Abstract
Overnight exposure of Salmonella enterica serovar Typhimurium to sublethal amounts of Origanum vulgare essential oil (OV) and carvacrol (CAR) did not result in direct and cross-bacterial protection. Cells subcultured with increasing amounts of OV or CAR survived up to the MIC of either compound, revealing few significant changes in bacterial susceptibility.
TEXT
Stress response development in bacteria has received much attention in recent years, including concern from a food safety perspective (11). The main reason for investigating the stress response of food-related bacteria is based on the fact that traditional methods used for food preservation impose physical and chemical stress upon bacterial cells to limit their growth and survival (12).
Salmonella enterica serovar Typhimurium is among the major food-borne pathogens that are of public concern with respect to food safety, causing gastroenteritis outbreaks with different severities (19). S. Typhimurium encounters many diverse and extreme environments that induce a bacterial developmental response to combat the stress, such as extreme pH, salt, and reactive oxygen intermediates (12). This acquired tolerance can be related to various cellular physiological changes that occur through plasmid acquisition, mutation, synthesis of stress proteins, and modification of lipid membrane composition (16, 19).
Early studies showed that Origanum vulgare L. essential oil (OV) possesses strong antimicrobial activity against food-related pathogenic bacteria (3, 9, 10, 13), including Salmonella (1, 8, 14). Despite the use of essential oils (and their components) as potential antimicrobials in foods, there is a lack of information regarding the potential development of direct and cross-protection by bacteria following exposure to subinhibitory amounts of these compounds. This study assessed the development of direct and cross-protection in S. Typhimurium ATCC 14028 when the strain was exposed to subinhibitory concentrations of OV and of the predominant component of this essential oil, carvacrol (CAR) (2), in a meat-based medium.
OV was purchased from Aromalândia Ind. Com. Ltda. (Minas Gerais, Brazil). The compound CAR was purchased from Sigma-Aldrich (Sigma, France). Solutions (160 to 0.075 μl ml−1) of OV and CAR were prepared in nutrient broth (NB) using bacteriological agar (0.15 g 100 ml−1) as a stabilizing agent (4). Inocula of S. Typhimurium ATCC 14028 used in the assays (ca. 7 log CFU ml−1) were obtained as previously described (7). MIC values of OV and CAR were determined by macrodilution in NB (18). OV and CAR both display a MIC value of 1.25 μl ml−1 against S. Typhimurium ATCC 14028.
Development of direct and cross-bacterial protection was measured using cultures grown in a meat-based broth prepared as previously described (10). All assays were performed in triplicate on three separate occasions, and the results were expressed as an average of results of the assays. The significant differences (P < 0.05) were calculated by analysis of variance (ANOVA) followed by Tukey tests using the software SigmaStat 3.1.
For assessing the induction of bacterial direct protection, the strain was exposed overnight to subinhibitory concentrations (1/2 MIC and 1/4 MIC) of OV or CAR in meat broth at 35°C (6, 16). The induction of direct protection was determined by comparing the viable cell counts (log CFU ml−1) in the treated and untreated (cells not previously exposed to OV or CAR) suspensions upon further inoculation into meat broth at 35°C, to which the same stress agent was added at its MIC values. The overnight exposure of S. Typhimurium to subinhibitory amounts of either OV or CAR did not reveal an induction of bacterial direct protection, as demonstrated by the viable cell counts over 240 min of exposure (Fig. 1A and B). S. Typhimurium that had been previously challenged with sublethal concentrations of OV displayed a decrease, compared to nonadapted cells, in viable counts (P < 0.05) when the cells were further cultivated in growth medium to which the same compound (at its MIC) was added. Otherwise, there was no difference (P > 0.05) between the viable counts of untreated cells and cells adapted to CAR at 1/2 MIC and 1/4 MIC following further cultivation in growth medium to which the same compound (at its MIC) was added.
Fig 1.
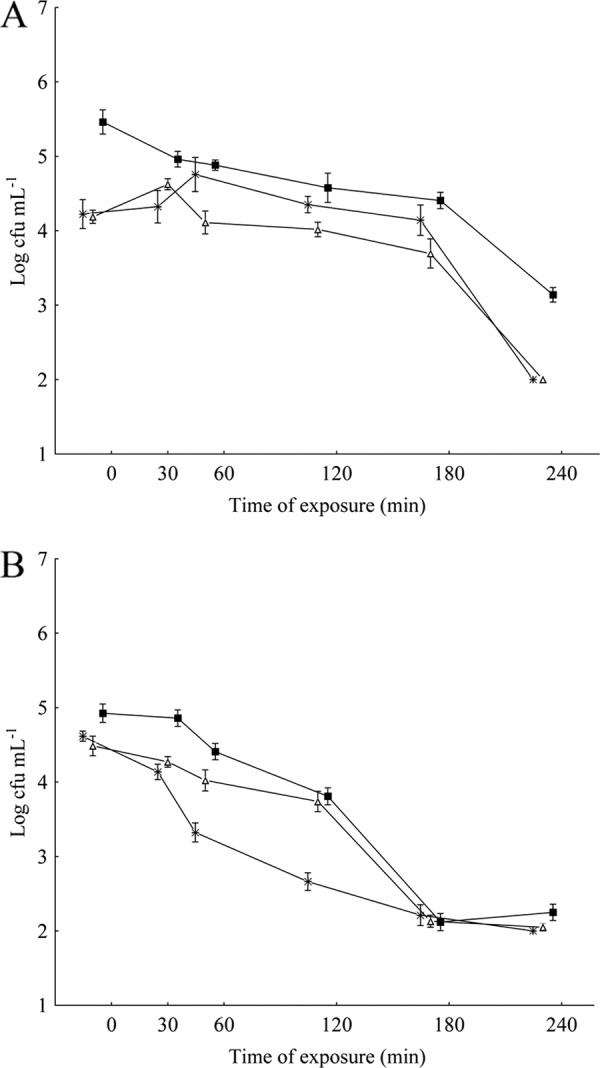
Viable counts of S. Typhimurium ATCC 14028 in meat broth to which the essential oil (at its MIC) was added following overnight exposure at 35°C to subinhibitory concentrations of O. vulgare L. essential oil (A) and carvacrol (B). ■, control (nonadapted cells); +, cells preadapted at an MIC of 2 to 0.625 μl ml−1; Δ, cells preadapted at an MIC of 4 to 0.312 μl ml−1.
Measurements of cross-protection induction were performed as previously described (5). S. Typhimurium cells were exposed overnight at 35°C to subinhibitory amounts of OV or CAR (1/2 MIC and 1/4 MIC), followed by exposure to other stress agents separately (45°C, pH 5.2, 5 g of NaCl 100 ml−1, conditions that modestly inhibited the growth of the assayed strain). The induction of bacterial cross-protection was measured by comparing viable cell counts (log CFU ml−1) in the treated and untreated samples following inoculation into growth medium either containing stressful additives or exposed to environmental stressful conditions (45°C). Consistent with the results of the bacterial direct protection assays, S. Typhimurium cells that were exposed overnight to sublethal concentrations of OV or CAR showed no induction of cross-protection to high temperature, lactic acid, or salt as determined by the viable cell counts throughout the 240 min of exposure (Fig. 2A to C and 3A to C). The cells submitted to preadaptation with OV or CAR at their 1/2 and 1/4 MICs revealed a decrease in viable counts (P < 0.05) upon further exposure to heterologous stressing agents in comparison with cells not previously adapted. These findings are interesting, as the development of homologous and heterologous resistance in S. Typhimurium has been well documented following the challenge of these cells with sublethal exposure to compounds or procedures classically used to control microorganisms in foods and food processing plants (11, 12, 16).
Fig 2.
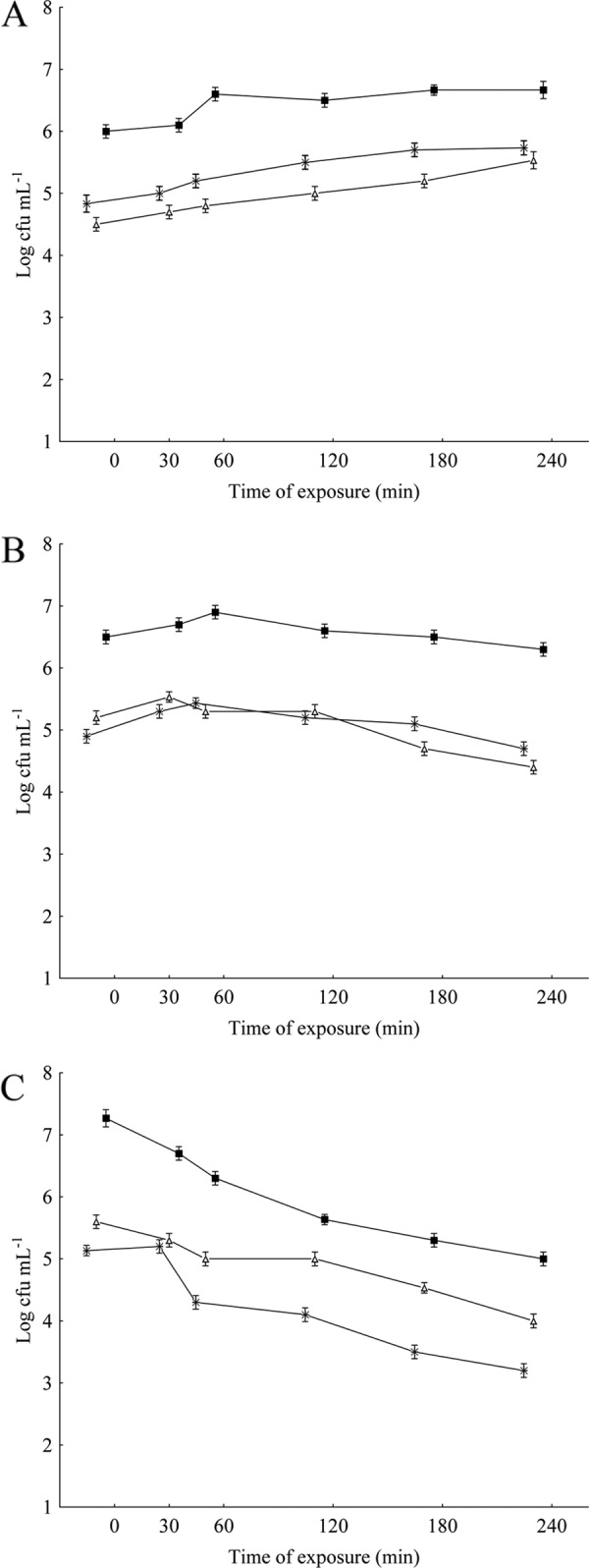
Viable cell counts of S. Typhimurium ATCC 14028 grown in meat broth incubated at high temperature (45°C) (A) or to which lactic acid (pH 5.2) (B) or NaCl (5 g 100 ml−1) (C) was added following overnight exposure at 35°C to subinhibitory concentrations of O. vulgare L. essential oil. ■, control (nonadapted cells); +, cells preadapted at an MIC of 2 to 0.625 μl ml−1; Δ, cells preadapted at an MIC of 4 to 0.312 μl ml−1.
Fig 3.
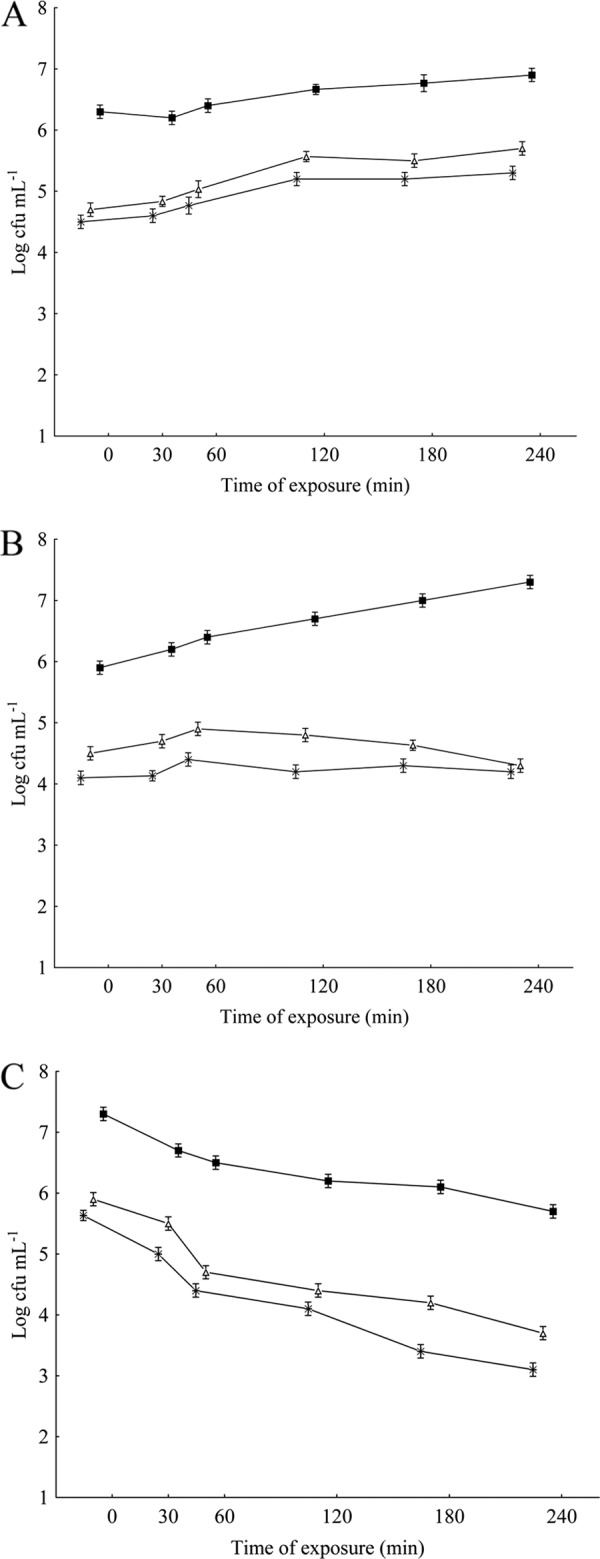
Viable counts of S. Typhimurium ATCC 14028 grown in meat broth incubated at high temperature (45°C) (A) or to which lactic acid (pH 5.2) (B) or NaCl (5 g 100 ml−1) (C) was added following overnight exposure at 35°C to subinhibitory concentrations of carvacrol. ■, control (nonadapted cells); +, cells preadapted at an MIC of 2 to 0.625 μl ml−1; Δ, cells preadapted at an MIC of 4 to 0.312 μl ml−1.
S. Typhimurium cells were exposed to increasing amounts of OV or CAR (1/16 MIC to 4× MIC) throughout successive 24-h habituation cycles (35°C) and monitored for viable cell detection. This procedure was repeated with bacteria exposed to increasing concentrations of the compounds until a concentration was reached at which no viable cells were detected (20). The assays of induction of tolerance following exposure to 24-h cycles in meat broth showed that S. Typhimurium was able to survive in broth to which the OV or CAR was added in concentrations up to their respective MIC values (1-fold increase in the original detected MIC value). These findings suggest minor changes (15, 17) in susceptibility in S. Typhimurium when cells are exposed to sublethal amounts of OV and CAR for a more prolonged time, and if adaptive measures were induced they were not sufficient to substantially alter antimicrobial susceptibility.
To the best of our knowledge, this is the first study investigating the development of adaptation or tolerance by a strain of S. Typhimurium, as determined by monitoring cell viability or growth behavior, following exposure to sublethal amounts of OV or CAR after short and more prolonged exposure times. These studies revealed that exposure to sublethal concentrations of these compounds did not induce direct or cross-protection to high temperature, lactic acid, or NaCl in S. Typhimurium ATCC 14028 when the cells were cultivated in a meat-based medium.
ACKNOWLEDGMENTS
We are grateful to the National Council of Scientific and Technological Development (CNPq, Brazil) for financial support and the Agency for Research Support of the Pernambuco State (FACEPE, Brazil) for a scholarship to Isabelle da Silva Luz.
Footnotes
Published ahead of print 27 April 2012
REFERENCES
- 1.Aligiannis N, Kalpoutzakis E, Mitaku S, Chino IB. 2001. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 49: 4168– 4170 [DOI] [PubMed] [Google Scholar]
- 2.Azeredo GA, Stamford TLM, Nunes PC, Gomes Neto NJ, de Souza EL. 2011. Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res. Int. 44: 1541– 1548 [Google Scholar]
- 3.Barros JC, et al. 2009. Interference of Origanum vulgare L. essential oil on the growth and some physiological characteristics of Staphylococcus aureus strains isolated from foods. LWT Food Sci. Technol. 42: 1139– 1143 [Google Scholar]
- 4.Bennis S, Chami F, Chami N, Bouchikhi T, Remmal R. 2004. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Let. Appl. Microbiol. 38: 454– 458 [DOI] [PubMed] [Google Scholar]
- 5.Boziaris IS, Chorianopoulos NG, Haroutounian SA, Nychas GJ. 2011. Effect of Satureja thymbra essential oil on growth-no growth interfaces of Listeria monocytogenes Scott A and Salmonella Enteritidis PT4, at various temperatures, pH, and water activities. J. Food Prot. 74: 45– 54 [DOI] [PubMed] [Google Scholar]
- 6.Brown JL, Ross T, McMeekin TA, Nichols PD. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37: 163– 173 [DOI] [PubMed] [Google Scholar]
- 7.Carson CF, Mee BJ, Riley TV. 2002. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 46: 1914– 1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadalioglu I, Evrendiek G. 2004. Chemical composition and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.) and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 52: 8255– 8260 [DOI] [PubMed] [Google Scholar]
- 9.D'Antuono LF, Galletti GC, Bochinni P. 2000. Variability of essential oil content and composition of Origanum vulgare L. population from a North Mediterranean area (Liguria Region, Northern Italy). Ann. Bot. 86: 471– 478 [Google Scholar]
- 10.de Oliveira CEV, Stamford TLM, Gomes Neto NJ, de Souza EL. 2010. Inhibition of Staphylococcus aureus in broth and meat broth using synergies of phenolics and organic acids. Int. J. Food Microbiol. 137: 312– 316 [DOI] [PubMed] [Google Scholar]
- 11.Di Pasqua R, Hoskins N, Betts G, Mauriello G. 2006. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 54: 2745– 27489 [DOI] [PubMed] [Google Scholar]
- 12.Dubois-Brissonnet F, Naïtali M, Mafu AA, Briandet R. 2011. Induction of fatty acid composition modifications and tolerance to biocides in Salmonella enterica serovar Typhimurium by plant-derived terpenes. Appl. Environ. Microbiol. 77: 906– 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilfoyle DE, Hirshfield IN. 1996. The survival benefit of sort-chain organic acids and the inducible arginine and lysine decarboxylase genes for Escherichia coli. Lett. Appl. Microbiol. 22: 393– 396 [DOI] [PubMed] [Google Scholar]
- 14.Gunduz GT, Gonul SA, Karapinar M. 2010. Efficacy of oregano oil in the inactivaction of Salmonella typhimurium on lettuce. Food Control 21: 513– 517 [Google Scholar]
- 15.Hammer KA, Carson CF, Riley TV. 2012. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 56: 909– 915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyer GJ, Johnson EA. 1993. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 59: 1842– 1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon M, Blair I, Moore J, McDowell D. 2007. Habituation to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia) is associated with reduced susceptibility to antibiotics in human pathogens. J. Antimicrob. Chemother. 59: 125– 127 [DOI] [PubMed] [Google Scholar]
- 18.Nostro A, et al. 2001. Effects of Helichrysum italicum extract on growth and enzymatic activity of Staphylococcus aureus. Int. J. Antimicrob. Agents 17: 517– 520 [DOI] [PubMed] [Google Scholar]
- 19.Theys TE, et al. 2008. Effect of pH, water activity and gel micro structure, including oxygen profiles and rheological characterization, on the growth kinetics of Salmonella Typhimurium. Int. J. Food Microbiol. 128: 67– 77 [DOI] [PubMed] [Google Scholar]
- 20.To MS, Favrin S, Romanova N, Griffiths MW. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 68: 5258– 5264 [DOI] [PMC free article] [PubMed] [Google Scholar]


