Abstract
Electron-accepting (electrotrophic) biocathodes were produced by first enriching graphite fiber brush electrodes as the anodes in sediment-type microbial fuel cells (sMFCs) using two different marine sediments and then electrically inverting the anodes to function as cathodes in two-chamber bioelectrochemical systems (BESs). Electron consumption occurred at set potentials of −439 mV and −539 mV (versus the potential of a standard hydrogen electrode) but not at −339 mV in minimal media lacking organic sources of energy. Results at these different potentials were consistent with separate linear sweep voltammetry (LSV) scans that indicated enhanced activity (current consumption) below only ca. −400 mV. MFC bioanodes not originally acclimated at a set potential produced electron-accepting (electrotrophic) biocathodes, but bioanodes operated at a set potential (+11 mV) did not. CO2 was removed from cathode headspace, indicating that the electrotrophic biocathodes were autotrophic. Hydrogen gas generation, followed by loss of hydrogen gas and methane production in one sample, suggested hydrogenotrophic methanogenesis. There was abundant microbial growth in the biocathode chamber, as evidenced by an increase in turbidity and the presence of microorganisms on the cathode surface. Clone library analysis of 16S rRNA genes indicated prominent sequences most similar to those of Eubacterium limosum (Butyribacterium methylotrophicum), Desulfovibrio sp. A2, Rhodococcus opacus, and Gemmata obscuriglobus. Transfer of the suspension to sterile cathodes made of graphite plates, carbon rods, or carbon brushes in new BESs resulted in enhanced current after 4 days, demonstrating growth by these microbial communities on a variety of cathode substrates. This report provides a simple and effective method for enriching autotrophic electrotrophs by the use of sMFCs without the need for set potentials, followed by the use of potentials more negative than −400 mV.
INTRODUCTION
Microbial electrosynthesis of fuels can be used to store intermittently produced renewable energy, such as wind and solar energy, as stable chemical bonds (19, 39). In this bioelectrochemical process, certain microorganisms, called electrotrophs, accept electrons directly from an electrode (36). The inorganic and organic products released are referred to as electrofuels if they can be used as fuels in engines or fuel cells. Microbial electrolysis cells (MECs) have been used to produce gaseous electrofuels such as hydrogen and methane (9, 32, 38). Other bioelectrochemical systems (BESs) have produced ethanol and butanol (28, 34). There is a growing interest in using such technologies to produce longer-chain hydrocarbon fuels and other higher-value products (23, 28, 30).
In many BESs, such as MECs, certain types of bacteria on the anode (called exoelectrogens) oxidize organic material to CO2 and protons and release electrons to the anode (3, 40). In MECs, the cathode reduction reaction is not spontaneous and an applied voltage more negative than −140 mV is needed to produce an appreciable volume of hydrogen gas (5, 40). In practice, −400 to −1,000 mV is usually applied to the circuit to overcome energy losses due to electrode overpotentials and the use of materials that increase internal resistance, such as ion exchange membranes and separators (18). Platinum is commonly used to catalyze hydrogen evolution, as it greatly reduces the cathode overpotential. However, platinum is expensive, nonrenewable, and ineffective for catalyzing CO2 reduction, and it is susceptible to poisoning by sulfur and carbon monoxide (6). Alternatives to platinum as a catalyst include several inorganic materials (stainless steel, nickel, and MoS2) or microorganisms (4, 7, 15, 21).
Biocathodes are of particular interest for producing organic electrofuels, since they do not require the use of metals and they are self-renewable. However, it is more difficult to obtain electron-accepting (electrotrophic) biofilms on cathodes under anoxic conditions than it is to produce exoelectrogenic biofilms on anodes (25). Electrical inversion of bioanodes to produce hydrogen-evolving MEC biocathodes in aqueous solution was first demonstrated by Rozendal et al. (32). They developed a hydrogenotrophic biofilm on the anode, using a chemical catholyte (ferricyanide), and then switched the electrode to change it to a hydrogen-producing biocathode using a chemical anolyte (ferrocyanide). This approach was effective in achieving biocathodic hydrogen production, but it requires the use of chemicals containing cyanide. Other methods to produce anoxic biocathodes have used complex, multistep acclimation processes and reactors that do not acclimate under spontaneous conditions (as they do in a microbial fuel cell [MFC]). These approaches have included periodic addition of hydrogen or organic substrates (19, 32, 39) and using a potentiostat or chemical solution and then switching the electrode from exoelectrogenic mode to electrotrophic production of hydrogen gas (16, 27). The biofilms that develop on anaerobic biocathodes under these conditions tend to be patchy compared to anodic biofilms. For example, anode biofilms of Geobacter sulfurreducens are relatively thick compared to single-cell-thick biofilms formed on cathodes in the absence of an organic source of carbon (26). Patchy, monolayer biofilms have also been reported for electrotrophic biofilms predominated by Anaeromyxobacter dehalogenans (35).
Biofilms that develop on electrodes in MFCs under oxic cathode conditions have been shown to sustain current generation when the functions of the electrodes are switched (the cathode, for example, becomes the anode) (7, 8, 35). This indicates that both exoelectrogenic and electrotrophic microorganisms can be maintained in the electrode biofilms when the cathode is switched from oxic to anoxic conditions. While this approach was successful for producing biocathodes for oxygen reduction (aerobic biocathodes), it was not clear if bioanodes would necessarily contain electrotrophic microorganisms that can function under anoxic conditions. Previous methods that achieved biocathodes that functioned under anoxic conditions generally used strictly anoxic conditions, suggesting that some types of MFCs might not be useful for creating anoxic biocathodes. Oxygen that diffuses into single-chamber, air-cathode MFCs through the cathode can adversely affect the growth of obligate anaerobes (2, 37). However, oxygen can also leak in through gaskets and fittings used for MFCs and MECs (43) and even small amounts of dissolved oxygen can prevent the growth of some obligate anaerobes. It was therefore reasoned that optimal growth of an electroactive biofilm for an anoxic biocathode would require maintenance of strictly anoxic conditions.
We tested the hypothesis that MFC anodes could be used to enrich diverse, electroactive biofilms that could subsequently be used to produce electrotroph-enriched biofilms by electrically inverting anodes to biocathodes by the use of sediment-type MFCs (sMFCs). In all previous biocathode studies, the electrodes were acclimated to a cell suspension rather than to the rich sediments as examined here. The use of an sMFC in which the anode is buried in an anoxic sediment provides consistent anaerobic culture conditions compared to electrodes suspended in fluid that could be exposed to dissolved oxygen during fluid replacement or to oxygen leaking into the reactor through gaskets and other seals. This approach based on an sMFC is notably different from previous approaches based on set potentials or the use of chemicals containing cyanide. We show here that this simple electrode inversion method based on acclimation in an sMFC produces biofilms capable of CO2 fixation and hydrogen or methane generation. This technique has additional potential to be used to produce and screen biofilms capable of electrofuel production.
MATERIALS AND METHODS
Establishment of sMFCs.
Chesapeake Bay sediment was collected from a near-shore sampling site off Sandy Point State Park (Annapolis, MD) and from a steel piling in the Baltimore Inner Harbor (Baltimore, MD) on 1 March 2011 and transported in a cooler to the laboratory. Two sediment-type microbial fuel cells (sMFCs) consisting of glass test tubes (1.8 cm wide by 15.0 cm deep) were filled to a depth of 5 cm with sediment from each site. Heat-treated graphite fiber brush anodes (Panex 33 160 K; Zoltek) (25 mm in diameter by 25 mm in length; 0.22-m2 surface area) were inserted 1 cm below the sediment surface. The anode was connected by an insulated titanium wire containing a resistor (1,000 Ω) to an upper air cathode positioned near the air-water interface at the upper opening of the test tube (Fig. 1). The air cathode was carbon cloth (7-cm2 projected area) with 30% polytetrafluoroethylene (PTFE) wet proofing (E-TEK) (type B) coated with a Pt catalyst layer (0.5 mg of Pt/cm2) on the side facing the anode. Each sMFC contained an Ag/AgCl reference electrode (RE-5B; BASi).
Fig 1.
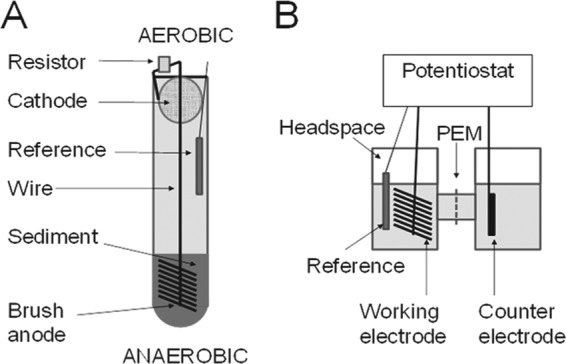
Two-step method used to first enrich (A) a diverse, electrically active sediment biofilm on an sMFC brush anode that was then transplanted to form (B) the working electrode of a dual-chamber, potentiostat-controlled BES, or microbial half-cell.
The sMFC startup medium (pH = 7.0, sterilized) contained a 50 mM phosphate-buffered saline (PBS) buffer (4.58 g/liter Na2HPO4, 2.45 g/liter NaH2PO4 · H2O, 0.31 g/liter NH4Cl, 0.13 g/liter KCl, and 10.0 g/liter NaCl) amended with 1 g/liter sodium acetate as a supplemental energy source and trace vitamins and minerals (9). Two reactors contained anodes set at a potential of EAn = 11 mV (versus the reference standard hydrogen electrode [SHE]) using a potentiostat (Uniscan model PG580; Buxton, United Kingdom) and an Ag/AgCl reference electrode (+211 mV versus SHE), while the other two reactors were operated without a set potential. The glass test tubes were covered with foil to prevent growth of oxygenic phototrophs and incubated for 3 months in a constant-temperature room (30°C). Deionized (DI) water was periodically added to replace water lost to evaporation. After 1-month intervals, the sMFC startup medium was replaced with fresh sMFC startup medium. Unless otherwise noted, electrode potentials are expressed versus the reference SHE.
Inversion into biocathodes.
After establishment of electroactive biofilms on the anodes, the brush electrodes were removed from sediments and gently rinsed with N2-degassed DI water in an anaerobic chamber to minimize carryover of residual material. Brush electrodes were then installed as the working electrodes (WE) in dual chamber, glass-bottle, two-chamber H-type BESs (Glasstron Inc., Vineland, NJ) containing 120 ml of N2-degassed PBS minimal medium per chamber. Each MEC chamber was 190 ml (working volume of 120 ml), and the chambers were connected by two sidearm joints 2.5 cm in diameter containing a cation exchange membrane (Nafion 117). The counterelectrode (anode) was a carbon rod (surface area, 13.8 cm2) (McMaster Carr, Cleveland, OH). An autoclave-sterilized BES was used as a negative control. Ethanol-sterilized Ag/AgCl reference electrodes were inserted into the WE chambers, and the BESs were degassed with N2/CO2 at a ratio of 80%/20% (vol/vol) for 15 min to remove oxygen and provide CO2 for electroautotrophic growth. The BESs were connected to a potentiostat (model MPG2; Biologic Instrument) for electrochemical examination.
Voltammetry and electrotrophic culture conditions.
Linear sweep voltammetry (LSV) was conducted to determine if electrodes could accept electrons. Electron uptake, displayed as a negative current, was measured at a scan rate of 1 mV/s, from 500 mV to −700 mV. Long-term patterns of electron uptake and electrotrophic growth were examined at weeklong intervals using set cathode potentials of −339 (days 0 to 7), −439 (days 7 to 14), and −539 mV (days 14 to 21). On days 7, 14, and 21, samples were obtained and the BESs were flushed with fresh N2/CO2 to remove all gases produced during the previous week (such as oxygen that might have accumulated from water splitting in the anode chamber). The electrical charge accepted for each biocathode was calculated from the measured current and converted into units of coulombs (C).
Chemical analysis.
Samples of the initial and final gases in the cathode headspace (200 μl; determinations performed in duplicate) were analyzed two times per week using an airtight syringe (Hamilton Samplelock syringe) (250 μl). H2, N2, and CH4 were analyzed using a gas chromatograph (GC) (model 2601B; SRI Instruments) equipped with a 3-m Molsieve 5A 80/100 column (Altech Associates, Inc., Bannockburn, IL) with argon as the carrier gas at 80°C. CO2 was measured on a second GC (model 310; SRI Instruments) using a 1-m silica gel column (Resteck Corporation, Bellefonte, PA) with helium as the carrier gas at 60°C. Both GCs used thermal conductivity detectors (TCDs) and had a detection limit of 0.01%. Prominent peaks suggestive of metabolites excreted into solution were assayed for using a Shiamadzu ultra-high-performance liquid chromatograph (HPLC; Restek Corporation, Bellefonte, PA) (250 mm by 4.6 mm; Allure organic acids; 5-μm column) using a phosphate buffer eluent (6.8 g/liter KH2PO4, pH 2.3) and a photodiode array detector. For the analysis, 1 ml of functional cathode suspension was recovered from the functional Bay or Harbor cathode chambers using a sterile syringe and filtered through a 0.22-μm-pore-size filter prior to analysis. The HPLC mobile-phase pH was adjusted with 50 mM KH2PO4, using H3PO4.
Microbial community analysis.
Sample clippings from functional brush biocathodes were collected using sterilized scissors in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). Gram-stained slides of sample smears were prepared and photographed using an Olympus BX-61 microscope equipped with a DP-72 digital camera. Total DNA was extracted from 0.2 g of the functional biocathode clippings by the use of a Power Soil DNA extraction kit (MO BIO Laboratories Inc., Carlsbad, CA). Total DNA was quantified using a spectrophotometer (model 2000C Nanodrop spectrophotometer; Thermo Scientific, Waltham, MA), and 16S rRNA genes were amplified using 16S primers inclusive for eubacteria and archaea (25). Clone libraries were generated using a TOPO TA cloning kit (Invitrogen Corp., Grand Island, NY). After heat shock transformation into DH5 Escherichia coli-competent cells and incubation at 37°C on an LB agar plate containing 50 μg/ml ampicillin plus X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 100 β-galactosidase-screened white colonies were subcultured overnight in 1.2 ml of LB broth per colony prior to plasmid Miniprep processing using an E-Z Fastfilter plasmid kit (Omega Bio-Tek, Inc., Norcross, GA). Sequencing of the 16S DNA clone libraries was carried out at the Pennsylvania State University Huck Institute of the Life Sciences Genomics Core facility using M13r primers. The nucleotide collection of the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi/) was searched using the BLASTn algorithm for sequence identification.
Bacterial colonies were isolated from electron-accepting brush cathodes by transferring carbon fibers onto minimal-medium agar plates and streaking for isolation using a platinum loop. Streaked plates were incubated in darkness for 2 weeks in an anaerobic chamber (98% N2/2% H2). To facilitate growth of autotrophs, a duplicate set of streak plates was incubated in an anaerobic jar containing an atmosphere of N2/CO2 (80%/20%). Pressurized H2 was supplied through a one-way valve as a source of reducing equivalents. After 2 weeks, plates were examined and colonies subcultured to fresh minimal-medium agar or tryptic soy agar (TSA) plates.
Sterile transfer of electrotrophic activity.
The viability of the enriched electrotrophic bacteria was determined through transfer of the cathode suspension (24 ml) into three additional sterilized BESs. Each BES reactor contained a different type of working electrode: carbon fiber brush (as described above), carbon rod (surface area, 13.8 cm2), or graphite plate (surface area, 18 cm2) (McMaster Carr, Cleveland, OH). The newly inoculated BESs were then operated at a set potential of −539 mV for 14 days.
RESULTS
Both the Chesapeake Bay sediment and Baltimore Inner Harbor sediment samples produced electrical current during operation as sMFCs, indicating successful initial enrichment of exoelectrogenic biofilms (see Fig. S1 in the supplemental material). The sMFC anodes were then placed into two-chamber BESs and analyzed using LSV. The Inner Harbor brush electrode acclimated without a set potential (Harbor N) in the sMFC was the only electrode to show a large negative current at potentials more negative than ∼400 mV (Fig. 2). There was no appreciable change in current for the sterile control, and there were only small changes in current for the Bay sample electrodes and the Harbor electrode originally operated at a set potential (Harbor SP) of 11 mV.
Fig 2.
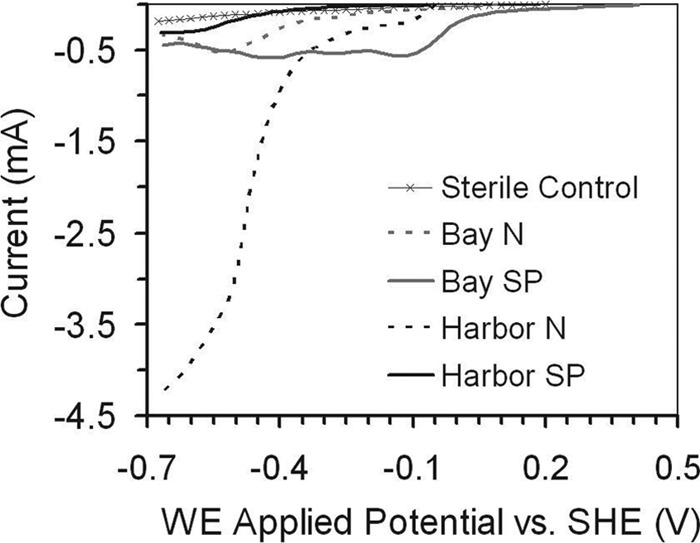
Capacity of sMFC-enriched electrodes to accept electrons at negative applied WE potentials as determined by linear sweep voltammetry (LSV).
The electroactive brush anodes were then electrically inverted to operate as cathodes at different set potentials in BESs. No appreciable current was measured for any of the electrodes after 1 week at a set potential of −339 mV (days 0 to 7). When the potential was set to −439 mV for days 7 to 14, there was sustained current in the Harbor N sample (Fig. 3A). The improved performance of this electrode was consistent with results based on LSV, which showed activity at potentials more negative than −400 mV. Between days 7 and 14, there was an increase in current from −0.15 mA to −0.45 mA (Fig. 3A), with the total electric charge transferred increasing from 25 C (day 11) to 84 C (day 14) (Fig. 3B). After subsequent operation at a set potential of −539 mV (days 14 to 21), the total electric charge transferred from the Harbor N sample increased (135 C). For the unpoised Bay sample (Bay N), a greater electric charge (16.5 C) also transferred at −539 mV (days 14 to 21) compared to the 0.7 C charge seen at −439 mV (days 7 to 14) (see Fig S2 and Table S1 in the supplemental material).
Fig 3.
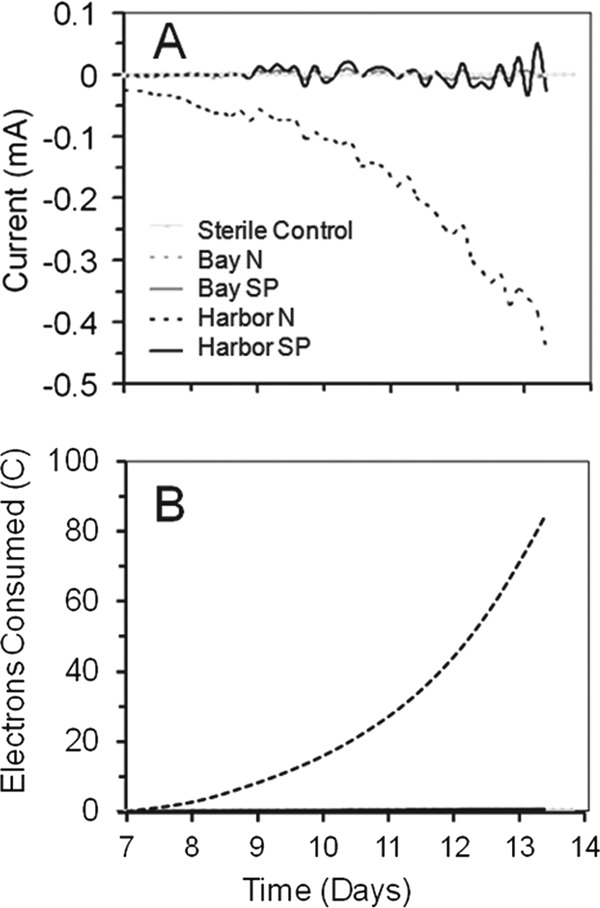
Development of negative current (A) and consumption of electrons in coulombs (B) at an applied WE potential of −439 mV in PBS minimal media degassed with an N2/CO2 ratio of 80%/20% (vol/vol) after approximately 7 days.
Electroautotrophic growth.
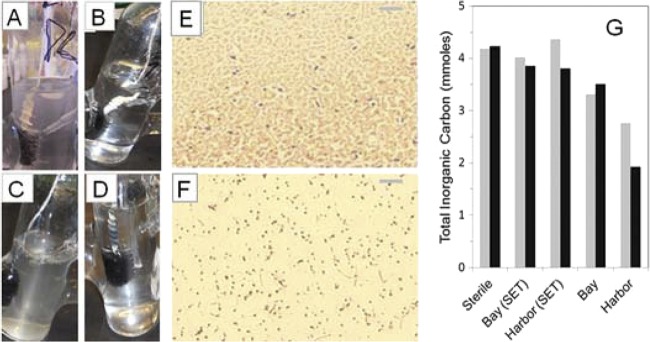
Based on visual observation of the cathode electrodes and suspension after day 14, microbial growth was observed for the Bay N and Harbor N samples, which had shown enhanced current, but not for the other samples. The Bay N cathode developed a visible biofilm with white, strand-like filaments trailing off the electrode, and the cathode suspension became mildly turbid (Fig. 4A). The Harbor N cathode did not develop a dense cathode biofilm, but the suspension became visibly turbid over the course of days 7 to 14. There were many bubbles observed at the air-liquid interface, consistent with the measured production of biogas (Fig. 4C). No gas bubbles were seen in any of the other reactors. This suggested autotrophic growth for the Harbor N and Bay N samples (electroautotrophic growth), as there was no added source of organic carbon, although some growth could have occurred due to organic matter carried over on the brush from the sediment. In the case of the Bay SP cathode, there was no apparent biofilm growth on the electrode, and the solution remained clear (Fig. 4B). However, a slightly positive gas pressure was detected upon insertion of a syringe into the Bay SP reactor, suggesting that some amount of biological activity could have been supported by carryover of organic matter. The Harbor SP cathode, which also did not produce current, showed some slight growth on the cathode, again suggesting some organic matter carryover (Fig. 4D). However, these were relatively small indications of growth compared to samples that exhibited current production. There was no apparent growth in the sterile control (see Fig. S3 in the supplemental material) and no growth in any of the anode compartments.
Fig 4.
(A to D) Patterns of cathode chamber growth after 1 week at −439 mV for (A) Bay N, (B) Bay SP, (C) Harbor N, and (D) Harbor SP samples. (E and F) Gram-positive bacteria (purple-stained cocci and rods) and Gram-negative (red-stained) bacteria were apparent in cathode chamber suspensions recovered from (E) Bay N samples or (F) Harbor N samples (×1,000 magnification; bar is 10 μm). (G) Total inorganic carbon remaining in the cathode chamber after 7-day incubations at either −439 mV (gray bars, day 7 to day 14) or −539 mV (black bars, day 14 to day 21). (Reactors were flushed with N2:CO2 [80:20] on days 7 and 14).
Microscopic examination of samples from the brush fibers of the two electrochemically active biocathodes (Harbor N and Bay N) revealed cultures richly populated with both Gram-positive and Gram-negative bacteria. Consistent with the observation of biofilm on the Bay N biocathode (Fig. 4A), smears prepared from the Bay N cathode suspension showed densely packed agglomerations of cells, most of which appeared to be Gram-negative rods (Fig. 4E). For the unpoised Harbor N sample, Gram-positive cocci or short rods were prevalent, although a diversity of other cell types was also present (Fig. 4F). Graphite fibers of the Harbor N cathode also appeared to have adherent biofilm 2 months after inversion (see Fig. S4 in the supplemental material).
Gas analysis indicated that there was CO2 removal and production of H2 and CH4 gases. Assuming conditions of equilibrium between the gas and liquid, there was an equivalent of 4.32 mmol of CO2 added to each reactor, with 0.61 mmol in the headspace and the balance in the solution phase (as dissolved carbon dioxide gas and bicarbonate; see detailed calculations in the supplemental material). Between days 7 and 14, there was a 40% decrease in the level of cathode chamber inorganic carbon for the current-accepting Harbor N sample and a 25% decrease for the Bay N sample, with biomass production in both relative to non-current-accepting reactors, supporting the possibility of autotrophic growth (Fig. 4G). The level of CO2 in the sterile control headspace was unchanged, ruling out gas leakage. The amount of CH4 that the Harbor N cathode produced (0.61 mmol) was less than the amount of CO2 removed (1.72 mmol), which could support methanogenesis derived from hydrogen gas or electrons from the cathode and CO2 (a 1:1 ratio for CH4 produced and CO2 used) (Fig. 5A). In addition, there were much smaller amounts of CH4 and H2 detected in the Harbor SP sample during startup (which did not produce appreciable current), suggesting minimal methanogenesis derived from organic matter carried over from sMFC operation. However, the total moles of electrons transferred during this period (0.87 mmol, days 7 to 14) by the Harbor N sample could only have resulted only in 0.11 mM CH4, suggesting that in this sample, methane production was enhanced by processes not associated with microbes taking in current or H2 gas (nonelectrotrophic processes).
Fig 5.
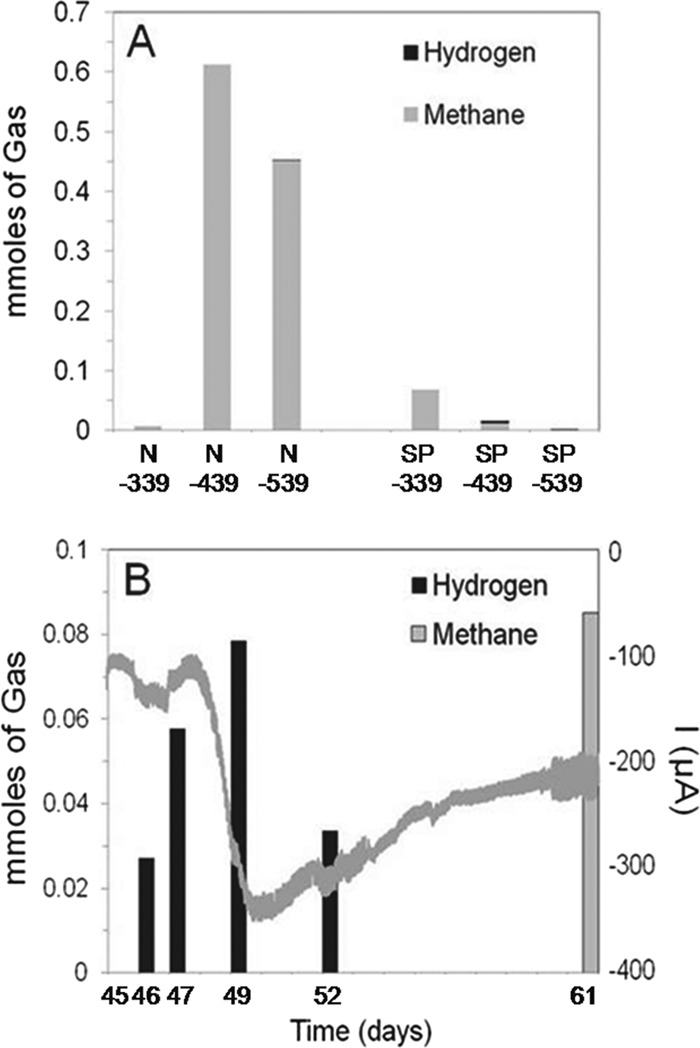
(A) Biogas composition of cathode chamber headspace for Harbor N and Harbor SP cathodes after 1 week of incubation at −339 mV (day 0 to day 7), −439 mV (day 7 to day 14), or −539 mV (day 14 to day 21). (B) Gases in the reactor headspace and current for the Harbor N reactor over a 16-day period (the headspace was initially flushed with N2 and CO2) after samples had been cultivated for 45 days (the gray undulating line represents electric current at an applied potential of −439 mV).
To further investigate this possible autotrophic growth of the Harbor N sample that accepted significant current, the sample was examined 45 days postinversion, when the impact of any original organic matter carryover would have been minimal (Fig. 5B). Over a 16-day period (after the reactor had been flushed with CO2 and N2), substantial hydrogen gas was evolved on day 46 and the volume then increased by day 49, but it declined on day 52, possibly due to consumption by microorganisms in the sample. When the sample was again examined on day 61, no H2 was detected, and there was 0.08 mmol of CH4 detected in the headspace. Under these conditions, there were sufficient electrons transferred (3.2 mmol) and inorganic carbon consumed (2.22 mmol) for production of up to 0.4 mmol of CH4, strongly supporting electrotrophic growth in this sample (Fig. 5B). No H2 or CH4 was detected in the cathode headspace of the sterile negative control after weeklong operation at any applied potentials, indicating that H2 was not formed abiotically through electrolysis. This activity of the Harbor N sample after long-term cultivation shows that this biocathode produced H2 gas and removed CO2, consistent with autotrophic growth.
To further demonstrate autotrophic growth by the Harbor N sample, cell suspensions were transferred to new reactors containing different types of cathodes (carbon fiber brush, carbon rod, or graphite plate) and examined for electrical current and growth. Electrical current was observed after a lag period of 4 days. The graphite plate electrode showed the highest consumption of current and power (based on the applied voltage and current) and current density (−93 μA, −83 mW m−2, −52 mA m−2) compared to the carbon fiber brush (−60 μA, −1 mW m−2, −1 mA m−2) and the carbon rod (−57 μA, −65 mW m−2, −41 mA m−2) (Fig. 6; see also Fig. S5 in the supplemental material). After 4 days, the counterelectrode (anode) potential was ∼1.2 V (versus SHE) for all three electrode materials (Fig. 6A, inset). All three cathode materials showed an increased electron uptake after approximately 4 days, with values of 64 C (graphite plate), 45 C (carbon fiber brush), and 40 C (carbon rod) (Fig. 6B, inset). These results represent daily rates of electron uptake of 16 C (graphite plate), 11 C (carbon fiber brush), and 10 C (carbon rod).
Fig 6.
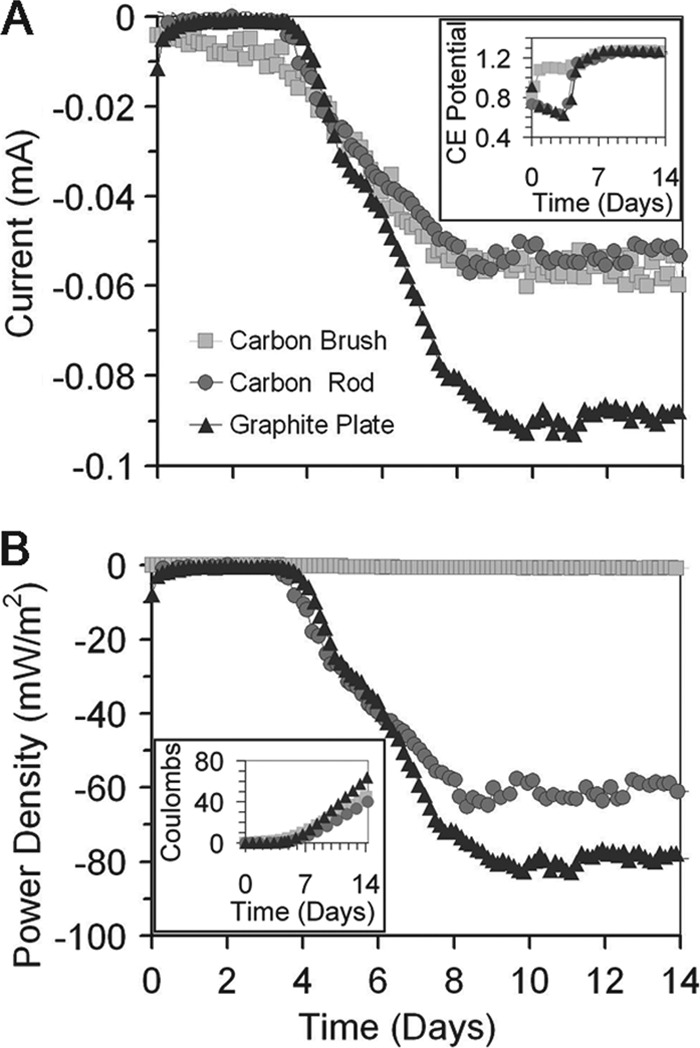
(A) Current uptake and counterelectrode (anode) potentials (shown in inset panel) and (B) power and electron consumption (shown in inset panel) after inoculation of biocathode cathode solution, obtained from the Harbor N suspension 45 days after its inversion from sMFC mode, into three sterile BES reactors, each with a different cathode material (carbon fiber brush, carbon rod, or graphite plate), at an applied potential of −539 mV. PBS medium was degassed with N2/CO2 at a ratio of 80%/20% (vol/vol) prior to inoculation.
Community analysis.
Genomic analysis of 16S rRNA gene clone libraries prepared from carbon fiber clippings of the biocathodes indicated a predominance of bacteria with sequences most similar to several Harbor N and Bay N chemolithoautotrophic bacteria. There were substantial differences in the microbial consortia on the two biocathodes with the highest current densities, consistent with macroscopic (visual) and microscopic (morphology and Gram staining) analysis. The Harbor N biocathode primarily (84% of 94 clones) consisted of bacteria most similar to just three species: Eubacterium limosum (i.e., Butyribacterium methylotrophicum), Desulfovibrio sp. A2, and Gemmata obscuriglobus (Fig. 7A). Nearly half (47%) of the total clones bore the closest homologic resemblance to E. limosum, a known chemolithoautotroph. Methanocorpusculum labreanum was also detected in the Harbor N sample, consistent with a previous report showing that the 338F/907R primer detects methanogenic archaea (25). The presence of this microorganism could explain the production of CH4 gas in these samples, but further analysis for other methanogens was not conducted with primers suitable to detect all archaea. This type of methanogen was not detected in the electron-accepting Bay N biocathode, consistent with the lack of biogas production by this sample. Analysis of the functional Bay biocathode indicated bacteria with the most similarity to G. obscuriglobus, also identified on the Harbor N cathode, and members of three different genera, Mesorhizobium (24%), Rhodococcus (22%), and Azospirillum (10%). A number of less prevalent bacteria were also detected (Fig. 7B).
Fig 7.
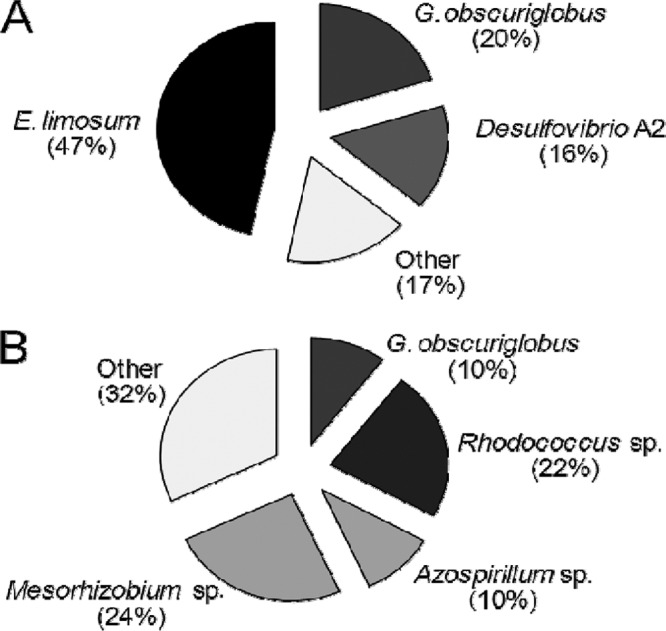
Clone library analysis of 16S rRNA genes extracted at day 61 from electrotrophically active biocathodes established from (A) Baltimore Inner Harbor sediment (n = 94) or (B) Chesapeake Bay sediment (n = 97).
DISCUSSION
While prior studies have shown that it can be difficult to obtain anaerobic biocathodes from natural assemblages of microorganisms (25), it was shown here that a simple two-step procedure based on initial acclimation of the electrode in an sMFC could produce a MEC biocathode with recoverable autotrophs (Fig. 1). The microbes on these biocathodes accepted electrons at potentials of −439 mV and −539 mV and grew and produced biomass, as determined on the basis of visual observation of a biofilm on the electrode or of suspension turbidity. H2 gas was produced, along with the consumption by the Harbor N cultures of CO2, which was subsequently converted to methane. The Bay N sample also produced current but did not produce H2 gas, indicating that the community that developed on this electrode was functionally different from that of the Harbor N biocathode. The Harbor SP cathode did not consume significant current and produced little methane, suggesting only residual activity of the biofilm supported by carryover of organic matter or decay of the existing biofilm (Fig. 5A).
Previous approaches to produce biocathodes either have used chemical solutions in the counterelectrode chamber to avoid oxygen (22) or have used a pure G. sulfurreducens culture (16). In an sMFC, dissolved oxygen typically does not penetrate more than ∼1 cm (31), resulting in an effective method for scavenging dissolved oxygen (47). Although a pure culture of G. sulfurreducens can successfully be used to generate H2 gas, it is not autotrophic; therefore, its growth cannot be sustained without the use of organic substrates. Interestingly, no sequences with similarity to any Geobacter species were identified in our clone libraries of either of the electrochemically active marine biocathodes. While H2 gas was produced by the autotrophic Harbor N biocathodes, methanogenesis was not inhibited and could have proceeded either directly by electron transfer via electromethanogenesis (9) or indirectly by hydrogenotrophic methanogenesis. Recoverable H2 gas was not detected for Bay N or SP samples. No organic compounds in filtrate from the electron-accepting biocathodes were detected in solution, based on an absence of measurable peaks in the HPLC chromatograms (data not shown), although we cannot rule out the possible presence of low concentrations of excreted organic carbon in the solution.
Electrode materials appeared to play a role in the maximum current densities of the biocathodes. When the same inoculum was used for three different types of cathodes, biocathodes on graphite (−93 μA) clearly outperformed the carbon brush (−83 μA) or a carbon rod (−52 μA) (Fig. 6). This higher current was produced despite the graphite having 64 times less total surface area than the carbon brush (see Fig. S5 in the supplemental material) (18-cm2 projected area for graphite, 1,150 cm2 for fibers on the carbon brush). These results are different from those seen when experiments were performed using these materials as MFC anodes, but the reasons for these differences are not known.
Microbes tentatively identified on functional biocathodes by 16S rRNA gene clone library analysis (Fig. 7) have been shown to interact directly (via contact) or indirectly (via excreted molecules) with cathodes. For example, hydrogenases of Rhodococcus on cathodes have been shown to mediate significantly higher rates of direct NADH reduction (in the absence of H2 gas formation) than those of Alcaligenes eutrophus (i.e., Ralstonia eutropha) (17). Microbes most similar to G. obscuriglobus were the most prominent on biocathodes, as this was the only organism detected in both Bay and Harbor biocathodes. G. obscuriglobus is a member of the Planctomycetes phylum and is related to the chemolithotrophic ammonium-oxidizing ammanox bacteria (14). However, known strains of G. obscuriglobus are heterotrophic, suggesting that the microbe detected might grow symbiotically with other cells in the biocathode consortia. Some members of the Planctomycetes possess methanopterin and methanofuran cofactors akin to those which catalyze C1 transfer reactions in methylotrophs and archeal methanogens (1, 10, 11). Mesorhizobium and Azospirillum species are root-associated bacteria that were conspicuous on the Bay biocathode (Fig. 7B). It was recently reported that fixation of carbon by Azospirillum species could play a role in the sequestration of carbon into soil (45).
An organism most closely related to a copper-resistant sulfate-reducing bacterium (SRB), Desulfovibrio species A2, was prominent in the Harbor biocathode (24). Desulfovibrio species are rarely autotrophic but typically possess hydrogenases and are common electrode biofilm constituents in BESs (18, 20). In Desulfovibrio desulfuricans, membrane enzymes link oxidative metabolism to extracellular electron transfer (42). Desulfovibrio biofilms on graphite electrodes have been shown to accept electrons and reduce the overpotential for hydrogen formation by about 200 mV (12, 44). Since pelagic cells were prevalent in the unpoised sample, extracellular electron mediators, such as hydrogen, might have served as electron shuttles. Soluble mediators might also be involved, although HPLC analysis comparing active samples to inactive samples failed to detect a conspicuous buildup of peaks indicative of soluble mediators or other metabolites (see Fig. S6 in the supplemental material).
Bacteria bearing greatest genotypic similarity to E. limosum and Desulfovibrio A2 represented nearly two-thirds (63%) of the clones in the highly electrotrophically active unpoised Harbor N sample (Fig. 7). The symbiosis known to exist between these organisms as relates to biocorrosion (i.e., oxidation) of metal might play a role in electron uptake and electrotrophic growth. Desulfovibrio SRBs are notorious for accelerating corrosion (29) through increased electron uptake from metals (29, 33). Since biologically mediated electron uptake from cathodes is necessary for electrotrophic growth, corrosive, metal-resistant Desulfovibrio species, such as Desulfovibrio A2, might facilitate electrotrophic activity. When D. vulgaris is cocultured with the autotroph E. limosum on metal in minimal media, corrosion is accelerated (13). In BES cathode biofilms, E. limosum might similarly accelerate the rate of electron uptake from cathodes by SRBs. From the standpoint of electrofuel production, E. limosum is of interest, since this autotroph can produce butanol (7), although no soluble biofuels were detected here. Follow-up studies are under way to identify pure cultures and cocultures capable of producing electrofuels.
In conclusion, this technique of inversion of an sMFC bioanode to a BES biocathode provides an effective method for enriching electrotrophic bacteria associated with gas-phase CO2 removal. This method may be useful for biofuel production and, more particularly, electrofuel synthesis or electrotroph screening. Since this approach is predicated on use of both an upper aqueous phase and a lower semisolid or granular phase, it is likely not limited to use with sediments. Indeed, biocathodes have been shown to develop well in beds of activated carbon and graphite when aided with carbon brushes (41, 46). Future studies are planned to explore if this strategy can function as a general method to enrich an exoelectrogenic-electrotrophic community and, when the electrodes are inverted, whether only the autotropic-electrotrophic community can thrive.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the divers of Specialty Underwater Services (Curtis Bay, MD) for obtaining samples from Baltimore Harbor. DNA sequencing was performed by the Huck Institute Genomic Core facility at Penn State. Justin Tokash and Jay Regan provided technical advice, and Xiuping Zhu provided help with the HPLC analysis.
Financial support under the Advanced Research Projects Agency Energy (ARPA-E) is gratefully acknowledged.
Footnotes
Published ahead of print 18 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bauer M, et al. 2004. Archaea-like genes for C1-transfer enzymes in Planctomycetes: phylogenetic implications of their unexpected presence in this phylum. J. Mol. Evol. 59:571–586 [DOI] [PubMed] [Google Scholar]
- 2.Butler CS, Nerenberg R. 2010. Performance and microbial ecology of air-cathode microbial fuel cells with layered electrode assemblies. Appl. Microbiol. Biotechnol. 86:1399–1408 [DOI] [PubMed] [Google Scholar]
- 3.Call D, Logan BE. 2008. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 42:3401–3406 [DOI] [PubMed] [Google Scholar]
- 4.Call DF, Merrill MD, Logan BE. 2009. High surface area stainless steel brushes as cathodes in microbial electrolysis cells. Environ. Sci. Technol. 43:2179–2183 [DOI] [PubMed] [Google Scholar]
- 5.Call DF, Wagner RC, Logan BE. 2009. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 75:7579–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chae KJ, et al. 2009. A solar-powered microbial electrolysis cell with a platinum catalyst-free cathode to produce hydrogen. Environ. Sci. Technol. 43:9525–9530 [DOI] [PubMed] [Google Scholar]
- 7.Cheng KY, Ho G, Cord-Ruwisch R. 2010. Anodophilic biofilm catalyzes cathodic oxygen reduction. Environ. Sci. Technol. 44:518–525 [DOI] [PubMed] [Google Scholar]
- 8.Cheng KY, Ho G, Cord-Ruwisch R. 2011. Novel methanogenic rotatable bioelectrochemical system operated with polarity inversion. Environ. Sci. Technol. 45:796–802 [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Xing D, Call DF, Logan BE. 2009. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 43:3953–3958 [DOI] [PubMed] [Google Scholar]
- 10.Chistoserdova L. 2011. Modularity of methylotrophy, revisited. Environ. Microbiol. 13:2603–2622 [DOI] [PubMed] [Google Scholar]
- 11.Chistoserdova L, et al. 2004. The enigmatic planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol. Biol. Evol. 21:1234–1241 [DOI] [PubMed] [Google Scholar]
- 12.Croese E, Pereira MA, Euverink GJW, Stams AJM, Geelhoed JS. 2011. Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl. Microbiol. Biotechnol. 92:1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling NJE, Brooks SA, Phelps TJ, White DC. 1992. Effects of selection and fate of substrates supplied to anaerobic bacteria involved in the corrosion of pipe-line steel. J. Ind. Microbiol. 10:207–215 [Google Scholar]
- 14.Fuerst JA, Sagulenko E. 2011. Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. Microbiol. 9:403–413 [DOI] [PubMed] [Google Scholar]
- 15.Geelhoed JS, Hamelers HVM, Stams AJM. 2010. Electricity-mediated biological hydrogen production. Curr. Opin. Microbiol. 13:307–315 [DOI] [PubMed] [Google Scholar]
- 16.Geelhoed JS, Stams AJM. 2011. Electricity-assisted biological hydrogen production from acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 45:815–820 [DOI] [PubMed] [Google Scholar]
- 17.Gros P, Zaborosch C, Schlegel HG, Bergel A. 1996. Direct electrochemistry of Rhodococcus opacus hydrogenase for the catalysis of NAD+ reduction. J. Electroanal. Chem. 405:189–195 [Google Scholar]
- 18.Jeremiasse AW, Hamelers HVM, Buisman CJN. 2010. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 78:39–43 [DOI] [PubMed] [Google Scholar]
- 19.Jeremiasse AW, Hamelers HVM, Croese E, Buisman CJN. 2012. Acetate enhances startup of a H2 producing microbial biocathode. Biotechnol. Bioeng. 109:657–664 [DOI] [PubMed] [Google Scholar]
- 20.Liu W, et al. 2012. Characterization of microbial communities during anode biofilm reformation in a two-chambered microbial electrolysis cell (MEC). J. Biotechnol. 157:628–632 doi:10.1016/j.jbiotec.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Logan BE. 2010. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 85:1665–1671 [DOI] [PubMed] [Google Scholar]
- 22.Logan BE, Murano C, Scott K, Gray ND, Head IM. 2005. Electricity generation from cysteine in a microbial fuel cell. Water Res. 39:942–952 [DOI] [PubMed] [Google Scholar]
- 23.Lovley DR, Nevin KP. 2011. A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr. Opin. Biotechnol. 22:441–448 [DOI] [PubMed] [Google Scholar]
- 24.Mancini S, Abicht HK, Karnachuk OV, Solioz M. 2011. Genome sequence of Desulfovibrio sp. A2, a highly copper resistant, sulfate-reducing bacterium isolated from effluents of a zinc smelter at the Urals. J. Bacteriol. 193:6793–6794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita M, et al. 2011. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2:e00159–11 doi: 10.1128/mBio.00159-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nevin K, et al. 2008. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 10:2505–2514 [DOI] [PubMed] [Google Scholar]
- 27.Nevin KP, et al. 2011. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 77:2882–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peguin S, Delorme P, Goma G, Soucaille P. 1994. Enhanced alcohol yields in batch cultures of Clostridium acetobutylicum using a three-electrode potentiometric system with methyl viologen as electron carrier. Biotechnol. Lett. 16:269–274 [Google Scholar]
- 29.Pérez EJ, Cabrera-Sierra R, González I, Ramírez-Vives F. 2007. Influence of Desulfovibrio sp. biofilm on SAE 1018 carbon steel corrosion in synthetic marine medium. Corros. Sci. 49:3580–3597 [Google Scholar]
- 30.Rabaey K, Rozendal RA. 2010. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 8:706–716 [DOI] [PubMed] [Google Scholar]
- 31.Revsbech NP, Sorensen J, Blackburn TH, Lomholt JP. 1980. Distribution of oxygen in marine sediments measured with microelectrodes. Limnol. Oceanogr. 25:403–411 [Google Scholar]
- 32.Rozendal RA, Jeremiasse AW, Hamelers HVM, Buisman CJN. 2008. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 42:629–634 [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Sharma C, Lata S. 2011. Microbial influenced corrosion due to Desulfovibrio desulfuricans. Anti-Corros. Methods Mater. 58:315–322 [Google Scholar]
- 34.Steinbusch KJJ, Hamelers HVM, Schaap JD, Kampman C, Buisman CJN. 2010. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ. Sci. Technol. 44:513–517 [DOI] [PubMed] [Google Scholar]
- 35.Strycharz SM, Gannon SM, Boles AR, Franks AE, Nevin KP, Lovley DR. 2010. Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ. Microbiol. 2:289–294 [DOI] [PubMed] [Google Scholar]
- 36.Strycharz SM, et al. 2011. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80:142–150 [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Hu Y, Bi Z, Cao Y. 2009. Improved performance of air-cathode single-chamber microbial fuel cell for wastewater treatment using microfiltration membranes and multiple sludge inoculation. J. Power Sources 187:471–479 [Google Scholar]
- 38.Sun M, et al. 2008. An MEC-MFC-coupled system for biohydrogen production from acetate. Environ. Sci. Technol. 42:8095–8100 [DOI] [PubMed] [Google Scholar]
- 39.Villano M, De Bonis L, Rossetti S, Aulenta F, Majone M. 2011. Bioelectrochemical hydrogen production with hydrogenophilic dechlorinating bacteria as electrocatalytic agents. Bioresour. Technol. 102:3193–3199 [DOI] [PubMed] [Google Scholar]
- 40.Wagner RC, Call DF, Logan BE. 2010. Optimal set anode potentials vary in bioelectrochemical systems. Environ. Sci. Technol. 44:6036–6041 [DOI] [PubMed] [Google Scholar]
- 41.Wei J, Liang P, Cao X, Huang X. 2011. Use of inexpensive semicoke and activated carbon as biocathode in microbial fuel cells. Bioresour. Technol. 102:10431–10435 [DOI] [PubMed] [Google Scholar]
- 42.Wu X, et al. 2011. A role for microbial palladium nanoparticles in extracellular electron transfer. Angew. Chem. Int. Ed. Engl. 50:427–430 [DOI] [PubMed] [Google Scholar]
- 43.Yang Q, Feng Y, Logan B. 2012. Using cathode spacers to minimize reactor size in air cathode microbial fuel cells. Bioresour. Technol. 110:273–277 doi:10.1016/j.biortech.2012.01.121 [DOI] [PubMed] [Google Scholar]
- 44.Yu L, Duan J, Zhao W, Huang Y, Hou B. 2011. Characteristics of hydrogen evolution and oxidation catalyzed by Desulfovibrio caledoniensis biofilm on pyrolytic graphite electrode. Electrochim. Acta 56:9041–9047 [Google Scholar]
- 45.Yuan H, Ge T, Chen C, O'Donnell AG, Wu J. 2012. Microbial autotrophy plays a significant role in the sequestration of soil carbon. Appl. Environ. Microbiol. 78:2328–2336 doi:10.1128/AEM.06881-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G, et al. 2011. Improved performance of microbial fuel cell using combination biocathode of graphite fiber brush and graphite granules. J. Power Sources 196:6036–6041 [Google Scholar]
- 47.Ziebis W, Huettel M, Forster S. 1996. Impact of biogenic sediment topography on oxygen fluxes in permeable seabeds. Mar Ecol. Prog. Ser. 140:227–237 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.