Abstract
Conventionally, two consecutive enzymatic reactions catalyzed by γ-glutamylcysteine synthetase and glutathione synthetase are most commonly used for glutathione production. Here we demonstrate that bacterial bifunctional GshF can be used for glutathione production in a eukaryotic system without accumulation of the intermediate γ-glutamylcysteine.
TEXT
Glutathione (γ-glutamylcysteinylglycine; GSH) is the predominant nonprotein thiol present in living organisms (16). It plays an important role in many physiological functions and has a number of diverse applications in the food, pharmaceutical, and cosmetic industries (12, 18). GSH is industrially produced by fermentation or enzymatic synthesis. By either process, GSH is synthesized through two consecutive enzymatic reactions catalyzed by γ-glutamylcysteine synthetase (γ-GCS, encoded by gshA in Escherichia coli, or GSHI in Saccharomyces cerevisiae) and GSH synthetase (GS, encoded by gshB in E. coli or GSHII in S. cerevisiae), which are widely present in eukaryotes and Gram-negative bacteria. These two enzymes, especially those derived from E. coli and S. cerevisiae, have been overexpressed in various model microorganisms to generate genetically engineered strains with higher GSH production capabilities. Previous studies have reported the overexpression of the E. coli gshA and gshB genes in Lactococcus lactis (13), Clostridium acetobutylicum (23), S. cerevisiae (17), and E. coli (8, 14). Overexpression of the S. cerevisiae GSHI and GSHII genes in S. cerevisiae (5, 20) and Pichia pastoris (6) has also been reported.
While researchers have focused mainly on improving GSH productivity, one fact that is often overlooked is accumulation of the intermediate metabolite, i.e., the dipeptide γ-glutamylcysteine (γ-GC), during these processes. For instance, when the gshA and gshB genes from E. coli were overexpressed in L. lactis (13), the intracellular molar ratio of γ-GC to GSH reached 9:1 in chemically defined medium and 1:1 in complex medium (M17 broth). Overexpression of the E. coli gshA and gshB genes in C. acetobutylicum also led to the intracellular accumulation of a significant amount of γ-GC (25% of the GSH produced; molar ratio) (23). When GSHI and GSHII from S. cerevisiae were overexpressed in P. pastoris, accumulation of γ-GC was also observed (this study; unpublished data). The accumulation of γ-GC might be attributed to the imbalanced activities of γ-GCS and GS. γ-GC accumulation is an undesirable feature in the production of GSH on an industrial scale, as it not only reduces the GSH yield but also increases the cost of GSH purification.
Recently, a novel bifunctional enzyme possessing both γ-GCS and GS activities (termed γ-GCS-GS or GshF, encoded by gshF) was identified and characterized in some Gram-positive bacteria, e.g., Streptococcus agalactiae (9, 19), Listeria monocytogenes (7), and Pasteurella multocida (19, 21). The emergence of this novel enzyme provides an alternative approach to solve the problem of γ-GC accumulation, because the colocalization of γ-GCS and γ-GS modules in GshF is expected to increase the effective concentration of γ-GC, thereby increasing the catalytic rate of the second enzymatic reaction. GshF also has other potential advantages over the conventional γ-GCS/GS systems when used for GSH production. First, the feedback inhibition of γ-GCS activity by GSH is considered a physiological mechanism to prevent the overaccumulation of GSH (1, 2). GshF from S. agalactiae and Streptococcus thermophilus was shown to be insensitive to an increased concentration of GSH (9, 11). Therefore, the use of GshF might be able to maintain the GSH biosynthesis rate even in the presence of a high concentration of GSH. Second, the manipulation and engineering of a single gene is supposed to be easier than the manipulation of two genes. To date, the value of GshF in the biotechnological production of GSH has not been fully exploited. Only recently, Li et al. (11) have successfully expressed GshF from S. thermophilus in E. coli. A recombinant E. coli strain with GshF activity was used for the enzymatic production of GSH using three precursor amino acids. However, due to the low ATP regeneration efficiency of E. coli, this process was coupled with an extraneous ATP regeneration system of S. cerevisiae, making further process optimization complex.
The objective of this study was to introduce bacterial GshF into eukaryotic cells so that the strong ATP-generating ability of the eukaryotic system can be used for one-step GSH biosynthesis. The methylotrophic yeast P. pastoris was chosen as the host because it had several attractive features beneficial for high-level GSH production, such as a low nutrient requirement and ease of growth to a high cell density (2a, 3, 4). The GshF proteins, from L. monocytogenes, S. agalactiae, and Lactobacillus plantarum, were each cloned and expressed in P. pastoris GS115. The GSH production capabilities and other characteristics of the resulting recombinant strains were then determined. The data demonstrated that bacterial bifunctional GshF can be used for GSH production in a eukaryotic system.
Cloning of gshF genes derived from bacteria.
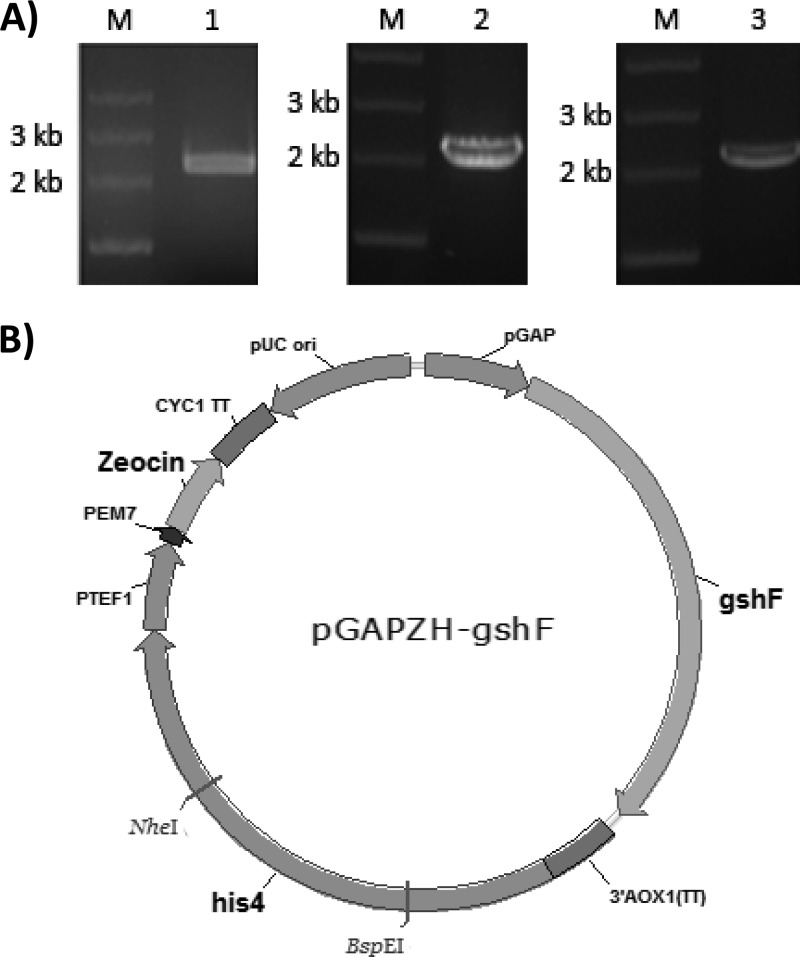
Lm-gshF, Sa-gshF, and Lp-gshF, derived from L. monocytogenes, S. agalactiae, and L. plantarum, respectively, were amplified by PCR from the respective genomic DNA. The sequences of the primers used and other PCR-related information are summarized in Table 1. The Lm-gshF gene was obtained from L. monocytogenes strain ATCC 19114-3 genomic DNA using LmgshF-F and LmgshF-R, creating ClaI and NotI sites (underlined in Table 1), respectively. Similarly, Sa-gshF and Lp-gshF were amplified using SagshF-F/SagshF-R and LpgshF-F/LpgshF-R, respectively. Three fragments of the expected size (around 2.3 kb) were thus obtained (Fig. 1A). The nucleotide sequence of Lm-gshF showed 99% identity with those of L. monocytogenes M7, L99, and HCC23. The nucleotide sequence of Sa-gshF had 100% identity with those of S. agalactiae NEM316, S. agalactiae 2603V/R and A909. The nucleotide sequence of Lp-gshF had 100% identity with that of L. plantarum WCFS1.
Table 1.
PCR-related information for cloning of gshF genes
| Gene name | Source | Primer pair used for amplificationa | Gene size (bp) | Closest homologue(s) (% homology) |
|---|---|---|---|---|
| Lm-gshF | L. monocytogenes | LmgshF-F, 5′-AGATCGATACCATGATAAAACTTGATATGAC-3′; LmgshF-R, 5′-CAGCGGCCGCTTAGTCAAATAAGAAATCTAA-3′ | 2,346 | L. monocytogenes M7, L99, HCC23 (99) |
| Sa-gshF | S. agalactiae | SagshF-F, 5′-AGATCGATACCATGATTATAGATCGACTGTTACAAAGAAGCCACTCTCATCTAC-3′; SagshF-R, 5′-CTAGCGGCCGCTTAGCCTAAGGAACTCTTTTTAGTTA-3′ | 2,276 | S. agalactiae NEM316, 2603V/R, A909 (100) |
| Lp-gshF | L. plantarum | LpgshF-F, 5′-AGATCGATACCATGGAATTAGATGCCGTTGGTA-3′; LpgshF-R, 5′-CAGCGGCCGCTTAATCTTCATTTTTAAACAATGC-3′ | 2,256 | L. plantarum WCFS1 (100) |
ClaI and NotI sites are underlined.
Fig 1.
Cloning and expression of gshF genes from three different Gram-positive bacteria in P. pastoris GS115. (A) PCR amplification of the three gshF genes. Lanes M, molecular size markers; lane 1, L. monocytogenes gshF; lane 2, S. agalactiae gshF; lane 3, L. plantarum gshF. (B) Schematic representation of the construction of a recombinant expression vector containing the gshF genes derived from Gram-positive bacteria.
Construction of recombinant strains.
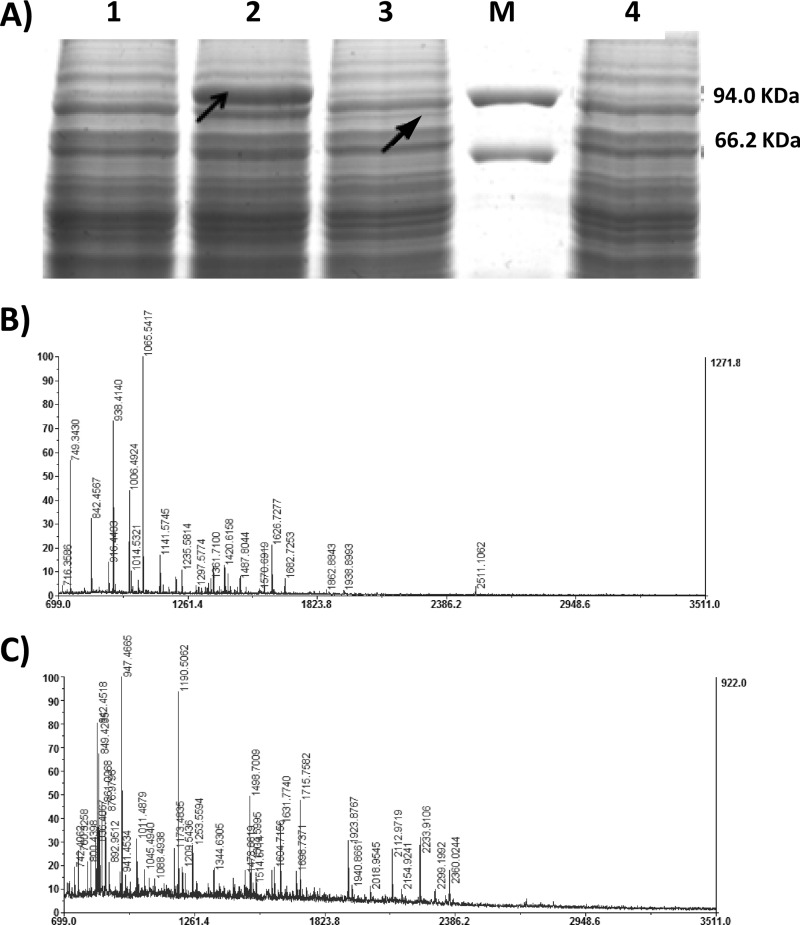
The nucleotide sequence of HIS4 was amplified from plasmid pPIC9k by PCR using oligonucleotide primers his4-F (5′-CTAGATCTATGACATTTCCCTTGCTACCTGCA-3′ [BglII site underlined]) and his4-R (5′-CTGGATCCTTAAATAAGTCCCAGTTTCTC-3′ [BamHI site underlined]). All of the strains and plasmids used and generated in this study are summarized in Table 2. The 2,921-bp product obtained was inserted into the BamHI site of the vector pGAPZαA, yielding plasmid pGAPZαAH. The ClaI/NotI PCR fragments of Lm-gshF, Sa-gshF, and Lp-gshF were cloned into the pGAPZαAH expression vector to form pGAPZH-Lmgsh, pGAPZH-Sagsh, and pGAPZH-Lpgsh, respectively. A schematic representation of the recombinant expression vectors is shown in Fig. 1B. Three recombinant P. pastoris strains, GS115/LmgshF, GS115/SagshF, and GS115/LpgshF, were then generated by transforming linearized pGAPZH-Lmgsh, pGAPZH-Sagsh, and pGAPZH-Lpgsh, respectively, into P. pastoris GS115. The results of gshF gene expression are shown in Fig. 2A. The predicted molecular masses of the L. monocytogenes, S. agalactiae, and L. plantarum GshF proteins were 88.8, 86.3, and 83.0 kDa, respectively. A strongly overexpressed protein band (indicated by the left arrow) was observed at the expected position on SDS-PAGE of L. monocytogenes cell lysate, and a weak band (indicated by the right arrow) was obtained with L. plantarum cell lysate. The bands indicated by arrows in lanes 2 and 3 of Fig. 2A were excised and subjected to matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis (15). Peptide mass fingerprinting results confirmed that the proteins in the two bands (from left to right) were GshF proteins from L. monocytogenes HCC23 and L. plantarum WCFS1, respectively (Fig. 2B and C). For S. agalactiae GshF, however, neither discernible protein band could be seen on SDS-PAGE and Coomassie blue staining, nor could its peptides be detected by MS analysis (data not shown). Therefore, although the three gshF genes were expressed by exactly the same strategy in P. pastoris, their expression levels varied from around 7% of the total soluble protein (quantified by the Gel-Pro Analyzer program [Media Cybernetics]) for GshF from L. monocytogenes to not detectable for that from S. agalactiae. We hypothesized that the codon usage biases of the three gshF genes may account for their different expression levels in P. pastoris.
Table 2.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | TaKaRa | |
| P. pastoris GS115 | his4− Mut+ | Invitrogen |
| S. cerevisiae S288c | Our lab | |
| L. monocytogenes | Our lab | |
| S. agalactiae | Our lab | |
| L. plantarum | Our lab | |
| GS115/LmgshF | GS115 integrated with recombinant plasmid pGAPZH-LmgshF | This study |
| GS115/SagshF | GS115 integrated with recombinant plasmid pGAPZH-SagshF | This study |
| GS115/LpgshF | GS115 integrated with recombinant plasmid pGAPZH-LpgshF | This study |
| GS115/Sc288c | GS115 integrated with recombinant plasmid pGAPZH-ScGSH | This study |
| Plasmids | ||
| pGAPZαA | Vector for constitutive secreted protein expression; Zeor | Invitrogen |
| pPIC9k | Vector for inducible secreted protein expression; HIS4; Kanr Ampr | Invitrogen |
| pGAPZαAH | pGAPZαA-based expression vector carrying HIS4 fragment from pPIC9k; Zeor | This study |
| pGAPZH-Scgsh1 | pGAPZαAH containing gsh1 from S. cerevisiae S288c; Zeor | This study |
| pGAPZH-Scgsh2 | pGAPZαA containing gsh2 from S. cerevisiae S288c; Zeor | This study |
| pGAPZH-ScGSH | pGAPZαAH containing gsh1 and gsh2 from S. cerevisiae S288c; Zeor | This study |
| pGAPZH-LmgshF | pGAPZαAH containing gshF from L. monocytogenes; Zeor | This study |
| pGAPZH-LpgshF | pGAPZαAH containing gshF from L. plantarum; Zeor | This study |
| pGAPZH-SagshF | pGAPZαAH containing gshF from S. agalactiae; Zeor | This study |
Fig 2.
Expression of gshF genes of L. monocytogenes, S. agalactiae, and L. plantarum in P. pastoris. (A) GshF expression analysis by SDS-PAGE. Recombinant P. pastoris cells were harvested after culture for 42 h in YPD. Cell lysate samples were subjected to 10% SDS-PAGE and then stained with Coomassie blue. Lane 1, GS115(control); lane 2, GS115/lmgshF; lane 3, GS115/LpgshF; lane 4, GS115/SagshF; lane M, protein molecular size markers. The arrows indicate the positions of GshF bands. (B) Peptide mass fingerprint of overexpressed bands in cell lysate of GS115/lmgshF (indicated by the left arrow in panel A). A MASCOT score of >82 (the default MASCOT threshold for such searches) was accepted as significant (P < 0.05). (C) Peptide mass fingerprint of overexpressed bands in cell lysate of GS115/LpgshF (indicated by the right arrow in panel A). A MASCOT score of >82 was accepted as significant (P < 0.05).
Synthesis of GSH using recombinant P. pastoris expressing GshF.
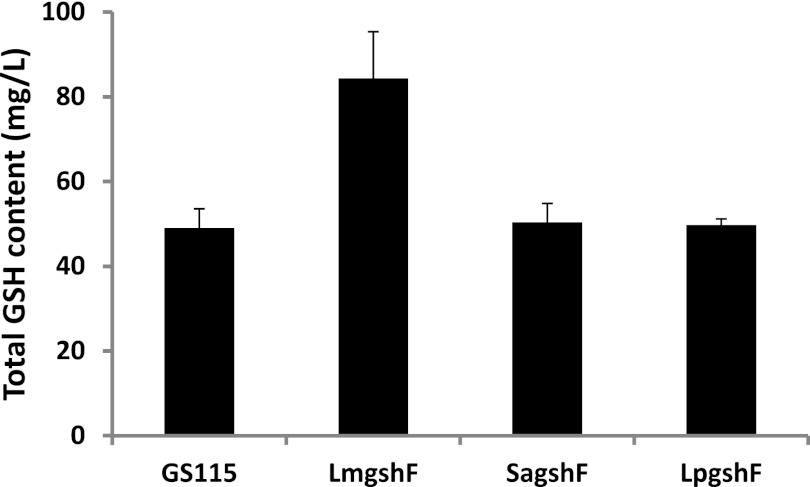
To evaluate the GSH synthesis capabilities of GS115/LmgshF, GS115/SagshF, and GS115/LpgshF, three positive clones (confirmed by colony PCR) of each strain, together with control strain GS115, were cultured in YPD liquid medium (2% glucose, 1% yeast extract, 2% peptone) for 2 days, and the intracellular GSH content was extracted from the cells with 40% ethanol (22) and determined using the monobromobimane fluorescent labeling and high-performance liquid chromatography methods (23). Figure 3 shows that strain GS115/LmgshF had significantly improved GSH synthesis capability, compared with that of strain GS115. The intracellular GSH level was increased by almost 80%. No significant increase in intracellular GSH content was observed in strains GS115/SagshF and GS115/LpgshF. These results were not unexpected, because the GshF expression level of GS115/LmgshF was significantly higher than that of the other two strains.
Fig 3.
Determination of GSH contents of recombinant P. pastoris strains expressing bacterial gshF genes. Recombinant P. pastoris cells were harvested after culture for 2 days in YPD.
Comparison of GSH synthesis capabilities of recombinant P. pastoris strains expressing L. monocytogenes gshF and S. cerevisiae GSHI/GSHII.
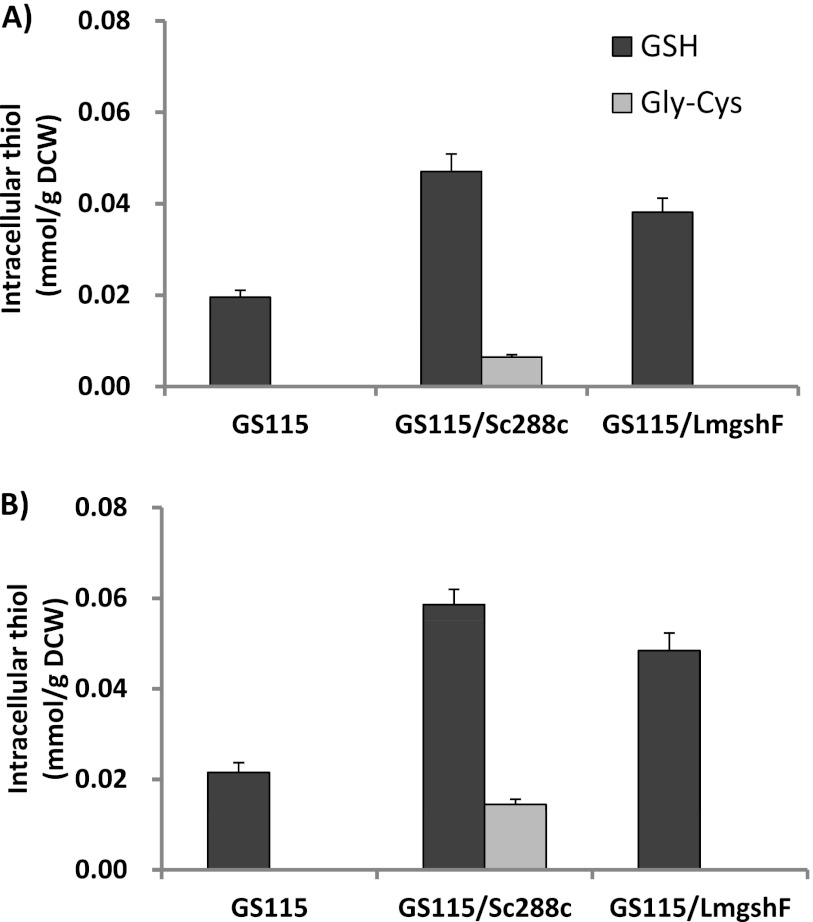
In order to further evaluate the potential of bacterial GshF for GSH biosynthesis and the potential of reducing γ-GC accumulation, we compared the thiol-producing profile of strain GS115/LmgshF with that of another P. pastoris recombinant strain expressing the S. cerevisiae GSHI and GSHII genes (Fig. 4). The strain expressing the GSHI and GSHII genes of S. cerevisiae Sc288c was generated by exactly following the strategy of Fei et al. (6) and designated GS115/Sc288c in this work. Strains GS115, GS115/LmgshF, and GS115/Sc288c were cultured in YPD medium for 42 h, and their intracellular thiol concentration profiles were determined. No difference in the growth rates of these three strains was observed (data not shown). Figure 2A shows that the intracellular γ-GC content of GS115/Sc288c accounted for 11.2% of the GSH synthesized (molar ratio). This increased to 20.1% when three amino acid precursors (glycine, cysteine, and glutamic acid) were added (Fig. 2B). In contrast, as we hypothesized, no intracellular γ-GC was detected in GS115/LmgshF in the absence or presence of precursor amino acids. Although the catalytic efficiency of GshF seems slightly lower than that of GSHI/GSHII, as the GSH synthesized by GS115/LmgshF is around 81 to 83% of that synthesized by GS115/Sc288c, this problem could be solved by increasing the GshF protein expression level in P. pastoris or improving the GshF turnover activity by directed evolution.
Fig 4.
Evaluation of GSH synthesis capability of recombinant P. pastoris strains expressing L. monocytogenes gshF and S. cerevisiae GSHI/GSHII with and without feeding with three amino acid precursors. A, without amino acid precursors; B, with glycine, glutamate, and cysteine. Each amino acid at 5 mM was fed to the flask 1 h before the harvesting of cells.
Conclusion.
In order to test the possibility of using bacterial bifunctional GshF for GSH production in a eukaryotic system, three GshF proteins from the Gram-positive species L. monocytogenes, S. agalactiae, and L. plantarum were each cloned and expressed in the methylotrophic yeast P. pastoris GS115. Only L. monocytogenes GshF displayed significant protein expression and catalytic activity in P. pastoris. Compared with that of S. cerevisiae GSHI and GSHII, the expression of GshF in P. pastoris could significantly reduce the accumulation of the intermediate metabolite γ-GC.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (grant 30560006) and the Knowledge Innovation Program of the Chinese Academy of Sciences (grant KSCX2-EW-G-15-03).
We thank Shutao Sun (Institute of Microbiology, Chinese Academy of Sciences) for her assistance with MALDI-TOF MS analysis.
Footnotes
Published ahead of print 18 May 2012
REFERENCES
- 1. Biterova EI, Barycki JJ. 2009. Mechanistic details of glutathione biosynthesis revealed by crystal structures of Saccharomyces cerevisiae glutamate cysteine ligase. J. Biol. Chem. 284:32700–32708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biterova EI, Barycki JJ. 2010. Structural basis for feedback and pharmacological inhibition of Saccharomyces cerevisiae glutamate cysteine ligase. J. Biol. Chem. 285:14459–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a. Cregg JM, et al. 2009. Expression in the yeast Pichia pastoris. Methods Enzymol. 463:169–189 [DOI] [PubMed] [Google Scholar]
- 3. Daly R, Hearn MT. 2005. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 18:119–138 [DOI] [PubMed] [Google Scholar]
- 4. Damasceno LM, Huang CJ, Batt CA. 2012. Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 93(1):31–39 [DOI] [PubMed] [Google Scholar]
- 5. Fan X, et al. 2004. Increasing glutathione formation by functional expression of the gamma-glutamylcysteine synthetase gene in Saccharomyces cerevisiae. Biotechnol. Lett. 26:415–417 [DOI] [PubMed] [Google Scholar]
- 6. Fei L, Wang Y, Chen S. 2009. Improved glutathione production by gene expression in Pichia pastoris. Bioprocess. Biosyst. Eng. 32:729–735 [DOI] [PubMed] [Google Scholar]
- 7. Gopal S, et al. 2005. A multidomain fusion protein in Listeria monocytogenes catalyzes the two primary activities for glutathione biosynthesis. J. Bacteriol. 187:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gushima H, Miya T, Murata K, Kimura A. 1983. Construction of glutathione-producing strains of Escherichia coli B by recombinant DNA techniques. J. Appl. Biochem. 5:43–52 [PubMed] [Google Scholar]
- 9. Janowiak BE, Griffith OW. 2005. Glutathione synthesis in Streptococcus agalactiae. One protein accounts for gamma-glutamylcysteine synthetase and glutathione synthetase activities. J. Biol. Chem. 280:11829–11839 [DOI] [PubMed] [Google Scholar]
- 10. Reference deleted. [Google Scholar]
- 11. Li W, Li Z, Yang J, Ye Q. 2011. Production of glutathione using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus expressed in Escherichia coli. J. Biotechnol. 154:261–268 [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Wei G, Chen J. 2004. Glutathione: a review on biotechnological production. Appl. Microbiol. Biotechnol. 66:233–242 [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Hugenholtz J, Sybesma W, Abee T, Molenaar D. 2005. Using Lactococcus lactis for glutathione overproduction. Appl. Microbiol. Biotechnol. 67:83–90 [DOI] [PubMed] [Google Scholar]
- 14. Liao XY, Shen W, Chen J, Li Y, Du GC. 2006. Improved glutathione production by gene expression in Escherichia coli. Lett. Appl. Microbiol. 43:211–214 [DOI] [PubMed] [Google Scholar]
- 15. Mao S, et al. 2010. Proteome reference map and comparative proteomic analysis between a wild type Clostridium acetobutylicum DSM 1731 and its mutant with enhanced butanol tolerance and butanol yield. J. Proteome Res. 9:3046–3061 [DOI] [PubMed] [Google Scholar]
- 16. Meister A, Anderson ME. 1983. Glutathione. Annu. Rev. Biochem. 52:711–760 [DOI] [PubMed] [Google Scholar]
- 17. Ohtake Y, et al. 1989. Expression of the glutathione synthetase gene of Escherichia coli B in Saccharomyces cerevisiae. J. Ferment. Bioeng. 68:390–394 [Google Scholar]
- 18. Sies H. 1999. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 27:916–921 [DOI] [PubMed] [Google Scholar]
- 19. Stout J, De Vos D, Vergauwen B, Savvides SN. 2012. Glutathione biosynthesis in bacteria by bifunctional GshF is driven by a modular structure featuring a novel hybrid ATP-grasp fold. J. Mol. Biol. 416:486–494 [DOI] [PubMed] [Google Scholar]
- 20. Tezuka H, Otake Y, Yabuchi S, Kimura H. 30 November 1987. Novel recombinant plasmid participating in glutathione synthesis and production of glutathione using yeast containing said recombinant plasmid. Japanese patent JP62275685. [Google Scholar]
- 21. Vergauwen B, De Vos D, Van Beeumen JJ. 2006. Characterization of the bifunctional gamma-glutamate-cysteine ligase/glutathione synthetase (GshF) of Pasteurella multocida. J. Biol. Chem. 281:4380–4394 [DOI] [PubMed] [Google Scholar]
- 22. Xiong ZQ, et al. 2009. Efficient extraction of intracellular reduced glutathione from fermentation broth of Saccharomyces cerevisiae by ethanol. Bioresour Technol. 100:1011–1014 [DOI] [PubMed] [Google Scholar]
- 23. Zhu L, Dong H, Zhang Y, Li Y. 2011. Engineering the robustness of Clostridium acetobutylicum by introducing glutathione biosynthetic capability. Metab. Eng. 13:426–434 [DOI] [PubMed] [Google Scholar]