Abstract
In the course of screening for virus-host systems in extreme thermal environments, we have isolated a strain of the hyperthermophilic archaeaon Acidianus hospitalis producing unusual filamentous particles with a zipper-like appearance. The particles were shown to represent a secreted form of a genuine cellular enzyme, tetrathionate hydrolase, involved in sulfur metabolism.
TEXT
Thermal aquatic areas with temperatures above 80°C are common habitats of archaea and their astoundingly diverse viruses (6). Due to frequent difficulties associated with isolation and cultivation of pure archaeal strains, studies of the viral diversity often rely on successful establishment of enrichment cultures from environmental samples (7). While exploring the viral community associated with a culture enriched in crenarchaeon Acidianus hospitalis, we have observed a variety of unique virus-like particles (9). One particle type was shown to represent infectious virions of a new virus, Acidianus filamentous virus 1 (AFV1) (2). The nature of other particles, however, remained unknown. The most unusual among them were filamentous particles with a surface demonstrating a zigzag-like periodic pattern, zipper-like particles (ZLPs) (9).
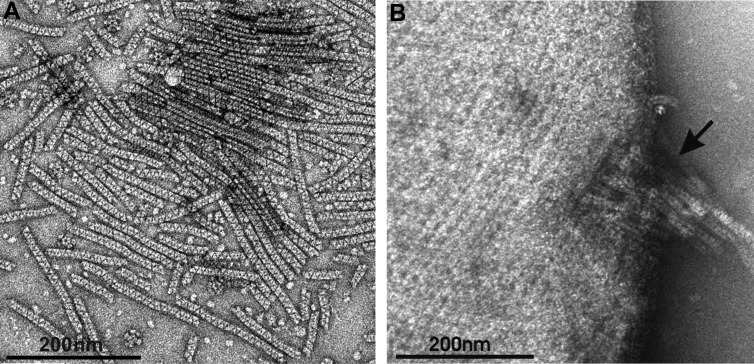
To study the ZLPs, we have isolated a producer strain, Acidianus hospitalis YS8, from the enrichment culture (see the supplemental material). The ZLPs were collected and purified from cell-free culture supernatant of A. hospitalis YS8 by sequential filtration through filters with pore sizes of 0.80, 0.45, and 0.20 μm, followed by concentration of ZLPs on ultrafilters with the exclusion size of 100 kDa (see the supplemental material). The purified ZLPs appeared as cylindrical particles uniform in their width, 15 ± 1 nm, and variable in length, 100 to 200 nm (Fig. 1A). The periodic zigzag-like pattern on the particle surface (Fig. 1A) most likely resulted from regular assemblage of identical structural units.
Fig 1.
Negative contrast electron micrographs of the ZLPs. (A) Purified ZLPs; (B) extrusion of ZLPs from a cell of A. hospitalis (indicated by an arrow).
By electron microscopic analysis of the growing cells of A. hospitalis YS8, we could observe extrusion from cells of ZLPs (Fig. 1B). The amount of ZLPs, estimated as described in the supplemental material, increased after treatment of the cells with mitomycin C (final concentration of 5%, vol/vol) and UV light (7 min in the layer of 3 mm) and by freezing cells in liquid nitrogen and their rapid thawing (not shown). The results suggested that the ZLP production may be under the general stress response regulatory network in A. hospitalis. Consistently, when the cells were allowed to adapt to the growth conditions, the presence of the ZLPs in the culture supernatant decreased to nondetectable levels (see the supplemental material).
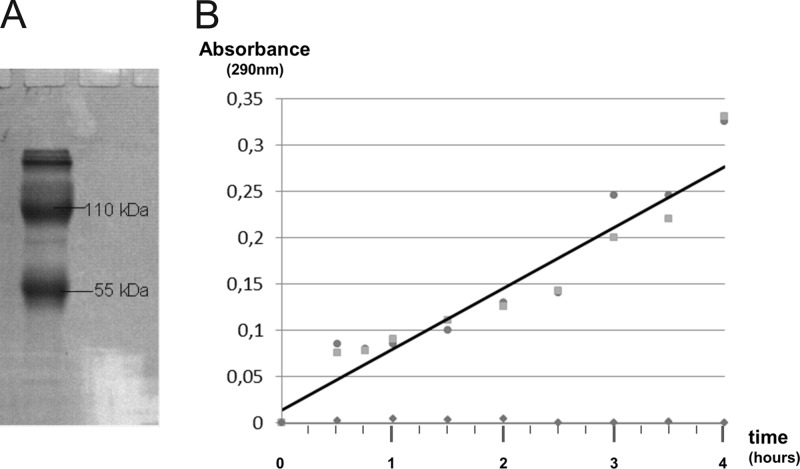
The possibility to induce the production of ZLPs with mitomycin and UV irradiation was in line with our initial assumption that ZLPs represent genuine viruses (9). However, upon examination of the molecular constituents of the purified ZLPs, no nucleic acids—neither DNA nor RNA—could be isolated from purified particles by phenol extraction (10). An SDS-PAGE analysis revealed two major protein bands with apparent molecular masses of 55 ± 5 and 110 ± 10 kDa as well as three minor bands of proteins larger than 200 kDa (Fig. 2A). N-terminal sequencing of proteins from both the 55- and 110-kDa bands revealed an identical sequence (PIVYTY). Thus, the larger 110-kDa protein was apparently a dimer of the 55-kDa protein, while the larger proteins likely represent multimers of the 110-kDa dimer.
Fig 2.
Protein composition of the ZLPs and their enzymatic activity. (A) SDS-PAGE of purified ZLP preparation; molecular masses of the major proteins are indicated. (B) Tetrathionate hydrolase activity of the ZLPs; the absorbance at 290 nm of the assay mixture, from duplicate experiments (indicated with squares and circles), is plotted over time. The average values are shown by a black line. The results of the control experiment in the absence of tetrathionate are shown with diamond symbols.
The N-terminal sequence enabled identification of the ZLP-coding gene on the genome of A. hospitalis W1 (11). The gene was annotated as coding for tetrathionate hydrolase (TetH; GenBank accession number YP_004458846) (11). Notably, an orthologue of the ZLP-forming protein from Acidianus ambivalens (99% identical), a very close relative of A. hospitalis, has been recently characterized biochemically and confirmed to possess the predicted activity (8). TetH is one of the key players in sulfur metabolism, oxidizing tetrathionate into thiosulfate and sulfate (8). The N-terminal sequence of the ZLP-forming protein matched to the Pro22-Tyr27 residues of the annotated tetH gene product, indicating that the protein is subjected to N-terminal processing. This is consistent with previous reports on the presence of the N-terminal signal sequence in bacterial and archaeal TetH proteins (4, 8).
The near-identity of the ZLP protein to the TetH from A. ambivalens suggested that the ZLPs are not viruses but rather represent homomultimers of TetH. To investigate whether such filamentous particles might represent a physiologically relevant form of TetH, we tested the biochemical activity of the purified ZLPs. The TetH activity was measured in a continuous assay by monitoring the increase in absorbance (λ = 290 nm) resulting from accumulation of long-chain sulfur intermediates as described previously (3). The assay mixture contained 1 M ammonium sulfate (pH 3.0), 1 mM sodium tetrathionate, and 20 μl of ZLP preparation (see the supplemental material), in 0.1 ml volume. The presence of purified ZLPs in the assay mixture resulted in hydrolysis of the tetrathionate, as documented by a continuous increase in the absorbance at 290 nm over 4 h of incubation at 70°C (Fig. 2B). The experiment was conducted in duplicate and resulted in an increase of the A290 by 0.35 units. No changes in the A290 were observed in the control experiment in the absence of tetrathionate (Fig. 2B), excluding a possibility that the increase in absorbance was due to solubilization of the ZLP constituent protein. Similarly, when ZLPs were omitted, no changes in the absorption at 290 nm were detected (not shown). Thus, we confirm that the secreted ZLPs possess the tetrathionate hydrolase activity. Notably, secretion of TetH from A. ambivalens and Acidithiobacillus ferrooxidans has been reported previously (1, 8). However, this is the first time that TetH is shown to form virus-like particles.
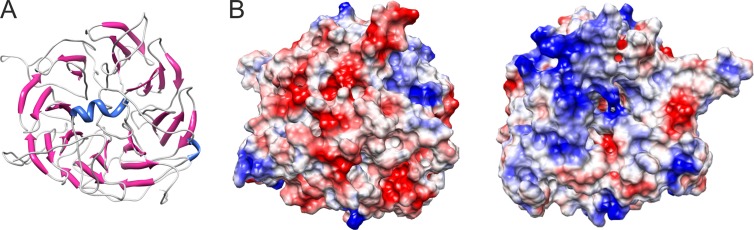
To gain insights into the remarkable ability of TetH from A. hospitalis to assemble into filamentous structures, we built a structural model of the ZLP-forming protein using I-TASSER (12). Similarly to the TetH from A. ambivalens (8), A. hospitalis TetH was found to adopt an eight-bladed β-propeller topology (Fig. 3A). Analysis of the electrostatic surface charge distribution revealed an overall opposite charge on the two faces of the disc-shaped molecule (Fig. 3B), suggesting that electrostatic interaction-mediated head-to-tail stacking of the TetH building blocks might play a role in ZLP formation. It should be noted, however, that stacking of the TetH monomers, with an estimated diameter of ∼4.5 nm (Fig. 3A), would be insufficient to produce ZLPs with the diameter of 15 nm (Fig. 1A). Consequently, the “stacks” used for ZLP formation are likely to consist of TetH multimers. Interestingly, electron microscopy data suggest that the assembly of ZLPs occurs prior to or concomitantly with the extrusion of the filaments from A. hospitalis cells (Fig. 1B).
Fig 3.
Structural model of the TetH monomer from A. hospitalis. (A) Ribbon representation of the eight-bladed β-propeller topology of TetH viewed down the central axis of the disc-shaped molecule. The model is colored according to the secondary structure elements: α-blue, red; β-strands, magenta; coils, gray. (B) TetH model colored according to the electrostatic surface potential following Coulomb's law. The color scale is from −7 (red) to +7 (blue) kcal/(mol · e). The view on the left corresponds to the orientation depicted in panel A, while the one on the right shows a flipside of the molecule. The figure was prepared in UCSF Chimera (5).
In the present study, we have demonstrated that filamentous particles previously assumed to correspond to archaeal viruses (9) in fact represent a secreted form of a cellular enzyme, TetH, involved in sulfur metabolism. Consequently, caution should be taken when exploring and interpreting the diversity of virus-like particles in different environments. Our results also pave a way for further structural and biochemical studies, which should reveal a detailed mechanism of assembly and secretion as well as the physiological role of the remarkable ZLP structures.
Supplementary Material
Footnotes
Published ahead of print 25 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Beard S, Paradela A, Albar JP, Jerez CA. 2011. Growth of Acidithiobacillus ferrooxidans ATCC 23270 in thiosulfate under oxygen-limiting conditions generates extracellular sulfur globules by means of a secreted tetrathionate hydrolase. Front. Microbiol. 2:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bettstetter M, Peng X, Garrett RA, Prangishvili D. 2003. AFV1, a novel virus infecting hyperthermophilic archaea of the genus Acidianus. Virology 315:68–79 [DOI] [PubMed] [Google Scholar]
- 3. de Jong GA, Hazeu W, Bos P, Kuenen JG. 1997. Polythionate degradation by tetrathionate hydrolase of Thiobacillus ferrooxidans. Microbiology 143:499–504 [DOI] [PubMed] [Google Scholar]
- 4. Kanao T, Kamimura K, Sugio T. 2007. Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans. J. Biotechnol. 132:16–22 [DOI] [PubMed] [Google Scholar]
- 5. Pettersen EF, et al. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- 6. Pina M, Bize A, Forterre P, Prangishvili D. 2011. The archeoviruses. FEMS Microbiol. Rev. 35:1035–1054 [DOI] [PubMed] [Google Scholar]
- 7. Prangishvili D. 2006. Hyperthermophilic virus-host systems. Methods Microbiol. 35:331–347 [Google Scholar]
- 8. Protze J, et al. 2011. An extracellular tetrathionate hydrolase from the thermoacidophilic archaeon Acidianus ambivalens with an activity optimum at pH 1. Front. Microbiol. 2:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rachel R, et al. 2002. Remarkable morphological diversity of viruses and virus-like particles in hot terrestrial environments. Arch. Virol. 147:2419–2429 [DOI] [PubMed] [Google Scholar]
- 10. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 11. You XY, et al. 2011. Genomic analysis of Acidianus hospitalis W1 a host for studying crenarchaeal virus and plasmid life cycles. Extremophiles 15:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.