 × 102 | |
A total of 126 unique yeast isolations were identified in this study, for a total of 28 yeast species. H. uvarum was the predominant yeast and was ubiquitous across all sample types, with 59 individual isolations. P. terricola was the next most common, with 10 individual isolations, although it was more common in raspberry samples. This was followed closely by P. kluyveri, with 9 isolations. All other species were found on 5 or fewer occasions (see Table S1 in the supplemental material).
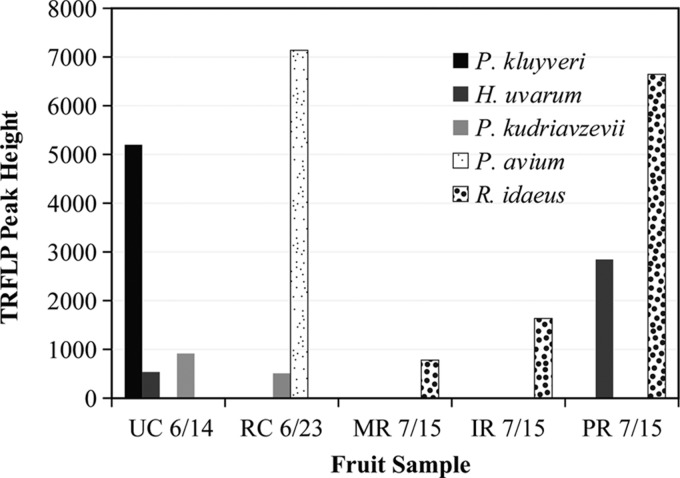
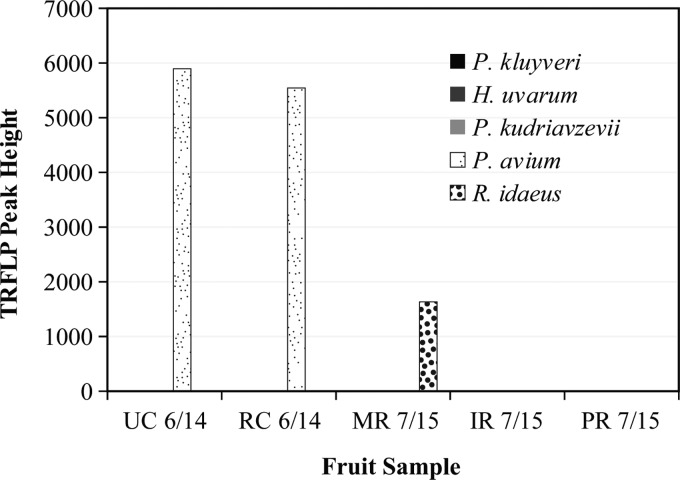
TRFLP yeast community profiles for early-season infested and uninfested fruit juices were able to detect species of yeast in three of the infested samples (Fig. 1). The infested cherry sample of unknown variety collected on 14 June 2011 had a high abundance of P. kluyveri, with H. uvarum detected at a lower abundance. The infested Royal Ann cherry sample had a low abundance of Pichia kudriavzevii. The only infested raspberry sample in which yeast was detected was the Pacifica variety, which had a high abundance of H. uvarum. The fruit's DNA was detected in all infested fruit samples, and only P. kluyveri in the infested cherry sample was in higher abundance than the sample's fruit DNA. No yeasts were detected in any uninfested fruit samples, and fruit DNA was prevalent (Fig. 2).
Fig 1.
DdeI fragment fluorescent peak heights and identifications for infested fruit. Fruit samples were the following: UC, unknown cherry; RC, Royal Ann cherry; MR, Maravilla raspberry; IR, Isabel raspberry; and PR, Pacifica raspberry. Yeast species were Pichia kluyveri, Hanseniaspora uvarum, and Pichia kudriavzevii. Fruit species were Prunus avium and Rubus idaeus.
Fig 2.
DdeI fragment fluorescent peak heights and identifications for uninfested fruit. Fruit samples were the following: UC, unknown cherry; RC, Royal Ann cherry; MR, Maravilla raspberry; IR, Isabel raspberry; PR, Pacifica raspberry. Yeast species were Pichia kluyveri, Hanseniaspora uvarum, and Pichia kudriavzevii. Fruit species were Prunus avium and Rubus idaeus.
DISCUSSION
The most frequently identified yeast species from adult and larval D. suzukii were H. uvarum, Metschnikowia pulcherrima, P. terricola, and P. kluyveri. H. uvarum is considered a widespread yeast species; however, it and other apiculate yeasts are most frequently isolated from mature fruits, early stages of wine fermentation, fermentative spoilage, and Drosophila (19, 20, 21, 31). M. pulcherrima is also often associated with feeding and breeding sites of insects, and yeasts isolated from Drosophila were once thought to be predominantly from the genus Pichia (16, 38). For both larvae and adults, the most commonly identified yeast species was H. uvarum, suggesting that there is an association between D. suzukii and H. uvarum.
Infested and uninfested cherry fruit juice contained a broader diversity of yeasts, although still being dominated by the fruit- and insect-associated species that were mentioned above. Ubiquitous phylloplane and soil yeasts were more common in the uninfested fruit juice isolations, with the yeast-like fungus A. pullulans and Cryptococcus spp. predominating. A. pullulans has been termed a plant-pathogenic fungus (11), and Cryptoccus spp. are considered to be generalist yeasts that are frequently isolated from the phylloplane (16). These yeasts can utilize a broad variety of carbon sources (16). Infested cherries predominately contained the yeast species H. uvarum and Metschnikowia spp., which utilize a narrower range of carbon sources (16). Uninfested and infested raspberry communities were much more similar both in composition and in quantity of yeasts in the sample, with Pichia spp. and Hanseniaspora spp. dominating. Theoretically, once a cherry has been infested with D. suzukii and yeast introduced into the mesocarp, larvae can travel throughout the fruit, thus yeasts can infest the entire fruit mesocarp and reach a high population. On the contrary, larval travel and, thus, yeast growth within a raspberry may be limited to the individual damaged drupelet, although larval D. suzukii movement in various fruits remains unknown. As fungicides are often applied to control plant-pathogenic fungi in raspberries, differences in fungicide use in organic versus conventional fields are a confounding factor. Organic fungicides have been found to reduce the overall yeast population and shift the microbial population toward the yeast-like fungus A. pullulans (10).
TRFLP analysis of yeast communities within infested versus uninfested fruit juice revealed yeast species present at detectable levels in a few of the samples. Unfortunately, the ITS primers also amplify plant DNA, which was present at a high level in fresh fruit juice and was often more abundant than yeast DNA. Detection of yeast by TRFLP analysis in fruit juice does not seem to correlate well with the culture-based methods. Samples with higher yeast CFU did not necessarily have high yeast TRFLP peaks, and P. kudriavzevii was not identified in any of the fruit juice yeast cultures. This could be due to sampling from the fruit dilutions or could be a limitation of the TRFLP database, which may not be able to distinguish all relevant species of Pichia. Despite these contradictions, the development of a primer that is more specific for yeast than plant DNA would allow TRFLP to become a useful analytical tool for yeast community comparisons.
Knowledge of Drosophila preference for yeast substrates has been instrumental in the development of trap attractants for Drosophila spp. (5). For more than 70 years, banana mash fermented by baker's yeast has been used to attract Drosophila spp. (24, 25, 30), and Drosophila spp. are common nontarget captures in Torula yeast-based food lures for tephritid fruit flies (18). Common synthetic volatiles for Drosophila spp. attraction included chemicals released by yeast fermentation of fruits, such as ethanol, acetic acid, methyl acetate, ethyl acetate, acetaldehyde, and n-propanol (4, 14, 15, 41). One disadvantage of using synthetic volatiles is that carbon dioxide, a fermentation product produced in live yeast baits, is not released (28).
A specific and highly attractive lure is an important part of integrated pest management strategies. Initial trap designs for monitoring D. suzukii utilized apple cider vinegar, grape wine, a baker's yeast and sugar water mixture, or a vinegar/wine mixture as trap bait (17, 40). A major problem with current baits is that other Drosophila species comprise a large part of trap captures, making it difficult to process traps, especially for non-entomologists who may have difficulties distinguishing D. suzukii from other Drosophila species. A live yeast bait with a yeast species that is specifically associated with D. suzukii could alleviate this problem and could be both more attractive and more selective for D. suzukii. The predominant yeast species associated with other temperate Drosophila species are not as heavily skewed to one species as we have seen with D. suzukii (13). Our work suggests H. uvarum as a species with which D. suzukii has a specific association, making it a good candidate species for a more attractive and selective lure. Further studies are needed to verify its long-range attractiveness to adult flies.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Louie Yang and Nicholas Bokulich for their advice on the manuscript. We also thank Elaine Chow, Ngoc-Lien Nguyen, Heather Wilson, and Doris Yu for their efforts in data collection.
Footnotes
Published ahead of print 11 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 1:2320–2325 [DOI] [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 3.Anagnostous C, LeGrand EA, Rohlfs M. 2010. Friendly food for fitter flies? Influences of dietary microbial species on food choices and parasitoid resistance in Drosophila. Oikos 119:533–541 [Google Scholar]
- 4.Barrows WM. 1907. Reactions of pomace fly to odorous substances. J. Exp. Zool. 4:515–540 [Google Scholar]
- 5.Birmingham AL, et al. 2011. A new trap and lure for Drosophila melanogaster (Diptera: Drosophilidae). J. Econ. Entomol. 104:1018–1023 [DOI] [PubMed] [Google Scholar]
- 6.Bokulich NA, Hwang CF, Liu S, Boundy-Mills K, Mills DA. 2011. Profiling the yeast communities of wine fermentation using terminal restriction fragment length polymorphism analysis. Am.J. Enol. Vitic. doi:10.5344/ajev.2011.11077
- 7.Bolda MP, Goodhue RE, Zalom FG. 2010. Spotted wing drosophila: potential economic impact of a newly established pest. Giannini Foundation Agric. Econ. 2010:5–8 [Google Scholar]
- 8.Chippindale AK, Leroi AM, Kim SB, Rose MR. 1993. Phenotypic plasticity and selection in Drosophila life history evolution. 1. Nutrition and the cost of reproduction. J. Evol. Biol. 6:171–173 [Google Scholar]
- 9.Chippindale AK, Leroi AM, Saing H, Borash DJ, Rose MR. 1997. Phenotypic plasticity and selection in Drosophila life history evolution. 2. Diet, mates and the cost of reproduction. J. Evol. Biol. 10:269–293 [Google Scholar]
- 10.Comitini F, Ciani M. 2008. Influence of fungicide treatments on the occurrence of yeast flora associated with wine grapes. Ann. Microbiol. 58:489–493 [Google Scholar]
- 11.Cooke WB. 1959. An ecological life history of Aureobasidium pullulans (de Bary) Arnaud. Mycopathologia 12:1–45 [DOI] [PubMed] [Google Scholar]
- 12.Cooper DM. 1960. Food preferences of larval and adult Drosophila. Evolution 14:41–55 [Google Scholar]
- 13.Heed WB, Starmer WT, Miranda M, Miller MW, Phaff HJ. 1976. An analysis of the yeast flora associated with cactiphilic Drosophila and their host plants in the Sonoran Desert and its relation to temperate and tropical associations. Ecology 57:151–160 [Google Scholar]
- 14.Hoffman AA. 1985. Interspecific variation in the response of Drosophila to chemicals and fruit odors in a wind tunnel. Aust. J. Zool. 33:451–460 [Google Scholar]
- 15.Hunter SH, Kaplan HM, Enxmann EV. 1937. Chemicals attracting Drosophila. Am. Nat. 71:575–581 [Google Scholar]
- 16.Kurtzman CP, Fell JW, Boekhout T. (ed). 2011. The yeasts: a taxonomic study, 5th ed Elsevier, London, United Kingdom [Google Scholar]
- 17.Landolt PJ, Adams T, Rogg H. 2011. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera:Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. doi:10.1111/j.1439-0418.2011.01646.x.
- 18.Leblanc L, Vargas RI, Rubinoff D. 2010. A comparison of nontarget captures in BioLure and liquid protein food lures in Hawaii. Proc. Haw. Entomol. Soc. 42:15–22 [Google Scholar]
- 19.Miller MW, Phaff HJ. 1962. Successive microbial populations in Calimyrna figs. Appl. Microbiol. 10:394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morais PB, Rosa CA, Hagler AN, MendoÇA-Hagler LC. 1995. Yeast communities as descriptors of habitat use by the Drosophila fasciola subgroup (replete group) in Atlantic rain forests. Oecologia 104:45–51 [DOI] [PubMed] [Google Scholar]
- 21.Morais PB, Martins MB, Klaczko LB, Mendoça-Hagler LC, Hagler AN. 1995. Yeast succession in the Amazon fruit Parahancornia amapa as resource partitioning among Drosophila species. Appl. Environ. Microbiol. 61:4251–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakeshott JG, Vacek DC, Anderson PR. 1989. Effects of microbial floras on the distributions of five domestic Drosophila species across fruit resources. Oecologia 78:533–541 [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell K. 1992. Ribosomal DNA internal transcribed spacers are highly divergent in the phytopathogenic ascomycete Fusarium sambucinum (Gibberella pulicaris). Curr. Genet. 22:213–220 [DOI] [PubMed] [Google Scholar]
- 24.Phaff HJ, Miller MW, Recca JA, Shifrine M, Mrak EM. 1956. Yeasts found in the alimentary canal of Drosophila. Ecology 37:533–538 [Google Scholar]
- 25.Reed MR. 1938. The olfactory reactions of Drosophila melanogaster Meigen to the products of fermenting bananas. Physiol. Zool. 11:317–325 [Google Scholar]
- 26.Rohlfs M, Kurschner L. 2010. Saprophagous insect larvae, Drosophila melanogaster, profit from increased species richness in beneficial microbes. J. Appl. Entomol. 134:667–671 [Google Scholar]
- 27.Rosa CA, Ganter P. (ed). 2006. The yeast handbook: biodiversity and ecophysiology of yeasts, p 303–369 Springer-Verlag, Berlin, Germany [Google Scholar]
- 28.Simchoni M, Hagalil M, Shinitzky M, Shmaryshu K. 2003 Feb; Insect trap. U.S. patent 6,516,599. [Google Scholar]
- 29.Simmons FH, Bradley TJ. 1997. An analysis of resource allocation in response to dietary yeast in Drosophila melanogaster. J. Insect. Physiol. 43:1039–1052 [DOI] [PubMed] [Google Scholar]
- 30.Spencer WP. 1950. The Drosophila of Jackson Hole, Wyoming–a taxonomic and ecological survey. Am. Midland Nat. 43:79–87 [Google Scholar]
- 31.Spencer DM, Spencer JFT, Figueroa L, Heluane H. 1992. Yeasts associated with rotting citrus fruits in Tucumán, Argentina. Mycol. Res. 96:891–892 [Google Scholar]
- 32.Starmer WT. 1981. A comparison of Drosophila habitats according to the physiological attributes of the associated yeast communities. Evolution 35:35–53 [DOI] [PubMed] [Google Scholar]
- 33.Starmer WT, Aberdeen V. 1990. The nutritional importance of pure and mixed cultures of yeasts in the development of Drosophila mulleri larvae in Opuntia tissues and its relationship to host plant shifts, p 145–160. In Barker JSF, Starmer WT, MacIntyre RJ. (ed), Ecological and evolutionary genetics of Drosophila. Plenum, New York, NY [Google Scholar]
- 34.Starmer WT, Fogleman JC. 1986. Coadaptation of Drosophila and yeasts in their natural habitat. J. Chem. Ecol. 12:1037–1055 [DOI] [PubMed] [Google Scholar]
- 35.Starmer WT, Peris F, Fontedevila A. 1988. The transmission of yeasts by Drosophila buzzatii during courtship and mating. Anim. Behav. 36:1691–1695 [Google Scholar]
- 36.Steele RH. 1986. Courtship feeding in Drosophila subobscura. I. The nutritional significance of courtship feeding. Anim. Behav. 34:1087–1098 [Google Scholar]
- 37.Steele RH. 1986. Courtship feeding in Drosophila subobscura. II. Courtship feeding by males influences female mate choice. Anim. Behav. 34:1099–1108 [Google Scholar]
- 38.Vacek DC, Starmer WT, Heed WB. 1979. Relevance of the ecology of Citrus yeasts to the diet of Drosophila. Microb. Ecol. 5:43–49 [DOI] [PubMed] [Google Scholar]
- 39.Vacek DC, East PD, Barker JSF, Soliman MH. 1985. Feeding and oviposition preferences of Drosophila buzzatii for microbial species isolated from its natural environment. Biol. J. Linn. Soc. 24:175–187 [Google Scholar]
- 40.Walsh DB, et al. 2011. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Int. Pest Manag. 2:G1–G7 [Google Scholar]
- 41.West AS. 1961. Chemical attraction for adult Drosophila species. J. Econ. Entomol. 54:677–681 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.