Abstract
We analyzed DNA microarrays to identify highly expressed genes during stationary-phase growth of Synechocystis sp. PCC 6803. Many identified genes are on endogenous plasmids, with copy numbers between 0.4 and 7 per chromosome. The promoters of such genes will be useful for synthetic biology applications with this phototrophic host.
TEXT
Bacterial cultures enter stationary phase when either nutrient limitation or a buildup of growth by-products ceases cell division. However, this does not necessarily imply that cells become metabolically inactive. Artificial “leaves” have been constructed from Rhodopseudomonas palustris cells embedded in latex that can produce H2 photoheterotrophically for over 5 months without cell growth (5). Pfic in Escherichia coli was recently used to produce a high titer of a bacteriotoxin at stationary phase without any inducer and during exponential phase without detectable growth-limiting toxin during exponential phase (1).
Cultures and microarray analysis.
We used DNA microarray analysis to identify genes and promoters active during stationary phase in Synechocystis sp. PCC 6803. This naturally competent cyanobacterium is a widely used host organism for autotrophic synthetic biology (3, 4, 13, 18). Synthetic biology is an emerging field in which genes, promoters, and other units of genetic code either taken from across the diversity of life or created entirely from scratch are mixed and matched in a host organism (chassis) to improve existing cellular functions or create entirely new ones. Such studies demand the use of a wide variety of promoters that are active under different conditions in the chassis organism.
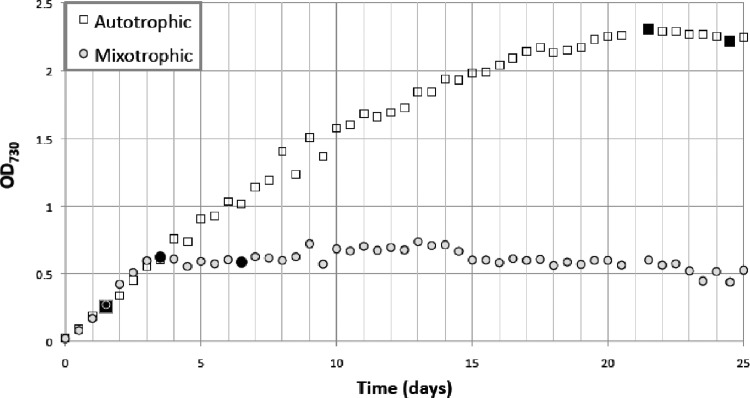
We grew replicate cultures of Synechocystis PCC 6803 in BG11 medium bubbled with air plus 5% CO2 (autotrophic) or with air plus 5 mM glucose (mixotrophic). The temperature was maintained at 30°C, and light intensity was 100 μE m−2 s−1 from cool white fluorescent lamps. Cell growth was monitored by measurement of the optical density at 730 nm on a BioTek μQuant plate reader (BioTek, VT). Cultures were sampled for microarrays in exponential phase and twice during stationary phase (Fig. 1) and analyzed as described previously (20). Briefly, 2 replicate microarrays were analyzed for each of 2 replicate cultures for each nutritional condition. Data were LOWESS normalized by using the MATLAB bioinformatics toolbox. Normalized probe intensities were grouped by genes and t tested to determine significant up- or downregulation (P < 0.05).
Fig 1.
Growth curves. Time points sampled for nucleic acid analysis are indicated with filled symbols.
SPPS.
To quantify the activity of potential promoters at stationary phase, we calculated a stationary-phase promoter score (SPPS) for each open reading frame (ORF), based on the following equation: SPPS = log2(fold change) + log2(normalized expression).
The changes were averaged across nutritional conditions. Normalized expression was the mean normalized intensity of all microarray spots corresponding to a gene at stationary phase divided by the mean normalized intensity for all genes at stationary phase.
Many of the genes with the highest SPPS are located on endogenous plasmids, especially pSysA, pCA2.4, and pCC5.2 (Table 1). The upstream sequences of these genes are listed in Table 2. The genome of Synechocystis PCC 6803 includes 1 circular chromosome of 3.57 Mb, 4 larger plasmids of 44 to 120 kb (pSysA, pSysG, pSysM, and pSysX), and 3 smaller plasmids of 2.4 to 5.2 kb (pCA2.4, pCB2.4, and pCC5.2) (10, 11, 22, 24, 25). Although the plasmids of Synechocystis PCC 6803 have undergone limited study, plasmid-borne genes are required for glucose tolerance (9) and carry genes for a 2-component system responsive to low oxygen (21). The 3 smaller plasmids contain only 10 ORFs, and repA on pCA2.4 is the only one with an annotated function (16).
Table 1.
Top genes, ranked by stationary-phase promoter score
| ORF | Replicon | Annotation | Stationary-phase promoter score | Normalized expression level | Fold changes (autotrophic/mixotrophic) |
|---|---|---|---|---|---|
| slr9003 | pCC5.2 | Unknown | 8.53 | 7.46 | 1.76/0.38 |
| pSysA_116 | pSysA | Unknown | 7.37 | 3.82 | 4.19/2.90 |
| slr9002 | pCC5.2 | Unknown | 6.87 | 4.41 | 0.44/4.47 |
| sll9006 | pCC5.2 | Unknown | 6.41 | 4.24 | 1.02/3.31 |
| pSysA_145 | pSysA | Unknown | 6.40 | 3.53 | 4.02/1.72 |
| sll1982 | Chromosome | Putative transposase | 6.32 | 4.85 | 2.28/0.66 |
| slr9101 | pCA2.4 | Replication protein A | 6.31 | 6.55 | 0.28/−0.77 |
| ssr9005 | pCC5.2 | Unknown | 6.03 | 3.46 | −0.14/5.28 |
| pSysA_39 | pSysA | Unknown | 5.97 | 3.44 | 2.89/2.17 |
| sll5036 | pSysM | Sulfide-quinone reductase | 5.94 | 2.40 | 3.61/3.47 |
| pSysA_27 | pSysA | Unknown | 5.92 | 3.32 | 2.96/2.24 |
| ssl9001 | pCC5.2 | Unknown | 5.90 | 3.58 | 0.05/4.59 |
| sll8019 | pSysG | Unknown | 5.84 | 4.42 | 2.03/0.81 |
| pSysA_25 | pSysA | Unknown | 5.83 | 3.17 | 2.99/2.33 |
| slr0915 | Chromosome | Putative endonuclease | 5.68 | 3.81 | 1.93/1.81 |
| pCA24_1 | pCA2.4 | Unknown | 5.61 | 3.88 | −0.01/3.47 |
| pSysA_24 | pSysA | Unknown | 5.60 | 3.11 | 2.81/2.18 |
| ssr9004 | pCC5.2 | Unknown | 5.44 | 3.07 | −0.48/5.22 |
| pSysA_34 | pSysA | Unknown | 5.44 | 2.52 | 3.10/2.72 |
| pSysA_22 | pSysA | Unknown | 5.24 | 2.37 | 3.03/2.72 |
Table 2.
Upstream sequences of high-scoring SPPS genes
| ORF | Upstream sequence |
|---|---|
| slr9003a | TCCAACAAAAAAAAGCTTTTCAGGAGGGAATTAAGATTGCTGCAGTAAAAAACGTAAGAAGTTTAGTTGACGCTAAAAAACTTACCTACAGACAATAACCCGGCCCAAAAAGCCAACAAAATACTTCAAAAATATTGTCTCTACTGTAGCTCTAAAAATTCCCAAAAGAAAAGCGGTCAACTCTTGAACCCGAGACCGCT |
| pSysA_116 | ATATTTCTGGACGTGGAATGACTCTTGTTCAGGTTTATGACACTGTCAGCAATTGAGATACTTTTGCTGATCGTTTCAGTCCCCTAACGGGGAAAAGAGGGTGTTGAACGAGCCATGGGGTCACTATTGACCACCTCAGGCGCGTTTCAGTCCCCTGACGGGGAAAAGAGGGTGTTGAACCTTGGAAGAAGTTTCCCCTT |
| slr9002 | ACGCACGATGACGTATGACCCTTTTAGCACGGTAGGGAGCGTGATAATCTTCTGCAACACCTATATAGTATTGTTGCGATCGCGAGCGATGGCGTATGACCGGCAATAAGCTACACTGCGCCGATTCCAGCAAAGATAATCCCCTAAGCAACGCAATAATCTTCTGCGAACCTTATATAAGGTTCTGCATATAACGCACG |
| sll9006 | TTGGCTAGGGAATCCTTGGAAAATTCCCCTATCCCGGTAAGGAATCTTTCAAAGCCCAATACTTTAAGGAAGTGAACGGGGACGGTGAGAGTCTTCCGGCTAGCATCCTTGGCACAAACCTTTCCATCTTCCCCGGCCAAATTTTGGAGCCTTTGGCTGTCCCTTGCCTGTAAAAATTCTGCACCGGTGGTGAAGTAATA |
| pSysA_145 | ATATTTCTGGACGTGGAATGACTCTTGTTCAGGTTTATGACACTGTCAGCAATTGAGATACTTTTGCTGATCGTTTCAGTCCCCTAACGGGGAAAAGAGGGTGTTGAACGAGCCATGGGGTCACTATTGACCACCTCAGGCGCGTTTCAGTCCCCTGACGGGGAAAAGAGGGTGTTGAACCTTGGAAGAAGTTTCCCCTT |
| sll1982 | TCCCTGGCAATTACGCTCAAAACGCAACTCTCGATTGTTCAAACAGAGTTGATAAAACTGCTCATCGGAAAGGGATAGGCTGTCAAGTTTGACGGTTATGGCGGCGGGCATTGGCTTTACCCCAAAGGCATTGATGTGGCATTGGCTCCCATTCTAAGTGATGTTTACGGTGACAGAATACCTATCGCTCTTCTATCATT |
| slr9101b | AAATATGGCATTTCATCTTTTCAGGTTTCCCCAAGGTTTCAACTTTCCTCCTTATATTTATTACTGAGGGATAAGTCGCGGATGACAAAATTTGCTGAAACCCTTACCAGATAAGGCATAGAAGCCTATTGACAAAGTAGAAACCCTCTAGCTAAGCTTTGAGTGTCACTTCAAAAACTCAATATCTAGAGGGCTCCAAG |
| ssr9005a | TAAGGATGAAGTGCAGGGCATTATTGACCGCTACAGGGAAGACTTACTGGCAGGAAGACAGCTCCAAGATGTTCCCAGCTCCTACGAGGTCAAAACGGCGATCGCCATTCTGACGGAGGCACTCAGCCTTAAAGCCAATGCCGGTGGAGCCATCAAAGCAAAAATCAGAGAAGCCCTAGCTATCTTGGAAAGGAACTGAA |
| pSysA_39 | GGATTAGTAGAAGGAAAGATTGCCGACCGTTCAGGACAATATGCTGGGAGCGATGGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGCTGTTGGCGAAACAGGAAAAATCTACGTAACAGAAGAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGAAGTATTAAAGGAGTTGGGCGGTGGCTAGTAACA |
| sll5036 | ATGCCTTCTTTTGCTAGGGAATCCTTTACTAATCCGATAGCTTCATCAAATCCTACCAGCAATTTTTTACTAAAGTAATACATTTTATATCTCTGATTTTTATTGAACTAGTCCTTGCCAAATCGAACAAGCACCAATATTATAAGAATATAACTACATAGTTGTATTCGTCAATAGTTTTTGGGGGGAGGGAGTTTAAA |
| pSysA_27 | CTTCAGCAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTAGACATTGTTGAAGAACTTGGACTGATGGAAGAAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGAACGGGCTGGGTCTGGAACCGGAATTCGTGTCTATTGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTCAAGTCTTC |
| ssl9001 | TGCGTGTCAGCAACAGACCTATAGGTGTTGCAGTAGATTATCGCGTTCCCTACCGTGCTAGCTTTGCTCTGATAGGGTCAATGTGGTTTTTTGCCGGTCATACTTCATAACGCTTTTCTACGGGAGTACCCTAGGGTAGTCGCAGTAGATTATCGTGCTTCCCAGCGTGCTAAAACTGGCTTGATAGGGGCAATGTAGGT |
| sll8019 | TTAAGAGAGGTAATTAACCTAACTTAACAAGAACATCGAGTTCTTAACGTACACCCCAGAAAAAGTTAAAGCCACCTGGCAAAGCGTGTTTCTCAGGCACGCCACAGGTAGCTACACAGACTAAAATCTTATGTTGTTAGTGTAGCATGCCAATTTGCCGGATAGCTCCTCCTGGGAAAAATTAGGAGAGTATCTAAGCA |
| pSysA_25 | CTTCAGCAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTAGACATTGTTGAAGAACTTGGACTGATGGAAGAAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGAACGGGCTGGGTCTGGAACCGGAATTCGTGTCTATTGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTCAAGTCTTC |
| slr0915 | GTTGTGACCATTGCAGTAAAGTACCGCCCGTATACTTCGAAAATCCCTAAAATTCTTACTCTTCAGTGCATAGACTATGGGATGAATCTGCCCTAAAAATAAAGTTTGGCAAAAATTCCCCCGATCAGTTATGATATTCGAAGCGACGCGGGATAGAGCAGTCTGGTAGCTCGTCGGGCTCAGGTCGCAAGATGTAAACC |
| pCA24_1b | TATGGTCATTCAACGCCCCCTAATTAGTCCCTAAACCCTGCCAAATATGGCATTTCATCTTTTCAGGTTTCCCCAAGGTTTCAACTTTCCTCCTTATATTTATTACTGAGGGATAAGTCGCGGATGACAAAATTTGCTGAAACCCTTACCAGATAAGGCATAGAAGCCTATTGACAAAGTAGAAACCCTCTAGCTAAGCT |
| pSysA_24c | GGATTAGTAGAAGGAAAGCCACCACCACCACCACGATTAATCAGCAATTATTAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGCTGATAGATCGTAGCGGAATGCTATGGGATGCCTTAGTTGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTTCTGAGGTTCTTTCTAAAATTCTTCCCTATATT |
| ssr9004a | ACTAAGCGACATTATGGCCCCGCCAATATTGACCCCGATGGGCGATCGGCTATTTTTTCCCGGTGGTTTGAGCGGGATTCTATTTTGTATCACTCTGATACCGTATCCACTGAATCCTTATTAATTAATCAAGCCTAGGAACTGGATACCAAAAACAGGGAGTTATGATGGGAATATAATCCCGTTAACAGGCTAAACCC |
| pSysA_34 | TCGCCGGGATTAGTAGAAGGAAAGACCACTCGACGATCAGATCTTCATCAATCAACCGAAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTCGGAGCCAGGCCCACCAAAAGCCGCTTGGTAGGATTGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGCCGACTTTTGGATCGATTAGAATCCGACG |
| pSysA_22c | CCTTAGTTGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGTTCTGAGGTTCTTTCTAAAATTCTTCCCTATATTTTGGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGCCACATTCATCGCTACAGACTTAGAAGGTAGTTACGAGTTTCAGTCCCGATCGCCGGGATTAGTAGAAGGAAAGCTAGCCT |
This ORF appears to be in an operon, with slr9003 at the 5′ end.
The predicted ORFs slr9101 and PCA24_1 overlap, and the latter includes an additional 42 nucleotides at its 5′ end.
This ORF appears to be in an operon, with pSysA_24 at the 5′ end.
Most genes on pSysA, pSysG, and pSysM were upregulated during stationary phase under either nutritional condition. Under mixotrophic conditions, nearly all genes on the smaller plasmids (12/14) were also upregulated (Table 3).
Table 3.
Genes up- or downregulated at stationary phase by replicons
| Replicon | Total no. of ORFs in replicon | No. of up- or downregulated genes |
|||
|---|---|---|---|---|---|
| Autotrophic |
Mixotrophic |
||||
| Up | Down | Up | Down | ||
| Chromosome | 3,239 | 792 | 1,278 | 658 | 1,245 |
| pSysA | 134 | 105 | 2 | 123 | 2 |
| pSysG | 52 | 48 | 1 | 44 | 1 |
| pSysM | 141 | 104 | 9 | 95 | 13 |
| pSysX | 114 | 51 | 31 | 42 | 14 |
| pCA2.4 | 4 | 1 | 2 | 3 | 1 |
| pCB2.4 | 4 | 0 | 4 | 4 | 0 |
| pCC5.2 | 6 | 3 | 2 | 5 | 0 |
In terms of function, our results agree with those of previous studies of the exponential-to-linear growth transition in Synechocystis PCC 6803 (2) and E. coli (6), which found that photosynthesis (in Synechocystis) and energy production processes (in both strains) were downregulated. However, the largest category of regulated genes in our study was that of unknown and hypothetical genes. Despite their unknown functions, the promoters of these genes are expected to serve useful roles in synthetic biology studies (15, 19).
Plasmid copy numbers.
Because plasmid copy numbers often increase during stationary phase, we were interested in testing whether this phenomenon might explain the observed upregulation of plasmid genes (Table 4). We measured plasmid copy numbers per chromosome via quantitative PCR (12). The 3 smaller plasmids had higher copy numbers, in the range of ∼3 to 7 at stationary phase under autotrophic conditions and at both exponential and stationary phases under mixotrophic growth conditions. The copy numbers of the 4 larger plasmids ranged from ∼0.3 to 1.2 per chromosome and varied less with growth phase. Copy numbers of pSysA, pSysM, and pSysX were about twice as high during mixotrophic growth as during autotrophic growth, but copy numbers were only slightly higher for pSysG.
Table 4.
Effects of nutritional condition and growth phase on plasmid copy number per chromosome
| Replicon | Copy no./chromosome (mean ± SD) |
|||
|---|---|---|---|---|
| Autotrophic |
Mixotrophic |
|||
| Exponential | Stationary | Exponential | Stationary | |
| pSysA | 0.34 ± 0.00 | 0.33 ± 0.00 | 0.64 ± 0.01 | 0.60 ± 0.01 |
| pSysG | 0.64 ± 0.01 | 0.54 ± 0.01 | 0.72 ± 0.02 | 0.83 ± 0.01 |
| pSysM | 0.33 ± 0.00 | 0.31 ± 0.00 | 0.69 ± 0.01 | 0.49 ± 0.01 |
| pSysX | 0.65 ± 0.01 | 0.66 ± 0.01 | 1.24 ± 0.02 | 1.09 ± 0.01 |
| pCA2.4 | 0.75 ± 0.01 | 5.41 ± 0.10 | 6.26 ± 0.06 | 7.39 ± 0.09 |
| pCB2.4 | 0.40 ± 0.00 | 2.46 ± 0.02 | 3.74 ± 0.04 | 2.68 ± 0.02 |
| pCC5.2 | 0.93 ± 0.01 | 3.72 ± 0.04 | 6.02 ± 0.05 | 7.33 ± 0.07 |
Copy numbers of pSysA, pCA2.4, and pCC5.2, the plasmids containing the highest-scoring SPPS genes, did not increase at stationary phase under any of the nutritional conditions, indicating that expression levels of such genes are controlled both at the gene dosage and transcriptional levels. For synthetic biology applications, the flexibility afforded by a range of available gene copy numbers and promoter specificities will serve as a benefit, since higher-copy-number plasmids have been associated with growth deficits, lower productivity, and lower inducibility (8). High-copy-number plasmids from E. coli have been modified for use in Synechocystis PCC 6803 (7) and have copy numbers between ∼1 (14) and ∼3 (17) per chromosome (10 to 30 per cell). These plasmids can be maintained with antibiotics, in contrast to endogenous cyanobacterial plasmids, which have higher copy numbers and can be modified to contain heterologous genes and maintained based on essential sequences they carry (23).
Thus, we have identified genes upregulated during the transition to stationary phase under various nutritional conditions in Synechocystis PCC 6803. These genes are mostly carried on plasmids, whose copy numbers range between ∼0.4 and 7 per chromosome. The transcriptional behaviors of the promoters of these genes may make them useful for synthetic biology applications where expression is desired only at stationary phase, to maximize production while not interfering with cell growth during the exponential phase. The higher copy numbers of these plasmids relative to the chromosome may also make them useful insertion sites for heterologous genes.
ACKNOWLEDGMENTS
We thank Thanura Elvitigala for valuable advice and other members of the Pakrasi lab for collegial discussions.
This research was supported by funding from the Office of Science (BER), U.S. Department of Energy, and from the Consortium for Clean Coal Utilization at Washington University.
Footnotes
Published ahead of print 25 May 2012
REFERENCES
- 1. Cao Y, Xian M. 2011. Production of phloroglucinol by Escherichia coli using a stationary-phase promoter. Biotechnol. Lett. 33:1853–1858 [DOI] [PubMed] [Google Scholar]
- 2. Foster J, Singh A, Rothschild L, Sherman L. 2007. Growth-phase dependent differential gene expression in Synechocystis sp. strain PCC 6803 and regulation by a group 2 sigma factor. Arch. Microbiol. 187:265–279 [DOI] [PubMed] [Google Scholar]
- 3. Fu P. 2009. Genome-scale modeling of Synechocystis sp. PCC 6803 and prediction of pathway insertion. J. Chem. Technol. Biotechnol. 84:473–483 [Google Scholar]
- 4. Gao Q, Wang W, Zhao H, Lu X. 2012. Effects of fatty acid activation on photosynthetic production of fatty acid-based biofuels in Synechocystis sp. PCC6803. Biotechnol. Biofuels 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gosse J, Engel B, Hui J, Harwood C, Flickinger M. 2010. Progress toward a biomimetic leaf: 4,000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 26:907–918 [DOI] [PubMed] [Google Scholar]
- 6. Haddadin F, Harcum S. 2005. Transcriptome profiles for high-cell-density recombinant and wild-type Escherichia coli. Biotechnol. Bioeng. 90:127–153 [DOI] [PubMed] [Google Scholar]
- 7. Huang H, Camsund D, Lindblad P, Heidorn T. 2010. Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 38:2577–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones K, Kim S, Keasling J. 2000. Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab. Eng. 2:328–338 [DOI] [PubMed] [Google Scholar]
- 9. Kahlon S, et al. 2006. A putative sensor kinase, Hik31, is involved in the response of Synechocystis sp. strain PCC 6803 to the presence of glucose. Microbiology 152:647–655 [DOI] [PubMed] [Google Scholar]
- 10. Kaneko T, et al. 2003. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 10:221–228 [DOI] [PubMed] [Google Scholar]
- 11. Kaneko T, et al. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109–136 [DOI] [PubMed] [Google Scholar]
- 12. Lee C, Ow D, Oh S. 2006. Quantitative real-time polymerase chain reaction for determination of plasmid copy number in bacteria. J. Microbiol. Methods 65:258–267 [DOI] [PubMed] [Google Scholar]
- 13. Lindberg P, Park S, Melis A. 2010. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 12:70–79 [DOI] [PubMed] [Google Scholar]
- 14. Marraccini P, Bulteau S, Cassier-Chauvat C, Mermet-Bouvier P, Chauvat F. 1993. A conjugative plasmid vector for promoter analysis in several cyanobacteria of the genera Synechococcus and Synechocystis. Plant Mol. Biol. 23:905–909 [DOI] [PubMed] [Google Scholar]
- 15. Miksch G, et al. 2005. Libraries of synthetic stationary-phase and stress promoters as a tool for fine-tuning of expression of recombinant proteins in Escherichia coli. J. Biotechnol. 120:25–37 [DOI] [PubMed] [Google Scholar]
- 16. Nakao M, et al. 2010. CyanoBase: the cyanobacteria genome database update 2010. Nucleic Acids Res. 38:D379–D381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng W, Zentella R, Wang Y, Taylor J, Pakrasi H. 2000. phrA, the major photoreactivating factor in the cyanobacterium Synechocystis sp. strain PCC 6803 codes for a cyclobutane-pyrimidine-dimer-specific DNA photolyase. Arch. Microbiol. 173:412–417 [DOI] [PubMed] [Google Scholar]
- 18. Niederholtmeyer H, Wolfstadter B, Savage D, Silver P, Way J. 2010. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl. Environ. Microbiol. 76:3462–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimada T, et al. 2004. Classification and strength measurement of stationary-phase promoters by use of a newly developed promoter cloning vector. J. Bacteriol. 186:7112–7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A, et al. 2008. Integration of carbon and nitrogen metabolism with energy production is crucial to light acclimation in the cyanobacterium Synechocystis. Plant Physiol. 148:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Summerfield T, Nagarajan S, Sherman L. 2011. Gene expression under low-oxygen conditions in the cyanobacterium Synechocystis sp. PCC 6803 demonstrates Hik31-dependent and -independent responses. Microbiology 157:301–312 [DOI] [PubMed] [Google Scholar]
- 22. Xu W, McFadden B. 1997. Sequence analysis of plasmid pCC5.2 from cyanobacterium Synechocystis PCC 6803 that replicates by a rolling circle mechanism. Plasmid 37:95–104 [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, et al. 2011. Expression of genes in cyanobacteria: adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002. Photosynth. Res. Protoc. 684:273–293 [DOI] [PubMed] [Google Scholar]
- 24. Yang X, McFadden B. 1994. The complete DNA sequence and replication analysis of the plasmid PCB2.4 from the cyanobacterium Synechocystis PCC 6803. Plasmid 31:131–137 [DOI] [PubMed] [Google Scholar]
- 25. Yang X, McFadden B. 1993. A small plasmid, pCA2.4, from the cyanobacterium Synechocystis sp. strain PCC 6803 encodes a rep protein and replicates by a rolling circle mechanism. J. Bacteriol. 175:3981–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]