Abstract
Physical cold atmospheric surface microdischarge (SMD) plasma operating in ambient air has promising properties for the sterilization of sensitive medical devices where conventional methods are not applicable. Furthermore, SMD plasma could revolutionize the field of disinfection at health care facilities. The antimicrobial effects on Gram-negative and Gram-positive bacteria of clinical relevance, as well as the fungus Candida albicans, were tested. Thirty seconds of plasma treatment led to a 4 to 6 log10 CFU reduction on agar plates. C. albicans was the hardest to inactivate. The sterilizing effect on standard bioindicators (bacterial endospores) was evaluated on dry test specimens that were wrapped in Tyvek coupons. The experimental D23°C values for Bacillus subtilis, Bacillus pumilus, Bacillus atrophaeus, and Geobacillus stearothermophilus were determined as 0.3 min, 0.5 min, 0.6 min, and 0.9 min, respectively. These decimal reduction times (D values) are distinctly lower than D values obtained with other reference methods. Importantly, the high inactivation rate was independent of the material of the test specimen. Possible inactivation mechanisms for relevant microorganisms are briefly discussed, emphasizing the important role of neutral reactive plasma species and pointing to recent diagnostic methods that will contribute to a better understanding of the strong biocidal effect of SMD air plasma.
INTRODUCTION
Conventional methods for sterilization of medical devices, like wet/dry heat, irradiation, or chemical gases, have several drawbacks. The material properties, such as molecular weight, volume, and morphology, of sensitive devices, including polymeric biomaterials, can be negatively altered (2, 18, 30, 36). This can influence the physical and biological performance of the medical device (36), leading to material failure (2). Some sterilization methods require a 120°C operating temperature, which causes the degradation of thermolabile medical devices. Other limitations are the need for vacuum chambers in common plasma sterilization methods (16) and the use of toxic gases, like formaldehyde or ethylene oxide (1, 11, 16).
Cold atmospheric plasma (CAP) technology does not have these disadvantages (10, 23, 26, 48). The plasmas operate under atmospheric conditions below 40°C. CAP is a weakly ionized gas. Only a small fraction of gas atoms and molecules, which are the main carriers of heat, collide with electrically generated highly energetic electrons. This results in further excitation, ionization, and dissociation, while the plasma remains “cold.” CAP specifications permit the disinfection or sterilization of thermosensitive materials (10, 25) and allow in vivo applications, opening a new and larger spectrum of possible applications. The first devices developed have already proven their bactericidal properties in vitro (12, 31, 46, 47), ex vivo (34), and in vivo (17, 19, 20).
Therefore, CAP is a promising tool for surface decontamination and hand disinfection in public health and hospital care. Despite all the progress in the last few decades, hospital-acquired infections (HAIs) continue to arise. A total of 1.7 million HAIs with 99,000 associated deaths were reported in the United States in 2002 (7, 39), and 307,000 surgical-site infections were reported in European hospitals in 2008 (15). The major concern involves the substantial decline in the antimicrobial susceptibility of pathogens. This includes methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and, more recently, extended-spectrum beta-lactamase-producing Gram-negative bacteria, such as Escherichia coli (15). From an economic point of view, HAIs are associated with considerable costs for health care systems. It is estimated that they increase morbidity, mortality, illness, and direct costs by approximately 30 to 100% (9, 28). Therefore, new strategies are needed for the prevention and treatment of HAIs.
We investigated a CAP device using surface microdischarge (SMD) plasma (38). Our plasma discharge is produced at atmospheric pressure using ambient air. The weakly ionized plasma contains electrons and positive and negative ions. Many chemical reactions take place during the plasma discharge. New neutral plasma-chemical species are created. Excitation processes that result in the emission of photons are favored. Charged, excited, and neutral reactive plasma species are of particular interest for the SMD plasma-cell surface interaction. As the main components of air are nitrogen and oxygen, the reactive species are mainly composed of reactive oxygen species (ROS), involving ozone and hydroxide radicals, and reactive nitrogen species (RNS), involving nitrogen oxides.
In this study, different microorganisms were treated with SMD air plasma. On one hand, the study was focused on vegetative bacteria, which are associated with HAIs. They include Gram-negative strains of E. coli, Burkholderia cepacia, and Pseudomonas aeruginosa, as well as Gram-positive strains of staphylococci, enterococci, and corynebacteria and vegetative cells of spore-forming bacilli. As a representative of fungi, the eukaryotic yeast Candida albicans was tested.
On the other hand, the efficacy of SMD plasma was investigated on bacterial endospores, which are used as bioindicators for the validation of sterilization processes on different materials (metal, glass, and polymeric surfaces), which were wrapped in Tyvek coupons, little envelopes containing the spore carrier and sealed with a gas-permeable Tyvek sheet and an impermeable polymer film (Fig. 1, no. 1). The tested bacterial endospores include spores of Geobacillus stearothermophilus and Bacillus spp.
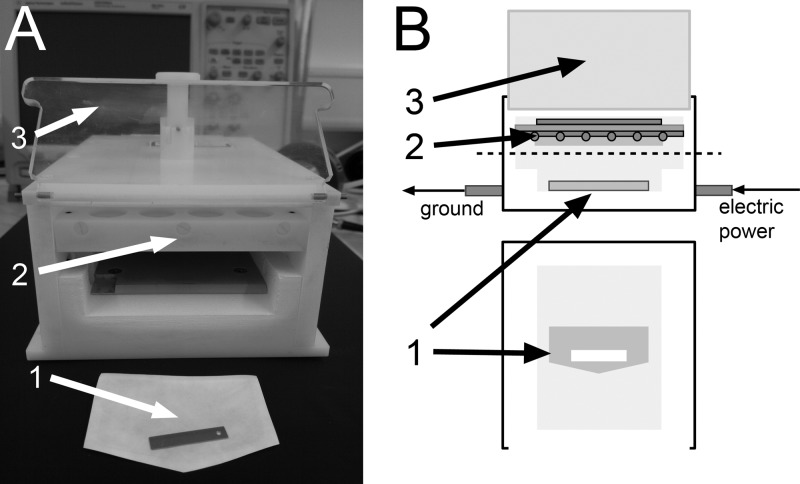
Fig 1.
SMD plasma device. Shown are a photograph (A) and a frontal view and cross-section drawing (B). 1, spore test sample; 2, plasma electrode system; 3, lid.
MATERIALS AND METHODS
The SMD plasma device.
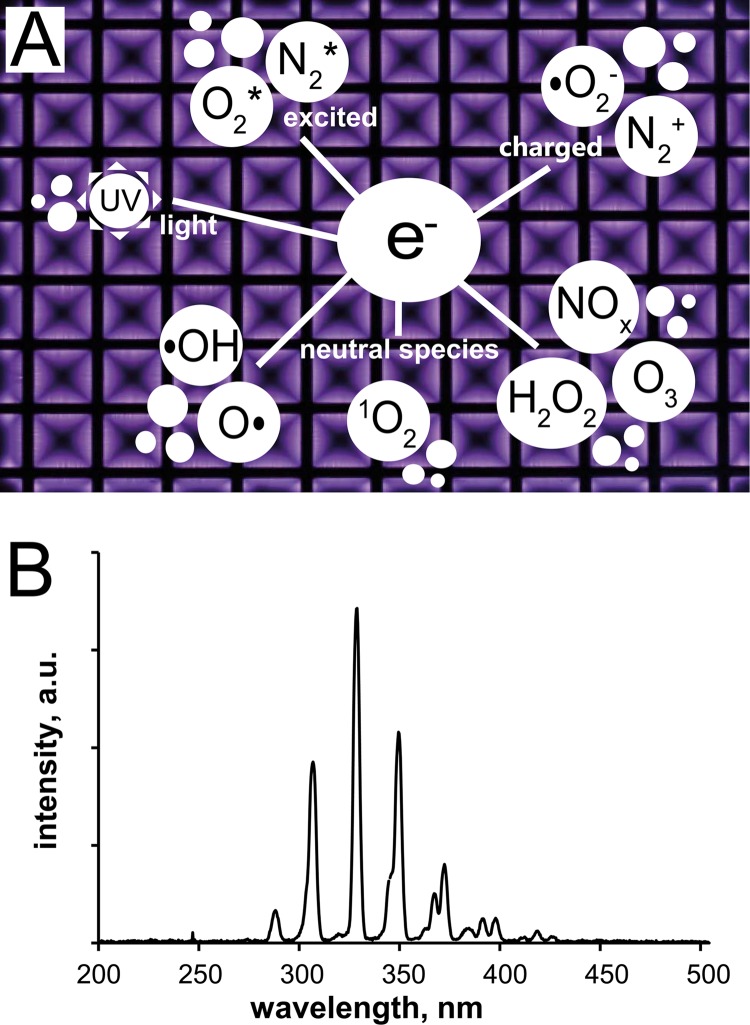
In the present study, the CAP discharge in ambient air was based on surface microdischarge technology. The setup of the plasma device is illustrated in Fig. 1. It is made of polyoxymethylene and has a front opening that can be closed by a transparent lid. The spatial dimensions of the interior (90 by 126 mm with adjustable height) allow the insertion of an endospore test sample or agar plate. Plasma is ignited above the sample at the electrode system. The electrode consists of two plates (here, solid brass and stainless steel mesh) separated by a dielectric (here, 0.5-mm-thick polytetrafluorethylene [PTFE] film). The mesh, with 1.0-cm square holes, is grounded, whereas the brass plate is powered. Application of a 1-kHz sinusoidal low frequency and a 10-kV peak-to-peak (10-kVpp) high voltage by an alternating current power supply that includes a function generator (HM8150 [Hameg Instruments, Germany] or 8202 [Voltcraft, Germany]) and an amplifier (PM 04015 or 10/10B-HS; Trek) is sufficient to generate microdischarges on the dielectric surface and the grounded mesh. These microdischarges are little filaments produced by the electrical power input that are created and broken down continuously. The power density of the plasma discharge at 1 kHz and 10 kVpp is 35 mW/cm2. These power parameters were set constant for all experiments. The SMD plasma emits purple light that is dominated mainly by the excitation of nitrogen molecules in air. The homogeneous plasma discharge, including a scheme of reactive chemical components, is shown in Fig. 2A. The optical emission spectrum of the SMD plasma was measured (UV/VIS Minispectrometer C10082CA; Hamamatsu, Japan). The spectrum in Fig. 2 shows the presence of UV light (λ > 280 nm), but almost no UVC light, which could result in DNA (λ = 260 nm) and protein (λ = 280 nm) damage in cells by absorption, is generated. Absolute UV power density measurements (C8026/H8025; Hamamatsu, Japan; UVC broadband filter; 1-min integration time; 10 cycles) do not exceed 50 μW/cm2. The temperature inside the device during plasma discharge was monitored (K102 thermocouple; Voltcraft, Germany). It increased slowly at 0.2°C/min for permanent plasma discharge.
Fig 2.
SMD air plasma at 1 kHz and 10 kVpp. (A) Discharge pattern and prominent plasma species. (B) Optical emission spectrum of the discharge dominated by excited N2.
Experimental setup for the treatment of microorganisms. (i) Vegetative bacteria and C. albicans.
The SMD air plasma experiments were conducted with various vegetative microorganisms (Table 1). Agar plates were used as substrates to allow comparison with previous experimental data and due to the instability of HAI-associated pathogen survival on inanimate surfaces (24). Müller-Hinton agar or Columbia blood agar plates, depending on the nutritional needs of the strain isolate, were dried for 1 h in ambient air. Bacterial suspensions were prepared in 0.9% NaCl and adjusted to a McFarland density of 0.5 (2 × 108 CFU/ml). A 10−5 dilution of each suspension was prepared through three dilution steps (100→10−2→10−4→10−5) for the negative control and for calculation of the initial bacterial density. One hundred microliters of each suspension was pipetted and immediately smeared on agar plates. After a 30-min drying period in ambient air, the inoculated agar plates were placed in the center of the plasma device, treated sequentially for 30 s with plasma, and removed. The device was closed for every treatment and ventilated for 30 s between treatments in order to equilibrate the conditions. The distance between the generated microdischarges and the agar surface was 8 mm. The treated agar plates and negative controls were incubated at 35 ± 2°C in an appropriate atmosphere overnight and for up to 36 h for Corynebacterium and C. albicans. Afterwards, the surviving CFU were counted. Each treatment was repeated three times for reproducibility. The results are shown as CFU reduction curves with log10(NR) on the ordinate plotted against the treatment time. Reduction was calculated as follows: log10(NR) = log10(N0) − log10(NS), where NR is the number of reduced cells, N0 is the number of the initial population, and NS is the number of surviving cells after plasma treatment of a given strain (NR/N0/NS are expressed as CFU).
Table 1.
Vegetative microorganisms used in this study
| Microorganism | Strain no. or sourcea | Substrate typeb |
|---|---|---|
| Gram-negative bacteria | ||
| E. coliK12 | DSM 11250 | MH |
| E. coli | ATCC 9637 | MH |
| B. cepacia | ATCC 25416 | MH |
| P. aeruginosa | ATCC 27853 | MH |
| Gram-positive bacteria | ||
| S. aureus | ATCC 25923 | MH |
| MRSA | Laboratory strain | MH |
| Staphylococcus epidermidis | DSM 3269 | MH |
| Enterococcus faecalis | ATCC 29212 | MH |
| VRE | Laboratory strain | MH |
| Enterococcus mundtii | ATCC 43186 | MH |
| Bacillus cereus | Laboratory strain | MH |
| B. pumilus | ATCC 27142 | MH |
| C. difficile | Laboratory strain | CBA |
| Group A Streptococcus pyogenes | ATCC 12344 | CBA |
| Corynebacterium jeikeium | ATCC 43734 | CBA |
| Fungus | ||
| C. albicans | ATCC 90028 | MH |
ATCC, American Type Culture Collection; DSM, German Collection of Microorganisms and Cell Cultures; laboratory strain, strain isolated at Hospital Munich Schwabing, Munich, Germany, from infected patients.
MH, Müller-Hinton agar; CBA, Columbia blood agar.
(ii) Bacterial endospores.
The bacterial endospores of G. stearothermophilus (ATCC 7953), Bacillus subtilis (DSM 13019), Bacillus atrophaeus (ATCC 9372), and Bacillus pumilus (ATCC 27142) were used as bioindicators for the validation of atmospheric SMD air plasma as a sterilizing agent. The bioindicator samples were provided and analyzed quantitatively by Simicon GmbH (Munich, Germany) according to DIN EN ISO 14937 (5), which is the standard for the validation of sterilization processes of medical devices, and according to DIN EN ISO 11737-1 (4) for the microbiological determination of surviving CFU on products. Initially, 100 μl of spore suspension containing 2 × 106 spores in deionized water was pipetted and dried on one side of a sterile test specimen (30 by 6 mm), for instance, stainless steel. The inoculated test specimen was wrapped according to medical packaging technology with Tyvek on the endospore-facing side and with impermeable polyethylene film on the back. Other test specimens were PTFE (Goodfellow), polyvinylchloride (PVC) (Goodfellow), and glass (Menzel, Germany). The spores were treated with CAP, leaving the samples inside the Tyvek coupon, which had already been shown to be permeable for plasma (13, 21).
For experiments, the sample was placed with the Tyvek at the top in the center of the SMD device. The treatments lasted 1 min, 3 min, and 5 min. Every spore treatment was repeated at least three times for reproducibility. Each sample was placed in a separate plastic bag after treatment, and the bag was zipped and sent to Simicon GmbH by mail on the same day. There, the spores were resuspended in 7 to 10 ml tryptic soy broth (TSB) medium by 10 min of ultrasonication. The recovery of viable spores followed a dilution series (10−1, 10−3, and 10−5). In the 10−1 or 10−3 dilutions, 100 μl of the dilution was distributed on tryptic soy agar. In the 10−5 dilutions, 1 ml was filled together with liquid tryptic soy agar in a petri dish, where the agar could harden afterwards. The inoculated agar plates were incubated overnight at 35 ± 2°C for Bacillus spp. The incubation temperature for G. stearothermophilus was 56 ± 2°C. The CFU were counted immediately after incubation. The results are shown as CFU reduction curves and decimal reduction times (D values).
(iii) D value calculation and sterility assurance level (SAL) of spore reduction.
The characteristic D value was determined from the data for plasma-treated endospores by the following equation: t = DT [log10(N0) − log10(NS)] = DT [log10(NR)], where t is the treatment time in minutes and DT is the D value in minutes for a single decimal reduction at the treatment temperature T. Here, T was room temperature (∼23°C) at ambient pressure. Data points were selected assuming 1st-order kinetics. Average values were used for calculation. Treatment times were determined as specific SALs that were equivalent to 6 log10 and 12 log10 reduction (43). An overkill with 12 log10 reduction means that a bacterial spore survives the plasma treatment with a predicted probability of 10−6.
RESULTS
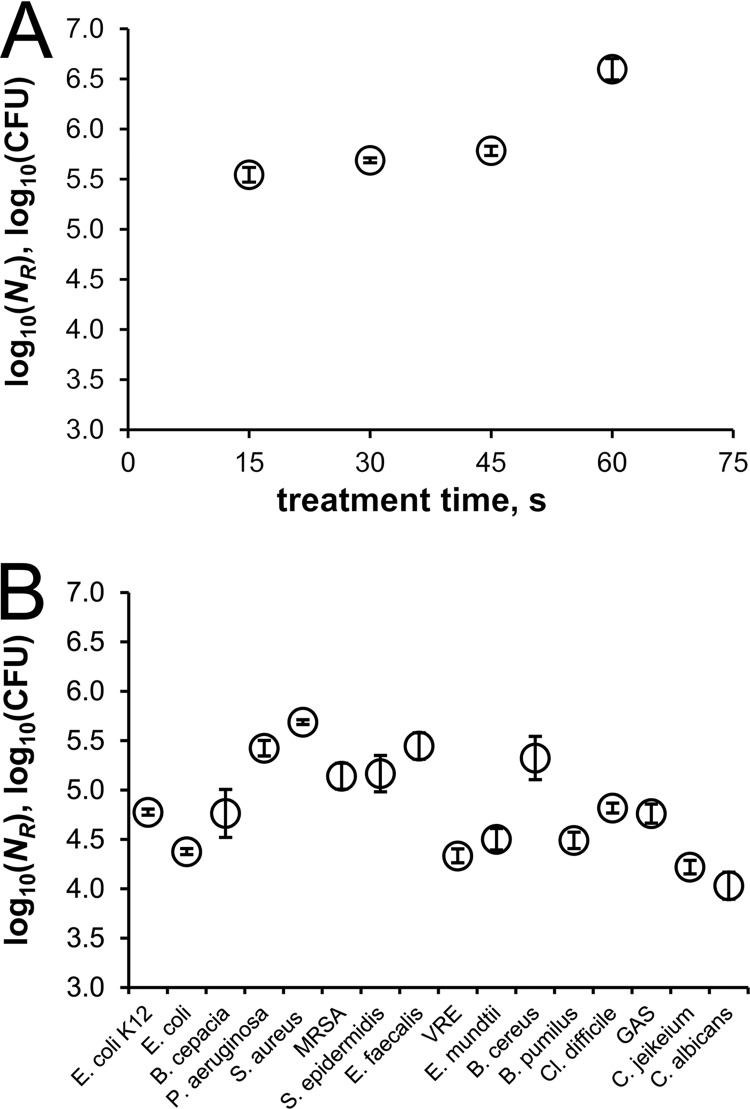
The effect of SMD air plasma on vegetative microorganisms was tested. Figure 3 shows the results of these treatments. Figure 3A shows a kinetic study of the inactivation of S. aureus (ATCC 25923). Within 15 s of plasma exposure, a 5 log10 CFU reduction was obtained, and a slight increase in the reduction was observed with longer treatments; 60 s was sufficient to reduce S. aureus by 6 log10. This kinetic behavior can be represented in terms of a reduction rate for other vegetative microorganisms in this study, while there were differences in the total reduction numbers. These distinctions are demonstrated in Fig. 3B, where the results for 15 bacterial strains and C. albicans exposed for 30 s to plasma are plotted. An average reduction of approximately 5 log10 CFU was achieved. The effect of 30 s of plasma treatment varied among the tested strains from at least 4 log10 to nearly 6 log10 reduction. MRSA was inactivated less efficiently than its drug-sensitive relative S. aureus, which showed the highest reduction by the CAP treatment. E. coli (ATCC 9637) was the most resistant strain among the Gram-negative bacteria. C. albicans cells were more resistant than all bacterial strains.
Fig 3.
Reduction of plasma-treated vegetative microorganisms at 1 kHz and 10 kVpp. (A) Kinetic study of S. aureus. (B) Thirty-second treatments. The bars indicate standard errors (n = 3).
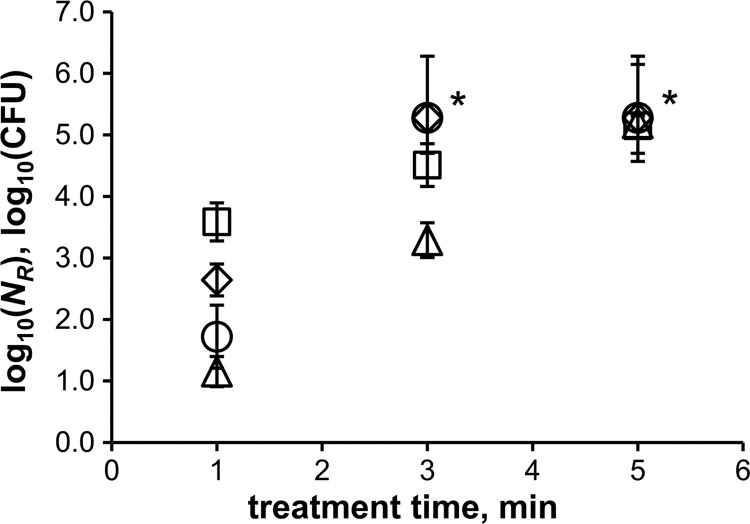
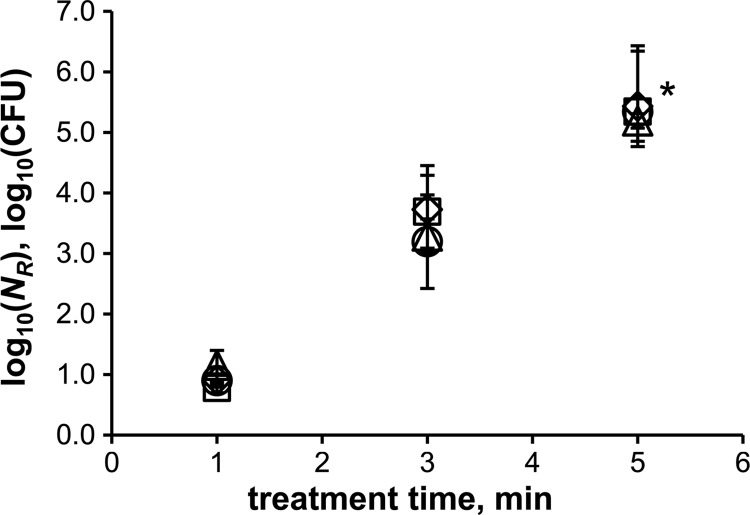
CAP treatments of more robust bacterial endospores showed that the SMD air plasma also strongly affects the survivability of spores. The reduction rates are shown in Fig. 4. The differences between the strains are most obvious after 1 min of plasma treatment. B. subtilis spores were reduced by nearly 4 log10 after 1 min, whereas G. stearothermophilus spores were reduced by only 1 log10. The inactivation rates of B. atrophaeus and B. pumilus were intermediate. Inactivation of at least 5.3 log10 (total inactivation) was obtained after a plasma treatment time of 3 min for Bacillus sp. spores and 5 min for G. stearothermophilus spores. The detection limit possibly prevented higher reduction values. G. stearothermophilus spores were least efficiently inactivated by SMD plasma among all tested strains. Therefore, all further experiments were conducted with this spore type. The influence of the specimen material on the inactivation was investigated using glass, PVC, and PTFE, in addition to conventional stainless steel. The results are shown in Fig. 5. It clearly shows that the same reduction rate can be observed independent of the specimen material.
Fig 4.
Reduction of plasma-treated bacterial endospores at 1 kHz and 10 kVpp. △, G. stearothermophilus; ○, B. pumilus; ♢, B. atrophaeus; □, B. subtilis; *, detection limit. The error bars indicate standard errors (n ≥ 3).
Fig 5.
Reduction of plasma-treated G. stearothermophilus spores at 1 kHz and 10 kVpp. △, stainless steel; ○, PTFE; ♢, PVC; □, glass; *, detection limit. The error bars indicate standard errors (n ≥ 3).
The D values of the bacterial endospores inactivated with SMD air plasma and their reference values with conventional sterilization methods are shown in Table 2. B. subtilis spores have a D value of only 0.3 min, while G. stearothermophilus spores have a D value of 0.9 min, which is still markedly lower than the minimum standard D value for reference H2O2 sterilization provided by the certified company Simicon GmbH. Table 2 also illustrates the plasma treatment time that is necessary to inactivate the initial inoculated spore load by 6 log10 and by a theoretical 12 log10.
Table 2.
D values and sterility assurance levels of bacterial endospores (CAP treatment and reference methods)
| Bioindicator | Strain | D23°Cvalue (min) | SAL (min) for spore reduction by: |
Reference method | D valuea (min) | |
|---|---|---|---|---|---|---|
| 6 log10 | 12 log10 | |||||
| G. stearothermophilus | ATCC 7953 | 0.9 | 5.7 | 11.4 | H2O2 | 4.2b |
| B. pumilus | ATCC 27142 | 0.5 | 3.2 | 6.5 | Gamma irradiation | – |
| B. atrophaeus | ATCC 9372 | 0.6 | 3.4 | 6.8 | Ethylene oxide | 3.0c,e |
| B. subtilis | DSM 13019 | 0.3 | 1.7 | 3.3 | Dry heat | 2.8d,e |
All D values for reference sterilization methods were provided by Simicon GmbH; −, data is not available.
D60°C of H2O2 (6.0 mg/liter; saturated steam; 60°C).
D54°C of ethylene oxide (600 mg/liter; 54°C).
D160°C of dry heat (160°C).
Devices were used according to the standard regulation DIN EN ISO 18472:2006-10 (3).
DISCUSSION
The antimicrobial effects of SMD air plasma on various vegetative microorganisms and bacterial endospores were demonstrated. Thirty seconds of SMD air plasma was very effective against different types of vegetative cells and led to a reduction of 4 log10 to 6 log10 CFU. The only eukaryotic cell, C. albicans, was the hardest to inactivate. Research in the area of resistance mechanisms against biocides has focused on bacteria and to a lesser extent on fungi (41, 42). In contrast to bacteria, the fungal cell wall consists of chitin/cellulose fibrils within a polysaccharide matrix. C. albicans can take distinct shapes depending on the environment and growth phase, e.g., at low pH, its cell wall is thicker than at neutral pH. It is known that its filamentous form contains more chitin in the cell wall than its yeast form (blastoconidia) and that C. albicans produces chlamydospores, which are dormant forms possessing a thicker cell wall than the vegetative cell. These properties can contribute to higher biocide resistance but were not measured in the overnight cultures used in the experiments in this study. In addition, C. albicans has a nucleus containing a diploid genome with many noncoding regions (introns) and sheltered by the nuclear membrane as an additional diffusion barrier, which contribute to higher resistance to DNA damage (37). Transcription processes take place in the nucleus. The presence of specialized cell organelles, like mitochondria and unique ribosomes, may enable C. albicans to be less sensitive to biocides (37). In addition to planktonic cell inactivation, encouraging results of successfully inactivated biofilms of C. albicans have been obtained recently with a similar SMD device (35). In this study, no clear difference in inactivation between Gram-negative (e.g., E. coli) and Gram-positive (e.g., Clostridium difficile) bacteria was observed. The data here suggest that there is no selectivity by or protection from CAP based on the bacterial cell wall structure. The wide spread of the reduction levels among all vegetative strains is remarkable but cannot be clearly ascribed to specific characteristics of a microorganism like properties that result in higher persistence against chemical (antibiotic agents, etc.) or physical (desiccation, heat, or irradiation) environmental stress. The effect of SMD air plasma is not influenced by mechanisms of microbial resistance to antibiotics (innate or acquired). This is reasonable, since SMD air plasma consists of a mixture of various reactive species (UV photons, electric field, neutral reactive species, etc.) that contribute to the plasma inactivation process of microorganisms. At atmospheric pressure, the most harmful UV components, such as vacuum UV or UVC (λ < 280 nm), which could cause intrinsic photodesorption or DNA damage, are missing or are generated to a only small extent, respectively. The UV light of SMD air plasmas is mainly emitted from N2 molecules with power densities below 50 μW/cm2, which is not expected to directly affect inactivation (27). UVB and UVA can still play a role by influencing the plasma chemistry. Plasma discharges create charged particles and an electrical field. It is proposed that electrical forces affect the cell membrane, which might cause electrostatic disruption or at least permeabilization for a very short time (29). As a consequence, plasma-derived ROS/RNS molecules, like reactive free radicals (NO, OH, and superoxide) or strong oxidizing agents (H2O2 and O3), might penetrate into the microorganism. Further chemical reactions can take place inside the cytoplasm. Then, all agents oxidize cellular proteins or microbial DNA. ROS/RNS can violate the integrity of the microbial cell structure by lipid peroxidation, resulting in membrane damage (6). This has been suggested for plasma-derived ROS/RNS, as well (40). Pompl et al. pointed out that the bactericidal effect of CAP is evident before morphological changes indicate cell wall disruption of S. aureus (40). This suggests that a more complex inactivation mechanism exists in which different plasma species may create a synergistic effect by alternating in-cell processes and, thus, are responsible for disinfection.
Bacterial endospores possess robust physical barriers and no metabolic activity that could be influenced by chemically aggressive species. Endospores are highly resistant to environmental stress, including heat, UV, gamma irradiation, desiccation, mechanical disruption, and toxic chemicals, such as strong oxidizers or pH-changing agents (44, 45). Their remarkable resistance has made them useful bioindicators for the validation of potential disinfecting and sterilizing agents. The results show that SMD air plasma inactivated spores rapidly. Bacillus spp. were reduced in 3 min by 5 log10. Their D values stayed below the standard reference D values, and 5.3 min or 10.6 min was sufficient to reduce the microbial load by 6 log10 or 12 log10, respectively (Table 2). Interestingly, it took only two times longer to reduce B. pumilus (ATCC 27142) spores by 6 log10 than to reduce its vegetative form. Spores of G. stearothermophilus were more resistant to SMD air plasma than spores of Bacillus spp. G. stearothermophilus spores are commonly used as standard bioindicators for H2O2 sterilization. The D value, with a D23°C of 0.9 min calculated for the SMD plasma reduction of G. stearothermophilus, is more than 4 times lower than the standard D60°C of 4.2 min for H2O2 sterilization. The influences of different nonmetal test materials on the inactivation of G. stearothermophilus were evaluated in this study, as well. The same inactivation rate was observed for all tested materials. Mahfoudh et al. found that polymeric materials treated with O3, a major component of SMD air plasma, can exhibit indirect sporicidal action against spores of B. atrophaeus (ATCC 9372) (32, 33). However, they applied very high doses of O3 (approximately 4,000 ppm), whereas in this study, O3 levels were 8 times lower at approximately 500 ppm. They tested with very long treatment times (a few hours) and emphasized that the sporicidal effect of O3-treated chemically stable polymers like PTFE was absent. The same observation that the specimen material does not play a significant role in inactivation was made in this study. In the case of direct exposure of spores, Eto et al. suggested that ozone and other neutral reactive species in humidified air have an important effect on the sporicidal activity of SMD air plasma (14). Yardimci and Setlow investigated the sporicidal effect of UV in plasmas. They found that plasma has a killing effect even in the absence of UV (49). This correlates with the data from our experimental SMD plasma setup, since wrapping with Tyvek blocks the UV photons from the plasma discharge. Functional damage of microbial macromolecules, including enzymes and membranes, can be caused by reactive plasma species that diffuse into the spore, where they cause inactivation that hinders spore germination. Cortezzo et al. suggested that the treatment of B. subtilis spores with oxidizing agents leads to damage of spore proteins, specifically proteins of the inner membrane, whose integrity is essential for spore viability (8). ROS in atmospheric SMD air plasma might have a similar effect. However, there is still a lack of knowledge about the exact inactivation mechanism of CAP in killing spores, since highly diverse plasma species are hard to measure appropriately at this time. The synergy effects of various SMD air plasma species for the inactivation of vegetative planktonic cells, as well as for environmentally more robust bacterial endospores, were considered. The development of tools that can identify and quantify relevant plasma species is required, in combination with biochemical studies, for the investigation of the inactivation mechanism for microorganisms evoked by CAP. Therefore, physiological studies that combine different methods for the provision of multimodal information, e.g., on a single bacterial spore in situ, are supportive (22), as described recently for wet-heat inactivation dynamics (50). CAP studies of mutants that lack specific proteins that confer resistance are especially necessary for better understanding.
It can be concluded that SMD plasma devices that operate in ambient air are able to efficiently inactivate bacterial and yeast pathogens, which often cause HAIs, as well as bacterial endospores on dry inanimate surfaces. In the case of spores, inactivation was achieved more rapidly than with standard sterilization methods. This is one important reason why SMD plasma could contribute to improve hygienic care in hospitals by efficient and safe surface disinfection, sterilization of sensitive medical devices, and disinfection of hands in the future. The specific characteristics of CAP make this innovative technology superior to conventional methods. Prospective clinical studies with larger devices will give better information on the suitability of the SMD plasma technology in hygienic practice.
ACKNOWLEDGMENTS
We thank Toni Seis from Simicon GmbH (Munich, Germany) for microbiological assistance with spore experiments. We thank Anindita Mitra for her microbiological expertise in discussions, Katinka Hartmann for the initial contact with Simicon GmbH, and Bernd Steffes for technical support.
This work was conducted within the Ph.D. Program Medical Life Science and Technology (Graduate School of the Technische Universität München, Munich, Germany) and was carried out in the framework of the Plasma Health Care Project, a collaboration initiated by the Max Planck Institute for Extraterrestrial Physics. The experimental work was performed at the Max Planck Institute for Extraterrestrial Physics and the Department of Microbiology, Hospital Munich Schwabing, Munich, Germany.
Footnotes
Published ahead of print 11 May 2012
REFERENCES
- 1. Angerer J, Bader M, Kramer A. 1998. Ambient and biochemical effect monitoring of workers exposed to ethylene oxide. Int. Arch. Occup. Environ. Health 71:14–18 [DOI] [PubMed] [Google Scholar]
- 2. Athanasiou KA, Niederauer GG, Agrawal CM. 1996. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 17:93–102. [DOI] [PubMed] [Google Scholar]
- 3.Beuth Verlag GmbH 2006. DIN EN ISO 18472:2006. Sterilization of health care products, biological and chemical indicators, test equipment. Beuth Verlag GmbH, Berlin, Germany [Google Scholar]
- 4.Beuth Verlag GmbH 2009. DIN EN ISO 11737-1:2006+AC:2009. Sterilization of medical devices, microbiological methods, part 1: determination of a population of microorganisms on products. Beuth Verlag GmbH, Berlin, Germany [Google Scholar]
- 5.Beuth Verlag GmbH 2010. DIN EN ISO 14937:2009. Sterilization of health care products. General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices. Beuth Verlag GmbH, Berlin, Germany [Google Scholar]
- 6. Bielski BHJ, Arudi RL, Sutherland MW. 1983. A study of the reactivity of perihydroxy radical/superoxide ion with unsaturated fatty acids. J. Biol. Chem. 258:4759–4761 [PubMed] [Google Scholar]
- 7.CDC 2007. Public health report. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 8. Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J. Appl. Microbiol. 97:838–852 [DOI] [PubMed] [Google Scholar]
- 9. Cosgrove SE, Carmeli Y. 2003. The impact of antimicrobial resistance on health and economic outcome. Clin. Infect. Dis. 36:1433–1437 [DOI] [PubMed] [Google Scholar]
- 10. Ehlbeck J, et al. 2011. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 44:013002 [Google Scholar]
- 11.Environmental Protection Agency 1997. Health effects assessment summary tables (HEAST). U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 12. Ermolaeva SA, et al. 2011. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 60:75–83 [DOI] [PubMed] [Google Scholar]
- 13. Eto H, Ono Y, Ogino A, Nagatsu M. 2008. Low-temperature internal sterilization of medical plastic tubes using a linear dielectric barrier discharge. Plasma Process Polym. 5:269–274 [Google Scholar]
- 14. Eto H, Ono Y, Ogino A, Nagatsu M. 2008. Low-temperature sterilization of wrapped materials using flexible sheet-type dielectric barrier discharge. Appl. Phys. Lett. 93:221502 [Google Scholar]
- 15.European Centre for Disease Prevention and Control 2010. Annual epidemiological report on communicable diseases in Europe. European Centre for Disease Prevention and Control, Stockholm, Sweden: [PubMed] [Google Scholar]
- 16. Feldman LA, Hui HK. 1997. Compatibility of medical devices and materials with low-temperature hydrogen peroxide gas plasma. Med. Device Diagn. Ind. Mag. 12:57–62 [Google Scholar]
- 17. Heinlin J, et al. 2011. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 25:1–11 [DOI] [PubMed] [Google Scholar]
- 18. Holy CE, Cheng C, Davies JE, Shoichet MS. 2001. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 22:25–31 [DOI] [PubMed] [Google Scholar]
- 19. Isbary G, Morfill GE, Zimmermann J, Shimizu T, Stolz W. 2011. Cold atmospheric plasma: a successful treatment of lesions in Hailey-Hailey disease. Arch. Dermatol. 147:388–390 [DOI] [PubMed] [Google Scholar]
- 20. Isbary G, et al. 2010. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 163:78–82 [DOI] [PubMed] [Google Scholar]
- 21. Kelly-Wintenberg K, et al. 1998. Room temperature sterilization of surfaces and fabrics with a one atmosphere uniform glow discharge plasma. J. Ind. Microbiol. Biotechnol. 20:69–74 [DOI] [PubMed] [Google Scholar]
- 22. Kong L, Zhang P, Wang G, Yu J, Setlow P, Li YQ. 2011. Characterization of bacterial spore germination using phase-contrast and fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat. Protoc. 6:625–639 [DOI] [PubMed] [Google Scholar]
- 23. Kong MG, et al. 2009. Plasma medicine: an introductory review. New J. Phys. 11:115012 [Google Scholar]
- 24. Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laroussi M. 1996. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 24:1188–1191 [Google Scholar]
- 26. Laroussi M. 2005. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process. Polym. 2:391–400 [Google Scholar]
- 27. Laroussi M, Leipold F. 2004. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 233:81–86 [Google Scholar]
- 28. Leape LL, et al. 1991. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N. Engl. J. Med. 324:377–384 [DOI] [PubMed] [Google Scholar]
- 29. Leduc M, Guay D, Leask RL, Coulombe S. 2009. Cell permeabilization using a non-thermal plasma. New J. Phys. 11:115021 [Google Scholar]
- 30. Lerouge S, Tabrizian M, Wertheimer MR, Marchand R, Yahia L. 2002. Safety of plasma-based sterilization: surface modifications of polymeric medical devices induced by Sterrad and Plazlyte processes. Biomed. Mater. Eng. 12:3–13 [PubMed] [Google Scholar]
- 31. Li Y-F, Shimizu T, Zimmermann JL, Morfill GE. 2011. Cold atmospheric plasma for surface disinfection. Plasma Process. Polym. doi:10.1002/ppap.201100090.
- 32. Mahfoudh A, Poncin-Épaillard F, Moisan M, Barbeau J. 2010. Effect of dry-ozone exposure on different polymer surfaces and their resulting biocidal action on sporulated bacteria. Surf. Sci. 604:1487–1493 [Google Scholar]
- 33. Mahfoudh A, Barbeau J, Moisan M, Leduc A, Segúin J. 2009. Biocidal action of ozone-treated polystyrene surfaces on vegetative and sporulated bacteria. Appl. Surf. Sci. 256:3063–3072 [Google Scholar]
- 34. Maisch T, et al. 2012. Decolonisation of MRSA, S. aureus and E. coli by atmospheric plasma using a porcine skin model in vitro. PLoS One 7:e34610 doi:10.1371/journal.pone.0034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maisch T, et al. 2012. Contact-free inactivation of Candida albicans biofilm by cold-atmospheric air plasma. J. Appl. Environ. Microbiol. doi:10.1128/AEM.07235-11. [DOI] [PMC free article] [PubMed]
- 36. Matthews IP, Gibson C, Samuel AH. 1994. Sterilisation of implantable devices. Clin. Mater. 15:191–215 [DOI] [PubMed] [Google Scholar]
- 37. McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morfill GE, Shimizu T, Steffes B, Schmidt H-U. 2009. Nosocomial infections: a new approach towards preventive medicine using plasmas. New J. Phys. 11:115019 [Google Scholar]
- 39. Pollack A. 27 February 2010. Rising threat of infections unfazed by antibiotics, p B1. New York Times. http://www.nytimes.com/2010/02/27/business/27germ.html.
- 40. Pompl R, et al. 2009. The effect of low-temperature plasma on bacteria as observed by repeated AFM imaging. New J. Phys. 11:115023 [Google Scholar]
- 41. Russell AD, Furr JR, Maillard J-Y. 1997. Microbial susceptibility and resistance to biocides. ASM News 63:481–487 [Google Scholar]
- 42. Russell AD, et al. 2004. Russell, Hugo and Ayliffe's principles and practice of disinfection, preservation and sterilization. Blackwell, Oxford, United Kingdom [Google Scholar]
- 43. Rutala WA. 1998. Principles of disinfecting patient-care items, p 133–149. In Rutala WA. (ed), Disinfection, sterilization and antiseptics in health care. Polyscience Publishers, New York, NY [Google Scholar]
- 44. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 45. Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol. 15:172–180 [DOI] [PubMed] [Google Scholar]
- 46. Shimizu T, Zimmermann JL, Morfill GE. 2011. The bactericidal effect of surface micro-discharge plasma under different ambient conditions. New J. Phys. 13:023026 [Google Scholar]
- 47. Shimizu T, et al. 2010. Characterization of low-temperature microwave plasma treatment with and without UV light for disinfection. Plasma Process Polym. 7:288–293 [Google Scholar]
- 48. Stoffels E, Sakiyama Y, Graves DB. 2008. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 36:1441–1457 [Google Scholar]
- 49. Yardimci O, Setlow P. 2010. Plasma sterilization: opportunities and microbial assessment strategies in medical device manufacturing. IEEE Trans. Plasma Sci. 38:973–981 [Google Scholar]
- 50. Zhang P, Kong L, Wang G, Setlow P, Li Y-Q. 2011. Monitoring the wet-heat inactivation dynamics of single spores of Bacillus species by using Raman tweezers, differential interference contrast microscopy, and nucleic acid dye fluorescence microscopy. J. Appl. Environ. Microbiol. 77:4754–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]