Abstract
Recent studies have suggested that the topical application of probiotic bacteria can improve skin health or combat disease. We have utilized a primary human keratinocyte culture model to investigate whether probiotic bacteria can inhibit Staphylococcus aureus infection. Evaluation of the candidate probiotics Lactobacillus reuteri ATCC 55730, Lactobacillus rhamnosus AC413, and Lactobacillus salivarius UCC118 demonstrated that both L. reuteri and L. rhamnosus, but not L. salivarius, reduced S. aureus-induced keratinocyte cell death in both undifferentiated and differentiated keratinocytes. Keratinocyte survival was significantly higher if the probiotic was applied prior to (P < 0.01) or simultaneously with (P < 0.01) infection with S. aureus but not when added after infection had commenced (P > 0.05). The protective effect of L. reuteri was not dependent on the elaboration of inhibitory substances such as lactic acid. L. reuteri inhibited adherence of S. aureus to keratinocytes by competitive exclusion (P = 0.026). L. salivarius UCC118, however, did not inhibit S. aureus from adhering to keratinocytes (P > 0.05) and did not protect keratinocyte viability. S. aureus utilizes the α5β1 integrin to adhere to keratinocytes, and blocking of this integrin resulted in a protective effect similar to that observed with probiotics (P = 0.03). This suggests that the protective mechanism for L. reuteri-mediated protection of keratinocytes was by competitive exclusion of the pathogen from its binding sites on the cells. Our results suggest that use of a topical probiotic prophylactically could inhibit the colonization of skin by S. aureus and thus aid in the prevention of infection.
INTRODUCTION
Humans live in constant contact with a multitude of microorganisms. The gut is by far the most heavily colonized environment of the human body, and the gut microbiota play multiple roles in normal physiology, including nutrient sequestration (2) and development of normal immune responses (20).
Among the normal gut microbiota are the so-called probiotic bacteria. Ingestion of these has been claimed to prevent or treat gastrointestinal disorders such as antibiotic-associated diarrhea (45) and inflammatory bowel disease (40). The mechanisms underlying these effects are largely unknown. However, studies have suggested that probiotics can inhibit colonization of the gut by pathogens. In vitro work has suggested that probiotics use various mechanisms to inhibit pathogens, including direct competition for binding sites on epithelial cells (10) and competition with pathogenic bacteria for nutrients. Probiotic organisms are also able to produce inhibitory substances such as bacteriocins (11) and organic acids (23) that can kill or limit the growth of pathogens. Selected probiotics, such as Lactobacillus plantarum 299v, have been shown to upregulate mucin production by epithelial cells, thereby preventing pathogen attachment (31). Probiotics may also produce biosurfactants that allow attachment of the probiotic while inhibiting attachment of pathogenic bacteria to cells (41).
In contrast to the gut, very little is known about the normal interactions between the skin microflora and the epidermis. Recent work suggests that skin commensals may also be able to limit the colonization of the skin by pathogenic bacteria (22, 46). Studies also suggest that certain skin diseases (such as acne vulgaris and atopic dermatitis) may be associated with disruptions to the normal microflora (3, 5, 7). Therefore, the idea that the skin microflora may be modulated using specific skin commensals to promote health or inhibit disease has received some attention (28, 29). However, the skin commensal bacteria can also be pathogenic under certain circumstances (14). By contrast, probiotics are generally regarded as safe (GRAS) and therefore could potentially be used topically if they have therapeutic value (15). So far, the limited amount of research in this area suggests that conventional probiotic bacteria may be of significant value when used on the skin. For example, topical application of a Bifidobacterium longum reuter lysate has been shown to induce clinical improvement of “reactive skin.” This is skin that is more sensitive to physical changes such as atmospheric temperature and to chemical changes such as seen with topically applied products (18). Application of the B. longum lysate to the skin of volunteers decreased sensitivity and transepidermal water loss (TEWL) after tape stripping. Additionally, application of the lysate to skin ex vivo reportedly decreased signs of inflammation such as vasodilation, edema, and tumor necrosis factor alpha (TNF-α) release (18). Topical application of Lactobacillus plantarum has also been demonstrated to improve tissue repair in a burned mouse model and to prevent infection in chronic leg ulcers and burns in humans (38, 39, 44). However, in general, the mechanisms underlying these effects are unknown.
Staphylococcus aureus is a transient colonizer of skin predominantly in the moist, warm regions of the body such as the groin, axilla, and anterior nares (26). Up to 60% of people are intermittent carriers, while 20% of people may be stably colonized (26). While normal carriage is asymptomatic, S. aureus may invade tissues (e.g., through broken skin) where it causes diseases ranging from the relatively minor impetigo and scalded skin syndrome to life-threatening conditions such as septicemia (27). Furthermore, S. aureus infection is often a secondary phenomenon in skin with underlying conditions such as atopic dermatitis (25).
Since probiotics can inhibit pathogen attachment to epithelial cells in the gut, we considered that, if the same is true in skin, conventional probiotics such as lactobacilli may have utility in the prevention of infection. In this study, we have investigated the relative ability of three probiotic strains, Lactobacillus reuteri ATCC 55730, Lactobacillus rhamnosus AC413, and Lactobacillus salivarius UCC118, to inhibit the effects of S. aureus upon normal primary human epidermal keratinocytes (NHEK). We have shown that L. reuteri can protect NHEK from the effects of S. aureus. Furthermore, the mechanism appears to be through the ability of the probiotic to inhibit pathogen attachment to NHEK.
MATERIALS AND METHODS
Growth of bacteria.
Probiotic bacteria were grown routinely to stationary phase in Wilkins-Chalgren broth (WCB) (Oxoid) at 37°C in a Mark 3 anaerobic work station (Don Whitley Scientific, Shipley, United Kingdom). S. aureus was grown aerobically at 37°C in nutrient broth (Oxoid). Culture densities were adjusted spectrophotometrically with medium to contain the number of bacteria required. For experiments utilizing keratinocytes, bacteria were washed twice in 0.85% NaCl solution, centrifuged, and reconstituted in keratinocyte medium. Selective agar was used in some experiments. This was mannitol salt agar (MSA; Oxoid) or de Man-Rogosa-Sharpe agar (MRS) for S. aureus or lactobacilli, respectively. For experiments utilizing heat-killed bacteria, L. reuteri was centrifuged and resuspended in 0.4% glucose and heat inactivated by placing in a water bath at 85°C for 45 min. Samples were Gram stained to ensure that lysis of bacterial cells had not occurred and plated onto growth medium to ensure that they had been killed. For experiments using probiotic lysates, 10 ml of 108 CFU/ml L. reuteri was centrifuged, washed, concentrated in 1 ml keratinocyte medium, and lysed using a bead beater (FastPrep FP120; Thermo Electron Corporation). Samples were filter sterilized to remove any whole bacteria remaining. Approximately 100 μl of this lysate was used to treat keratinocytes.
Mammalian cell culture.
Normal human epidermal keratinocytes (Promocell, Heidelberg, Germany) were maintained in keratinocyte basal medium (Promocell, Heidelberg, Germany) containing a supplement mix (bovine pituitary extract 0.004 mg/ml, epidermal growth factor [recombinant human] 0.125 ng/ml, insulin [recombinant human] 5 μg/ml, hydrocortisone 0.33 μg/ml, epinephrine 0.39 μg/ml, and holotransferrin [human] 10 μg/ml) and 0.06 mM CaCl2 (Promocell, Heidelberg, Germany) without antibiotics. Medium was substituted twice weekly and cells were cultured at 37°C in a humid atmosphere of 5% CO2. Cells were detached once grown to 80% confluence and reseeded at approximately 5 × 103 cells/cm2. For experiments using differentiated cells, calcium chloride in the medium was increased to 1.8 mM, and the cells were grown in this for 24 h prior to experimentation.
Bacterial competition assay.
Aliquots (100 μl) of overnight cultures of L. reuteri and S. aureus were inoculated into fresh 10-ml broths (both as axenic cultures and as cocultures). The pH and optical density of cultures was measured at 0 and 48 h. At regular intervals (3, 6, 24, 30, and 48 h), bacteria were counted by serial dilution plate counts using selective and nonselective agars. At 48 h, BacLite Live/Dead stains were performed according to the manufacturer's instructions (Invitrogen).
Production of inhibitory substances by L. reuteri.
Inhibition of the growth of S. aureus by L. reuteri was determined using well diffusion assays, as described by Holder and Boyce (21). For inhibition studies, L. reuteri was grown in either MRS medium (Oxoid) or WCB (Oxoid) anaerobically at 37°C to stationary phase. Whole-cell (WC) cultures of L. reuteri grown in WCB (1 g/liter glucose) or MRS (20 g/liter glucose) were used. In other experiments, cells were sedimented in a microcentrifuge at 15,000 × g for 5 min, and the cell-free supernatant (CFS) was extracted for use. Well diffusion plates were incubated at 37°C for 48 h, and zones of inhibition were measured using a ruler. To measure the production of organic acids by L. reuteri, the pH of WC and CFS was measured. In a separate experiment to determine whether L. reuteri produced any other inhibitory substances, cell-free supernatants were neutralized using 1 M NaOH prior to use in the well diffusion assay. To determine if L. reuteri produced organic acids in cell culture, the pH of infected cell cultures was measured.
Measurement of keratinocyte viability.
NHEK cells were grown in 24-well tissue culture plates to confluence. Cell culture medium was replaced with medium containing the indicated concentration of bacteria. For preexposure and postexposure experiments, NHEK were treated with L. reuteri for periods of between 1 and 4 h prior to the addition/after the addition of S. aureus. In all experiments, cells were incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 h. The medium was then removed and cells were washed with sterile phosphate-buffered saline (PBS). Cells were detached using trypsin (0.4%)-EDTA (0.3%) (Promocell), and cell viability was determined by trypan blue exclusion assay (43).
Measurement of S. aureus viability.
To determine if L. reuteri or NHEK were able to inhibit the growth of S. aureus in cell culture, cells were grown to confluence in a 24-well plate. These were infected with either S. aureus or S. aureus and L. reuteri together. In parallel, wells without any cells also had these combinations added to determine the effect of the keratinocytes themselves on staphylococcal viability. After 24 h of exposure, the medium was removed and centrifuged to pellet extracellular bacteria. The cells were then trypsinized, and 500 μl of 0.25% Triton X-100 (Sigma-Aldrich) in PBS was added for approximately 30 min to lyse the keratinocytes. The well contents were then combined with the cell pellet and serial dilution plate counts using MSA performed to determine the total number of viable staphylococci.
Adhesion assays for L. reuteri, L. salivarius, and S. aureus.
Confluent differentiating NHEK were exposed to either the probiotic or S. aureus for 1 h. After incubation, cells were washed three times in PBS to remove nonadherent bacteria. The cells were trypsinized and serial dilution plate counts were performed to assess the number of adherent bacteria. Selective agar was used for growth of staphylococci. In separate experiments, cells were exposed to L. reuteri 1 h before the addition of S. aureus (exclusion), at the same time (competition), or 30 min after S. aureus infection had begun (displacement) to determine whether L. reuteri could inhibit S. aureus adhesion to keratinocytes. To determine whether L. reuteri could inhibit invasion of keratinocytes by staphylococci, cells were exposed to either S. aureus or S. aureus and L. reuteri for 1 h. Cells were washed three times in sterile PBS to remove nonadherent bacteria, and the growth medium was replaced with medium containing 100 μg/ml gentamicin (Sigma-Aldrich) for 2 h. Cells were then washed three times in sterile PBS and trypsinized and lysed in 0.25% Triton X-100 for 30 min to release internalized bacteria. Bacteria in lysates were counted using serial dilutions. In experiments utilizing conditioned medium (CM), NHEK were exposed to L. reuteri and incubated for 1 h at 37°C in 5% CO2. The CM from the exposed cells was removed and centrifuged at 15,000 × g for 3 min to pellet the bacterial cells, and the CM filtered through a 0.22-μm-pore-size filter (Millipore). In experiments exploring the role of the α5β1 integrin in S. aureus adhesion, NHEK were preexposed to 60 μg/ml of an anti-α5β1 integrin antibody (Millipore) for 1 h prior to infection of cells. An alternative method for assessing the number of adherent bacteria was utilized for dead preparations of L. reuteri. Cells were grown on LabTek chamber slides (Thermo-Scientific). Adhesion assays were performed as usual, using adjusted amounts of bacteria. After the cells were exposed to bacteria, cells were washed twice in PBS and fixed in methanol for 20 min before performing a Gram stain. The number of adherent bacteria per 100 cells was then assessed using a Keyence All-in-One fluorescence microscope.
Statistical analyses.
All experiments were performed a minimum of three times, with three replicates each time. For experiments comparing two treatments, Student's t test was used. For experiments comparing two or more treatments, a one-way analysis of variance (ANOVA) with blocking by experiment and post hoc Tukey's test was utilized. Dose-response and competition assays were analyzed using linear regression and 2-way ANOVA, respectively. Results were considered significant if P values were <0.05.
RESULTS
S. aureus-induced cell death in primary human keratinocytes.
We initially characterized the effects of S. aureus on keratinocyte viability by performing trypan blue exclusion assays on undifferentiated NHEK incubated with S. aureus at different doses. At a concentration of 105 CFU/ml S. aureus, only 49.4% of keratinocytes were viable after 24 h of infection (see Fig. S1 in the supplemental material). This was reduced in a dose-dependent manner such that only 3.3% of keratinocytes were viable at concentrations of 108 CFU/ml S. aureus (see Fig. S1). Approximately 106 organisms is a physiologically relevant concentration that might be found, e.g., in a skin wound infection (6). At this concentration, 30.5% of keratinocytes were viable after 24 h of infection. This concentration was therefore used in further assays.
L. reuteri protects keratinocytes from S. aureus-induced cell death.
The ability of three strains of lactobacilli (L. reuteri ATCC 55730, L. rhamnosus AC413, and L. salivarius UCC118) to protect keratinocytes from the effects of S. aureus was investigated (Fig. 1a). Infection of NHEK with 108 S. aureus cells reduced viability to 8.8%. In contrast to S. aureus, incubation of NHEK with 108 lactobacilli did not result in significant reduction in cell viability (Fig. 1a; also see Fig. S2 in the supplemental material). Coinfection with S. aureus and either L. rhamnosus or L. reuteri resulted in increased keratinocyte viability at 24 h of either 42.7% or 53.1% (P = 0.0012 and P < 0.0001), respectively. However, coinfection of keratinocytes with S. aureus and L. salivarius did not result in a significant elevation in viability compared to NHEK infected with S. aureus (P = 0.052) (Fig. 1a). Since L. reuteri was more protective than the other two strains, we further investigated the effects of L. reuteri by determining whether the protective effect of L. reuteri was dose dependent. However, there was no further significant increase in keratinocyte viability using L. reuteri at 105, 106, 107, or 108 CFU/ml (P > 0.05) (Fig. 1b). Furthermore, the same level of protection was observed in cells that were completely undifferentiated (i.e., grown in low-calcium medium) or differentiated by growing them for 24 h in medium containing 1.8 mM calcium (see Fig. S3 in the supplemental material). We also investigated whether the protective effects of the probiotic were dependent on live bacteria. Although heat-killed L. reuteri did not protect NHEK from S. aureus, an L. reuteri lysate afforded a significant protective effect (P = 0.01) (Fig. 1c and d, respectively). Further experiments utilized 108 CFU/ml of live L. reuteri cells (multiplicity of infection [MOI] of 1,000).
Fig 1.
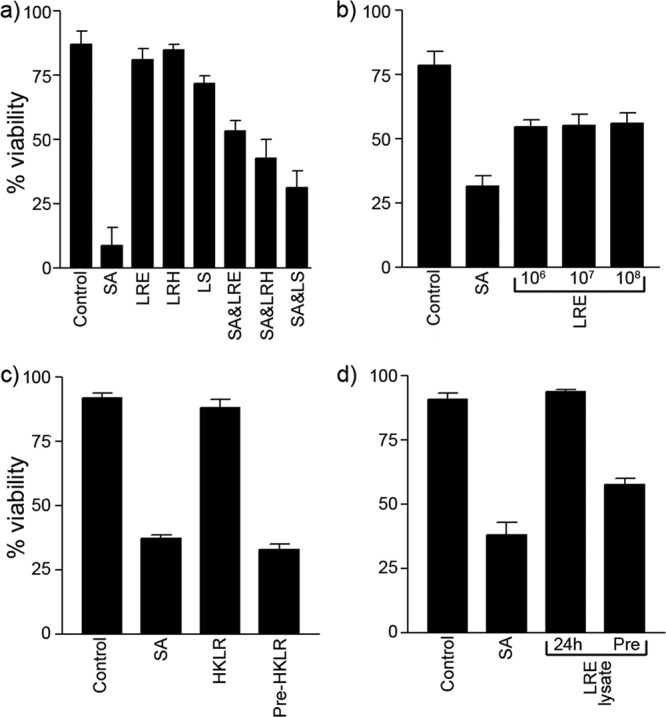
Lactobacilli protect undifferentiated keratinocytes from the cytotoxic effects of S. aureus. (a) Percentage of viability for uninfected control NHEK (86.9% ± 5.1%) and NHEK infected with 108 CFU/ml of S. aureus (SA) (8.8% ± 7.1%), L. reuteri (LRE) (80.8% ± 4.5%), L. rhamnosus (LRH) (84.8% ± 2.1%), L. salivarius (LS) (71.7% ± 2.9%), S. aureus and L. reuteri (SA&LRE) (53.1% ± 4.2%), S. aureus and L. rhamnosus (SA&LRH) (42.7% ± 7.4%), and S. aureus and L. salivarius (SA&LS) (31.1% ± 6.5%). (b) Viability of cells infected with either 106 CFU/ml of S. aureus (SA) (31.6% ± 4.1%), S. aureus and 106 CFU/ml of L. reuteri (54.8% ± 2.7%), S. aureus and 107 CFU/ml of L. reuteri (55.4% ± 4.3%), and S. aureus and 108 CFU/ml of L. reuteri (56.2% ± 4.1%). (c) Keratinocytes exposed to S. aureus (SA) had a reduced viability of 37.4% ± 1.3% compared to controls (92% ± 1.9%), while keratinocytes exposed to heat-killed L. reuteri (HKLR) had little change to their viability (88.3% ± 3.3%). Keratinocytes preexposed to heat-killed L. reuteri (Pre-HKLR) had viabilities similar to those of S. aureus-exposed keratinocytes (33% ± 2.1%). (d) L. reuteri lysate (LRE lysate) protected keratinocytes, P = 0.01. Cells exposed to S. aureus (SA) alone had significantly lower viability (38.3% ± 4.7%) than cells preexposed to a lysate of L. reuteri (Pre) (57.7% ± 2.4%). Results are expressed as mean ± standard error of the mean (SEM).
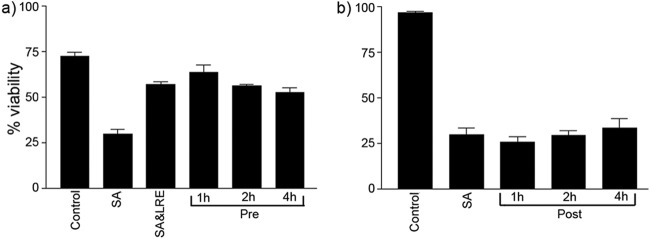
Protective effects of L. reuteri are time dependent.
We next investigated whether the timing of L. reuteri application relative to S. aureus infection was critical to the protective effect of the probiotic. We assessed the viability of differentiated keratinocytes exposed to L. reuteri 1, 2, or 4 h prior to or following the commencement of infection with S. aureus for 24 h. Preexposed keratinocytes had viabilities similar to those of coinfected cells (P > 0.05) (Fig. 2a). However, keratinocytes exposed to L. reuteri 1, 2, or 4 h after S. aureus infection had commenced had viabilities similar to those of cells exposed to S. aureus (P > 0.05) (Fig. 2b).
Fig 2.
L. reuteri protects keratinocytes only if added prior to infection with S. aureus. (a) Percentage of viability was significantly higher in cells that were coinfected (SA&LRE) (57.1% ± 2%, P = 0.0016) and preexposed (Pre) for 1 h (63.6% ± 5.6%, P = 0.0003), 2 h (56.1% ± 1.2%, P = 0.0022), and 4 h (52.4% ± 4.1%, P = 0.0066) with L. reuteri compared to S. aureus (SA)-infected cells (29.8% ± 3.8%). (b) There was no significant difference between the viability of cells treated with L. reuteri 1 h (25.8% ± 0.6%), 2 h (29.3% ± 2.6%), or 4 h (33.6% ± 4.9%) after infection had begun (Post) and cells that had been infected with S. aureus (SA) alone (29.9% ± 3.4%) (P > 0.05). Both experiments utilized differentiating NHEK. Results are expressed as the mean ± SEM.
L. reuteri does not inhibit growth of S. aureus.
We hypothesized that the mechanism(s) underlying the protective effects of S. aureus might be due to direct effects of the probiotic on the pathogen. However, competition assays showed no significant reduction in either L. reuteri or S. aureus viability during coculture for 48 h (see Fig. S4 in the supplemental material) compared to axenic cultures (P > 0.05). Additionally, in keratinocyte culture assays, the total number of viable staphylococci was also unaffected by the presence of the probiotic L. reuteri. S. aureus grown in the absence of cells had a viable count of 8.0 (log CFU). S. aureus grown in the presence of differentiating keratinocytes had a viable count of 8.6 ± 0.2 (mean ± standard error of the mean [SEM]). S. aureus coincubated with L. reuteri in the absence of cells had a viable count of 8.4 ± 0.4, while S. aureus coincubated with L. reuteri in the presence of cells had a viable count of 8.0 ± 0.2. There was no significant difference found between groups (P = 0.34). A live-dead count of the coculture at 48 h also demonstrated no significant reduction in bacterial cell viability (data not shown). Furthermore, although we established that L. reuteri could produce organic acid that inhibited pathogen growth on agar plates (data not shown), when we measured the pH of keratinocyte media infected with S. aureus, L. reuteri, and both simultaneously for 24 h, there was no significant difference in the pH between treatments (see Fig. S5 in the supplemental material). However, since keratinocyte medium is buffered, acid could be produced but not in quantities sufficient to detect by measuring pH changes. Therefore, to eliminate the possibility of acid-induced S. aureus death, we compared the numbers of S. aureus in keratinocyte medium grown with or without L. reuteri (see above). There was no significant difference in numbers of S. aureus under these conditions.
L. reuteri inhibits adhesion and invasion of staphylococci to keratinocytes.
One of the mechanisms by which probiotics may protect gut epithelia is by inhibition of pathogenic adhesion to epithelial cells. Therefore, we explored whether L. reuteri could inhibit the adhesion of S. aureus to NHEK.
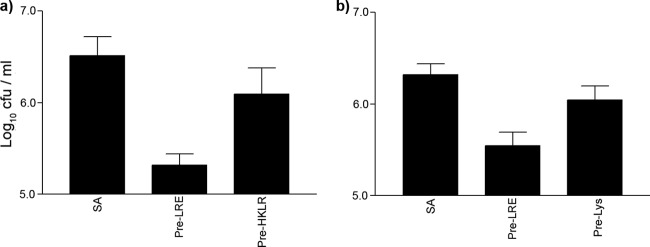
The graph in Fig. 3a demonstrates that both L. reuteri and S. aureus adhered to NHEK. Next we established whether L. reuteri could exclude or compete with S. aureus for binding sites by adding the probiotic either before or at the same time as the pathogen. The graphs in Fig. 3b and c demonstrate a reduction in adhesion of S. aureus to NHEK when L. reuteri was added before or at the same time as the addition of the pathogen (P = 0.026 or P = 0.0078, respectively). However, if L. reuteri was added after S. aureus, the probiotic did not prevent the pathogen from adhering to NHEK (P > 0.05) (Fig. 3d). Furthermore, gentamicin protection assays demonstrated that the probiotic inhibited S. aureus invasion of NHEK (P = 0.009) (Fig. 3e). Our results showed that heat-killed L. reuteri did not significantly inhibit staphylococcal adhesion (P > 0.05) (Fig. 4a). However, probiotic lysates were able to inhibit staphylococcal adhesion (P = 0.032), though not to the same extent as live L. reuteri (P = 0.0002). NHEK had approximately 5.6 ± 0.2 log CFU staphylococci adhered when exposed to live L. reuteri compared to 6.0 ± 0.2 log CFU when exposed to lysates (Fig. 4b).
Fig 3.
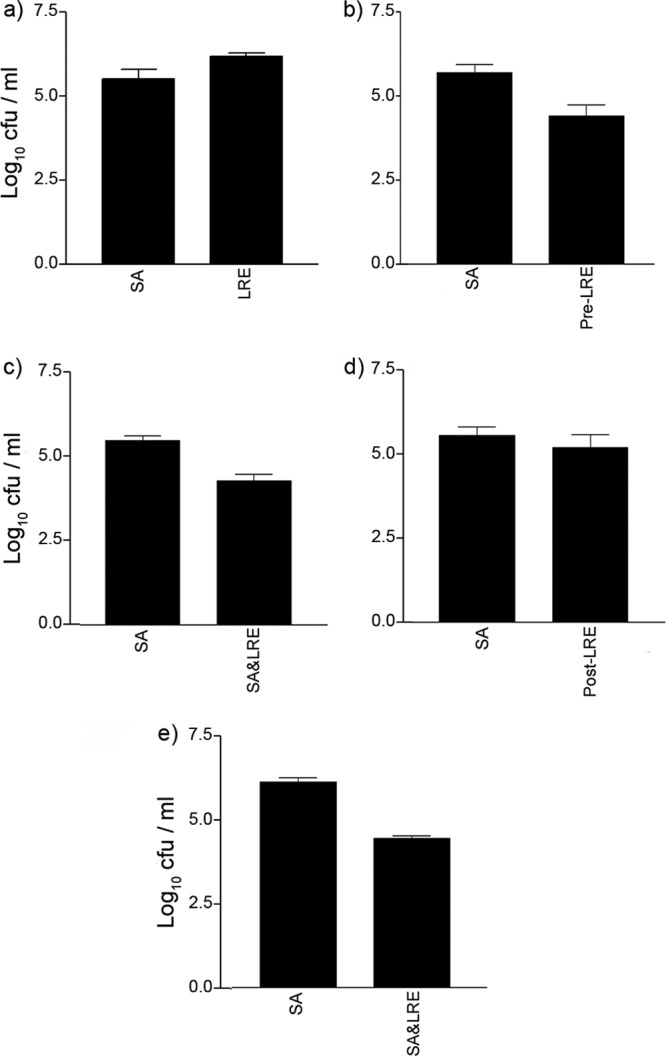
L. reuteri inhibits staphylococcal adhesion and invasion of keratinocytes. (a) S. aureus (SA) adhered at approximately 5.5 ± 0.3 log10 CFU/ml and L. reuteri (LRE) adhered at approximately 6.2 ± 0.1 log10 CFU/ml. (b) Exclusion. Cells preexposed to L. reuteri before S. aureus (Pre-LRE) infection had significantly fewer staphylococci adhered to them (4.4 ± 0.4 log10 CFU) than cells infected with S. aureus (SA) alone (5.7 ± 0.2 log10 CFU). (c) Competition. Cells coinfected with L. reuteri (SA&LRE) had significantly fewer staphylococci adhered to them (4.4 ± 0.4 log10 CFU) than cells infected with S. aureus (SA) alone (5.6 ± 0.1 log10 CFU). (d) Displacement. There was no significant difference in the number of adherent staphylococci on cells exposed to L. reuteri after S. aureus infection had begun (Post-LRE) (5.3 ± 0.5 log10 CFU), compared to cells infected with S. aureus (SA) alone (5.7 ± 0.3 log10 CFU, P = 0.47). (e) Cells coinfected with L. reuteri had significantly fewer internalized staphylococci (4.5 ± 0.1 log10 CFU) than cells infected with S. aureus alone (6.1 ± 0.1 log10 CFU). All experiments utilized differentiating NHEK. Results are expressed as the mean ± SEM.
Fig 4.
Heat-killed L. reuteri was not able to inhibit staphylococcal adhesion to keratinocytes, but lysates could. (a) The number of staphylococci adherent to NHEK when applied alone (SA) (6.5 ± 0.2 log10 CFU), when added after NHEK were preexposed to live L. reuteri (Pre-LRE) (5.3 ± 0.1 log10 CFU), and when preexposed to heat-killed L. reuteri (Pre-HKLR) (6.1 ± 0.3 log10 CFU). Cells exposed to untreated L. reuteri had significantly fewer staphylococci bound to them (P = 0.003), but cells exposed to heat-killed L. reuteri had no significant difference in adherent staphylococci (P = 0.09). (b) The number of staphylococci adherent to NHEK when applied alone (SA) (6.3 ± 0.1 log10 CFU) was significantly more than when they were added after NHEK were preexposed to live L. reuteri (Pre-LRE) (5.6 ± 0.2 log10 CFU, P = 0.0002) and when preexposed to lysates of L. reuteri (Pre-Lys) (6.0 ± 0.2 log10 CFU, P = 0.032). All experiments utilized differentiating NHEK. Results are expressed as the mean ± SEM.
L. salivarius UCC118 does not prevent S. aureus from adhering to keratinocytes.
We considered that the protective effects of L. reuteri might be connected to its ability to inhibit pathogen adhesion to NHEK. To further explore this, we assessed whether inhibition of S. aureus binding to NHEK resulted in a decrease in its toxicity. S. aureus can adhere to cells by means of a fibronectin-binding protein (FnBP) which interacts with extracellular fibronectin (24, 34). Fibronectin itself binds to the keratinocyte receptor, the α5β1 integrin. As shown in previous studies (25), pretreatment of cells with a blocking antibody to α5β1 integrin resulted in decreased S. aureus adhesion. Therefore, we treated NHEK with the α5β1 blocking antibody and investigated its ability to inhibit adherence and toxicity to NHEK (Fig. 5a and b). The blocking antibody significantly inhibited S. aureus adhesion to NHEK (P = 0.007), while it also appeared to inhibit the ability of S. aureus to induce cell death in NHEK (P = 0.03). Next, we investigated the ability of L. salivarius, which did not protect NHEK (Fig. 1a), to inhibit S. aureus adhesion. Keratinocytes exposed to L. salivarius and S. aureus were found to have similar numbers of adherent staphylococci (4.5 ± 0.2 log CFU) as keratinocytes exposed to S. aureus only (4.9 ± 0.2 log CFU) (P > 0.05) (Fig. 6a). Furthermore, L. salivarius did not adhere to NHEK as efficiently (5.6 ± 0.1 log CFU) as L. reuteri (6.7 ± 0.1 log CFU) (P = 0.005) (Fig. 6b).
Fig 5.
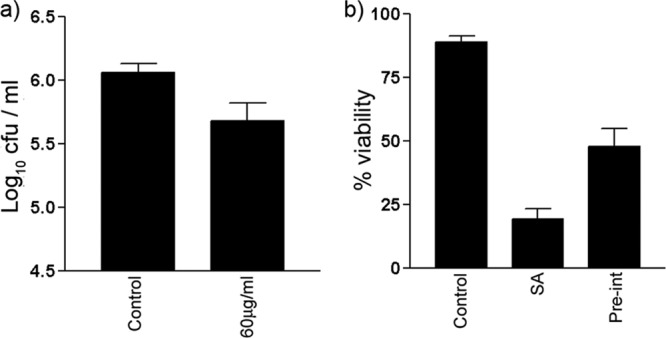
S. aureus utilizes α5β1 integrin to bind to keratinocytes, and blocking of this integrin is sufficient to protect keratinocytes from the effects of S. aureus. (a) Significantly fewer staphylococci adhered to cells treated with 60 μg/ml blocking antibody (5.7 ± 0.1 log10 CFU) than to untreated control keratinocytes (6.1 ± 0.1 log10 CFU, P = 0.007). (b) Significantly more keratinocytes were viable after infection with S. aureus for 24 h if preexposed to an anti-α5β1 integrin (Pre-int) antibody prior to infection (47.9% ± 6.0%) compared to keratinocytes infected with S. aureus alone (SA) (19.3% ± 2.0%, P = 0.03). All experiments utilized differentiating NHEK. Results are expressed as the mean ± SEM.
Fig 6.
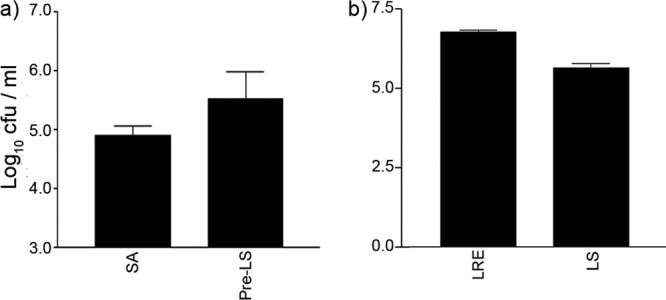
L. salivarius was not able to inhibit staphylococcal adhesion to keratinocytes. (a) Keratinocytes preexposed to L. salivarius (Pre-LS) had a similar number of adherent staphylococci (5.5 ± 0.5 log10 CFU) as cells exposed to S. aureus alone (SA) (4.9 ± 0.2 log10 CFU). (b) L. reuteri (LRE) adhered significantly better (6.7 ± 0.1 log10 CFU) than L. salivarius (LS) (5.6 ± 0.1 log10 CFU, P = 0.005). All experiments utilized differentiating NHEK. Results are expressed as mean ± SEM.
DISCUSSION
In this study, we have made a preliminary step toward characterizing the utility of probiotics for topical use. We have investigated the ability of three strains of lactobacilli with previously reported probiotic potential in the gut and mouth (8, 36, 42) to protect keratinocytes from the effects of the common skin pathogen S. aureus.
The application of S. aureus at physiologically relevant concentrations killed 69.5% of NHEK within 24 h. In contrast, none of the strains of lactobacilli significantly affected the viability of NHEK under the same conditions, suggesting that probiotics were well tolerated by keratinocytes. When NHEK were exposed to both pathogen and L. reuteri, the probiotic protected the cells from the effects of the pathogen, as indicated by the significantly higher percentage of viability of S. aureus-infected NHEK in the presence versus absence of L. reuteri (Fig. 1a; also see Fig. S2 in the supplemental material). The protective effect was also observed with lysates of L. reuteri but not heat-killed L. reuteri cells (Fig. 1d and e). Furthermore, the protective effects of the probiotic were variable between different species because the protection afforded by L. reuteri was greater than that provided by L. rhamnosus. In contrast, L. salivarius did not provide keratinocytes with any significant protection from S. aureus (Fig. 1a).
While to our knowledge, this is the first evidence showing the ability of a probiotic to protect keratinocytes from the pathogenic effects of S. aureus in vitro, L. fermentum reportedly may inhibit S. aureus infection of surgical implants and abscess formation in rats (16). Probiotics have previously been shown to exert protective effects against pathogen-induced cell death in other epithelia. For example, L. rhamnosus, L. reuteri, and L. oris were shown to inhibit Streptococcus pyogenes-induced cell death of pharyngeal epithelial cells (32). Probiotic cell-free supernatants have also been shown to be protective, since L. delbrueckii supernatants protected enterocytes from Clostridium difficile pathogenicity (4).
The protective effect of L. reuteri against S. aureus-induced cell death of keratinocytes could result from several different mechanisms. Lactobacilli produce organic acids and bacteriocins that can have direct antimicrobial effects on pathogens. Although we could demonstrate in diffusion assays that L. reuteri could produce acid, this does not appear to be responsible for the protective effects observed because the pH of the keratinocyte culture medium did not change in the presence of lactobacilli (see Fig. S5 in the supplemental material). Similarly, we did not detect any other antimicrobial activity since, in a competition assay, the growth rate and productivity of S. aureus in the presence of L. reuteri were identical to those of axenic cultures (see Fig. S4 in the supplemental material).
At least part of the mechanism by which L. reuteri protects keratinocytes appears to be linked to the ability of L. reuteri to prevent adhesion of S. aureus. Several lines of evidence point to this. First, S. aureus was less toxic to the cells when adhesion was inhibited using an integrin-blocking antibody (Fig. 5a and b). Second, lysates of L. reuteri that inhibit adhesion were also protective (Fig. 1d and 4b). On the other hand, heat-killed L. reuteri did not protect keratinocytes (Fig. 1c). Third, strains of lactobacilli that did not inhibit S. aureus adhesion (L. salivarius) (Fig. 6a) did not protect keratinocytes (Fig. 1a).
The timing of L. reuteri addition to the cells appears to be critical to its protective effects. When L. reuteri was added before or at the same time as S. aureus, NHEK were protected from the effects of the pathogen (Fig. 2a). However, if the probiotic was added after the pathogen, the levels of keratinocyte cell death were similar to that seen in cultures not treated with probiotic (Fig. 2b). Taken together, our data point to a mechanism of protection by L. reuteri involving competitive exclusion of S. aureus from keratinocyte binding sites.
How S. aureus induces cell death in keratinocytes is a complex process. In general, the first step in S. aureus pathogenesis is adherence to keratinocytes prior to invasion (25). Staphylococcal adhesins known to be involved in adhesion to keratinocytes include fibronectin-binding proteins (FnBPs) (25, 34), protein A, clumping factors, and coagulase (33). Teichoic acid mediates binding of S. aureus to nasal epithelial cells, though whether this is true for keratinocytes is not known (1). However, integrin blocking did not wholly inhibit binding of S. aureus to NHEK, suggesting that more than one adhesin is used (Fig. 5a).
Our study and others (25) have shown that blocking fibronectin receptors on the keratinocytes inhibits the adhesion of S. aureus (Fig. 5a). Interestingly, in separate experiments (results not shown), we could not show the involvement of this integrin in the binding of L. reuteri to NHEK. Indeed, neither protease nor glycosidase treatment of keratinocytes was able to prevent L. reuteri from binding to cells (data not shown). However, whatever the mechanism is by which the probiotic binds to cells, it involves a heat-labile molecule, as heat-killed L. reuteri do not adhere to keratinocytes as efficiently (see Fig. S6 in the supplemental material) or protect them from S. aureus (Fig. 1c).
To date, there are no published reports on the ability of probiotics to adhere to epidermal keratinocytes. Adhesion of probiotics to epithelia elsewhere has been suggested to occur via collagen binding proteins (19), mucus binding proteins (35), lipoteichoic acids (17), and S-layer proteins (9, 12). The relevant adhesins may be proteinacious, carbohydrate, or a combination of the two, and more than one adhesin may be in use. L. reuteri inhibited staphylococcal adhesion to keratinocytes when cells were preexposed to the probiotic (exclusion of binding sites) (Fig. 3b) and coinfected with S. aureus (competition for binding sites) (Fig. 3c) but not when applied after staphylococcal infection had begun (displacement from binding sites) (Fig. 3d). Others have shown that competitive inhibition/exclusion is often strain dependent, while displacement is generally not as effective or may require longer to occur (30, 47). Our results suggest that L. reuteri does not displace existing S. aureus from keratinocytes and support the finding that L. reuteri cannot protect keratinocytes from S. aureus cytotoxicity if added after infection has commenced. Some evidence has emerged to suggest that probiotics may have a synergistic effect on each others' adhesion, suggesting that an investigation into the effect of combinations of probiotics may be of interest in the future (37).
It seems likely that L. reuteri employs multiple mechanisms to attach to host epithelia and may use several strategies to prevent S. aureus from adhering. It is possible that L. reuteri may influence the expression of adhesive structures on the surfaces of keratinocytes, an area which has yet to be explored. Previous work has shown that probiotic strains L. rhamnosus GG and L. plantarum 299v can induce upregulation of genes involved in mucin expression in the intestine, which in turn inhibits the adhesion of enteropathogenic Escherichia coli (31). In a study in 1999, application of the lactic acid bacterium Streptococcus thermophilus to keratinocytes in vitro and skin in vivo resulted in increased ceramide production (13).
We have provided evidence that a probiotic has a protective effect against the pathogenic effects of S. aureus on keratinocyte viability in vitro. These results, in agreement with those of others, suggest that a topical probiotic for skin could exclude pathogenic organisms and therefore prevent infections. The topical administration of L. plantarum to burns in mice has been shown to inhibit Pseudomonas aeruginosa colonization of burns, promote healing, and modify the inflammatory response (44). This organism has also been compared to and found to be as effective as traditional silver suphadiazine treatments of burn wounds for limiting the bacterial load of wounds and healing in vivo (39). While further investigations are required to determine the full effect probiotics have on the skin, our findings show that L. reuteri can protect keratinocytes from the pathogenic effects of S. aureus through exclusion and competition for binding sites. We suggest that the prophylactic use of probiotics or their lysates in barrier creams and soaps may aid in the fight against staphylococcal skin colonization and subsequent infections.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a studentship from the BBSRC to T.P.
Footnotes
Published ahead of print 11 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Aly R, Shinefield HR, Litz C, Maibach HI. 1980. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J. Infect. Dis. 141:463–465 [DOI] [PubMed] [Google Scholar]
- 2. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915–1920 [DOI] [PubMed] [Google Scholar]
- 3. Balma-Mena A, et al. 2011. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int. J. Dermatol. 50:682–688 [DOI] [PubMed] [Google Scholar]
- 4. Banerjee P, Merkel G, Bhunia A. 2009. Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog. 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bek-Thomsen M, Lomholt HB, Kilian M. 2008. Acne is not associated with yet-uncultured bacteria. J. Clin. Microbiol. 46:3355–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowler PG, Duerden BI, Armstrong DG. 2001. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 14:244–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhart CN, Burkhart CG. 2003. Microbiology's principle of biofilms as a major factor in the pathogenesis of acne vulgaris. Int. J. Dermatol. 42:925–927 [DOI] [PubMed] [Google Scholar]
- 8. Çaglar E, Kavaloglu Cildir Ergeneli S, Sandalli S, Twetman NS. 2006. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol. Scand. 64:314–318 [DOI] [PubMed] [Google Scholar]
- 9. Chen X, et al. 2007. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol. 115:307–312 [DOI] [PubMed] [Google Scholar]
- 10. Coconnier MH, et al. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 110:299–306 [DOI] [PubMed] [Google Scholar]
- 11. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 12. Deepika G, Charalampopoulos D, Allen IL, Sima S, Geoffrey MG. 2010. Surface and adhesion properties of lactobacilli. Adv. Appl. Microbiol. 70:127–152 [DOI] [PubMed] [Google Scholar]
- 13. Di Marzio L, Cinque B, De Simone C, Cifone MG. 1999. Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo. J. Invest. Dermatol. 113:98–106 [DOI] [PubMed] [Google Scholar]
- 14. Edmond MB, et al. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239–244 [DOI] [PubMed] [Google Scholar]
- 15.FAO/WHO 2002. Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
- 16. Gan B, Kim J, Reid G, Cadieux P, Howard J. 2002. Lactobacillus fermentum RC-14 inhibits Staphylococcus aureus infection of surgical implants in rats. J. Infect. Dis. 185:1369–1372 [DOI] [PubMed] [Google Scholar]
- 17. Granato D, et al. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like caco-2 cells. Appl. Environ. Microbiol. 65:1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guéniche A, et al. 2010. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 19:e1–e8 doi:10.1111/j.1600-0625.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- 19. Heinemann C, et al. 2000. Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol. Lett. 190:177–180 [DOI] [PubMed] [Google Scholar]
- 20. Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. 1996. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology 89:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holder IA, Boyce ST. 1994. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 20:426–429 [DOI] [PubMed] [Google Scholar]
- 22. Iwase T, et al. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349 [DOI] [PubMed] [Google Scholar]
- 23. Karska-Wysocki B, Bazo M, Smoragiewicz W. 2010. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Res. 165:674–686 [DOI] [PubMed] [Google Scholar]
- 24. Kintarak S, Nair SP, Speight PM, Whawell SA. 2004. A recombinant fragment of the fibronectin-binding protein of Staphylococcus aureus inhibits keratinocyte migration. Arch. Dermatol. Res. 296:250–257 [DOI] [PubMed] [Google Scholar]
- 25. Kintarak S, Whawell SA, Speight PM, Packer S, Nair SP. 2004. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 72:5668–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kluytmans J, Van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krut O, Utermohlen O, Schlossherr X, Krönke M. 2003. Strain-specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates. Infect. Immun. 71:2716–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai Y, et al. 2010. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 130:2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai Y, et al. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YK, Puong KY, Ouwehand AC, Salminen S. 2003. Displacement of bacterial pathogens from mucus and caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52:925–930 [DOI] [PubMed] [Google Scholar]
- 31. Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 276:G941–G950 [DOI] [PubMed] [Google Scholar]
- 32. Maudsdotter L, Jonsson H, Roos S, Jonsson A-B. 2011. Lactobacilli reduce cell cytotoxicity caused by Streptococcus pyogenes by producing lactic acid that degrades the toxic component lipoteichoic acid. Antimicrob. Agents Chemother. 55:1622–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mempel M, et al. 1998. Role of Staphylococcus aureus surface-associated proteins in the attachment to cultured HaCaT keratinocytes in a new adhesion assay. J. Invest. Dermatol. 111:452–456 [DOI] [PubMed] [Google Scholar]
- 34. Mempel M, et al. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943–951 [DOI] [PubMed] [Google Scholar]
- 35. Miyoshi Y, Okada S, Uchimura T, Satoh E. 2006. A mucus adhesion promoting protein, MapA, mediates the adhesion of Lactobacillus reuteri to caco-2 human intestinal epithelial cells. Biosci. Biotechnol. Biochem. 70:1622–1628 [DOI] [PubMed] [Google Scholar]
- 36. Nikawa H, et al. 2004. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int. J. Food Microbiol. 95:219–223 [DOI] [PubMed] [Google Scholar]
- 37. Ouwehand AC, Isolauri E, Kirjavainen PV, Tölkkö S, Salminen SJ. 2000. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lactobacillus delbrueckii subsp. bulgaricus. Lett. Appl. Microbiol. 30:10–13 [DOI] [PubMed] [Google Scholar]
- 38. Peral MC, Huaman Martinez MA, Valdez JC. 2009. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 6:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peral MC, Rachid MM, Gobbato NM, Martinez MAH, Valdez JC. 2010. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 16:281–286 [DOI] [PubMed] [Google Scholar]
- 40. Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635–639 [DOI] [PubMed] [Google Scholar]
- 41. Ron EZ, Rosenberg E. 2001. Natural roles of biosurfactants. Environ. Microbiol. 3:229–236 [DOI] [PubMed] [Google Scholar]
- 42. Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. 1997. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J. Pediatr. Gastroenterol. Nutr. 24:399–404 [DOI] [PubMed] [Google Scholar]
- 43. Strober W. 1997. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. May:Appendix 3B. doi:10.1002/0471142735.ima03bs21. [DOI] [PubMed]
- 44. Valdéz JC, Peral MC, Rachid M, Santana M, Perdigón G. 2005. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 11:472–479 [DOI] [PubMed] [Google Scholar]
- 45. Vanderhoof JA, et al. 1999. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J. Pediatr. 135:564–568 [DOI] [PubMed] [Google Scholar]
- 46. Wanke I, et al. 2011. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Invest. Dermatol. 131:382–390 [DOI] [PubMed] [Google Scholar]
- 47. Zarate G, Nader-Macias ME. 2006. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 43:174–180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.