Abstract
Targeted gene deletion is now available for molecular genetic research of dermatophytes, and the physiological roles of several genes have been elucidated. However, this method cannot be applied to essential genes, which can be potential drug targets. To overcome this limitation, we have developed a conditional gene knockdown system using a copper-responsive promoter. The promoter sequence of the copper transporter gene CTR4 (PCTR4) and that of the copper efflux pump gene CRP1 (PCRP1) derived from Trichophyton rubrum were examined for their response to copper in Arthroderma vanbreuseghemii. PCTR4 was demonstrated to repress expression of a reporter gene in the presence of copper, while the activity of PCRP1 was induced by addition of copper. Importantly, PCTR4 regulated the gene expression more tightly. Furthermore, when PCTR4 was applied to regulate the expression of the endogenous genes ERG1 and TRP5, their conditional mutants exhibited decreased growth activity under the repressive conditions. These results suggest that the PCTR4-based gene regulation system represents a powerful tool for identification and characterization of a broad range of genes, including essential genes, in dermatophytes.
INTRODUCTION
Dermatophytes are a group of filamentous fungi that cause superficial infection (so-called ringworm) in humans and animals. Due to their ability to utilize keratin, they colonize keratinized tissues, such as the skin, nails, and hair. Unlike deep-seated mycoses, e.g., aspergillosis and candidiasis, dermatophytosis is rarely fatal but severely decreases the quality of life of patients. More than 20% of the world's population is estimated to be infected with dermatophytes (12). Therefore, management of dermatophyte infection is a global concern, because currently available antifungal agents do not provide satisfactory results in many cases (11).
Basic tools for molecular genetic studies of dermatophytes have been developed in the last few years, including improved genetic transformation methods (1, 2, 31, 32, 33). In addition, genome sequence databases of seven dermatophyte species became available from the Broad Institute (http://www.broadinstitute.org/annotation/genome/dermatophyte_comparative). Considering genetic relatedness among dermatophytes (16), their genome sequence databases are useful for identification of homologs in strains of other dermatophytic species, including our experimental strain of Arthroderma vanbreuseghemii, one of the teleomorphs of the Trichophyton mentagrophytes complex. These technical advances have made it possible to conduct the functional analyses of several nonessential genes by efficient targeted gene deletion (6, 7, 10, 30).
Conditional gene knockdown methods are often used for identification and evaluation of essential genes, but no such methods have been reported for use in dermatophytes. Conditional gene knockdown systems have been established for some fungi, including the Aspergillus nidulans alcA promoter system (25), the nitrogen-regulated promoter system (pNiiA) in Aspergillus fumigatus (14), and the Escherichia coli tetracycline (Tet) resistance operon-based system in A. fumigatus, Candida albicans, and Candida glabrata (19, 24, 29). In the dimorphic fungus Histoplasma capsulatum, the promoter region of the copper efflux pump CRP1 was shown to function as a copper-inducible promoter (8). Conversely, expression of the copper transporter CTR3 was repressed in the presence of copper. In Cryptococcus neoformans, the promoter of the copper transporter CTR4 (a homolog of H. capsulatum CTR3) was demonstrated to be an excellent copper-repressive promoter and to be suitable for validation of essential genes (4, 15, 22).
The present study was performed to establish a conditional gene knockdown system for use in dermatophytes. For this purpose, we focused on copper-responsive promoters for the following reasons: (i) Histoplasma spp. are phylogenetically related to dermatophytes; (ii) copper-based systems are simple and do not require exogenous trans-acting factors such as tTA or rtTA in the Tet system; and (iii) copper-based systems are thought to be widely applicable, including in animal models, unlike other systems regulated by nutritional sources, such as carbon or nitrogen.
In this study, two copper-responsive promoters, PCTR4 and PCRP1, derived from Trichophyton rubrum were isolated. The lacZ reporter gene was used to evaluate their applicability as conditional promoters in A. vanbreuseghemii. The results of a β-galactosidase assay suggested that both PCTR4 and PCRP1 were copper-responsive promoters. Furthermore, PCTR4 was shown to function as a copper-repressive promoter, allowing tight regulation of essential genes. Here, we report a conditional gene knockdown system in dermatophytes. We expect this PCTR4-based conditional gene knockdown system to facilitate analysis of essential genes and thus advance molecular genetic research in dermatophytes.
MATERIALS AND METHODS
Strains and culture media.
The strains used in this study are listed in Table 1. A. vanbreuseghemii TmL28 was used as a host strain for transformation experiments throughout this study. The strain lacks its homolog of human DNA ligase 4 and is deficient in the nonhomologous end-joining pathway, which enables homologous recombination to occur efficiently (2). Conidial formation of TmL28 was induced at 28°C using modified solid 1/10 Sabouraud dextrose agar (SDA) (0.1% [wt/vol] Bacto peptone, 0.2% [wt/vol] glucose, 0.1% [wt/vol] KH2PO4, and 0.1% [wt/vol] MgSO4 · 7H2O) (28). After transformation, test strains were maintained on solid MOPS ([N-morpholino]propanesulfonic acid)-buffered RPMI 1640 medium (RPMI 1640A; 0.165 M 3-[N-morpholino]propanesulfonic acid, pH 7.0, 10.4 g/liter RPMI 1640). To test the effects of copper on the phenotypes of test strains, copper sulfate (CuSO4) was added at 0.1 to 100 μM. To deplete copper ions from media completely, bathocuproine disulfonate (BCS) (Dojin, Kumamoto, Japan), a copper ion-specific chelator, was added at 10 μM.
Table 1.
Fungal and bacterial strains used in this study
| Organism and strain | Description or genotype | Source or reference |
|---|---|---|
| A. vanbreuseghemii | ||
| TIMM2789 | Wild type | 2 |
| TmL28 | ΔTmLIG4::nptII | 2 |
| PCTR4lacZ | ΔTmLIG4::nptII ΔTmku80::Pch::hph::TtrpC::PCTR4::lacZ::TtrpC | This study |
| PCRP1lacZ | ΔTmLIG4::nptII ΔTmku80::Pch::hph::TtrpC::PCRP1::lacZ::TtrpC | This study |
| PLESSlacZ | ΔTmLIG4::nptII ΔTmku80::Pch::hph::TtrpC::lacZ::TtrpC | This study |
| PCTR4ERG1 | ΔTmLIG4::nptII Pch::hph::TtrpC::PCTR4::ERG1 | This study |
| PCTR4TRP5 | ΔTmLIG4::nptII Pch::hph::TtrpC::PCTR4::TRP5 | This study |
| A. tumefaciens EHA105 | Carrying Ti plasmid pEHA105 | 13 |
Agrobacterium tumefaciens strain EHA105 (13) for A. tumefaciens-mediated transformation (ATMT) was maintained as described previously (33).
Southern hybridization analyses.
The growing mycelia from each dermatophyte strain were collected after incubation on SDA for 3 days at 28°C. The mycelia were frozen and ground under liquid nitrogen with a Multi-Beads shocker (Yasui Kikai, Osaka, Japan) at 1,800 rpm for 10 s, and this step was repeated 10 times. Total DNA was extracted as described previously (9). For Southern blotting, the total DNA samples were digested with an appropriate restriction enzyme, separated by electrophoresis on 0.8% (wt/vol) agarose gels, and transferred onto Hybond N+ membranes (GE Healthcare Limited, Buckinghamshire, United Kingdom). Southern blotting hybridization was performed using the ECL direct nucleic acid labeling and detection system (GE Healthcare Limited) according to the manufacturer's instructions.
Preparation of PCTR4 and PCRP1 from T. rubrum total DNA.
To identify a Trichophyton rubrum homolog of C. neoformans CTR4 (GenBank accession number AAW43046), the T. rubrum CBS118892 genome sequence database was searched using the local BLASTp (protein-protein BLAST) provided by the Broad Institute. A region of approximately 1.3 kb upstream of the start codon of the identified gene TERG_01401 was amplified from T. rubrum total DNA by PCR with a pair of primers, PCTR4-F/BamHI and PCTR4-R (Table 2). The obtained fragment was subcloned into pUC118, sequenced, and used as a putative repressive promoter (PCTR4). Similarly, approximately 1.3 kb of upstream sequence of TERG_07477, a homolog of H. capsulatum CRP1 (GenBank accession number EGC43700), was amplified by PCR with two primers, PCRP1-F/BamHI and PCRP1-R, subcloned into pUC118, sequenced, and used as a putative inducible promoter (PCRP1).
Table 2.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| PCTR4-F/BamHI | ATGGATCCTCGTAGGTACGCC |
| PCTR4-R | ATGAATTCTTCGCAGGCTTTGTAC |
| PCRP1-F/BamHI | ATGGATCCGGAACTCGTTCTTCCATATC |
| PCRP1-R | ATGAATTCGGTGATAGACGGTTCAGTG |
| lacZ/PCTR4-R | AGCCGTTCATTTCGCAGGCTTTGTACTTTG |
| PCTR4/lacZ-F | AGCCTGCGAAATGAACGGCTCCGGAGCTTG |
| TtrpC/lacZ-R | GAGACCCGGGTTTTTGACACCAGACCAAC |
| lacZ/TtrpC-F | GTGTCAAAAACCCGGGTCTCGAGCAGCTAG |
| TtrpC-R/BamHI | TCGGGATCCAAAGAAGGATTACCTCTAAAC |
| PCTR4-F/KpnI | GCATGGTACCTCGTAGGTACGCCCTTCCGG |
| ERG1R/PCTR4-R | ACCATTGTAGTTCGCAGGCTTTGTACTTTG |
| PCTR4/ERG1R-F | GCCTGCGAACTACAATGGTTGTAGAGGCTC |
| ERG1R-R/SacI | GTTTGAGCTCATTATCTGGCCACCTTTGTT |
| ERG1L-F/SpeI | TCCAACTAGTGGAATTAGATAAATCTGGCC |
| ERG1L-R/SpeI | CCATACTAGTAACTTGTCGAAGGGGATAAA |
| TRP5R-F | CAATGGAGCAAATAAAGGAGAC |
| TRP5R-R | TGAATCCAATTAGGGCCTCTG |
| TRP5L-F | GAAAATCATTGGGAAGTATTC |
| TRP5L-R | TGAATGGTGTTGTATATGTTGTGTCG |
Restriction recognition sites are underlined.
Construction of transformation vectors.
To generate lacZ reporter strains, binary vectors pAgPCTR4lacZ and pAgPCRP1lacZ were constructed (Fig. 1A). For pAgPCTR4lacZ, PCTR4, E. coli lacZ (18), and the A. nidulans trpC terminator (TtrpC) (27) were amplified separately by PCR with primers PCTR4-F/BamHI and lacZ/PCTR4-R, PCTR4/lacZ-F and TtrpC/lacZ-R, and lacZ/TtrpC-F and TtrpC-R/BamHI, respectively (Table 2). These three fragments were gel purified and fused by overlap PCR (5) with the primer pair PCTR4-F/BamHI and TtrpC-R/BamHI. The resulting reporter cassette was subcloned into pUC118 and sequenced. The cassette was then excised from the plasmid by BamHI digestion and ligated into the binary vector pAg1-Tmku80/T (31) at the BamHI site to give pAgPCTR4lacZ. pAgPCRP1lacZ was constructed through the same procedure, except using PCRP1 instead of PCTR4. pAgPLESSlacZ, which carried a promoterless lacZ reporter cassette, was also constructed to generate a negative-control strain.
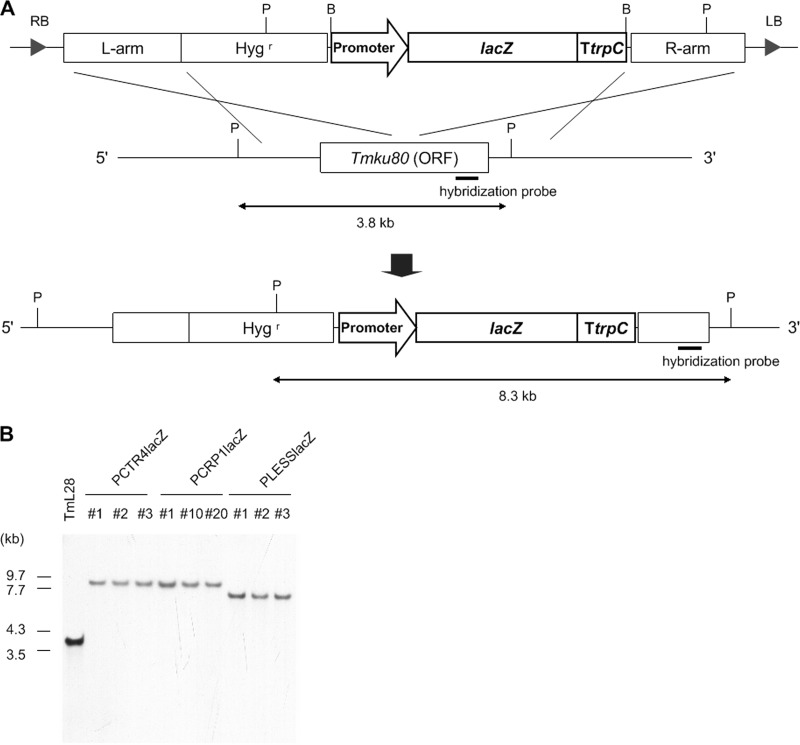
Fig 1.
Construction of lacZ reporter strains. The lacZ reporter cassette was integrated into the Tmku80 locus by ATMT and confirmed by Southern blotting. (A) Schematic representation of homologous recombination. Restriction sites and sizes of expected fragments on Southern blotting are shown. “Promoter” represents either PCTR4 or PCRP1. B, BamHI; P, PstI; Hygr, hygromycin B resistance cassette; L and R arms, homologous sequences for recombination at the Tmku80 locus; LB and RB, left and right borders, respectively. (B) Total DNA from each strain was digested with PstI and separated by gel electrophoresis. Southern blotting hybridization was performed as described in Materials and Methods. A partial fragment of Tmku80 393 bp in length was used as a hybridization probe.
Transformation vectors for production of conditional knockdown strains were constructed as follows. First, the nucleotide sequences of A. vanbreuseghemii ERG1 and TRP5 loci were determined by chromosome walking. For pAgPCTR4ERG1, a binary vector for construction of the PCTR4-ERG1 conditional mutant, approximately 2 kb of sequence beginning from the start codon of ERG1 (R arm) was amplified by PCR from A. vanbreuseghemii total DNA with the primer pair comprising PCTR4/ERG1R-F and ERG1R-R/SacI. PCTR4 and the R arm of ERG1 were then fused by overlap PCR with a pair of primers, PCTR4-F/KpnI and ERG1R-R/SacI. The resulting fragment was digested with KpnI/SacI and ligated into the corresponding site of the binary vector pAg1-hph (33). In the second step, an approximately 2-kb region upstream of the start codon of ERG1 (L arm) was amplified by PCR from A. vanbreuseghemii total DNA with the two primers ERG1L-F/SpeI and ERG1L-R/SpeI. The resulting fragment was digested with SpeI and inserted into the SpeI-digested vector to give pAgPCTR4ERG1. pAgPCTR4TRP5, a binary vector for construction of the PCTR4-TRP5 conditional mutant, was also constructed according to a similar procedure. The R arm and L arm for TRP5 were amplified by PCR with the primer pairs comprising TRP5R-F/TRP5R-R and TRP5L-F/TRP5L-R, respectively, and then assembled into a binary vector.
Transformation of A. vanbreuseghemii.
A. vanbreuseghemii was transformed by ATMT as described previously (33). After cocultivation, transformants were selected on SDA or RPMI 1640A containing 100 to 300 μg/ml hygromycin B. The desired transformants were confirmed by Southern blotting.
β-Galactosidase assays.
Quantitative β-galactosidase assays using cell extracts of reporter strains were performed as follows. Reporter strains were grown at 28°C for 5 days in RPMI 1640 broth in the presence or absence of copper. Mycelia were harvested by filtration using a cell strainer with 40 μM nylon mesh (Becton, Dickinson and Company, Sparks, MD) and stored at −80°C. Frozen mycelia were ground under liquid nitrogen with a Multi-Beads shocker at 2,200 rpm for 15 s, and this step was repeated 10 times. Mycelial powders were suspended in 300 μl of extraction buffer (100 mM sodium phosphate, pH 7.0, 10 mM β-mercaptoethanol, 10 mM KCl, 1 mM MgSO4, 0.1% Triton X-100, 10 mM EDTA) and mixed well. The extracts were centrifuged for 10 min at 20,000 × g to remove cell debris, and the supernatants were collected as crude protein extracts. Protein concentration was measured with a Pierce protein assay kit (660 nm; Thermo Scientific, Rockford, IL), using bovine serum albumin (BSA) as a standard.
An enzyme reaction was performed in 96-well microplates. Aliquots of cell extracts (20 μl, corresponding to approximately 1 μg of protein) were incubated with 150 μl of 1-mg/ml ο-nitrophenyl-β-d-galactopyranoside (ONPG) in Z buffer (100 mM sodium phosphate, pH 7.0, 50 mM β-mercaptoethanol, 10 mM KCl, 1 mM MgSO4) at 30°C until a yellow color developed (usually up to 30 min). Optical density at 405 nm (OD405) was then immediately measured with a Multiskan FC microplate reader (Thermo Scientific). Units were defined as numbers of OD405 units/min of reaction/mg of protein.
Real-time RT-PCR.
Strain PCTR4lacZ was grown in RPMI 1640 broth at 28°C for 5 days, and then CuSO4 was added to give a final concentration of 50 μM and culture was continued for a further 24 h. Mycelia were harvested by filtration and ground into powder as described above. Total RNA was extracted using an RNeasy plant minikit (Qiagen, Hilden, Germany) and was treated with DNase I (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using a high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA) and stored at −30°C until use. Real-time reverse transcription-PCR (RT-PCR) was performed using Fast SYBR green PCR master mix on an ABI PRISM 7500 Fast real-time-PCR system (Applied Biosystems) under the standard conditions recommended by the manufacturer. A dissociation curve of the PCR-amplified products was plotted to confirm the absence of nonspecific products and primer dimers. Primers were designed using Primer Express software (Applied Biosystems). The relative expression level of lacZ after addition of CuSO4 was calculated using the standard curve method and was normalized relative to the 18S rRNA level.
Phenotypic analysis of conditional mutants.
Aliquots of 30 μl of conidial suspensions (containing 3 × 104 conidia) of each conditional mutant were spotted onto solid medium supplemented with 0 to 50 μM CuSO4 or 10 μM BCS and grown at 28°C for 4 days. RPMI 1640A for PCTR4ERG1 and yeast nitrogen base without copper (YNB; ForMedium, Norfolk, United Kingdom) supplemented with 1% glucose and amino acids for PCTR4TRP5 were used as the basal medium.
SEM.
TmL28 and PCTR4ERG1 were grown at 28°C for 2 days as described above, except with a shorter duration of culture. Agar blocks containing single colonies were excised and fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) at 4°C for 24 h. After a thorough washing with the buffer, the samples were postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4) at 4°C for 20 h, dehydrated through a graded series of acetone, and transferred into isoamyl acetate. The samples were dried in a critical-point drying chamber (HCP-1; Hitachi, Tokyo, Japan) using liquid carbon dioxide, coated with osmium tetroxide using an osmium plasma coater (OPC60A; Filgen, Inc., Aichi, Japan), and observed by high-resolution field emission-type scanning electron microscopy (SEM) (JSM-7500F; JEOL, Tokyo, Japan) at an acceleration voltage of 1 kV.
Nucleotide sequence accession numbers.
The newly determined sequences of ERG1 and TRP5 loci from A. vanbreuseghemii were deposited in the GenBank database under accession numbers AB690298 and AB690299, respectively.
RESULTS
Isolation of copper-responsive promoters from the T. rubrum genome.
When we started this study, the whole-genome sequence of A. vanbreuseghemii was not available. Instead, as dermatophytes share closely related genetic backgrounds, we searched the genome sequence database of T. rubrum CBS118892 for a homolog of C. neoformans CTR4 or H. capsulatum CRP1. The results of protein-protein BLAST yielded one candidate for each gene in the T. rubrum genome. The gene TERG_01401 showed 36% identity to C. neoformans CTR4, and the gene TERG_07477 showed 56% identity to H. capsulatum CRP1. An upstream sequence of approximately 1.3 kb of each homologous gene was amplified from T. rubrum total DNA by PCR, and these sequences were designated PCTR4 and PCRP1.
To confirm that the PCTR4 and PCRP1 fragments can function as copper-repressive or -inducible promoters in A. vanbreuseghemii, their copper dose-responses were examined with A. vanbreuseghemii transformants harboring a β-galactosidase (lacZ) reporter cassette. Three types of reporter cassette were constructed and introduced into TmL28 by ATMT (Fig. 1A), yielding PCTR4lacZ, PCRP1lacZ, and PLESSlacZ (Table 1). Southern blotting confirmed that reporter cassettes were successfully integrated into the Tmku80 locus (Fig. 1B). Confirmed clones of PCTR4lacZ (strains #1, #2, and #3), PCRP1lacZ (#1, #10, and #20), and PLESSlacZ (#1) were further analyzed by a quantitative β-galactosidase assay.
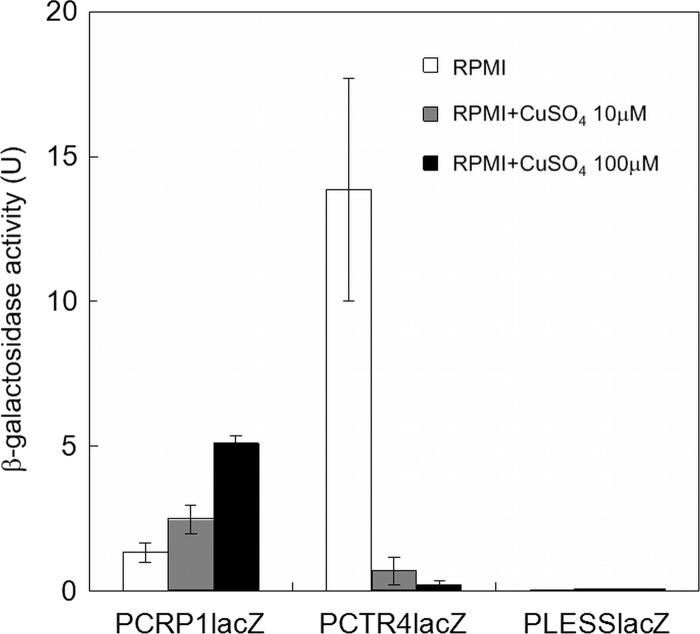
These reporter strains were grown for 5 days in RPMI 1640 broth in the presence or absence of copper and assayed for β-galactosidase activity. As shown in Fig. 2, both PCTR4 and PCRP1 were shown to function as copper-responsive promoters in A. vanbreuseghemii. PCTR4lacZ exhibited marked repression of β-galactosidase activity when cultured in the presence of ≥10 μM CuSO4. Maximum repression was observed at 100 μM, where the activity was repressed approximately 70-fold compared with that in the absence of copper. In contrast, β-galactosidase activity of PCRP1lacZ was induced in a copper dose-dependent manner. The activity in the presence of 100 μM CuSO4 was approximately 4-fold higher than that in the absence of copper. Note that higher leakage activity was observed for PCRP1lacZ, i.e., enzyme activity of PCRP1lacZ in the absence of CuSO4 was higher than that of PCTR4lacZ in the presence of 100 μM CuSO4. PLESSlacZ showed a very low level of β-galactosidase activity, suggesting that A. vanbreuseghemii does not have intrinsic β-galactosidase activity. None of the tested strains showed apparent growth impairment even in the presence of 100 μM CuSO4 (data not shown). These results indicated that PCTR4 would be more suitable for tight regulation of gene expression. Therefore, we focused on PCTR4 in the subsequent studies.
Fig 2.
Copper-dependent responses of PCTR4 and PCRP1. Strains PCTR4lacZ, PCRP1lacZ, and PLESSlacZ were grown for 5 days in RPMI 1640 broth supplemented with 0, 10, or 100 μM CuSO4. The mean β-galactosidase activity is shown in units (U) on the y axis. Error bars indicate standard deviations for three clones.
Time course study of the PCTR4-lacZ reporter strain.
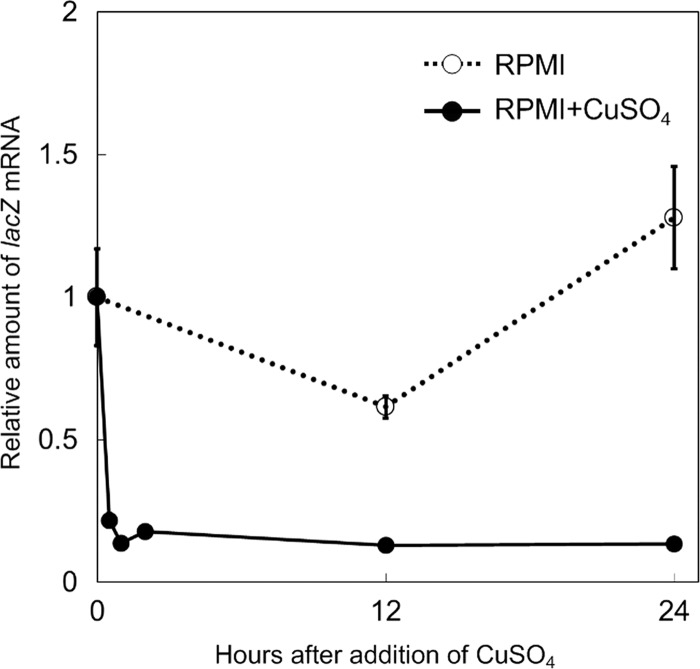
To characterize PCTR4 in detail, the time course of the copper response of PCTR4lacZ was evaluated by real-time RT-PCR. PCTR4lacZ was grown for 5 days in RPMI 1640 broth in the absence of copper (inducing conditions), after which 50 μM CuSO4 was added. The levels of lacZ mRNA were quantified at 0.5, 1, 2, 12, and 24 h after application of CuSO4. As shown in Fig. 3, the lacZ gene expression level rapidly decreased to approximately 20% of the initial level within 0.5 h after administration of CuSO4. The minimal level of expression (approximately 10% of the initial level) was observed at 12 h and lasted for 24 h after addition of CuSO4.
Fig 3.
Time course of transcriptional repression by PCTR4. Strain PCTR4lacZ 1 was grown in RPMI 1640 broth (inducing conditions) for 5 days, and then 50 μM CuSO4 was added. Samples were collected at 0.5, 1, 2, 12, and 24 h after addition of copper. Real-time RT-PCR was performed as described in Materials and Methods. The expression levels of lacZ at each time point are indicated as relative fold changes compared to the level at time zero (addition of CuSO4). Error bars indicate standard deviations for triplicate measurements from a representative experiment.
Regulation of endogenous ERG1 and TRP5 genes by PCTR4.
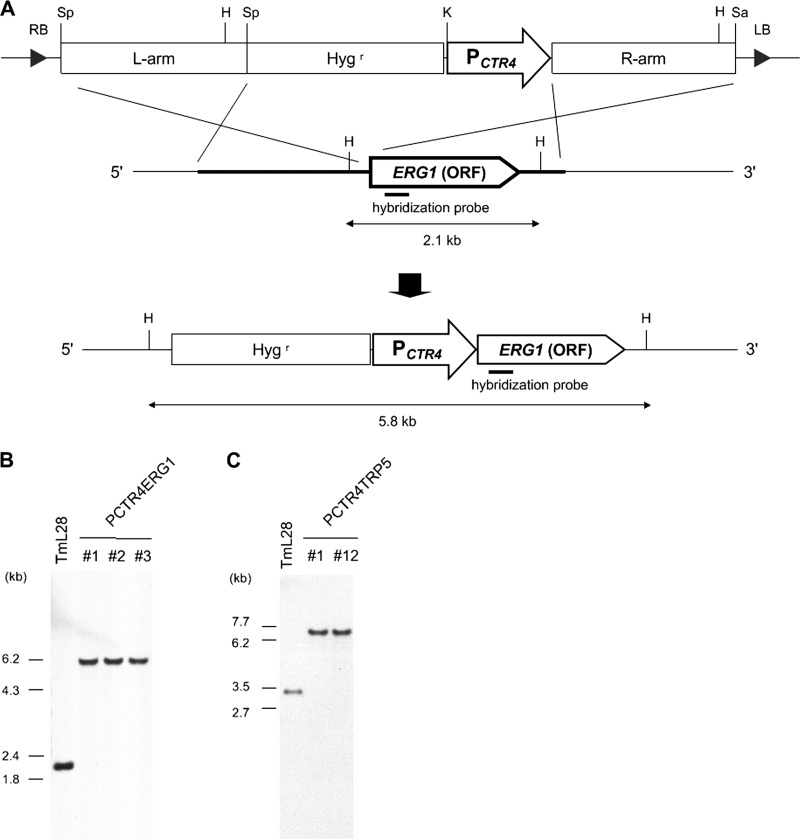
From the above-described experimental results, PCTR4 was expected to be a potential tight conditional promoter. Here, we applied PCTR4 to regulate the expression of specific endogenous genes in A. vanbreuseghemii. For this purpose, ERG1 and TRP5 were chosen as target genes. ERG1, which encodes squalene epoxidase involved in the ergosterol biosynthesis pathway, is a target molecule of antifungal agents such as terbinafine (26) and was expected to be essential for cell growth. TRP5 encodes tryptophan synthase in the tryptophan biosynthesis pathway, and its downregulation caused tryptophan auxotrophy in A. fumigatus (14). Two binary vectors for conditional regulation of the expression of these genes were constructed and introduced into TmL28 by ATMT (Fig. 4A), yielding PCTR4ERG1 and PCTR4TRP5 (Table 1). Southern blotting confirmed that PCTR4 was correctly inserted directly upstream of the target genes (Fig. 4B and C).
Fig 4.
Construction of conditional mutants of endogenous ERG1 or TRP5. PCTR4 was inserted directly upstream of target genes by ATMT and confirmed by Southern blotting. (A) Schematic representation of homologous recombination at the ERG1 locus. Restriction sites and sizes of expected fragments on Southern blotting are shown. Sp, SpeI; K, KpnI; Sa, SacI; H, HindIII; Hygr, hygromycin B resistance cassette; L arm, 5′ homologous sequence 2 kb upstream of the start codon of ERG1; R arm, 2-kb 3′ homologous sequence beginning at the start codon of ERG1; ORF, open reading frame. Parts of host DNA which are homologous to the L and R arms are shown as a thick line. (B) Total DNA from each strain was digested with HindIII and separated by gel electrophoresis. Southern blotting hybridization was performed as described in Materials and Methods. A partial fragment of ERG1 (length, 415 bp) or (C) TRP5 (length, 414 bp) was used as a hybridization probe.
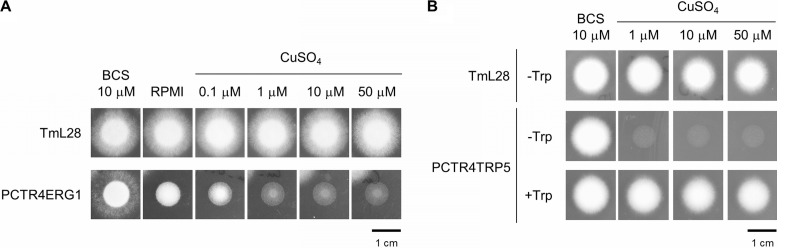
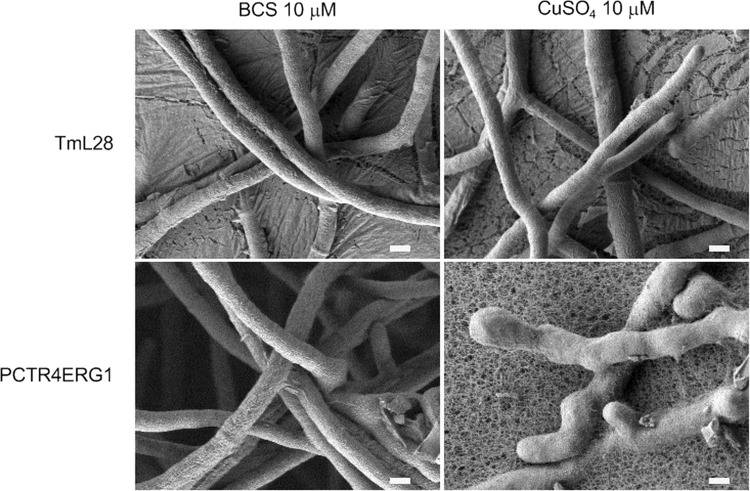
Strain PCTR4ERG1 was grown at 28°C for 4 days on RPMI 1640A in the presence or absence of copper. As shown in Fig. 5A, growth of PCTR4ERG1 was significantly repressed on RPMI 1640A supplemented with ≥0.1 μM CuSO4. Growth was also mildly suppressed on RPMI 1640A without any supplementations of copper, implying the existence of a trace amount of copper in the agar, as RPMI 1640 is a synthetic copper-free medium. However, addition of a copper ion-specific chelator, BCS, restored the growth of PCTR4ERG1 to the level of the host strain TmL28. These macroscopic observations were supported by SEM analysis. Short, irregularly shaped hyphae were observed in PCTR4ERG1 grown in the presence of 10 μM CuSO4 (Fig. 6). Swelling and bending were seen frequently over the whole length of the hyphae. On the other hand, long elongated hyphae with smooth surfaces were observed in PCTR4ERG1 grown in the presence of 10 μM BCS as well as in TmL28 under both conditions (Fig. 6).
Fig 5.
Growth phenotypes of conditional mutants. Conidia were spotted onto solid medium supplemented with the indicated concentrations of CuSO4 or 10 μM BCS and grown at 28°C for 4 days. Two or three clones for each mutant were tested, and the results for one representative clone are shown. (A) Strain PCTR4ERG1 1 was grown on RPMI 1640A. (B) Strain PCTR4TRP5 12 was grown on YNB-based solid medium with (+Trp) or without (−Trp) tryptophan.
Fig 6.
Microscopic analysis by SEM. PCTR4ERG1 1 and TmL28 were grown on RPMI 1640A supplemented with 10 μM CuSO4 or 10 μM BCS at 28°C for 2 days. Agar blocks containing single colonies were excised, and SEM samples were prepared as described in Materials and Methods. Bars = 1 μm.
Clear phenotypic differences were also observed in strain PCTR4TRP5. After incubation at 28°C for 4 days, PCTR4TRP5 grew independent of tryptophan on solid YNB (without tryptophan [−Trp]) under inducing conditions, supplemented with 10 μM BCS. On the other hand, growth was markedly repressed on solid YNB (−Trp) medium in the presence of ≥1 μM CuSO4 (Fig. 5B). This auxotrophy was restored by adding tryptophan to the growth medium.
These results suggested that in both conditional mutants, the transcriptional activity of PCTR4 under inducible conditions was sufficiently high to support their growth and that residual activity under repressive conditions was sufficiently low to impair growth.
DISCUSSION
There has been steady development of molecular tools for genetic studies of dermatophytic species, including selectable markers (1, 32), transformation methods (2, 31, 33), and genome sequence databases. However, conditional knockdown systems, which are indispensable for proving that a gene is essential, have yet to be developed because no conditional promoters have been reported. In the present study, we demonstrated that PCTR4 and PCRP1 could function effectively as copper-responsive conditional promoters in A. vanbreuseghemii. Furthermore, analyses of conditional mutants of endogenous genes suggested that the PCTR4-based conditional knockdown system can be useful for identification of essential genes.
In previous studies, promoters of CTR4 and CRP1 genes were shown to be copper responsive in C. neoformans (22) and H. capsulatum (8), respectively. To evaluate the applicability of these promoters in A. vanbreuseghemii, cognate promoters of CTR4 and CRP1 homologs in T. rubrum were isolated. The criteria for choosing T. rubrum-derived promoters were (i) the availability of the full genome sequence of T. rubrum, (ii) its genetic relatedness to A. vanbreuseghemii, and (iii) avoidance of the introduction of endogenous sequences with knockdown cassettes, therefore increasing homologous recombination efficiency. The isolated promoter sequences, PCRP1 and PCTR4, were tested for their responses to copper by a β-galactosidase reporter assay. As shown in Fig. 2, PCRP1 showed inducible characteristics in accordance with copper concentration, while PCTR4 was repressive, consistent with previous reports (8, 22). Despite the phylogenetic relatedness of the two genera Histoplasma and Trichophyton, the levels of induction of PCRP1 were considerably lower than those reported to occur in H. capsulatum, which reached as high as 125-fold (8), whereas only 4-fold induction was observed in the present study (Fig. 2). Similarly, fold repression of PCTR4 was not as high as that reported to occur in C. neoformans (22). On the other hand, residual activity under repressive conditions was lower in PCTR4 than in PCRP1. Therefore, PCTR4 can regulate gene expression more tightly than PCRP1 in A. vanbreuseghemii, suggesting that PCTR4 is more reliable for genetic studies.
Real-time RT-PCR was used to study the kinetics of PCTR4 under repressive conditions. The expression level of lacZ decreased within 30 min after administration of copper (Fig. 3). This rapid response gives the PCTR4 knockdown system an advantage over other systems regulated by nutrient sources, which require several hours. Monteiro and De Lucas reported that in A. fumigatus, ammonium regulation of the niiA promoter requires 36 h to significantly decrease triA expression level, while the alcA promoter repressed triA expression after 18 h (17). Thus, the tightness and promptness of the response vary depending on the promoter. Furthermore, transcriptional repression by PCTR4 lasted for at least 24 h after addition of copper (Fig. 3), which may be due to excessive copper ions in liquid medium provided by high and essentially unaltered levels of copper during the testing period.
The ability of PCTR4 to cause phenotypic changes in A. vanbreuseghemii through its tight regulation of the endogenous genes ERG1 and TRP5 was confirmed. As shown in Fig. 5, the levels of PCTR4ERG1 and PCTR4TRP5 growth were markedly reduced due to repression of ERG1 and TRP5, as the growth of their parental strain, TmL28, was unaffected in the presence of copper. Scanning electron microscopy of PCTR4ERG1 showed short hyphae with swelling (Fig. 6) similar to the ultrastructural changes caused by terbinafine (20). When growing hyphae of T. mentagrophytes were treated with 2 ng/ml terbinafine, swollen hyphal tips were observed. Furthermore, severely damaged hyphae were observed microscopically with a higher terbinafine concentration of 20 ng/ml (20). Terbinafine has been shown to have fungicidal activity against dermatophytes (3, 23). However, no fungicidal effects caused by the repression of ERG1 expression were observed even when PCTR4ERG1 was grown under high (50 μM or higher) concentrations of copper (Fig. 5A and data not shown). One possible reason is that transcriptional repression by PCTR4 was not as stringent as enzymatic inhibition by terbinafine, and thus, leakage of PCTR4 promoter activity might allow PCTR4ERG1 to grow slowly. Another explanation is that the fungicidal effect and hyphal disruption were due to off-target effects of terbinafine. Further studies are therefore required for clarification.
The PCTR4-based system can potentially participate in elucidation of dermatophytosis mechanisms under various conditions and particularly in vivo studies, due to its highly sensitive responses to copper (at a level as low as 0.1 μM CuSO4) (Fig. 5A). In addition, the parental strain of the host cells used in our knockdown system, A. vanbreuseghemii TIMM2789, is a zoophilic strain of T. mentagrophytes complex, and this strain has been used in a guinea pig model of tinea pedis (21, 28). Therefore, we hopefully expect that functional analyses of genes can be performed in vivo by infecting animals and treating them with copper-containing agents.
The applicability of any conditional knockdown system is highly correlated with the expression level of its promoter, whether it is sufficiently high to support growth of a mutant under inducible conditions or low enough to cause phenotypic changes under repressive conditions. Furthermore, the expression level of a conditional promoter may vary from one locus to another. The successful application of the PCTR4-based system to ERG1 and TRP5 loci suggested that this PCTR4-based conditional knockdown system can be applied to most genes. Several other genes are currently being tested in our laboratory.
In conclusion, the two copper-responsive promoters PCTR4 and PCRP1 are the first to be reported for dermatophytes. The applicability and tightness of PCTR4 in A. vanbreuseghemii were confirmed for two loci, ERG1 and TRP5. Therefore, the PCTR4-based conditional knockdown system represents a potentially powerful tool for identification of essential genes. This system will contribute to further progress in molecular genetic research in dermatophytes.
ACKNOWLEDGMENTS
We thank Z. An for providing the binary vector pAg1 and K. J. Kwon-Chung for providing A. tumefaciens strain EHA105.
Footnotes
Published ahead of print 18 May 2012
REFERENCES
- 1. Alshahni MM, Makimura K, Yamada T, Takatori K, Sawada T. 2010. Nourseothricin acetyltransferase: a new dominant selectable marker for the dermatophyte Trichophyton mentagrophytes. Med. Mycol. 48:665–668 [DOI] [PubMed] [Google Scholar]
- 2. Alshahni MM, Yamada T, Takatori K, Sawada T, Makimura K. 2011. Insights into a nonhomologous integration pathway in the dermatophyte Trichophyton mentagrophytes: efficient targeted gene disruption by use of mutants lacking ligase IV. Microbiol. Immunol. 55:34–43 [DOI] [PubMed] [Google Scholar]
- 3. Arzeni D, et al. 1998. In vitro activity of terbinafine against clinical isolates of dermatophytes. Med. Mycol. 36:235–237 [PubMed] [Google Scholar]
- 4. Chayakulkeeree M, Rude TH, Toffaletti DL, Perfect JR. 2007. Fatty acid synthesis is essential for survival of Cryptococcus neoformans and a potential fungicidal target. Antimicrob. Agents Chemother. 51:3537–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davidson RC, et al. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 6. Fachin AL, Ferreira-Nozawa MS, Maccheroni W, Jr, Martinez-Rossi WNM. 2006. Role of the ABC transporter TruMDR2 in terbinafine, 4-nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J. Med. Microbiol. 55:1093–1099 [DOI] [PubMed] [Google Scholar]
- 7. Ferreira-Nozawa MS, et al. 2006. The pH signaling transcription factor PacC mediates the growth of Trichophyton rubrum on human nail in vitro. Med. Mycol. 44:641–645 [DOI] [PubMed] [Google Scholar]
- 8. Gebhart D, Bahrami AK, Sil A. 2006. Identification of a copper-inducible promoter for use in ectopic expression in the fungal pathogen Histoplasma capsulatum. Eukaryot. Cell 5:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Girardin H, Latge JP. 1994. DNA extraction and quantification, p 5–9 In Maresca B, Kobayashi GS. (ed), Molecular biology of pathogenic fungi, 2nd ed. Telos Press, New York, NY [Google Scholar]
- 10. Grumbt M, et al. 2011. Targeted gene deletion and in vivo analysis of putative virulence gene function in the pathogenic dermatophyte Arthroderma benhamiae. Eukaryot. Cell 10:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta AK, Cooper EA. 2008. Update in antifungal therapy of dermatophytosis. Mycopathologia 166:353–367 [DOI] [PubMed] [Google Scholar]
- 12. Havlickova B, Czaika VA, Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51(Suppl. 4):2–15 [DOI] [PubMed] [Google Scholar]
- 13. Hood EE, Gelvin SB, Melchers LS, Hoekema A. 1993. New Agrobacterium helper plasmids for gene transfer to plants. Trans. Res. 2:208–218 [Google Scholar]
- 14. Hu W, et al. 2007. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3:e24 doi:10.1371/journal.ppat.0030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kingsbury JM, McCusker JH. 2008. Threonine biosynthetic genes are essential in Cryptococcus neoformans. Microbiology 154:2767–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makimura K, et al. 1998. Phylogenetic classification of Trichophyton mentagrophytes complex strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J. Clin. Microbiol. 36:2629–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monteiro MC, De Lucas JR. 2010. Study of the essentiality of the Aspergillus fumigatus triA gene, encoding RNA triphosphatase, using the heterokaryon rescue technique and the conditional gene expression driven by the alcA and niiA promoters. Fungal Genet. Biol. 47:66–79 [DOI] [PubMed] [Google Scholar]
- 18. Nagahashi S, et al. 1997. Regulation by tetracycline of gene expression in Saccharomyces cerevisiae. Mol. Gen. Genet. 255:372–375 [DOI] [PubMed] [Google Scholar]
- 19. Nakayama H, et al. 1998. A controllable gene-expression system for the pathogenic fungus Candida glabrata. Microbiology 144:2407–2415 [DOI] [PubMed] [Google Scholar]
- 20. Nishiyama Y, et al. 1991. Ultrastructural changes induced by terbinafine, a new antifungal agent, in Trichophyton mentagrophytes. Jpn. J. Med. Mycol. 32:165–175 [Google Scholar]
- 21. Niwano Y, et al. 1998. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob. Agents Chemother. 42:967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ory JJ, Griffith CL, Doering TL. 2004. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21:919–926 [DOI] [PubMed] [Google Scholar]
- 23. Petranyi G, Meingassner JG, Mieth H. 1987. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob. Agents Chemother. 31:1365–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roemer T, et al. 2003. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50:167–181 [DOI] [PubMed] [Google Scholar]
- 25. Romero B, Turner G, Olivas I, Laborda F, De Lucas JR. 2003. The Aspergillus nidulans alcA promoter drives tightly regulated conditional gene expression in Aspergillus fumigatus permitting validation of essential genes in this human pathogen. Fungal Genet. Biol. 40:103–114 [DOI] [PubMed] [Google Scholar]
- 26. Ryder NS. 1992. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 126(Suppl. 39):2–7 [DOI] [PubMed] [Google Scholar]
- 27. Shiotani H, Tsuge T. 1995. Efficient gene targeting in the filamentous fungus Alternaria alternata. Mol. Gen. Genet. 248:142–150 [DOI] [PubMed] [Google Scholar]
- 28. Uchida K, Tanaka T, Yamaguchi H. 2003. Achievement of complete mycological cure by topical antifungal agent NND-502 in guinea pig model of tinea pedis. Microbiol. Immunol. 47:143–146 [DOI] [PubMed] [Google Scholar]
- 29. Vogt K, Bhabhra R, Rhodes JC, Askew DS. 2005. Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamada T, Makimura K, Abe S. 2006. Isolation, characterization, and disruption of dnr1, the areA/nit-2-like nitrogen regulatory gene of the zoophilic dermatophyte, Microsporum canis. Med. Mycol. 44:243–252 [DOI] [PubMed] [Google Scholar]
- 31. Yamada T, et al. 2009. Enhanced gene replacements in Ku80 disruption mutants of the dermatophyte, Trichophyton mentagrophytes. FEMS Microbiol. Lett. 298:208–217 [DOI] [PubMed] [Google Scholar]
- 32. Yamada T, et al. 2008. Genetic transformation of the dermatophyte, Trichophyton mentagrophytes, based on the use of G418 resistance as a dominant selectable marker. J. Dermatol. Sci. 49:53–61 [DOI] [PubMed] [Google Scholar]
- 33. Yamada T, et al. 2009. Agrobacterium tumefaciens-mediated transformation of the dermatophyte, Trichophyton mentagrophytes: an efficient tool for gene transfer. Med. Mycol. 47:485–494 [DOI] [PubMed] [Google Scholar]