Abstract
Acropora and Porites corals are important reef builders in the Indo-Pacific and Caribbean. Bacteria associated with mucus produced by Porites spp. and Acropora spp. from Caribbean (Punta Maroma, Mexico) and Indo-Pacific (Hoga and Sampela, Indonesia) reefs were determined. Analysis of pyrosequencing libraries showed that bacterial communities from Caribbean corals were significantly more diverse (H′, 3.18 to 4.25) than their Indonesian counterparts (H′, 2.54 to 3.25). Dominant taxa were Gammaproteobacteria, Alphaproteobacteria, Firmicutes, and Cyanobacteria, which varied in relative abundance between coral genera and region. Distinct coral host-specific communities were also found; for example, Clostridiales were dominant on Acropora spp. (at Hoga and the Mexican Caribbean) compared to Porites spp. and seawater. Within the Gammproteobacteria, Halomonas spp. dominated sequence libraries from Porites spp. (49%) and Acropora spp. (5.6%) from the Mexican Caribbean, compared to the corresponding Indonesian coral libraries (<2%). Interestingly, with the exception of Porites spp. from the Mexican Caribbean, there was also a ubiquity of Psychrobacter spp., which dominated Acropora and Porites libraries from Indonesia and Acropora libraries from the Caribbean. In conclusion, there was a dominance of Halomonas spp. (associated with Acropora and Porites [Mexican Caribbean]), Firmicutes (associated with Acropora [Mexican Caribbean] and with Acropora and Porites [Hoga]), and Cyanobacteria (associated with Acropora and Porites [Hoga] and Porites [Sampela]). This is also the first report describing geographically distinct Psychrobacter spp. associated with coral mucus. In addition, the predominance of Clostridiales associated with Acropora spp. provided additional evidence for coral host-specific microorganisms.
INTRODUCTION
Microorganisms are important to coral reef ecosystems through their roles in carbon/nitrogen cycling (43), coral nutrition (7), response to stress (12), and health and disease (6, 40). Coral bleaching and various coral diseases are increasing due to changes in environmental conditions that result in either an increase in abundance of pathogenic microbes or the expansion of ecological niches occupied by coral pathogens (32, 34, 36). Therefore, understanding the microbial communities associated with corals and how they vary in response to changing environmental conditions is important for understanding the future health of coral reefs.
Previous studies that used both culture-dependent and culture-independent methods demonstrated that coral-associated bacterial communities may be host species specific and differ from those dominating the surrounding reef water (4, 17, 39). Similar bacterial populations have also been found on the same coral species from geographically different locations, and different bacterial communities have been found on different coral species (38, 39). By using clone libraries and sequence analysis, Bourne and Munn (4) found that the majority of clones recovered from the coral tissue of Pocillopora damicornis were related to Gammaproteobacteria, while Alphaproteobacteria were dominant in the coral mucus, thus further supporting the hypothesis that specific bacterium-coral associations exist.
Although many studies have supported the hypothesis that corals harbor unique microbiota, inconsistencies across studies have raised many questions about the specificity and dynamics of associations between corals and microbes. One of the major limitations of these studies is that conventional cloning and sequencing methods do not allow for characterization of the microbial community beyond the most dominant taxa (47). However, current methodologies utilizing pyrosequencing have allowed for detection of rare taxa (45). These rare taxa remain largely unexplored, but they may be extremely important and may become more dominant in response to environmental changes (47).
Currently, little is known about the composition and structure of bacterial communities across reef bioregions and environmental gradients. The genera Acropora and Porites are dominant corals and important reef builders with representative species in both the Indo-Pacific and Caribbean (48). While acroporids are fast-growing, branching genera, Porites are submassive to massive flat corals with a hemispherical shape and slow growth rates. As a result, Porites tend to be longer lived, often for hundreds of years, and grow to large sizes (37, 48). The Caribbean Porites asteroides may grow up to 1 m but tends to form more numerous, smaller colonies, while Porites lutea from Indonesia grows up to a few meters (37). The Caribbean Acropora palmata grows up to 2 m, and Acropora formosa from Indonesia grows on average up to 1 m. Both genera occur in shallow, tropical reef environments, reef slopes, and in lagoons (48).
This study aimed to apply deep sequencing analysis to compare the bacterial community structures associated with the coral mucus produced by Porites astreoides and Acropora palmata from Mexican Caribbean reefs with those associated with the closely related Porites lutea and Acropora formosa found in Indonesia (southeast Sulawesi, Hoga and Sampela reefs). Such information will provide a better understanding of the diversity of microorganisms associated with these important coral species and further our understanding of coral physiology, health, and ecosystems.
MATERIALS AND METHODS
Site location and mucus collection.
A total of 54 mucus samples (derived from three regions on a colony, from triplicate living colonies) of Porites astreoides and Acropora palmata at Punta Maroma, Mexican Caribbean (20°43′N, 86°57′W; 18 samples) and the related coral species Porites lutea and Acropora formosa at Hoga (18 samples) and Sampela (18 samples), Sulawesi, Indonesia (123°46′E, 5°28′S) (Fig. 1) were sampled in July 2007 by using sterile syringes (as described by Ducklow and Mitchell [12]). The mucus samples were filtered using 0.22-μm filters, and the filters were stored at −20°C. Overlying seawater (two 500-ml volumes) adjacent to the coral colonies was also collected at a depth of 10 m, filtered through 0.22-μm filters, and stored at −20°C. The water temperature during collections at both sites was typically 28°C.
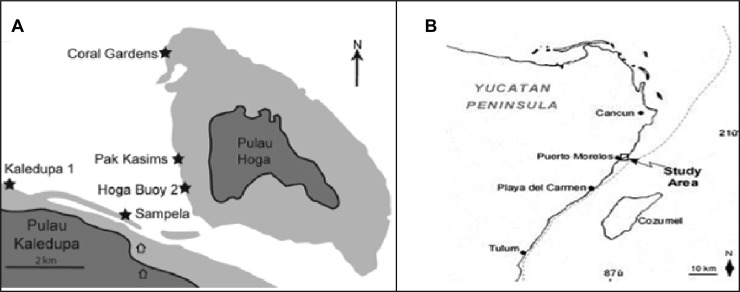
Fig 1.
Sampling sites. (A) Map of the Wakatobi National Park, Sulawesi, Indonesia, showing the Sampela and Hoga (Hoga buoy 2) reefs. (Reprinted from reference 19 with permission of the publisher.) (B) Map showing Punta Maroma on the northeastern coast of the Yucatan Peninsula, Mexico.
DNA extraction, 16S rRNA gene amplification, and DGGE analysis.
Total community DNA was extracted from the filtered seawater and mucus samples (from pooled colony regions on each colony replicate) by using a modified beadbeating method (26). Eubacterial 16S rRNA genes were PCR amplified using the primers for positions 341 to 534 in Escherichia coli (Table 1) (28). PCRs were performed in a GeneAmp PCR system 9700 thermocycler (Applied Biosystems) with the following mixtures (50-μl volumes): 1× buffer (Qiagen), 0.2 mM deoxynucleoside triphosphates (Fermentas), 0.4 μM each primer, 2.5 U Taq DNA polymerase (Qiagen), and approximately 25 ng of DNA (26). PCR cycling conditions were as follows: 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min, and then 72°C for 10 min and holding at 4°C. PCR products were analyzed using 1% (wt/vol) 1× TAE agarose gels (40 mM Tris-acetate, 1.0 mM EDTA; pH 8.0), stained with ethidium bromide (0.5 mg liter−1), and visualized under UV light by using the Gel-Doc system (Bio-Rad). Denaturing gradient gel electrophoresis (DGGE) was performed as described previously (26), except that gels were silver stained (30).
Table 1.
Summary of PCR primer sequences used in this study
| Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| pA | AGAGTTTGATCCTGGCTCAG | 15 |
| pH′ | AAGGAGGTGATCCAGCCGCA | 15 |
| 341 | CCTACGGGAGGCAGCAG | 28 |
| 534 | ATTACCGCGGCTGCTGG | 28 |
| GC clampa | CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGCCTACGGGAG | 28 |
Primer 341 contains a 40-nucleotide GC-rich sequence (GC clamp) for DGGE analysis.
Clone libraries.
PCR products were obtained using the primers pA/pH′ (Table 1) and cycling conditions previously described (15). The PCR products were ligated into the pGEM-T Easy vector (Promega) according to the manufacturer's instructions. Ligations were transformed into high-efficiency JM109 Escherichia coli cells (Promega) according to the manufacturer's instructions. Plasmids of transformed E. coli were purified using a plasmid purification kit (Qiagen) according to the manufacturer's instructions.
Sequencing.
16S rRNA gene sequencing was performed from selected clones by Geneservice Ltd., Cambridge, United Kingdom. Partial sequences (>500 bp) were obtained from clones using the primer pH′ (Table 1) (15). The closest phylogenetic relatives were compared with those in the GenBank database by using the Basic Local Alignment Search Tool (BLAST) network service (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) (1). Sequences were aligned with sequences from GenBank by using the RDP INFERNAL alignment tool (29). Analysis was performed using PHYLIP 3.4 (16) with Jukes-Cantor DNA distance correction and neighbor-joining methods (21, 42). Bootstrap analysis was based on 100 replicates, using SEQBOOT and CONSENSE (PHYLIP 3.4). Tree construction was performed using Treeview (WIN32; version 1.5.2) (31). For pyrosequencing, PCR products were obtained as described above using the primers F341-GC and 534 R (Table 1) (28), except that the forward primer had no GC clamp and a 5′ modification with a 454 amplicon adaptor followed by a unique 10-nucleotide barcode (2, 33). PCR products were quantified with a Nanodrop ND-1000 spectrophotometer, and replicate samples were pooled in equal amounts. Approximately 200 ng of pooled sample was analyzed by pyrosequencing by the NERC Biomolecular Analysis Facility.
Pyrosequence reads were analyzed using the QIIME pipeline and its associated modules (8). All sequences were checked for the presence of correct pyrosequencing adaptors, 10-bp barcodes, and taxon-specific primers, and any sequences containing errors in these primer regions were removed. In addition, sequences of <150 bp or >200 bp in read length, sequences with low quality scores (<20), and sequences containing homopolymer inserts were also removed from further analysis. All pyrosequence reads were clustered into operational taxonomic units (OTUs) by using the UClust algorithm (14). Representative sequences from each OTU were identified using the RDP classifier, which assigns taxonomic identities against the RDP database by using a naïve Bayesian classifier (49). Finally, all singletons were removed before further analysis.
Statistical analysis.
Similarity between DGGE profiles was calculated using binary data indicating the presence of particular bands (Jaccard's index) and a hierarchal cluster analysis constructed using Primer E software version 6 (9). Compositional changes in bacterial assemblages across coral species and sites were analyzed by nonmetric multidimensional scaling (NMDS) ordination of distance matrices calculated from the OTU pyrosequence-read matrix and using Jaccard's index (24, 25). Differences in composition between sites were assessed using permutation-based multivariate analysis of variance (PERMANOVA), based on the distance matrix calculated using Jaccard's index and using 10,000 randomizations. Geographic location (either the Mexican Caribbean, Sampela, or Hoga) and coral species/water sample (either Porites, Acropora, or seawater) were used as independent factors, but the interaction between these two factors was not explored.
Species diversity (number and relative abundance of OTUs) was calculated using the Shannon-Wiener index and compared between sites and corals by using a simple randomization test, based on 10,000 randomizations (46). This randomization approach was also used to compare Jaccard index results between coral species. The randomization approach used treats the entire community as a single data set and is an absolute statistical measure that does not require replication to produce probabilities. This approach is commonly employed in community ecology studies where full replication of sampled communities is impossible (25). All analyses were conducted in the R statistical language version 2.7.2 and using the R standard libraries and the community ecology analysis-specific package Vegan (version 2007; R Development Core Team).
Nucleotide sequence accession numbers.
Clone sequences were submitted to GenBank and assigned accession numbers HQ456683 to HQ456770.
RESULTS
Community and phylogenetic analyses.
Bacterial communities from coral and seawater samples were analyzed by 16S rRNA PCR-DGGE analysis (see Fig. S1 in the supplemental material). There were clear differences in DGGE profiles between corals and their geographical regions (see Fig. S1). Profiles were, however, similar between colony replicates, and so the replicates were pooled for detailed community analysis by pyrosequencing of the 16S rRNA gene. Libraries comprising a total of 9,353 sequences (once sequences of <150 bp or >200 bp in read length, sequences with low quality scores [<20], and sequences containing homopolymer inserts were removed) derived from pooled coral mucus samples produced by P. astreoides and A. palmata (Mexican Caribbean) and P. lutea and A. formosa (Hoga and Sampela, Indonesia) were analyzed (Table 2).
Table 2.
Bacterial assemblages based on 16S rRNA pyrosequencing libraries from coral mucus and seawater
| Phylum, class, or order | % of sequences (total n) in phylum, class, or order from sample areaa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Indonesia |
Mexican Caribbean |
||||||||
| HA (1,264) | HP (924) | HSW (1,083) | SA (1,110) | SP (1,169) | SSW (820) | MA (1,887) | MP (635) | MSW (461) | |
| Actinobacteria | 0 | 0.1 | 0 | 0 | 0.1 | 0.2 | 0.5 | 0.2 | 0.6 |
| Bacteroidetes | 0 | 0 | 6.2 | 5.8 | 2.3 | 4.1 | 0.1 | 0.2 | 0.4 |
| Firmicutes | 16.5 | 6.4 | 0 | 0.7 | 1.4 | 2.1 | 24.1 | 2.8 | 2.4 |
| Clostridiales | 16.0 | 5.4 | 0 | 0.2 | 1.1 | 0.1 | 7.2 | 0.2 | 0.2 |
| Bacilli | 0.2 | 1.0 | 0 | 0.5 | 0.3 | 2.0 | 16.7 | 2.7 | 2.2 |
| Unclassified Firmicutes | 0.2 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 |
| Fusobacteria | 0.6 | 1.2 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 |
| Proteobacteria | 4.4 | 2.4 | 20.0 | 59.0 | 54.3 | 67.7 | 28.3 | 14.0 | 43.2 |
| Alphaproteobacteria | |||||||||
| Caulobacterales | 0.1 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0.7 |
| Rhizobiales | 0.2 | 0 | 0.1 | 0.1 | 0.1 | 0.4 | 3.0 | 1.4 | 3.3 |
| Rhodobacterales (not including Silicibacter spp. or Sulfitobacter spp.) | 0.7 | 0.9 | 1.5 | 6.6 | 5.4 | 3.9 | 11.7 | 7.2 | 2.2 |
| Silicibacter spp. | 0 | 0.2 | 0 | 0 | 0 | 0.4 | 6.2 | 2.0 | 1.3 |
| Sulfitobacter spp. | 3.2 | 1.2 | 18.2 | 48.3 | 47.3 | 61.8 | 1.6 | 0.3 | 0.4 |
| Rhodospirillales | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 |
| Sphingomonadales | 0.2 | 0 | 0.2 | 4.0 | 1.4 | 1.1 | 1.2 | 0 | 31.9 |
| Unclassified Alphaproteobacteria | 0.2 | 0.1 | 0 | 0.3 | 0.2 | 0.1 | 4.5 | 3.0 | 3.5 |
| Betaproteobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 | 0 |
| Gammaproteobacteria | 69.7 | 59.2 | 70.1 | 31.2 | 35.2 | 23.6 | 37.0 | 76.1 | 26.7 |
| Alteromonadales | 0 | 0 | 0 | 0 | 0.4 | 0 | 0.3 | 9.1 | 0.4 |
| Oceanospirillales (not including Halomonas spp.) | 0.4 | 0.2 | 0.6 | 0.2 | 0 | 0 | 0.8 | 4.1 | 0.2 |
| Halomonas spp. | 2.0 | 2.0 | 3.4 | 0.6 | 1.9 | 3.4 | 5.6 | 49.0 | 0.4 |
| Pseudomonadales (not including Acinetobacter spp., Psychrobacter spp.) | 2.6 | 1.8 | 3.6 | 3.1 | 1.6 | 3.4 | 1.5 | 2.1 | 0.7 |
| Acinetobacter spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 | 6.9 | 12.8 |
| Psychrobacter spp. | 63.4 | 53.4 | 63.0 | 27.1 | 30.1 | 18.2 | 26.7 | 0.6 | 9.1 |
| Xanthomonadales | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 |
| Vibrionales | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unclassified Gammaproteobacteria | 1.3 | 1.6 | 0.3 | 0.2 | 0.4 | 0.6 | 2.0 | 3.1 | 1.1 |
| Verrucomicrobia; Verrucomicrobiales | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 |
| Cyanobacteria | 6.4 | 28.0 | 1.8 | 1.8 | 5.2 | 1.0 | 3.7 | 1.7 | 23.6 |
| Planctomycetes | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 |
| Unclassified bacteria | 1.8 | 2.7 | 0.5 | 0.1 | 0.3 | 0 | 3.0 | 1.1 | 2.4 |
Samples were from Acropora formosa at Hoga (HA), Porites lutea at Hoga (HP), seawater at Hoga (HSW), Acropora formosa at Sampela (SA), Porites lutea at Sampela (SP), seawater at Sampela (SSW), Acropora palmata at the Mexican Caribbean (MA), Porites astreoides at the Mexican Caribbean (MP), and seawater at the Mexican Caribbean (MSW). Numbers in bold indicate that the group represents >5% of the community.
NMDS ordination revealed distinct microbial communities associated with samples from geographically distinct regions (Table 2; Fig. 2). PERMANOVA results supported the NMDS ordination and showed that the compositions of the bacterial assemblages were significantly different between geographic sites (PERMANOVA, based on 10,000 randomizations; F1,8 = 2.08; P = 0.03), but not between coral species and seawater samples (F1,8 = 0.58; P = 0.88). The most noticeable result was that bacterial communities from the corals and seawater in the Mexican Caribbean were clearly distinct from those from Indonesia (Fig. 2). Although across all sites there was no significant difference between coral species in the composition of the associated bacterial assemblages (see above), by focusing only on data from the Mexican Caribbean we found significant compositionally distinct assemblages of bacteria from P. astreoides, A. palmata, and seawater samples (simple pairwise randomization test based on 10,000 randomizations; Jaccard's index, >0.89; P < 0.01 in all cases).
Fig 2.
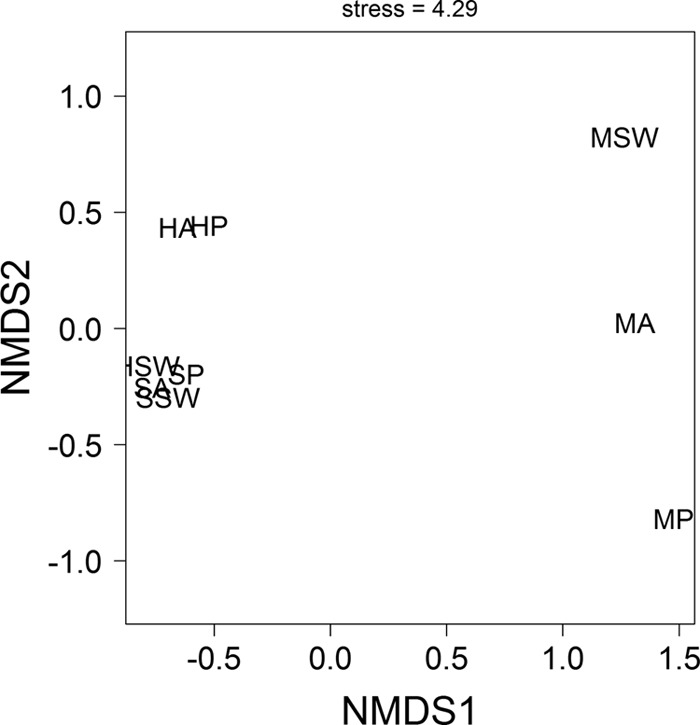
NMDS ordination of distance matrices calculated from the OTU pyrosequence read matrix and using Jaccard's index. Shown are the bacterial communities associated with Acropora formosa at Hoga (HA), Porites lutea at Hoga (HP), seawater at Hoga (HSW), Acropora formosa at Sampela (SA), Porites lutea at Sampela (SP), seawater at Sampela (SSW), Acropora palmata at the Mexican Caribbean (MA), Porites astreoides at the Mexican Caribbean (MP), and seawater at the Mexican Caribbean (MSW) based on the 454 pyrosequencing libraries.
Clustering and classification (Table 2) and diversity analysis (Table 2; Fig. 3A and B) of pyrosequencing libraries revealed geographically distinct coral-bacteria associations. In general, bacterial assemblages from the Mexican Caribbean corals were the most diverse (H′, 3.18 to 4.25), followed by samples from Hoga (H′, 3.25), with samples from Sampela containing the least diverse bacterial assemblages (H′, 2.54 to 2.64) (Fig. 3B). However, most samples had similar levels of bacterial diversity, and only in bacterial assemblages from the Mexican Caribbean Acropora samples were diversity levels significantly higher than those from other sites or corals (simple pairwise randomization test based on 10,000 randomizations; ΔH′, >0.87; P < 0.001 in all cases) (Fig. 3B). Analyses of diversity indices were supported by analysis of rarefied species richness, which provided quantitatively similar results but accounted for differences in sequencing intensities between samples (Fig. 3A).
Fig 3.
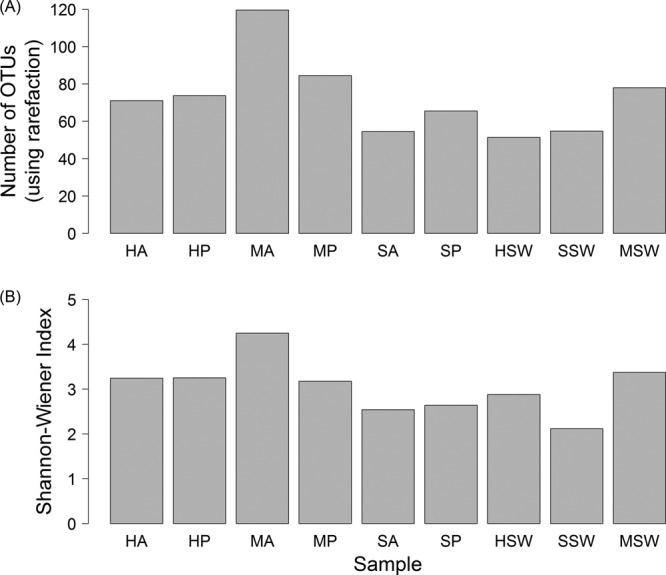
Bacterial community diversity based on the analysis of rarefied species (OTU) richness (A) and the Shannon-Wiener diversity index (B). OTUs were defined using the UClust algorithm with clustering at the 5% level and assigned taxonomic identities using the RDP classifier. Samples are from Acropora formosa at Hoga (HA), Porites lutea at Hoga (HP), seawater at Hoga (HSW), Acropora formosa at Sampela (SA), Porites lutea at Sampela (SP), seawater at Sampela (SSW), Acropora palmata at the Mexican Caribbean (MA), Porites astreoides at the Mexican Caribbean (MP), and seawater at the Mexican Caribbean (MSW).
Overall, Gammaproteobacteria dominated pyrosequencing libraries from Acropora (69.7%) and Porites (59.2%) (at Hoga) and from Porites (76.1%) (from the Mexican Caribbean) (Table 2). This was in contrast to Acropora and Porites libraries from Sampela, which were dominated by Alphaproteobacteria (comprising >54.3%). Bacterial communities that differed with coral genera (from either Indonesia or the Caribbean) were also observed. For example, within the Gammaproteobacteria, Halomonas spp. (49%) and Alteromonadales (9.1%) were predominant in Porites libraries (from the Mexican Caribbean), compared to Acropora at the same location and the corresponding Indonesian corals (<5.6%) (Table 2). Other differences in coral-associated bacteria included Clostridiales, which were more predominant from Acropora (16.0%) than from Porites (5.4%) (at Hoga). Similarly, in the Mexican Caribbean, 7.2% of sequences were related to Clostridiales from Acropora, compared to 0.2% from Porites libraries. In Hoga, cyanobacteria were predominant in Porites libraries (28%), compared to Acropora (6.4%) and the corresponding corals from Sampela and the Caribbean, where cyanobacteria were much rarer (<5.2%).
As with Indonesia, other distinct sequences were associated with the corals found in the Mexican Caribbean (Table 2). For example, there was a relative dominance of Firmicutes (24.1%), primarily bacilli (16.7%), found in Acropora libraries, but Firmicutes were much rarer (<2.8%) in the Porites libraries from the same region. Additionally, within the Rhodobacteriales, Silicibacter spp. were more dominant in the Mexican Caribbean Acropora libraries (6.2%) than Porites (2.0%) and the corresponding Indonesian coral libraries (<0.2%). In contrast, Sulfitobacter spp. dominated both coral libraries from Sampela (comprising >47.3%) compared to the corresponding coral libraries from Hoga and the Mexican Caribbean (<3.2%).
Similarities were also found with the coral-associated bacterial communities (from either Indonesia or the Caribbean) (Table 2). For example, Psychrobacter spp. were ubiquitous in all coral libraries (with the exception of Porites from the Mexican Caribbean). Psychrobacter spp. were also more dominant in Acropora (63.4%) and Porites (53.4%) from Hoga (compared to the corresponding corals in Sampela [27.1% and 30.1%, respectively] and Acropora [26.7%] from the Mexican Caribbean) (Table 2). Similarly, cyanobacteria (28%) were also more dominant in Porites than in Acropora libraries from Hoga (6.4%) and the corresponding coral libraries from Sampela (<5.2%) and the Mexican Caribbean (<3.7%).
Not surprisingly, there were also differences observed in seawater bacterial communities from Indonesia and the Mexican Caribbean (Table 2). For example, Gammaproteobacteria (70.1%) were dominant in seawater from Hoga, Indonesia, compared to seawater from Sampela (23.6%) and the Mexican Caribbean (26.7%). Conversely, Alphaproteobacteria (67.7%) were more dominant in seawater from Sampela, Indonesia, than in seawater from Hoga (20.0%) and the Mexican Caribbean (42.3%). In addition to Alpha- and Gammaproteobacteria, seawater from the Mexican Caribbean was dominated by Sphingomonadales (31.9%), Cyanobacteria (23.6%), and Acinetobacter spp. (12.8%).
Clone libraries.
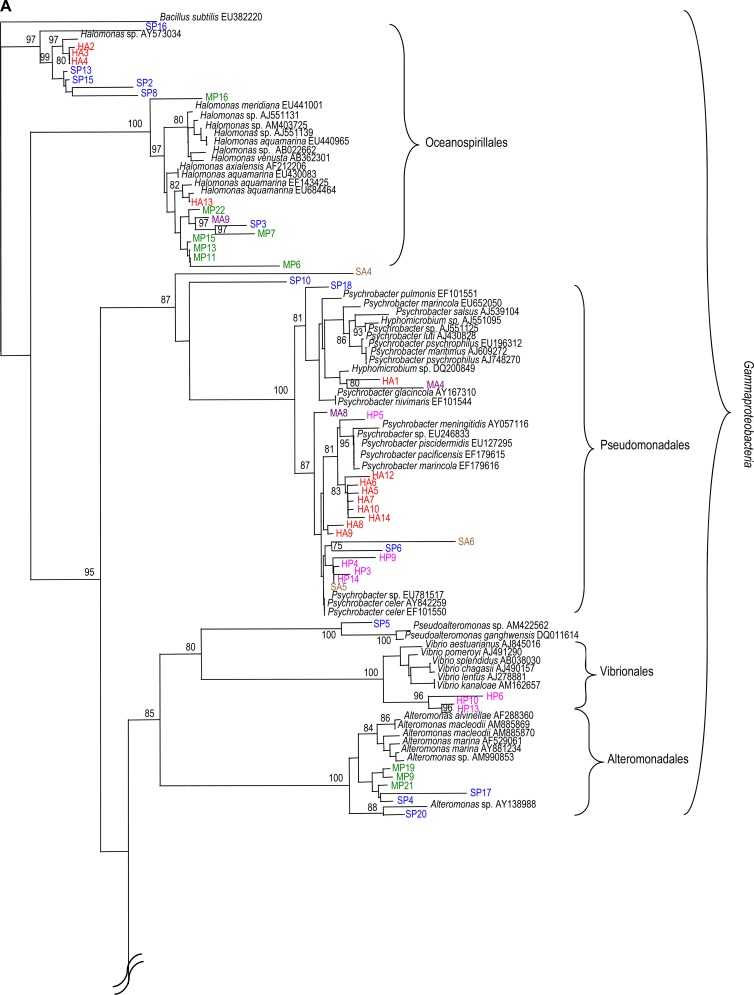
In order to obtain almost-full-length 16S rRNA gene sequences for phylogenetic analysis, clone libraries were generated from each coral sample (Fig. 4A and B). Libraries were screened by DGGE, and from a total of 317 clones, 106 partial 16S rRNA gene sequences (of >500 bp) were obtained (distributed relatively evenly across all samples). Generally, there was good agreement between clone and pyrosequencing libraries, with discrete sequence clusters associated with each coral. For example, sequences from Acropora and Porites (at Hoga) predominantly clustered within the Gammaproteobacteria (Fig. 4A), with discrete clusters associated with Psychrobacter spp. and Halomonas spp.
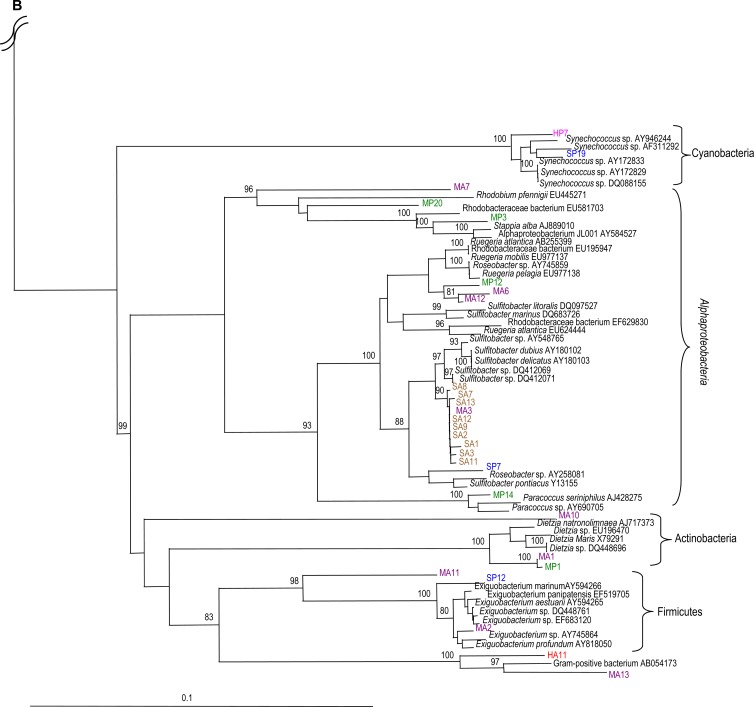
Fig 4.
Phylogenetic analysis of the 16S rRNA gene sequences from selected clones. Included are type strains obtained from GenBank. Sequence analysis was performed on common partial sequences (>500 bp) by using Jukes-Cantor DNA distance and neighbor-joining methods. Bootstrap values represent percentages from 100 replicates of the data; percentages of >80% are shown. Bar, 0.1 substitutions per nucleotide base. The 16S rRNA gene sequences from clones clustered within the Gammaproteobacteria (A) and within the Alphaproteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, Cyanobacteria, and uncultured bacteria (B). Clone sequences are color coded as follows: Acropora formosa at Hoga (HA; red), Porites lutea at Hoga (HP; pink), Acropora formosa at Sampela (SA; brown), Porites lutea at Sampela (SP; blue), Acropora palmata at the Mexican Caribbean (MA; purple), and Porites astreoides at the Mexican Caribbean (MP; green). Unique clone identifiers are shown following the species names.
In addition to Gammaproteobacteria, one clone (HP7) from Porites (Hoga) grouped with Synechococcus spp. within the Cyanobacteria, and two clones (HA11 and MA13) from Acropora (from Hoga and the Mexican Caribbean, respectively) clustered with an uncultured Gram-positive bacterium with strong bootstrap support (Fig. 4B). Sequences from Acropora (Sampela) were dominant within the Alphaproteobacteria, followed by Gammaproteobacteria (Fig. 4A and B). Dominant sequences from Acropora (Sampela) within the Alphaproteobacteria were related to Sulfitobacter spp., while dominant clone sequences from Porites (Sampela) affiliated with Gammaproteobacteria, including Halomonas spp., Alteromonas spp., and Psychrobacter spp. In addition to Gammaproteobacteria sequences, one clone (SP19) associated with Porites from Sampela clustered with Synechococcus spp. within the Cyanobacteria. Another clone (SP12) associated with Porites from Sampela clustered with Exiguobacterium spp. within the Firmicutes.
In comparison to clones from Indonesian corals, the clone libraries from Acropora and Porites from the Mexican Caribbean spanned several phyla, including Gamma- and Alphaproteobacteria, Firmicutes, Altermonadales, and Actinobacteria (Fig. 4A and B). In contrast to the dominance of Gammaproteobacteria in samples from Indonesia, only three clones from Acropora (Mexican Caribbean) clustered within the Gammaproteobacteria. In addition, two clones (MA2 and MA11) were closely related to Exiguobacterium spp. within the Firmicutes with strong bootstrap support (100%) (Fig. 4B).
Gammaproteobacteria (specifically, Halomonas spp. and Alteromonas spp.) dominated the Porites library (Mexican Caribbean), followed by Alphaproteobacteria. We also found that no clone sequences from the Porites library (Mexican Caribbean) clustered with Psychrobacter spp. In addition, two clones (MA1 and MP1; from Acropora and Porites libraries, respectively) had 99% sequence identity to Dietzia spp. within the Actinobacteria, and one clone (MP14) from the Porites library grouped with Paracoccus spp. with strong bootstrap support (100%).
Although clone libraries generally corroborated information from pyrosequencing libraries, some differences were observed, which may have been due to differences in sequencing efforts. For example, Alphaproteobacteria were dominant in the pyrosequencing library from Porites for Sampela (54.3%), but only one clone sequence (SP7) was found within the Alphaproteobacteria (Fig. 4B). Conversely, Vibrionales were rarer in the pyrosequencing library, while three clone sequences (HP6, HP10, and HP13) from Porites (Hoga) grouped with Vibrio spp. with strong bootstrap support (96%) (Fig. 4A).
DISCUSSION
The coral mucus samples yielded more diverse 16S rRNA pyrosequecing libraries than seawater. This finding, coupled with the fact that particular phylotypes were present in greater abundance, suggests discrete bacterial associations with their coral hosts. Here, we found a high relative abundance of Gammaproteobacteria sequences associated with Porites from the Mexican Caribbean, followed by Acropora from the Mexican Caribbean and Acropora and Porites from Sampela. Similar findings have been previously reported, whereby there was an association of Gammaproteobacteria with Porites astreoides from Panama and Bermuda (39). Furthermore, a high relative abundance of Gammaproteobacteria in the coral Montastrea cavernosa from the Mexican Caribbean has also been found (17). In our study, within the Gammaproteobacteria there was a predominance of sequences relating to Halomonas spp. associated with Porites and Acropora from the Mexican Caribbean; in addition, this is the first study to report an abundance of sequences relating to Psychrobacter spp. associated with both Acropora spp. and Porites spp.
In addition to Gammaproteobacteria, another study found Alphaproteobacteria to be the dominant microbial group within the coral mucus of Pocillopora damicornis from the Great Barrier Reef (4). More specifically, 36% of the clones were affiliated with Alphaproteobacteria and only 16% with Gammaproteobacteria (4). In our study, Alphaproteobacteria also dominated microbial communities associated with Acropora and Porites from Sampela and Acropora and Porites from the Mexican Caribbean.
It was previously suggested that mucus of different coral species enriches for different bacterial communities (12, 37). In our study, differences in the library compositions in the coral mucus of Porites spp. versus Acropora spp. were observed. A metagenomic analysis of the microbial community associated with the coral P. astreoides in Panama found that the most prominent bacteria were Proteobacteria (68%), followed by Firmicutes (10%), Cyanobacteria (7%), and Actinobacteria (6%) (50), similar to the findings in the present study. Interestingly, in the present study, within the Firmicutes, a high abundance of Clostridiales sequences was recovered from Acropora from Hoga and the Mexican Caribbean (compared to Porites and seawater samples from the same location), suggesting that Clostridiales are strongly associated with coral mucus from Acropora spp. in both regions. It is possible that the Clostridia spp. may play a role in the breakdown of complex carbon compounds present in the mucus produced by Acropora, although the available evidence remains inconclusive. Since clostridia are generally obligate anaerobes, it is possible that oxygen becomes depleted during complex carbon degradation, generating anaerobic or low-oxygen microniches within the thick mucus and facilitating their proliferation.
It is known that Porites spp. produce more and denser mucus than Acropora spp. (12), which may be attributable to its greater tolerance to sedimentation (7, 27). Furthermore, metagenomic studies of microbial communities associated with P. astreoides have shown that coral-associated bacteria possess a large number of genes for the uptake and processing of protein and sugars, reflecting the compounds found in coral mucus (50). However, it is also entirely possible that bacteria associated with other corals may possess similar genes.
The Hoga reef in this study has been designated a protected area (10, 11). The Sampela reef is in an enclosed lagoon, buffered from the Hoga-Kaledupa Channel by an outer reef wall and located approximately 400 m away from the Bajau village, which has a population of >1,500 people (3, 44). Due to continued human activities, the Sampela reef has low light availability and high sedimentation rates (10). Specifically, Sampela sedimentation rates are around 2-fold higher (11.5 mg cm−2 day−1) than at Hoga (<5 mg cm−2 day−1) (20). Consequently, corals at Sampela may be more likely to be stressed and produce more mucus (27).
It has also been suggested that coral bleaching and coral diseases may be more likely to occur in Hoga than Sampela due to high light exposure (5, 18), resulting in either an increase in abundance of pathogens or the expansion of ecological niches occupied by coral pathogens, like Vibrio spp. (32, 34, 36). In our study, three clone sequences relating to Vibrio spp. (from Porites at Hoga) were recovered, suggesting that the corals at Hoga may be more likely to become infected than those from Sampela, where low light penetration may account for the lack of Vibrio spp. Furthermore, Porites spp. may produce a higher disease prevalence than Acropora spp. (19). Sulfite-oxidizing bacteria have also been isolated from black band-diseased corals (12), and in our study, sequences relating to Sulfitobacter spp. were found in corals from Sampela.
In the Mexican Caribbean, where the reefs are enclosed in a lagoon (41), a much higher diversity of microorganisms was associated with coral mucus of Porites and Acropora compared to their Indonesian counterparts. Interestingly, two clone sequences, one from Acropora and one from Porites, were closely related to Actinobacteria. Similar findings have been obtained from the Red Sea coral Fungia scutaria, whereas Actinobacteria were cultured from the mucus of healthy corals (22, 23). In addition, our results revealed that the bacterial assemblages associated with corals in the Mexican Caribbean had significantly distinct compositions from those from Indonesia, as well significantly distinct communities between Acropora spp. and Porites spp. within the Mexican Caribbean. These findings highlight that not only are coral host species-specific effects structuring these bacterial assemblages, but also isolation by distance effects due to the relative geographic separation of the main sites contributes. Thus, adding further supporting evidence for the hypothesis that both the dispersal limitation and environmental gradients (biotic coral-host niche) affect the structure microbial communities (13).
It has previously been suggested that the coral host may obtain nutrients, such as nitrogen and phosphorus, from their associated microbial communities, and indeed, nitrogen-fixing bacteria have been identified within the coral holobiont (43). In our study, microorganisms closely affiliated with Synechococcus spp. (Cyanobacteria) were recovered from Acropora and Porites in Hoga and to a lesser extent from Porites at Sampela, and thus they may provide nitrogen to the coral host.
In conclusion, the microbes associated with mucus from Porites and Acropora spp. from the Mexican Caribbean and Indonesia (Hoga and Sampela) reefs were determined. The pyrosequence library composition associated with the mucus of Acropora spp. and Porites spp. was more diverse in the Mexican Caribbean than Indonesia. To our knowledge, this is also the first report describing geographically distinct Psychrobacter spp. associated with coral mucus. We found that different coral species harbored different bacterial sequences that were distinct from seawater, and some bacteria-coral relationships appeared to be host specific, such as for Clostridiales with Acropora spp. Since corals are increasingly faced with changing environmental conditions (35), characterization of coral-associated microbes and their interactions with the coral host is essential in order to better understand the dynamics of coral reef systems and their responses to environmental changes.
Supplementary Material
ACKNOWLEDGMENTS
We thank the University of Essex and the Society for General Microbiology for funding this research.
We also thank UNAM (Mexico), in particular the station at Puerto Morelos, and Paul Blanchon for facilitating fieldwork in Mexico. We also thank Operation Wallacea and David Smith, University of Essex, for facilitating the fieldwork at Hoga and Sampela.
Published ahead of print 25 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez LA, Exton DA, Timmis KN, Suggett DJ, McGenity TJ. 2009. Characterization of marine isoprene-degrading communities. Environ. Microbiol. 11:3280–3291 [DOI] [PubMed] [Google Scholar]
- 3. Bell JJ, Smith DJ. 2004. Ecology of sponge assemblages (Porifera) in the Wakatobi region, southeast Sulawesi, Indonesia: richness and abundance. J. Mar. Biol. Assoc. UK 84:581–591 [Google Scholar]
- 4. Bourne DG, Munn CB. 2005. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environ. Microbiol. 7:1162–1174 [DOI] [PubMed] [Google Scholar]
- 5. Boyett HV, Bourne DG, Willis BL. 2007. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar. Biol. 151:1711–1720 [Google Scholar]
- 6. Breitbart M, Bhagooli R, Griffin S, Johnston I, Rohwer F. 2005. Microbial communities associated with skeletal tumors on Porites compressa. FEMS Microbiol. Lett. 243:431–436 [DOI] [PubMed] [Google Scholar]
- 7. Brown BE, Bythell JC. 2005. Perspectives on mucus secretion in reef corals. Mar. Ecol. Prog. Ser. 296:291–309 [Google Scholar]
- 8. Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequence data. Nat. Methods 7:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke KR, Gorley RN. 2006. PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth, MA [Google Scholar]
- 10. Crabbe MJC, Smith DJ. 2002. Comparison of two reef sites in the Wakatobi Marine National Park (SE Sulawesi, Indonesia). Using digital image analysis. Coral Reefs 24:437–441 [Google Scholar]
- 11. Crabbe MJC, Karaviotis S, Smith DJ. 2004. Preliminary comparison of three coral reef sites in the Wakatobi Marine National Park (S.E. Sulawesi, Indonesia): estimated recruitment dates compared with Discovery Bay, Jamaica. Bull. Mar. Sci. 74:469–476 [Google Scholar]
- 12. Ducklow HW, Mitchell R. 1979. Bacterial populations and adaptations in the mucus layers on living corals. Limnol. Oceanogr. 24:715–725 [Google Scholar]
- 13. Dumbrell AJ, Nelson M, Dytham C, Helgason T, Fitter AH. 2010. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 4:337–345 [DOI] [PubMed] [Google Scholar]
- 14. Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461 [DOI] [PubMed] [Google Scholar]
- 15. Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC. 1989. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S-ribosomal RNA. Nucleic Acids Res. 17:7843–7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Felsenstein J. 1989. PHYLIP: Phylogeny Inference package (version 3.2). Cladistics 5:164–166 [Google Scholar]
- 17. Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. 2002. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl. Environ. Microbiol. 68:2214–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glynn PW. 1993. Coral reef bleaching: ecological perspectives. Coral Reefs 12:1–17 [Google Scholar]
- 19. Haapkylä J, Seymour AS, Trebilco J, Smith DJ. 2007. Coral disease prevalence and coral health in the Wakatobi Marine Park, southeast Sulawesi, Indonesia. J. Mar. Biol. Assoc. UK 87:1–12 [Google Scholar]
- 20. Haapkyla et al 2009. Spatio-temporal coral disease dynamics in the Wakatobi Marine National Park, South-East Sulawesi. Indones. Dis. Aquat. Organ. 87:105–115 [DOI] [PubMed] [Google Scholar]
- 21. Jukes TH, Cantor CR. 1969. Evolution of protein molecules, p 21–132 In Munro HN. (ed), Mammalian protein metabolism. Academic Press, New York, NY [Google Scholar]
- 22. Lampert Y, Kelman D, Dubinsky Z, Nitzan Y, Hill RT. 2006. Diversity of culturable bacteria in the mucus of the Red Sea coral Fungia scutaria. FEMS Microbiol. Ecol. 58:99–108 [DOI] [PubMed] [Google Scholar]
- 23. Lampert Y, et al. 2008. Phylogenetic diversity of bacteria associated with the mucus of Red Sea corals. FEMS Microbiol. Ecol. 64:187–198 [DOI] [PubMed] [Google Scholar]
- 24. Lozupone C, Hamady M, Knight R. 2006. UniFrac: an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 7:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maček I, et al. 2011. Local adaptation to soil hypoxia determines the structure of an arbuscular mycorrhizal fungal community in natural CO2 springs. Appl. Environ. Microbiol. 77:4770–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKew BA, Coulon F, Osborn AM, Timmis KN, McGenity TJ. 2007. Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary UK. Environ. Microbiol. 9:165–176 [DOI] [PubMed] [Google Scholar]
- 27. Meikle P, Richards GN, Yellowlees D. 1988. Structural investigations on the mucus from six species of coral. Mar. Biol. 99:187–193 [Google Scholar]
- 28. Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nawrocki EP, Eddy SR. 2007. Query-dependent banding (QDB) for faster RNA similarity searches. PLoS Comput. Biol. 3:e56 doi:10.1371/journal.pcbi.0030056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicol G, Tscherko D, Embley TM, Prosser JI. 2005. Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ. Microbiol. 7:337–347 [DOI] [PubMed] [Google Scholar]
- 31. Page RDM. 1996. TreeView: an application to display phylogenetic trees on personal computers. Bioinformatics 12:357–358 [DOI] [PubMed] [Google Scholar]
- 32. Pantos O, et al. 2003. The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis. Environ. Microbiol. 5:370–382 [DOI] [PubMed] [Google Scholar]
- 33. Parameswaran P, et al. 2007. A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Res. 35:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Remily ER, Richardson LL. 2006. Ecological physiology of a coral pathogen and the coral reef environment. Microb. Ecol. 51:345–352 [DOI] [PubMed] [Google Scholar]
- 35. Riegl B, Bruckner A, Coles SL, Renaud P, Dodge RE. 2009. Coral reefs: threats and conservation in an era of global change. Ann. N. Y. Acad. Sci. 1162:136–186 [DOI] [PubMed] [Google Scholar]
- 36. Ritchie KB. 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Progr. Ser. 322:1–14 [Google Scholar]
- 37. Ritchie KB, Smith GW. 1997. Physiological comparison of bacterial communities from various species of scleractinian corals, p 521–526 Proc. 8th Int. Coral Reef Symp. Smithsonian Tropical Research Institute, Balboa, Panama [Google Scholar]
- 38. Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N. 2001. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 20:85–91 [Google Scholar]
- 39. Rohwer F, Seguritan V, Azam F, Knowlton N. 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Progr. Ser. 243:1–10 [Google Scholar]
- 40. Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. 2007. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5:355–362 [DOI] [PubMed] [Google Scholar]
- 41. Ruìz-Renterìa F, van Tussenbroek BI, Jordán-Dahlgren E. 1998. Puerto Morelos, Quintana Roo, Mexico, p 57–66 In Kjerfve B. (ed), CARICOMP: Caribbean coral reef, seagrass and mangrove sites. UNESCO, Paris, France [Google Scholar]
- 42. Saitou N, Nei M. 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 43. Shashar N, Cohen Y, Loya Y, Sar N. 1994. Nitrogen-fixation (acetylene reduction) in stony corals: evidence for coral-bacteria interactions. Mar. Ecol. Progr. Ser. 111:259–264 [Google Scholar]
- 44. Smith DJ, Pilgrim S, Cullen L. 2007. Coral reefs and people, p 1081–1117 In Pretty J.et al (ed), Sage handbook on environment and society. Sage Publications, London, England [Google Scholar]
- 45. Sogin ML, et al. 2006. Microbial diversity in the deep sea and the underexplored ‘rare biosphere.’ Proc. Natl. Acad. Sci. U. S. A. 103:12115–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Solow AR. 1993. A simple test for change in community structure. J. Anim. Ecol. 62:191–193 [Google Scholar]
- 47. Sunagawa S, Woodley CM, Medina M. 2010. Threatened corals provide underexplored microbial habitats. PLoS One 5:1–6 doi:10.1371/journal.pone.0009554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wallace CC, Chen CA, Fukami H, Muir PR. 2007. Recognition of separate genera within Acropora based on new morphological, reproductive and genetic evidence from Acropora togianensis, and elevation of the subgenus Iso pora Studer, 1878 to genus (Scleractinia: Astrocoeniidae; Acroporidae). Coral Reefs 26:231–239 [Google Scholar]
- 49. Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wild C, et al. 2004. Degradation and mineralization of coral mucus in reef environments. Mar. Ecol. Progr. Ser. 267:159–171 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.