Abstract
The A-to-V mutation at position 111 (A111V) in the mechanosensitive channel NCgl1221 (MscCG) causes constitutive glutamate secretion in Corynebacterium glutamicum. Patch clamp experiments revealed that NCgl1221 (A111V) had a significantly smaller gating threshold than the wild-type counterpart and displayed strong hysteresis, suggesting that the gain-of-function mutation in the gating of NCgl1221 leads to the oversecretion of glutamate.
TEXT
Corynebacterium glutamicum is used worldwide for the industrial fermentative production of glutamate. Under biotin-limiting conditions, this organism secretes a large amount of glutamate (11). Reagents that result in a change in the cell surface structure, such as fatty acid ester surfactants and penicillin, induce glutamate secretion even in the presence of biotin (5). Recent studies have revealed that NCgl1221 (MscCG), a homolog of the mechanosensitive channel of small conductance (MscS) of Escherichia coli, functions as a glutamate exporter. Mutations in NCgl1221 lead to constitutive glutamate secretion, and disruption of the gene abolishes its secretion (8). NCgl1221 is localized at the plasma membrane (14) and has mechanosensitive channel activity when expressed in E. coli spheroplasts (2) and Bacillus subtilis provacuoles (4). These findings suggest that the mechanosensitive gating of NCgl1221 caused by membrane distortion triggers glutamate secretion.
In this study, to elucidate the molecular basis of the relationship between NCgl1221 gating and glutamate secretion, we investigated electrophysiological properties of the NCgl1221 (A111V) mutant protein, having an A-to-V mutation at position 111, which causes constitutive glutamate secretion (8). On the basis of amino acid sequence alignment between NCgl1221 and E. coli MscS, Ala111 in NCgl1221 was shown to be a residue corresponding to Ala106 or Ala110 in E. coli MscS. These alanine residues of MscS reside in the third transmembrane domain (TM3; residues 95 to 126), which forms an ion-conducting pore (1), and the mutations alter the gating threshold (3). Thus, we hypothesized that the A111V mutation in NCgl1221 alters the gating property of the channel.
The difference in gating between wild-type NCgl1221 and NCgl1221 (A111V) was examined by patch-clamping E. coli spheroplasts expressing these channels. The NCgl1221 gene was amplified by PCR from pVK9-NCgl1221 (8) and cloned into the expression vector pB10b (9) with the In-Fusion cloning kit (TaKaRa). The A111V mutation was created with a mutagenesis kit (Toyobo). E. coli spheroplasts were prepared as described previously (7). Strains PB113 (ΔmscS ΔmscK) (6) and MJF612 (ΔmscM ΔmscS ΔmscK ΔmscL) (10) harboring pB10b, pB10b-NCgl1221, or pB10b-NCgl1221 (A111V) were incubated to the exponential growth phase. NCgl1221 expression was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 30 min. Single-channel currents were recorded with inside-out excised patches. The pipette solution contained 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES-KOH (pH 7.2). The bath solution consisted of the pipette solution supplemented with 300 mM sucrose to stabilize the spheroplasts. Currents were amplified using an Axopatch 200B amplifier (Molecular Devices, California) and filtered at 2 kHz. Current recordings were digitized at 5 kHz using a Digidata 1322A interface with pCLAMP9 software (Molecular Devices). Negative pressure was applied to the patch membrane as mechanical stimulation using a high-speed pressure clamp (HSPC) apparatus (ALA Scientific Instruments).
When PB113 was transfected with an empty vector and an increasingly negative pressure was applied to the patch membrane by suction at constant ramp rate (Fig. 1A), endogenous E. coli MscL with ∼80 pA single-channel currents were elicited at approximately 200 mmHg (Fig. 1B). When transfected with a vector harboring wild-type NCgl1221, on the other hand, NCgl1221 started to open at approximately 60 mmHg (Fig. 1C, OPCG), and endogenous E. coli MscL was subsequently elicited at approximately 170 mmHg (OPL). The magnitude of single-channel currents of NCgl1221 was 5 pA. NCgl1221 was distinguished from MscL by the smaller single-channel current and lower activation threshold. When the negative pressure was reduced at the same ramp rate, E. coli MscL and wild-type NCgl1221 closed at pressures (CPL and CPCG) similar to those for the opening. In contrast, NCgl1221 (A111V) opened at a negative pressure lower than that for the wild type (Fig. 1C): NCgl1221 (A111V) opened at approximately 20 mmHg (OPCG) and E. coli MscL was subsequently elicited at approximately 130 mmHg (OPL). Interestingly, NCgl1221 (A111V) closed barely a few seconds after the complete release of the negative pressure (CPCG), while E. coli MscL closed at the same pressure (CPL) as OPL (Fig. 1D).
Fig 1.
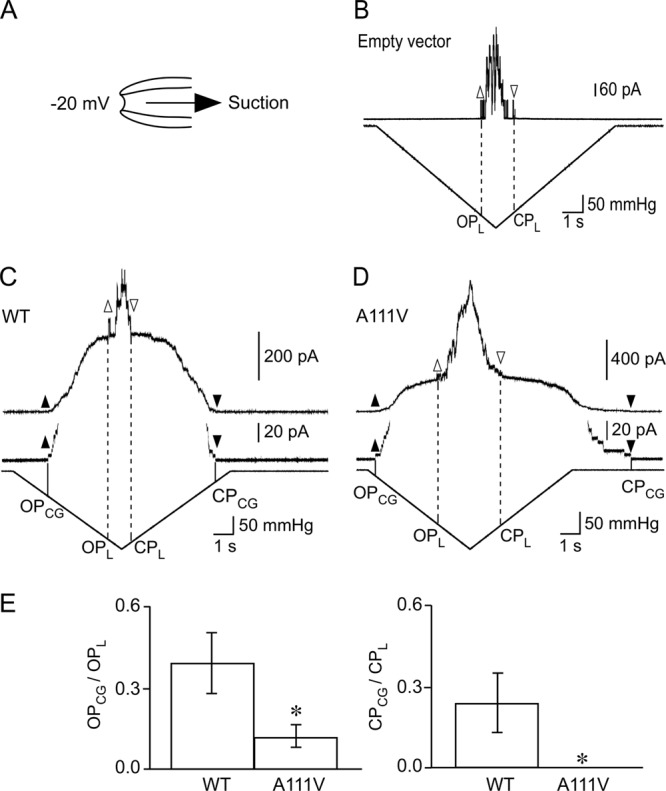
Electrophysiological analysis on gating of wild-type NCgl1221 and NCgl1221 (A111V). (A) Configuration of mechanosensitive current recording in E. coli spheroplasts. Negative pressure to the patch membrane was applied by suction with HSPC. All experiments were performed at holding potential of −20 mV. (B) Mechanosensitive currents in strain PB113 (mscS mscK) harboring an empty vector. Upper traces and lower traces show the current and the negative pressure applied to the patch membrane through the pipette, respectively. Mechanosensitive current recording in PB113 expressing wild-type NCgl1221 (WT) (C) and NCgl1221 (A111V) (D). Middle traces show vertical enlargements of the upper traces. Upward and downward arrowheads represent the start and end of channel activity, respectively, of NCgl1221 (black) and E. coli MscL (white). (E) Quantitative analysis of the threshold for gating in wild-type NCgl1221 and NCgl1221 (A111V). The ratio (OPCG/OPL) of the pressure required for the opening of E. coli MscL (OPL) to NCgl1221 (OPCG) is shown in the left panel, and the ratio (CPCG/CPL) of the pressure required for the closing of E. coli MscL (CPL) to NCgl1221 (CPCG) is shown in the right panel. *, P < 0.01, compared to wild-type NCgl1221 (Student's t test). Error bars represent standard deviations of the data of wild-type NCgl1221 (n = 8) and NCgl1221 (A111V) (n = 5).
To evaluate the changes in the gating threshold of NCgl1221, the ratio of the pressure required for the gating of E. coli MscL (PL) to NCgl1221 (PCG) was calculated using PL as an internal standard. This ratio rather than the pressure was used because mechanosensitive channels are activated by membrane tension (T), which is defined by the radius (r) of membrane curvature and magnitude of pressure (P) according to Laplace's law (T = Pr/2) (12), but the radius varies slightly from patch to patch. The use of the ratio allows for compensating the effect of the radius because the radius is expected to be the same when examined on identical patches. OPCG/OPL for the opening of NCgl1221 (A111V) (0.12 ± 0.04, n = 5) was significantly smaller than that of wild-type NCgl1221 (0.39 ± 0.11, n = 8) (Fig. 1E), suggesting that the A111V mutation makes NCgl1221 easy to open. While CPCG/CPL for the closing of wild-type NCgl1221 (0.24 ± 0.11, n = 8) did not differ statistically from OPCG/OPL for the opening, NCgl1221 (A111V) did not close during the presence of negative pressure in all experiments. The difference in the thresholds for opening and closing shows that the gating of NCgl1221 (A111V) has strong hysteresis, which is not evident in wild-type NCgl1221.
To exclude possible influence of MscM and MscL, we examined NCgl1221 in strain MJF612 (ΔmscM ΔmscS ΔmscK ΔmscL), lacking all four mechanosensitive channels cloned so far (10). NCgl1221 (A111V) expressed in MJF612 also opened at low pressure and displayed strong hysteresis, whereas wild-type NCgl1221 did not (Fig. 2). This result suggests that the change of gating kinetics in NCgl1221 (A111V) is not influenced by endogenous E. coli mechanosensitive channels.
Fig 2.
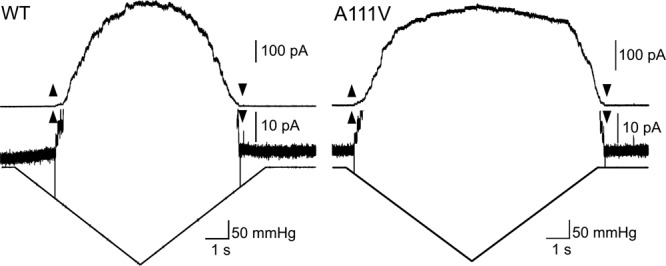
Mechanosensitive channel activity of wild-type NCgl1221 (left) and NCgl1221 (A111V) (right) in E. coli strain MJF612 (ΔmscM ΔmscS ΔmscK ΔmscL). The membrane current and its vertical enlargement and pressure are shown from the top to the bottom. Upward and downward arrowheads represent the start and end of the channel activity of NCgl1221, respectively. All experiments were performed at a holding potential of −20 mV.
E. coli MscS is a homoheptamer, each subunit of which has three transmembrane domains and a cytoplasmic domain (1). Replacement of conserved alanines in the pore region with glycine (A106G and A110G) decreases the gating threshold and results in a gain-of-function phenotype (3, 13). These alanines (knobs) are proposed to line up against the conserved glycines (holes) of an adjacent subunit and stabilize the closed state (3). Despite the steric importance, amino acid sequence alignment of TM3 between E. coli MscS and NCgl1221 shows that the conserved alanines and glycines present in E. coli MscS are poorly conserved in NCgl1221 (Fig. 3), suggesting that the low threshold of NCgl1221 may be due to the loose alignment of TM3.
Fig 3.
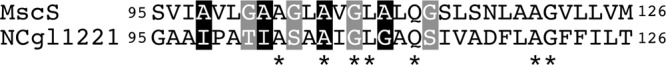
Amino acid sequence alignment of the transmembrane segment TM3 between E. coli MscS and NCgl1221. The positional regularities of conserved alanines (black) and glycines (gray) present in E. coli MscS are highlighted. Asterisks represent identical residues.
NCgl1221 is a mechanosensitive channel gated by membrane tension and functions not only for glutamate secretion but also for osmotic safety valves (2, 4). Thus, we propose that fluctuations in membrane tension caused by endogenous osmotic pressure activate NCgl1221 (A111V), which has a gating threshold significantly lower than that of wild-type NCgl1221. The strong hysteresis of the gating of NCgl1221 (A111V) possibly keeps this channel open, leading to constitutive glutamate secretion. Thus, the present study suggests that constitutive glutamate secretion by NCgl1221 (A111V) is tightly associated with the altered gating property of NCgl1221.
ACKNOWLEDGMENTS
We thank M. Wachi for general information, Ajinomoto Co., Inc., for the generous gift of pVK9-NCgl1221, and Ian Booth and Akiko Rasmussen for the gift of E. coli strain MJF612.
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas no. 21026009 (to H.I.) and no. 23120509 (to H.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Scientific Research B no. 21370017 (to H.I.) from the Japan Society for the Promotion of Science (JSPS), and Grant-in-Aid for JSPS Fellows no. 10J02008 (to Y.N.).
Footnotes
Published ahead of print 18 May 2012
REFERENCES
- 1. Bass RB, Strop P, Barclay M, Rees DC. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298:1582–1587 [DOI] [PubMed] [Google Scholar]
- 2. Börngen K, et al. 2010. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim. Biophys. Acta 1798:2141–2149 [DOI] [PubMed] [Google Scholar]
- 3. Edwards MD, et al. 2005. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat. Struct. Mol. Biol. 12:113–119 [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto K, et al. 2010. The protein encoded by NCgl1221 in Corynebacterium glutamicum functions as a mechanosensitive channel. Biosci. Biotechnol. Biochem. 74:2546–2549 [DOI] [PubMed] [Google Scholar]
- 5. Kim J, Hirasawa T, Saito M, Furusawa C, Shimizu H. 2011. Investigation of phosphorylation status of OdhI protein during penicillin- and Tween 40-triggered glutamate overproduction by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 91:143–151 [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21:5323–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinac B, Buechner M, Delcour AH, Adler J, Kung C. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 84:2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura J, Hirano S, Ito H, Wachi M. 2007. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl. Environ. Microbiol. 73:4491–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okada K, Moe PC, Blount P. 2002. Functional design of bacterial mechanosensitive channels. J. Biol. Chem. 277:27682–27688 [DOI] [PubMed] [Google Scholar]
- 10. Schumann U, et al. 2010. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc. Natl. Acad. Sci. U. S. A. 107:12664–12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiio I, Otsuka SI, Takahashi M. 1962. Effect of biotin on the bacterial formation of glutamic acid. I. Glutamate formation and cellular permeability of amino acids. J. Biochem. 51:56–62 [DOI] [PubMed] [Google Scholar]
- 12. Sukharev S. 2002. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang W, et al. 2008. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science 321:1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yao W, et al. 2009. Expression and localization of the Corynebacterium glutamicum NCgl1221 protein encoding an L-glutamic acid exporter. Microbiol. Res. 164:680–687 [DOI] [PubMed] [Google Scholar]


