Abstract
Viable ova of Ascaris lumbricoides, an indicator organism for pathogens, are frequently found in feces-derived compost produced from ecological toilets, demonstrating that threshold levels of time, temperature, pH, and moisture content for pathogen inactivation are not routinely met. Previous studies have determined that NH3 has ovicidal properties for pathogens, including Ascaris ova. This research attempted to achieve Ascaris inactivation via NH3 under environmental conditions commonly found in ecological toilets and using materials universally available in an ecological sanitation setting, including compost (feces and sawdust), urine, and ash. Compost mixed with stored urine and ash produced the most rapid inactivation, with significant inactivation observed after 2 weeks and with a time to 99% ovum inactivation (T99) of 8 weeks. Compost mixed with fresh urine and ash achieved a T99 of 15 weeks, after a 4-week lag phase. Both matrices had relatively high total-ammonia concentrations and pH values of >9.24 (pKa of ammonia). In compost mixed with ash only, and in compost mixed with fresh urine only, inactivation was observed after an 11-week lag phase. These matrices contained NH3 concentrations of 164 to 173 and 102 to 277 mg/liter, respectively, when inactivation occurred, which was below the previously hypothesized threshold for inactivation (280 mg/liter), suggesting that a lower threshold NH3 concentration may be possible with a longer contact time. Other significant results include the hydrolysis of urea to ammonia between pH values of 10.4 and 11.6, above the literature threshold pH of 10.
INTRODUCTION
Ecological sanitation is the practice of converting human excreta into compost and liquid fertilizer for beneficial reuse of the carbon and nutrients naturally occurring in feces and urine. Urine diversion dehydration toilets (UDDT) are specifically designed for the collection and production of human fecal compost and are an increasingly common sanitation alternative in developing countries. The compost produced from UDDT is a mix of feces and bulking materials that have been decomposed for at least 6 to 12 months. Common bulking materials include sawdust, rice husks, and ash and are used to cover fecal material, eliminate odors and insects, absorb moisture, and supplement carbon concentrations in the compost (6). While ecological sanitation has many advantages, including the provision of affordable sanitation and the production of free, organic compost, a potentially dangerous component shared by ecological toilets is the necessity for human handling of the excreta and compost. Pathogens are frequently found in “finished” ecological compost, and human contact with incompletely biodegraded excreta can cause diarrheal disease (6, 7).
The helminth Ascaris lumbricoides is commonly used as an indicator organism for pathogens in ecological compost due to its ubiquitous nature and the resistance of Ascaris ova to harsh environmental conditions, including desiccation and high pH (9). Variables such as temperature, time, pH, and moisture content have traditionally been considered to affect the viability of Ascaris ova (1, 7, 10, 12). In a study on pathogen inactivation in sewage sludge, Pecson and Nelson (10) reported a temperature threshold for thermal inactivation of Ascaris ova between 30 and 40°C, with higher temperatures yielding faster inactivation. Unfortunately, these temperatures are not consistently achieved in ecological toilets, where the internal compost temperature does not rise significantly above the ambient temperature (7). Research concerning the effects of time, pH, and moisture content on the rate of Ascaris inactivation during ecological composting has provided conflicting results, possibly indicating that some combination of these variables or other factors may affect inactivation rates (1, 12).
Other studies have considered the combined effects of ammonia and pH on Ascaris viability. Ammonia has been shown to be toxic to pathogens as NH3 gas, which is the dominant nitrogen species at pH values above the NH4+/NH3 pKa of 9.24 (16). In a study of sewage sludge, Pecson et al. (11) found that at 20°C and pH 12, the addition of 1,000 and 5,000 mg/liter ammonia decreased the time to 99% ovum inactivation (T99) by factors of 3.4 and 7.5, respectively, compared to sludge samples at pH 12 with no ammonia amendment. During this 80-day experiment, pH was adjusted using analytical-grade calcium hydroxide, and ammonia supplementation was achieved using granular NH4Cl. In a 35-day study focused on ecological sanitation, Nordin et al. (8) reported 100% inactivation of Ascaris ova after 22 days in source-separated feces adjusted with ash to pH 9.6, with a 1% (wt/wt) urea amendment (≈2,300 mg/liter). At pH 8.9, T99 was achieved after 35 days with a 2% urea amendment (≈4,600 mg/liter). The data also suggested a minimum threshold concentration of ≈20 mM NH3 for inactivation. In the study, urea was degraded and converted to ammonia using the enzyme urease prior to experimental use.
A challenge to pathogen inactivation in ecological sanitation is the limited resources available at the toilet sites; the use of analytical-grade chemicals to adjust the quantity and species of nitrogen or to ensure a high pH is not practical. Feces and urine are always present at ecological toilet sites, and ash is an available additive commonly used to eliminate odors and insects. The addition of 1 to 2 cups of ash after each fecal event has been observed to produce compost with pH values between 7.26 and 10.58 (average pH, 9.67), higher than the ammonia pKa (6). Fresh urine is typically neutral or slightly acidic and contains ≈6,000 g N/m3, mostly in the form of urea (15). After the hydrolysis of urea to ammonia, urine provides a natural source of ammonia. As hydrolysis occurs, urine becomes basic (pH ≈9) over a period of months. Kabdasli et al. (5) reported that the hydrolysis of urea to ammonia was retarded above a pH of 10 and that between pH 2 and 7.5, 24% of urea was hydrolyzed in 30 days.
The purpose of the research reported in this article was to test matrices for Ascaris ovum inactivation under environmental conditions present during ecological composting, using materials and quantities of materials commonly found at ecological toilets. The content of the matrices is based on previous Ascaris inactivation research that used laboratory chemicals, enzymes, moisture, and temperature manipulation to achieve inactivation (8, 11). The study was designed with a longer period (16 weeks) than previous experiments in order to observe the inactivation that can be achieved in a time frame similar to that needed for the conversion of feces and bulking materials to compost.
MATERIALS AND METHODS
Matrix materials.
Ecological compost and urine from single-chamber UDDT were collected from the Sumaj Huasi ecological toilet compost facility in El Alto, Bolivia. The compost was a mix of partially decomposed human feces and sawdust that had been batch composted for 12 weeks in an open-air environment. The urine had been stored for 6 weeks in a 5,000-liter closed storage container. Fresh human urine was collected and stored in plastic containers 1 day before experimental use. Hardwood ash was collected from baking ovens located in Cochabamba, Bolivia. Ascaris suum ova were extracted from slaughterhouse pig feces, washed, and counted, by following the procedure described by Bowman et al. (2). This procedure produced a solution containing ≈120,000 ova/ml, as determined by microscopic examination of aliquot samples. The solution was then diluted to 12,000 ova/ml for experimental use. A. suum ova have been demonstrated to behave similarly to A. lumbricoides ova in inactivation studies and are commonly used as a surrogate (4).
Matrix construction.
Six matrices were developed using compost, urine, and ash in order to observe their effects on the rate of Ascaris inactivation: matrix 1, compost plus deionized (DI) water; matrix 2, compost plus DI water plus ash; matrix 3, compost plus stored urine; matrix 4, compost plus stored urine plus ash; matrix 5, compost plus fresh urine; matrix 6, compost plus fresh urine plus ash. Matrix 1 served as a control, and matrices 2 to 6 simulated conditions that can easily be achieved during ecological composting. To make each matrix, ≈900 ml water or urine was added to 945 g compost (field moist) until the material was saturated (≈85%, wet weight.). Saturation was defined as the maximum amount of liquid the compost could hold without standing liquid. Saturated conditions were chosen to ensure uninhibited movement of ammonia throughout the matrix. Ash was added in the proportion of 100 ml ash/100 g compost (after liquid was added) to represent the amount that is normally added to UDDT in instances where users add ash as a bulking material after each fecal event (8). After the addition of ash, more water or urine was added to reachieve saturation.
Four hundred fifty grams of each matrix was placed inside 960-ml airtight plastic containers, in triplicate. Approximately 12,000 A. suum ova were pipetted into nylon mesh filter bags (5 by 5 cm; pore size, 30 μm), and six bags were inserted into each container, a methodology similar to that used by Nordin et al. (8). The bottoms of the bags, where the ova were located, were completely immersed in the matrix. The 18 containers were stored in a dark location at ambient temperatures (19.5 ± 1.5°C) for the duration of the 16-week experiment. This temperature was selected because it was significantly lower than the temperature threshold for ovum inactivation of 30 to 40°C reported by Pecson and Nelson (10), ensuring that any inactivation observed during this study was not caused by temperature. The temperature was also chosen because it represents a reasonable temperature in a temperate climate, where many UDDT are located.
Ascaris suum sampling and analysis.
After 1, 2, 4, 8, 12, and 16 weeks, one filter bag was extracted from each container, and the outside of the bag was rinsed with DI water to remove matrix particles that had adhered to the nylon mesh. After each filter bag was opened, the Ascaris ova were collected, incubated, and assessed for viability by following the Tulane method (2), using the following steps. Ova were rinsed through a 150-μm sieve and were collected on a 25-μm sieve before transfer to a petri dish. Formalin (0.5%) was added, and the ova were incubated for 4 weeks at 26 to 28°C. After incubation, 2 ml of 10% bleach was added to the petri dishes for 10 min to remove the opaque albuminous coating of the A. suum ova. Ova were subsequently examined microscopically at ×20 magnification for viability by following standard procedure; only ova with clearly defined larvae were considered viable (2, 8). Untreated ova from the same batch of A. suum ova used in the experiment were incubated and assessed to determine an average baseline viability of 80.6 ± 5.9%.
Data were analyzed and presented using Microsoft Excel 2007. Lag phases and T99 were calculated using a linear regression approach adapted from previous research (8, 10, 11). When no lag phase was apparent, a linear function with the lowest residual sum of squares was fit to the data to determine T99. When a period of no inactivation was observed at the beginning of the experiment (lag phase), the data were split into two groups: the lag phase and the inactivation phase. A linear regression function was fit to each group, and the intercept of these two functions was defined as a breakpoint between the lag phase and the inactivation phase. The timing of the breakpoint was used as the duration of the lag phase, and the regression function of the inactivation phase was used to determine T99. T99 values were calculated relative to the baseline viability of 80.6%.
Matrix analysis.
At the beginning of the experiment (0 weeks), samples of the raw materials (DI water, compost, stored urine, fresh urine, and ash) and the freshly mixed matrices were analyzed for moisture content, pH, total Kjeldahl nitrogen (TKN), NO3−, NO2−, and total ammonia (NH4+ and NH3) by using standard methods (3). At the end of the experiment (16 weeks), samples from all 18 containers were analyzed for the same parameters. At 4, 8, 12, and 16 weeks, samples from all containers were analyzed for pH and total ammonia.
To determine moisture content (wet weight), samples were oven dried at 100°C for 24 h. pH was measured in a 3:1 water-matrix slurry that was agitated for 30 min, using a Thermo Scientific Orion electrode (9165BNWP) and meter (230A). TKN was measured from samples that were air dried until the change in weight over a 24-h period was less than 1%. Extracts used to measure NO3−, NO2−, and total ammonia were prepared by mixing 5 g fresh sample with 50 ml 2 M potassium chloride (KCl). Slurries were agitated on a rotating shaker for 1 h and were filtered through 1-μm-nominal-diameter glass fiber filter paper (type A/E; Pall Corporation). Nitrate and nitrite were analyzed using cadmium reduction. Total ammonia was measured using a Thermo Scientific Orion NH4+ ion-selective electrode (9512BNWP) and meter (320 PerpHect LogR).
RESULTS AND DISCUSSION
Composition of matrix materials.
The pH values of the compost, stored urine, fresh urine, and ash used to make the matrices were 7.6, 9.0, 5.5, and 11.0, respectively. As expected, the compost pH was close to neutral; the fresh urine was acidic; and the stored urine and ash were basic. Total-ammonia concentrations (mg/liter) of the compost, stored urine, and fresh urine were 172.6, 3,957.7, and 326.8 mg/liter, respectively. As expected, compost contained a relatively low concentration of total ammonia; the stored urine contained a high concentration due to the hydrolysis of urea to ammonia; and the fresh urine contained a moderate concentration because the majority of nitrogen was most likely still in the form of urea.
Ascaris inactivation.
Data for total ammonia, pH, NH3, lag phase, and T99 for each matrix at 0, 4, 8, 12, and 16 weeks can be found in Table 1. Matrix 1 (control) contained <1 mg/liter NH3 at pH 7.2 to 7.6 and provided no significant inactivation during the 16-week experiment (Fig. 1). Matrices 4 and 6 resulted in the fastest and most complete inactivation of A. suum during the experiment. These matrices also had the highest sustained NH3 concentrations and pH values (Fig. 2 and 3). Inactivation in matrix 4 (stored urine plus ash) was fastest, with no recorded lag phase and a T99 of 7.5 weeks, compared to matrix 6 (fresh urine plus ash), with a lag phase of 4 weeks and a T99 of 14.9 weeks. Faster inactivation in matrix 4 may be attributed to a higher initial and sustained concentration of NH3 during the experiment. Furthermore, the initial NH3 concentration in matrix 6 was relatively low (505 mg/liter), most likely because urea hydrolysis had not yet occurred in the fresh urine, which may explain the observed 4-week lag phase.
Table 1.
Total ammonia, pH, ammonia gas, lag phase, and T99 for each matrixa
| Matrix | Wk 0 |
Wk 4 |
Wk 8 |
Wk 12 |
Wk 16 |
Lag phase (wks) | T99 (wks) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NHtot | pH | NH3 | NHtot | pH | NH3 | NHtot | pH | NH3 | NHtot | pH | NH3 | NHtot | pH | NH3 | |||
| 1 | 52 | 7.4 | 1 | 24 | 7.4 | 0 | 19 | 7.2 | 0 | 14 | 7.5 | 0 | 14 | 7.6 | 0 | NA | NA |
| 2 | 47 | 10.4 | 44 | 138 | 12.3 | 138 | 207 | 12.1 | 207 | 173 | 12.3 | 173 | 164 | 12.2 | 164 | 11.2 | 25.1 |
| 3 | 3,180 | 8.9 | 1,013 | 3,038 | 8.3 | 344 | 1,382 | 8.0 | 81 | 582 | 8.1 | 37 | 209 | 7.9 | 9 | NA | NA |
| 4 | 2,637 | 10.4 | 2,466 | 2,817 | 11.6 | 2,804 | 2,501 | 11.3 | 2,480 | 2,226 | 11.5 | 2,213 | NM | NM | NM | NA | 7.5 |
| 5 | 2,098 | 6.8 | 8 | 5,321 | 8.8 | 1,425 | 4,451 | 8.3 | 413 | 2,708 | 8.3 | 277 | 1,243 | 8.2 | 102 | 11.1 | 22.8 |
| 6 | 540 | 10.4 | 505 | 1,321 | 11.7 | 1,317 | 1,494 | 11.6 | 1,488 | 985 | 11.8 | 983 | 885 | 11.8 | 882 | 4.0 | 14.9 |
The chemical characteristics were measured each month for the duration of the experiment. Total-ammonia (NHtot) and NH3 concentrations are given in milligrams per liter. The matrices consist of compost and the following additional materials: matrix 1, DI water; matrix 2, DI water and ash; matrix 3, stored urine; matrix 4, stored urine and ash; matrix 5, fresh urine; matrix 6, fresh urine and ash. Week 16 values for matrix 4 were not measured (NM) because 100% inactivation of Ascaris ova had already been observed. NA, not applicable.
Fig 1.
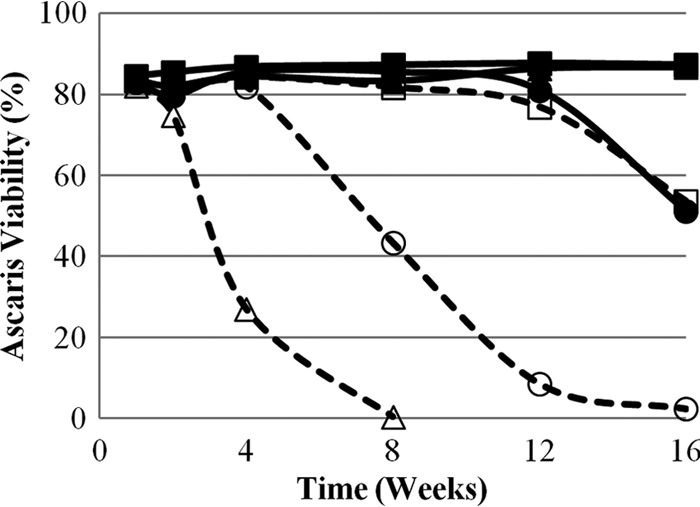
Ascaris suum viability in six ecological compost matrices during 16 weeks of experimental observation. Each data point represents the average value from triplicate measurements. The matrices consist of compost and the following additional materials: matrix 1, DI water (■); matrix 2, DI water and ash (□); matrix 3, stored urine (▲); matrix 4, stored urine and ash (△); matrix 5, fresh urine (●); matrix 6, fresh urine and ash (○). Open symbols and dashed lines represent matrices with ash. The pooled standard deviations of the triplicate measurements are 2.1 for matrix 1, 3.7 for matrix 2, 3.3 for matrix 3, 2.2 for matrix 4, 14.2 for matrix 5. and 6.6 for matrix 6.
Fig 2.
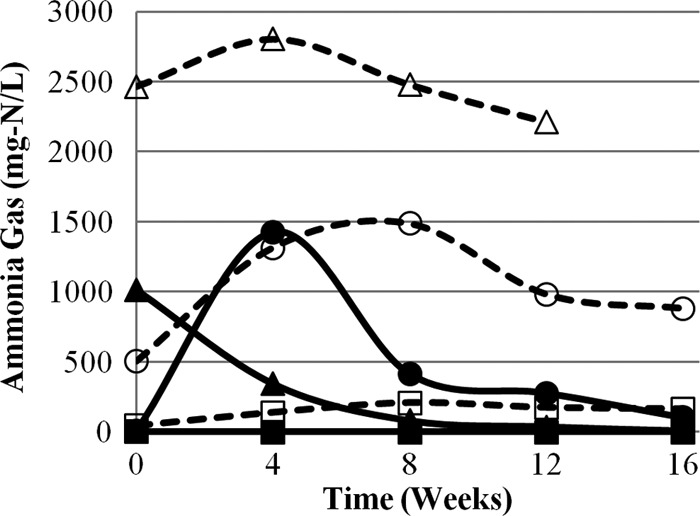
Ammonia gas (NH3) concentrations in six ecological compost matrices during 16 weeks of experimental observation. Each data point represents the average value from triplicate measurements. The matrices consist of compost and the following additional materials: matrix 1, DI water (■); matrix 2, DI water and ash (□); matrix 3, stored urine (▲); matrix 4, stored urine and ash (△); matrix 5, fresh urine (●); matrix 6, fresh urine and ash (○). Open symbols and dashed lines represent matrices with ash. The pooled standard deviations of the triplicate measurements are 0.1 for matrix 1, 14.3 for matrix 2, 19.0 for matrix 3, 201.2 for matrix 4, 224.6 for matrix 5, and 77.5 for matrix 6.
Fig 3.
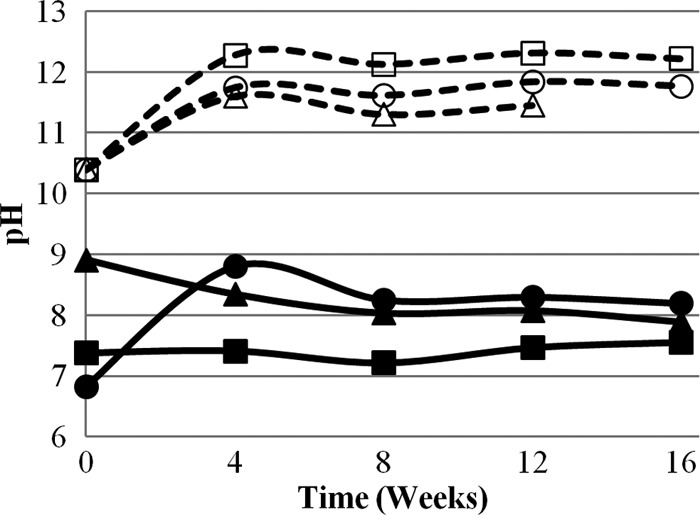
pH values in six ecological compost matrices during 16 weeks of experimental observation. Each data point represents the average value from triplicate measurements. The matrices consist of compost and the following additional materials: matrix 1, DI water (■); matrix 2, DI water and ash (□); matrix 3, stored urine (▲); matrix 4, stored urine and ash (△); matrix 5, fresh urine (●); matrix 6, fresh urine and ash (○). Open symbols and dashed lines represent matrices with ash. The pooled standard deviations of the triplicate measurements are 0.0 for matrix 1, 0.0 for matrix 2, 0.1 for matrix 3, 0.0 for matrix 4, 0.1 for matrix 5, and 0.0 for matrix 6.
Matrices 2 and 5 also provided conditions for Ascaris inactivation after a lag phase of approximately 11 weeks. Values for T99 were calculated to be 25.1 weeks for matrix 2 and 22.8 weeks for matrix 5. Matrix 2 had a high sustained pH (10.4 to 12.3) caused by the ash in the matrix, and relatively low concentrations of total ammonia (47 to 207 mg/liter) compared to other matrices that showed inactivation (Fig. 4). However, due to the high pH, almost all ammonia was in the form of NH3 and was sufficient to cause Ascaris inactivation after a lag phase. Conversely, matrix 5, which contained fresh urine and no ash, had the highest total-ammonia concentrations of all matrices from weeks 4 to 16 (1,243 to 5,321 mg/liter) but pH values below the ammonia pKa. Although the majority of ammonia was present as NH4+, there was sufficient NH3 to cause inactivation after an initial lag phase. When inactivation was recorded in matrices 2 and 5, NH3 concentrations were relatively similar in both matrices, with differences of only 104 mg/liter and 62 mg/liter at weeks 12 and 16, respectively.
Fig 4.
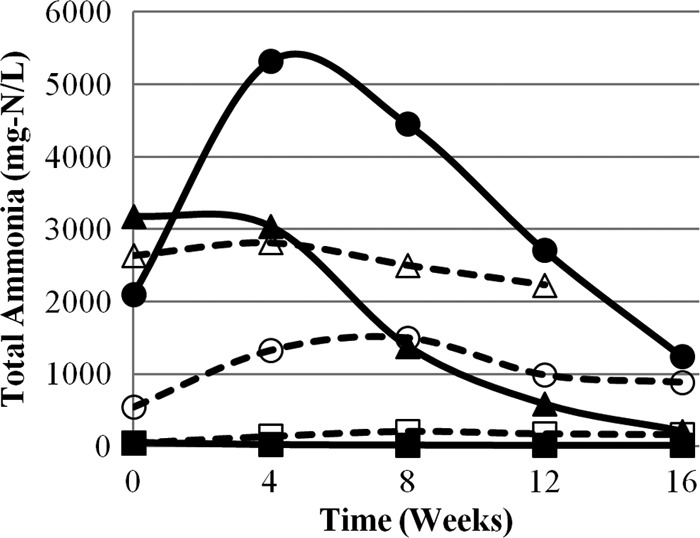
Total-ammonia concentrations in six ecological compost matrices during 16 weeks of experimental observation. Each data point is the average value from triplicate measurements. The matrices consist of compost and the following additional materials: matrix 1,= DI water (■); matrix 2, DI water and ash (□); matrix 3, stored urine (▲); matrix 4, stored urine and ash (△); matrix 5, fresh urine (●); matrix 6, fresh urine and ash (○). Open symbols and dashed lines represent matrices with ash. The pooled standard deviations of the triplicate measurements are 3.2 for matrix 1, 14.3 for matrix 2, 291.6 for matrix 3, 201.7 for matrix 4, 714.5 for matrix 5, and 78.1 for matrix 6.
Effects of NH3 on Ascaris inactivation.
Average A. suum inactivation over the 16 weeks of experimental observation was plotted against the average NH3 concentration for each matrix, showing a correlation (R2 = 0.87). The inactivation results compare closely to those observed by Pecson et al. (11). At 20°C and pH 12, they reported a T99 of 87 days (12.4 weeks) with 1,000 mg/liter ammonia and of 25 days (3.6 weeks) with 5,000 mg/liter ammonia (similar to the conditions and results of matrices 4 and 6). At pH 12, the background sludge in the study of Pecson et al. (11) contained 230 mg/liter ammonia, and a T99 of 230 days (32.9 weeks) was reported (similar to matrix 2). Nordin et al. (8) conducted 35-day inactivation experiments at 24°C and proposed a threshold concentration for Ascaris inactivation of 20 mM NH3 (280 mg/liter). Matrices 2 and 5 had NH3 concentrations below this threshold value during the period of observed inactivation (weeks 12 and 16), although the lag phase was over 11 weeks, more than double the length of that in the experiment by Nordin et al. These results indicate that the threshold NH3 concentration for Ascaris inactivation may be lower than 280 mg/liter when exposure times are longer, as they would be during the ecological composting process. Matrices 1 and 3 exhibited NH3 concentrations much lower than the suggested 280-mg/liter threshold for most of the experiment, and no inactivation was observed in these matrices. Matrix 3 had high concentrations of NH3 initially (≈1,000 mg/liter), but these concentrations dropped sharply over the course of the experiment, and no inactivation was observed. Matrix 5 exhibited high concentrations (≈1,400 mg/liter) 4 weeks into the experiment, but these concentrations dropped to moderate levels in the following weeks, and inactivation was not observed until after a lag phase of 11 weeks. These results demonstrate that the concentration of NH3 must be sustained for inactivation for occur.
It is noteworthy that the individual effects of ammonia and pH on ovum viability cannot be separated in this experiment, since the compost itself contained low concentrations of ammonia. To observe the effects of high pH alone, compost with no ammonia would be needed. A feces-derived compost without ammonia, however, is unrealistic in an ecological toilet setting, and any chemical treatment to remove the ammonia has the potential to introduce variability into the experiment. For this reason, untreated ecological toilet compost was used in the matrices.
Urea hydrolysis and pH.
It was hypothesized that in the matrix containing only compost and fresh urine, ammonia concentrations would increase over time from the hydrolysis of urea. Conversely, it was also hypothesized that hydrolysis would not occur when ash was included with compost and fresh urine, since the process would be inhibited by pH values of >10. In matrix 5 (fresh urine), between 0 and 4 weeks, concentrations of total ammonia increased from 2,098 to 5,321 mg/liter at pH 6.8 to 8.8, due to the hydrolysis of urea. This result fell within previously reported ranges for time (1 to 2 months) and pH (<10) for urea hydrolysis to occur (5, 8). However, in matrix 6 (fresh urine and ash), total-ammonia concentrations increased from 540 to 1,494 mg/liter at pH 10.4 to 11.7, from 0 to 8 weeks, which is above the reported pH threshold for urea hydrolysis. These data suggest that urea was hydrolyzed to ammonia at pH values above 10, although at a lower rate than that observed at lower pH values. Kabdasli et al. (5) and Nordin et al. (8) used observation times of 30 and 35 days, which may have been too short to observe a slow hydrolysis process. In matrix 6 (new urine and ash), ammonification of the organic nitrogen in the compost was ruled out as the sole source of the increase in total ammonia observed at week 8 because of the relatively large increase of 948 mg/liter, compared to 160 mg/liter in matrix 2 (DI water and ash), where there was no urea. The larger increase in matrix 6 indicates that the source of the majority of ammonia was urea, and not organic nitrogen present in the compost.
Anammox in ecological sanitation.
After 4 weeks, total-ammonia concentrations in matrices 3 and 5 began to decrease. Ammonia gas escaping through a leak in the airtight containers was ruled out, because both matrices exhibited relatively low pH values compared to the NH3/NH4+ pKa (9.24), indicating that the majority of the total ammonia was present in the form of ammonium salt. Concentrations of total ammonia, NO3−, NO2−, and TKN at the beginning and end of the experiment (weeks 0 and 16) were compared in an attempt to determine how the nitrogen was partitioning (Table 2). In all matrices, the concentrations of all four species of nitrogen decreased over time. However, in the matrices without ash (1, 3, and 5), the concentrations of total ammonia and NO3− decreased significantly more than in the matrices with ash (P < 0.02). The total ammonia was not lost as ammonia gas (as evidenced by the low pH values) or converted to NO3−, NO2−, or TKN (as evidenced by the decreased concentrations of these species); therefore, it is hypothesized that anaerobic ammonium oxidation, or anammox, may have been occurring.
Table 2.
Change in nitrogen species concentrations in the four compost matrices containing urine from the beginning (0 weeks) to the end (16 weeks) of experimental observationa
| Matrix | Concn (%) of: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total ammonia |
N-NO3 |
N-NO2− |
TKN |
|||||||||||||
| Initial | Final | SD | Difference | Initial | Final | SD | Difference | Initial | Final | SD | Difference | Initial | Final | SD | Difference | |
| 3 | 1.62 | 0.12 | 0.03 | −1.50 | 0.47 | 0.10 | 0.16 | −0.37 | 0.08 | 0.00 | 0.01 | −0.08 | 2.01 | 1.35 | 0.11 | −0.65 |
| 5 | 1.06 | 0.66 | 0.31 | −0.40 | 0.55 | 0.02 | 0.03 | −0.52 | 0.07 | 0.10 | 0.14 | 0.03 | 2.33 | 1.90 | 0.28 | −0.43 |
| 4 | 0.43 | 0.37 | 0.02 | −0.06 | 0.04 | 0.01 | 0.00 | −0.03 | 0.22 | 0.00 | 0.00 | −0.22 | 1.24 | 0.79 | 0.47 | −0.44 |
| 6 | 0.08 | 0.10 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 | 0.00 | 0.00 | −0.37 | 1.03 | 1.00 | 0.24 | −0.02 |
The initial value is the measurement of the bulk raw matrix, and the final value is the average of triplicate measurements. The matrices consist of compost and the following additional materials: matrix 1, DI water; matrix 2, DI water and ash; matrix 3, stored urine; matrix 4, stored urine and ash; matrix 5, fresh urine; matrix 6, fresh urine and ash. The standard deviations (SD) of the triplicate measurements are also provided.
The anammox reaction is the oxidation of NH4+ to N2 gas under anoxic conditions, a likely condition in the matrices, which had moisture contents of ≈85% (wet weight) (14). The anammox hypothesis is further supported by the observation of gas bubbles exclusively in matrices 3 and 5 during weeks 4 to 16 of the experiment. The bacteria responsible for anammox grow slowly and have an 11-day doubling time, which may explain the 1-month lag phase observed before the bubbles appeared and the concentrations of total ammonia began to decrease (13). The anammox bacteria are also hindered by pH values above 8.3, which may explain why matrices with ash did not display signs of anammox (14). It was unlikely that total ammonia was being converted to N2 gas via the nitrification/denitrification pathway, since nitrification occurs in environments that are aerobic and contain low concentrations of organic carbon. The matrices contained high water contents and materials that are composed of organic carbon (feces and sawdust), which would have inhibited nitrification. Direct measurements of the gas bubbles were not made due to a lack of appropriate equipment. This finding has implications for ecological sanitation, since anammox may serve as a mechanism by which total ammonia is lost from ecological compost, both reducing the potential effectiveness of pathogen inactivation and decreasing the nutrient quality of the compost.
Implications.
Ammonia gas concentration was positively correlated with inactivation of A. suum ova. Materials commonly available in ecological toilets were successful in causing 99% inactivation within 6 months. Stored urine and ash constituted the most effective combination of materials for inactivation; fresh urine and ash were also effective after a lag time of 4 weeks; and fresh urine alone, and ash alone, were effective after a lag time of approximately 11 weeks. These results have important implications for ecological sanitation technologies in which urine is separated from feces and stored outside the toilet structure. In stored urine, the urea is already converted to ammonia, and when ash is used as a bulking material, the stored urine can be added to the feces and ash to achieve greater levels of pathogen inactivation. The results demonstrate that adding ash alone (raising pH) without urine, which is common in ecological sanitation, does not achieve 99% A. suum inactivation after 4 months. Additionally, in ecological toilets that do not separate urine, the results demonstrate that fresh urine and ash can also be effective in pathogen inactivation after a lag phase. Ascaris ova are commonly encountered in ecological toilets that have been sealed for more than 6 months; however, the results of this research demonstrate that by using materials that are readily available in ecological toilets, a much faster ovum inactivation can be achieved.
ACKNOWLEDGMENTS
This material is based on work supported by the National Science Foundation under grant 0853097. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
We acknowledge Dwight Bowman of the College of Veterinary Medicine at Cornell University for his support of this research.
Footnotes
Published ahead of print 11 May 2012
REFERENCES
- 1. Austin LM, Duncker LC, Matsebe GN, Phasha MC, Cloete TE. 2005. Ecological sanitation—-literature review. WRC report no. TT 246/05. WRC, Pretoria, South Africa [Google Scholar]
- 2. Bowman DD, Little MD, Reimers RS. 2003. Precision and accuracy of an assay for detecting Ascaris eggs in various biosolid matrices. Water Res. 37:2063–2072 [DOI] [PubMed] [Google Scholar]
- 3. Carter MR. (ed). 1993. Soil sampling and methods of analysis. Lewis Publishers. Boca Raton, FL [Google Scholar]
- 4. Ghiglietti R, Rossi P, Ramsan M, Colombi A. 1995. Viability of Ascaris suum, Ascaris lumbricoides and Trichuris muris eggs to alkaline pH and different temperatures. Parassitologia 37:229–232 [PubMed] [Google Scholar]
- 5. Kabdasli I, et al. 2006. Nitrogen recovery by urea hydrolysis and struvite precipitation from anthropogenic urine. Water Sci. Technol. 53:305–312 [DOI] [PubMed] [Google Scholar]
- 6. McKinley JW, Parzen RE, Gusmán AM. Impact of climate and bulking materials on characteristics of compost from ecological toilets. J. Water Sanit. Hyg. Dev., in press [Google Scholar]
- 7. Moe C, Izurieta R. 2003. Longitudinal study of double vault urine diverting toilets and solar toilets in El Salvador, p 295–302. In Werner C, et al. (ed), EcoSan—closing the loop. Proceedings of the 2nd International Symposium on Ecological Sanitation, Lubeck, Germany. [Google Scholar]
- 8. Nordin A, Nyberg K, Vinnerås B. 2009. Inactivation of Ascaris eggs in source-separated urine and feces by ammonia at ambient temperatures. Appl. Environ. Microbiol. 75:662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Lorcain P, Holland CV. 2000. The public health importance of Ascaris lumbricoides. Parasitology 121:S51–S71 [DOI] [PubMed] [Google Scholar]
- 10. Pecson BM, Nelson KL. 2005. Inactivation of Ascaris suum eggs by ammonia. Environ. Sci. Technol. 39:7909–7914 [DOI] [PubMed] [Google Scholar]
- 11. Pecson BM, Barrios JA, Jimenez BE, Nelson KL. 2007. The effects of temperature, pH, and ammonia concentration on the inactivation of Ascaris eggs in sewage sludge. Water Res. 41:2893–2902 [DOI] [PubMed] [Google Scholar]
- 12. Schonning C, Stenström TA. 2004. Guidelines for the safe use of urine and faeces in ecological sanitation systems. EcoSanRes, SEI, Stockholm, Sweden [Google Scholar]
- 13. Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. 1998. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 50:589–596 [Google Scholar]
- 14. Strous M, Kuenen JG, Jetten M. 1999. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 65:3248–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Udert KM, Larsen TA, Biebow M, Gujer W. 2003. Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res. 37:2571–2582 [DOI] [PubMed] [Google Scholar]
- 16. Warren KS. 1962. Ammonia toxicity and pH. Nature 195:47–49 [DOI] [PubMed] [Google Scholar]


