Abstract
Osteoarticular brucellosis is the most common presentation of the active disease in humans. Loss of bone is a serious complication of localized bacterial infection of bones or the adjacent tissue, and brucellosis proved not to be the exception. The skeleton is a dynamic organ system which is constantly remodeled. Osteoblasts are responsible for the deposition of bone matrix and are thought to facilitate the calcification and mineralization of the bone matrix, and their function could be altered under infectious conditions. In this article, we describe immune mechanisms whereby Brucella abortus may invade and replicate within osteoblasts, inducing apoptosis, inhibiting mineral and organic matrix deposition, and inducing upregulation of RANKL expression. Additionally, all of these mechanisms contributed in different ways to bone loss. These processes implicate the activation of signaling pathways (mitogen-activated protein kinases [MAPK] and caspases) involved in cytokine secretion, expression of activating molecules, and cell death of osteoblasts. In addition, considering the relevance of macrophages in intracellular Brucella survival and proinflammatory cytokine secretion in response to infection, we also investigated the role of these cells as modulators of osteoblast survival, differentiation, and function. We demonstrated that supernatants from B. abortus-infected macrophages may also mediate osteoblast apoptosis and inhibit osteoblast function in a process that is dependent on the presence of tumor necrosis factor alpha (TNF-α). These results indicate that B. abortus may directly and indirectly harm osteoblast function, contributing to the bone and joint destruction observed in patients with osteoarticular complications of brucellosis.
INTRODUCTION
Osteoarticular brucellosis is the most common presentation of the active disease in humans, affecting up to 85% of patients. The three most common forms of osteoarticular involvement are sacroileitis, spondylitis, and peripheral arthritis (1, 11, 33, 34, 57). Loss of bone is a serious complication of localized bacterial infection of bones or the adjacent tissue. Although Brucella bacteria have the ability to induce bone loss and despite the fact that clinical and imaging aspects of osteoarticular brucellosis have been described widely, the mechanisms involved in this process have not yet been completely elucidated (33, 34).
The skeleton is a dynamic organ system which is constantly remodeled. These processes involve the coordinated effort of osteoblasts and osteoclasts (18). Together, these cells function to ensure healthy bone, giving strength and rigidity to the skeletal system. Osteoblasts are responsible for the deposition of bone matrix and are thought to facilitate the calcification and mineralization of the bone matrix. In contrast, osteoclasts drive the resorption of bone by acidification and the release of lysosomal enzymes (18).
We have recently partially deciphered potential mechanisms for the bone damage caused by Brucella. We first demonstrated that Brucella spp. can infect and survive within human osteoblasts and that this infection elicits the secretion of proinflammatory cytokines and chemokines, as well as matrix metalloproteases (MMPs), that might be involved in the osteoarticular manifestations of brucellosis (42). More recently, we have demonstrated that Brucella infection induced an increase in the number of osteoclasts (defined as pathological osteoclastogenesis), resulting in excessive bone resorption (12).
However, at present it has not been investigated whether Brucella infection may inhibit osteoblast differentiation and function. Therefore, this study was undertaken to investigate whether Brucella abortus infection inhibits osteoblast differentiation and function and by this contributes to bone loss. In particular, we examined signaling pathways (mitogen-activated protein kinases [MAPK] and caspases) involved in cytokine secretion, the expression of activating molecules, and cell death of osteoblasts.
In addition, considering the relevance of macrophages in intracellular Brucella survival and proinflammatory cytokine secretion in response to infection and taking into account that osteoblasts secrete monocyte chemoattractant protein 1 (MCP-1) in response to Brucella infection (42), we also investigated the role of these cells as modulators of osteoblast survival, differentiation, and function. Here, we present the results of this study.
MATERIALS AND METHODS
Bacterial culture.
Brucella abortus S2308 and its isogenic virB10 polar mutant (kindly provided by Diego Comerci) were grown overnight in 10 ml of tryptic soy broth (Merck, Buenos Aires, Argentina) with constant agitation at 37°C. Bacteria were harvested by centrifugation for 15 min at 6,000 × g at 4°C and washed twice in 10 ml of phosphate-buffered saline (PBS). The numbers of bacteria in stationary-phase cultures were determined by comparing the optical densities at 600 nm (OD600) with a standard curve obtained in our laboratory. To prepare inocula, cultures were diluted in sterile PBS to the desired bacterial concentration on the basis of the optical density readings, but the precise concentrations of inocula were determined by plating cells onto tryptic soy agar. All live Brucella manipulations were performed in biosafety level 3 facilities located at the Centro Nacional de Referencia para el SIDA, School of Medicine, University of Buenos Aires.
Cell culture.
Primary osteoblasts were isolated from newborn-mouse calvaria using the method described by Wong and Cohn (54). Briefly, calvaria were subjected to sequential 15-min digestions in an enzyme mixture containing 0.05% trypsin and 0.1% collagenase at 37°C. Cell fractions 3 to 5 were pooled and resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin (complete medium).
Cells were plated at a density of 5 × 105 cells/well, and the medium was changed 24 h later. For differentiation, alpha minimum essential medium (α-MEM) containing 10% FBS, 50 mg/ml ascorbic acid, and 4 mM b-glycerophosphate (differentiation medium) was used and the medium was changed every other day thereafter to day 30. All animal-related experiments were performed according to the rules and standards for the use of laboratory animals of the U.S. National Institutes of Health. Animal experiments were approved by the Ethical Committee of the IDEHU.
The mouse clonal MC3T3-E1 preosteoblastic cell line, a standard osteoblast cell line used routinely for the assessment of osteoblasts under different culture conditions (35, 38, 51), and murine macrophage-like J774.A1 cells (ATCC, Middlesex, United Kingdom) were used in some experiments. All cells were cultured in standard tissue culture flasks containing complete medium. The medium was replaced every 3 or 4 days, and after confluence, cells were harvested using trypsin and resuspended in complete medium.
Cellular infection.
Primary mouse osteoblasts and MC3T3-E1 cells were infected with B. abortus at different multiplicities of infection (MOI), and J774.A1 cells were infected at an MOI of 100. After the bacterial suspension was dispensed, the plates were centrifuged for 10 min at 2,000 rpm and then incubated for 2 h at 37°C under a 5% CO2 atmosphere. Cells were extensively washed with DMEM to remove extracellular bacteria and incubated in medium supplemented with FBS (10%) and with 100 μg/ml gentamicin and 50 μg/ml streptomycin to kill extracellular bacteria. At different times (see below), osteoblast cells were harvested to determine apoptosis or to measure osteocalcin, collagen, and calcium deposition or alkaline phosphatase (ALP) activity. Supernatants from J774.A1 cells were harvested at 24 h postinfection (p.i.) to be used as conditioned medium.
Stimulation with conditioned medium.
Culture supernatants from J774.A1 macrophages infected with B. abortus at an MOI of 100 were harvested at 24 h p.i., sterilized by filtration through a 0.22-μm nitrocellulose filter, and used to stimulate noninfected primary mouse osteoblasts or MC3T3.E1 cells. Supernatants were used diluted 1/2, 1/5, or 1/10 in complete medium. After 24 h, the cells were harvested to determine apoptosis, or they were assayed at 7, 14, or 30 days to measured osteocalcin, collagen, and calcium deposition or ALP activity.
Signaling pathway.
To study the potential involvement of different signaling pathways in the production of MCP-1, keratinocyte chemoattractant (KC), MMP-2, and MMP-9 and apoptosis by osteoblasts, pharmacological inhibitors (SB203580, a p38 MAPK inhibitor, and PD98059, an extracellular signal-regulated kinase 1 and 2 [ERK1/2] MAPK inhibitor) or vehicle (dimethyl sulfoxide [DMSO]) were added at the beginning of culture. Inhibitors (Calbiochem, San Diego, CA) were used at a concentration of 10 μM, based on previous reports (1, 27). Cell viability after incubation with these inhibitors was higher than 90%, as assessed by staining with trypan blue. To account for any possible effect of DMSO on cell viability, cell cultures not treated with the inhibitors were treated with the highest final concentration of DMSO used in these studies (0.01%), and the results were compared to those for cell cultures not exposed to DMSO. In addition, inhibitors do not have a toxic effect on bacterial survival since the levels of invasion and replication were similar to the levels in untreated cells.
p38 and ERK1/2 activation assessment by Western blotting.
Infected osteoblast cells or osteoblasts stimulated with 200 mM phorbol 12-myristate 13-acetate (PMA) (positive control) were lysed in ice-cold lysis buffer consisting of 20 mM HEPES, pH 8.5 mM EDTA, 10 mM EGTA, 5 mM NaF, 10% glycerol, 1 mM dithiothreitol, 400 mM KCl, 0.4% Triton X-100, 20 mM sodium β-glycerophosphate, and protease inhibitor cocktail (Sigma-Aldrich de Argentina S.A.). Lysates were incubated on ice for 10 min and cleared by centrifugation at 13,000 × g for 10 min. Protein concentrations were measured in the supernatants by the Bradford method (6), and equal amounts of proteins were loaded onto electrophoresis gels. After separation, proteins were transferred to a nitrocellulose membrane (GE, Little Chalfont, United Kingdom) and blocked for 1 h with 5% milk protein–0.1% Tween 20. Then, membranes were incubated with primary anti-MAPK antibodies (1:1,000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After washing, the membrane was incubated with peroxidase-conjugated secondary antibody (1:2,000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Protein bands were visualized using Hyperfilm ECL (GE, Little Chalfont, United Kingdom) enhanced chemiluminescence. The results from Western blotting were analyzed by densitometric analysis (Image J software; National Institutes of Health).
Zymography.
Gelatinase activity was assayed by the method of Hibbs et al. with modifications (17, 42). Briefly, proteins from osteoblast culture supernatants were separated on 10% SDS–PAGE gels containing 1 mg/ml gelatin (Sigma-Aldrich de Argentina S.A.). Following electrophoresis, gels were washed with 50 mM Tris-HCl, pH 7.5, 2.5% Triton X-100 for 30 min and 50 mM Tris-HCl, pH 7.5, 2.5% Triton X-100, 5 mM CaCl2, 1 μM ZnCl2 for 30 min and incubated with 50 mM Tris-HCl, pH 7.5, 2.5% Triton X-100, 10 mM CaCl2, 200 mM NaCl for 48 h at 37°C. This denaturation/renaturation step promotes MMP activity without proteolytic cleavage of pro-MMP. Gelatin activity was visualized by staining gels with 0.5% Coomassie blue and destaining with methanol-acetic acid.
Measurement of cytokine concentrations.
The secretion of interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), and monocyte chemotactic protein 1 (MCP-1) in the supernatants was quantified with an ELISA kit from BD, and chemokine keratinocyte chemoattractant (KC) and RANKL were quantified with ELISA kits from R&D Systems, Inc. (Minneapolis, MN).
Blocking of TNF-α.
Neutralization experiments were performed with an anti-TNF-α neutralizing antibody or its isotype control (both from BD Biosciences, San Diego, CA).
RANKL expression.
Osteoblasts were infected at MOIs of 100, 250, 500, or 1,000 or stimulated with supernatants from B. abortus-infected J774.A1 macrophages for 24 h. As a positive control, cells were infected with Staphylococcus aureus at an MOI of 100. At the end of culture, cells were washed and were lysed in ice-cold lysis buffer consisting of 20 mM HEPES, pH 8, 5 mM EDTA, 0.4% Triton X-100, and protease inhibitor cocktail (Sigma-Aldrich de Argentina S.A.). The resulting protein was removed and centrifuged at 10,000 × g for 10 min. RANKL was detected in culture supernatants and lysates using an ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Apoptosis assays.
Primary osteoblasts were infected with B. abortus or its isogenic virB10 polar mutant and were harvested 24 h later. Cells were washed, and the percentage of apoptotic cells was assessed by using the annexin V-FITC (fluorescein isothiocyanate) (Sigma-Aldrich) assay with fluorescence-activated cell sorting (FACS) analysis. Apoptosis was also assessed by FACS analysis using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay performed with the fluorescein-FragEL DNA fragmentation detection kit (Calbiochem, San Diego, CA). The percentage of apoptotic cells was assessed by fluorescence microscopy after labeling of the cells by the TUNEL assay or staining with Hoechst 33342 dye. A concentration of 4% paraformaldehyde (PFA) was used as a positive control for apoptosis.
To block caspase activity, osteoblasts (5 × 105 cells per well) seeded in 24-well plates were treated with or without 50 μM general caspase inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone; R&D Systems) for 2 h and then infected with B. abortus at an MOI of 100 and treated with 4% PFA or medium. Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342 dye after 24 h as described above.
Osteoblast differentiation.
Osteoblast differentiation includes the expression of genes involved in the specific function of osteoblasts to secrete mineral and organic matrix. To elucidate this, we studied phosphatase alkaline activity, including calcium, collagen, and osteocalcin deposition. On days 7, 14, and 30 of differentiation, cultures of osteoblast cells were seeded onto glass coverslips and were infected with B. abortus as described above (MOI of 100) or they were stimulated with supernatants from B. abortus-infected macrophages.
Assessment of collagen deposition by Sirius red staining.
Collagen deposition was quantified by using Sirius red (Sigma-Aldrich de Argentina S.A.), a strong anionic dye that binds strongly to collagen molecules (52). Sirius red was dissolved in saturated aqueous picric acid at a concentration of 0.1%. Bouin's fluid (for cell fixation) was prepared by mixing 15 ml saturated aqueous picric acid with 5 ml 35% formaldehyde and 1 ml glacial acetic acid.
Cell layers were extensively washed with PBS before they were fixed with 1 ml Bouin's fluid for 1 h. The fixation fluid was removed and the culture plates were washed 3 times with deionized water. The culture dishes were air dried before adding 1 ml Sirius red dye reagent. The cells were stained for 18 h with mild shaking. The stained cell layers were extensively washed with 0.01 N hydrochloric acid to remove all unbound dye. After rinsing, coverslips were mounted in PBS-glycerine (9:1 [vol/vol]) and were analyzed by light microscopy. For quantitative analysis, the stained material was dissolved in 0.2 ml 0.1 N sodium hydroxide by shaking for 30 min. The dye solution was transferred to microtiter plates, and the optical density (OD) measured with a microplate reader (Metertech, Inc., Taiwan) at 550 nm against 0.1 N sodium hydroxide as a blank.
Osteocalcin deposition.
To determine extracellular osteocalcin deposition, osteoblasts were seeded at a density of 1 × 104 cells/well in 24-well culture plates. At 7, 14, and 30 days, cells were fixed in 4% buffered formalin, followed by three 5-min washes in PBS. Cells were stained with antiosteocalcin antibody (sc-7449; Santa Cruz Biotechnology, Santa Cruz, CA), followed by goat anti-rabbit IgG conjugated to FITC at a dilution of 1:200 in 0.5% PBS–bovine serum albumin (Invitrogen, Carlsbad, CA).
Alizarin red S staining.
To determine calcium deposition, we used alizarin red staining. Osteoblast cells seeded onto glass coverslips were infected with B. abortus as described above (MOI of 100). On days 7, 14, and 30 of the culture's differentiation, osteoblasts were fixed in 4% PFA for 10 min at room temperature. The cells were washed with deionized water and stained with 2% (wt/vol) alizarin red S and were visualized by light microscopy or extracted to perform quantitative analysis.
Alkaline phosphatase staining.
On days 7, 14, and 30 of the culture's differentiation, ALP staining was carried out with BCIP (5-bromo-4-chloro-3-indolylphosphate)-NBT (nitroblue tetrazolium) solution (Sigma-Aldrich de Argentina S.A.) according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance, followed by the post hoc Tukey test, using GraphPad Prism 4.0 software. Data are represented as means ± standard deviations (SD).
RESULTS
B. abortus invades and multiplies in primary mouse osteoblastic cells.
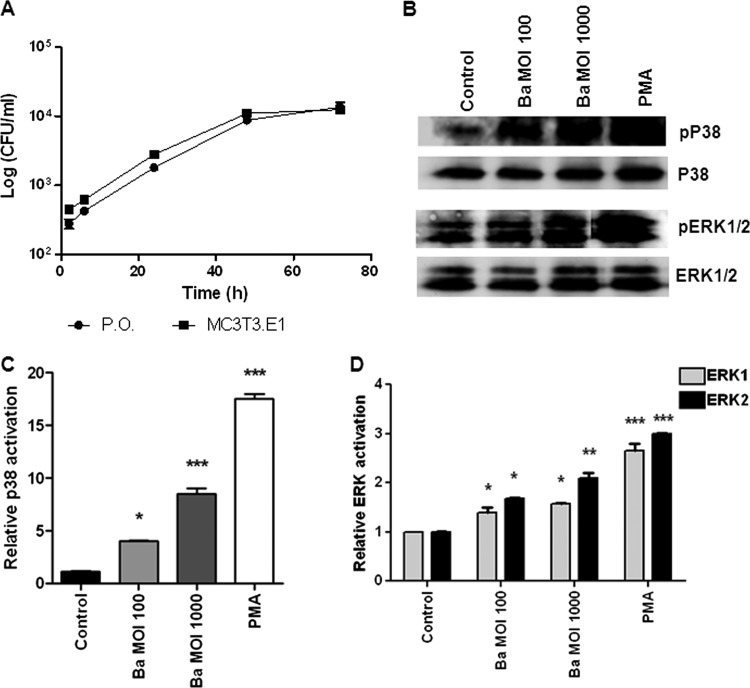
We have previously demonstrated that B. abortus may invade and replicate within human osteoblast cell lines (14, 42). Since immortalized cells may have phenotypical modifications with respect to normal cells, in the present work, we decided to expand these results to a primary osteoblast culture. To this end, we infected primary mouse osteoblasts from mouse calvaria. Infection experiments showed that B. abortus cells were internalized by primary mouse osteoblast cells and the MC3T3 murine cell line in vitro. The magnitude of the infection (intracellular CFU) was directly related to the MOI used, but both infection and intracellular replication were observed for MOIs as low as 100 (Fig. 1A). These results indicate that the ability of B. abortus to infect both murine osteoblast cell lines and primary cell cultures was similar to that previously found for human cell lines (14, 42), supporting their use for functional experiments.
Fig 1.
B. abortus infection in primary mouse osteoblasts induces p38 and ERK1/2 phosphorylation. (A) Infection and replication of B. abortus within primary mouse osteoblasts (P.O.) and the MC3T3 cell line. After infection at an MOI of 100, cells were incubated with antibiotics to kill extracellular bacteria. Cell lysates obtained at different times p.i. were plated on agar to determine intracellular CFU. (B) Activation of ERK1/2 and p38 MAPK in primary mouse osteoblasts. Cells were infected with B. abortus (Ba) at an MOI of 1,000 or 100 or treated with PMA as a positive control. MAPKs were determined by Western immunoblotting. (C and D) Densitometric analysis of results from two independent experiments performed as described for panel B. The data shown are from a representative experiment of five performed. P values for comparison with the results for uninfected cells (Control) are as follows: *, P < 0.1; **, P < 0.01; ***, P < 0.001.
B. abortus induces phosphorylation of p38 and ERK1/2 in osteoblasts.
Mitogen-activated protein kinases (MAPK) play a key role in the regulation of cell development, growth, and survival, as well as the regulation of proinflammatory cytokine production (25, 29). Thus, we explored the possibility that MAPK could play a role in mediating apoptosis and chemokine and MMP secretion. As a first step, we investigated whether p38 and ERK1/2 MAPK were phosphorylated in osteoblasts when these cells were infected with B. abortus. Phorbol myristate acetate (PMA) stimulation was used as a positive control. Upon infection of primary osteoblasts, B. abortus induced significant (P < 0.1) p38 and ERK1/2 phosphorylation when these cells were infected with MOIs of 100 and 1,000 (Fig. 1B). This indicates that the p38 and ERK1/2 MAPK pathway could be involved in the pathological responses induced by B. abortus infection of osteoblasts.
p38 and ERK1/2 signaling pathways are involved in the secretion of KC, MCP-1, and MMPs by B. abortus-infected primary osteoblasts.
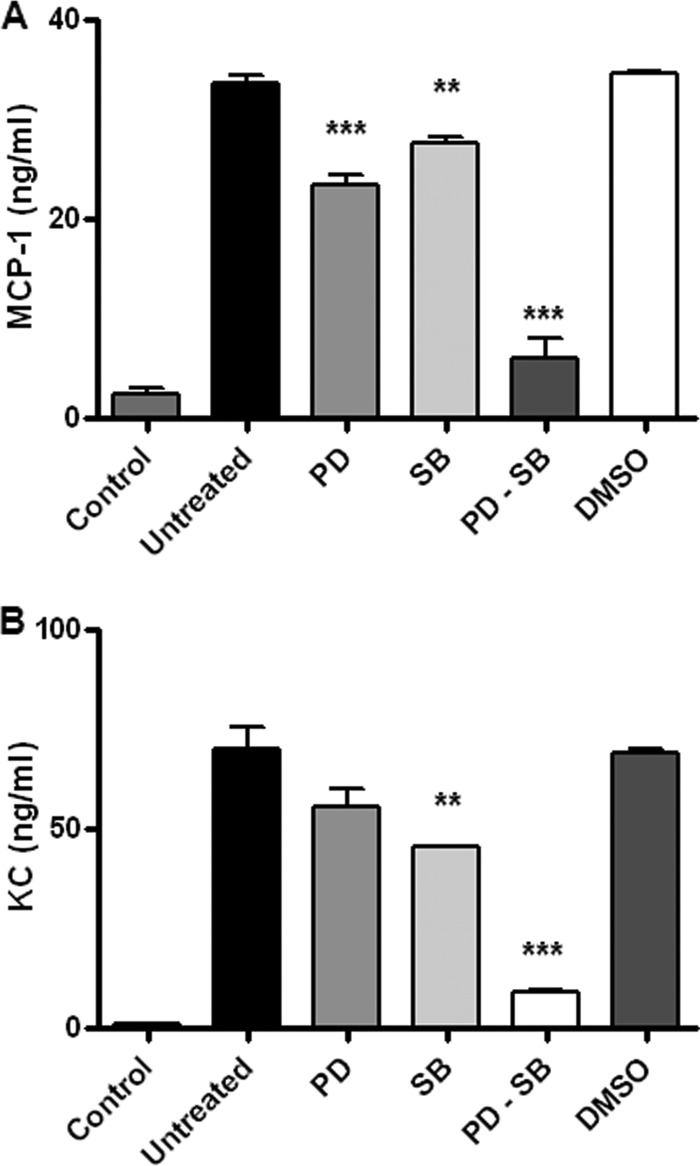
We next decided to investigate the ability of mouse osteoblasts to secrete chemokines and MMPs upon infection with B. abortus. In agreement with previous studies using human cell lines (14, 42), we found that primary osteoblasts and the MC3T3 cell line secrete MCP-1, KC, and MMPs in response to B. abortus infection. Moreover, we also investigated whether specific inhibition of p38 and ERK1/2 MAPK could inhibit KC and MCP-1 production.
Therefore, inhibition experiments on the p38 and ERK1/2 MAPK signaling pathways were performed with the specific inhibitors SB203580 and PD98059, respectively. As shown in Fig. 2, MCP-1 and KC were significantly inhibited (P < 0.01) by either the p38 or ERK1/2 inhibitor and completely abrogated when both inhibitors were used together, indicating that both the p38 and the ERK1/2 MAPK pathways participate in the production of chemokine mediators elicited by B. abortus infection.
Fig 2.
p38 and ERK1/2 signaling pathways are involved in the secretion of KC and MCP-1 by B. abortus-infected primary osteoblasts. Effects of SB23850 (SB), a p38 MAPK inhibitor, and PD98059 (PD), an ERK1/2 inhibitor, on B. abortus induction of MCP-1 (A) and KC (B), respectively, are shown. Levels of MCP-1 and KC production by primary mouse osteoblasts were measured at 48 h after infection with B. abortus at an MOI of 100 by ELISA. P values for comparison with results for untreated infected cells (Untreated) are as follows: **, P < 0.01; ***, P < 0.001. Data shown are from a representative experiment of five performed.
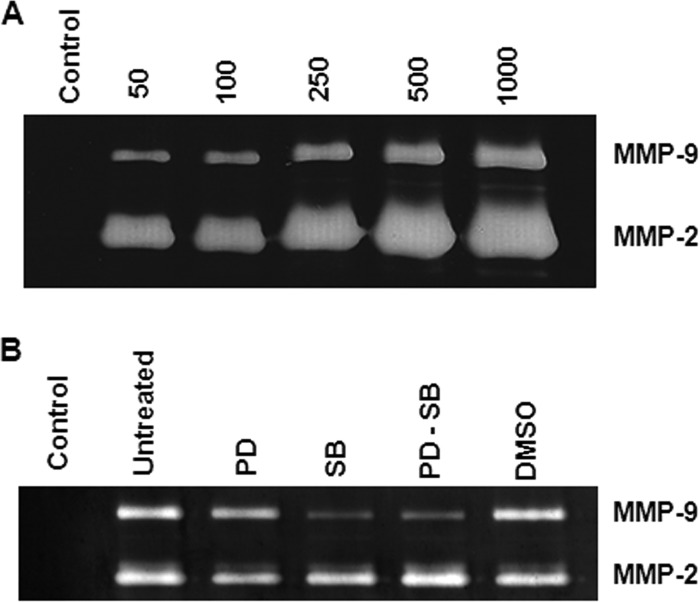
Infections of primary osteoblast cells with B. abortus were performed at MOIs of 50, 100, 250, 500, and 1,000. Significantly increased levels of MMP-2 and MMP-9 were detected at 48 h p.i. in the supernatants from infected cells (Fig. 3A). The p38 MAPK pathway participated in the secretion of MMP-9 induced by B. abortus infection since SB203580 inhibited MMP-9 production. In contrast, PD98059 was unable to inhibit MMP-9 production, indicating that the ERK1/2 MAPK pathway does not play a relevant role in the production of MMP-9. In addition, when both inhibitors were used, the inhibition of MMP-9 production was similar to that produced by SB203580, corroborating that MMP-9 production is mainly dependent on the p38 MAPK pathway and independent of the ERK1/2 pathway (Fig. 3B). In contrast, MMP-2 secretion was not affected by SB203580 or PD98059, indicating that p38 and ERK1/2 do not participate in the MMP-2 secretion induced by B. abortus infection (Fig. 2B).
Fig 3.
p38 signaling pathway is involved in MMP-9 secretion. (A) MMP-9 and MMP-2 production by primary mouse osteoblasts infected with B. abortus at different MOIs (50 to 1,000) or not infected (Control). (B) Effects of SB23850 (SB), a p38 MAP kinase inhibitor, and PD98059 (PD), an ERK1/2 inhibitor, on MMP production by B. abortus-infected (MOI of 100) primary mouse osteoblasts. Data shown are from a representative experiment of five performed.
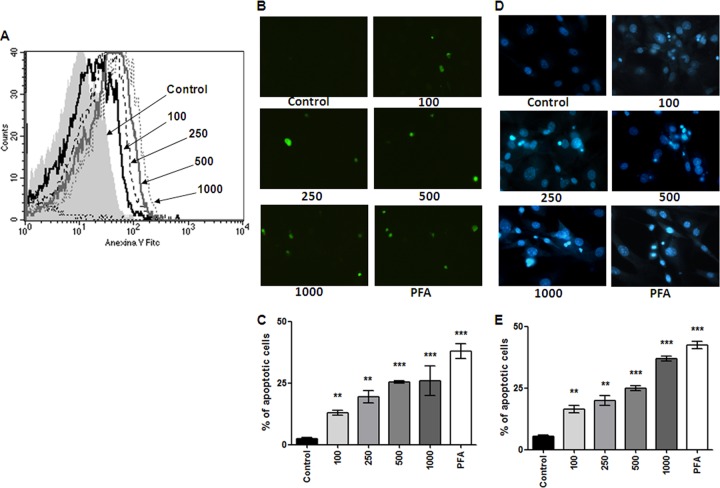
B. abortus infection induces osteoblast apoptosis.
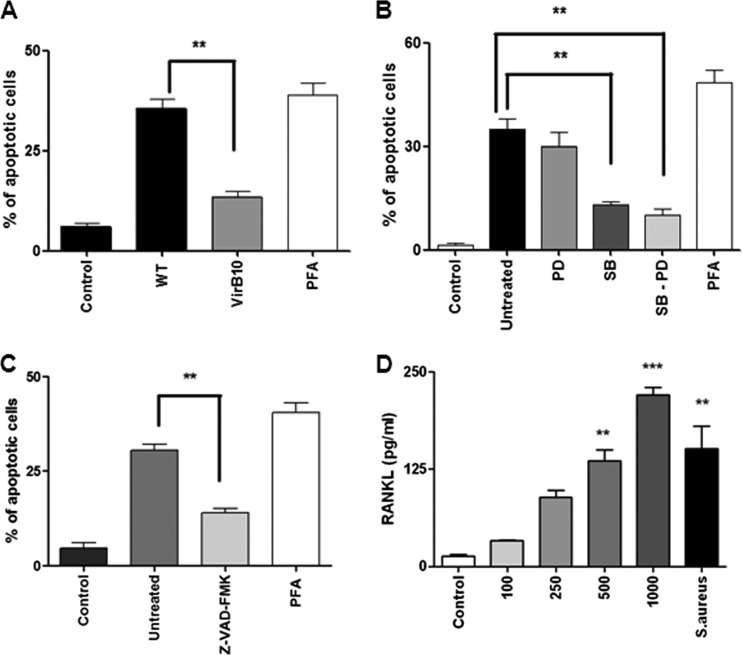
Osteoblast apoptosis is known to be involved in bone loss and results in the elimination of the cells responsible for matrix deposition (20, 36). We then investigated whether B. abortus could also induce osteoblast apoptosis. To test this hypothesis, primary mouse osteoblasts were infected with B. abortus, and after 24 h, the cells were stained with annexin V-phosphatidylinositol (PI) and analyzed by flow cytometry. Paraformaldehyde (PFA) at 4% was used as a positive control. B. abortus infection induced osteoblast apoptosis in an MOI-dependent manner (Fig. 4A). The occurrence of apoptosis was confirmed by TUNEL (Fig. 4B and D) and Hoechst 33342 staining (Fig. 4C and E). Apoptosis was dependent on the expression of a functional type four secretion system (T4SS), since the percentage of apoptotic cells did not differ significantly between osteoblast cells infected with a B. abortus virB10 polar mutant and uninfected controls (Fig. 5A). In addition, apoptosis was dependent on the p38 MAPK pathway since the phenomenon was significantly (P < 0.01) inhibited when we performed the infection in the presence of SB203580, a specific p38 inhibitor. On the contrary, there was not a significant difference in apoptosis levels between B. abortus-infected osteoblasts and B. abortus-infected osteoblasts in the presence of PD98059, a specific ERK1/2 inhibitor (Fig. 5B).
Fig 4.
B. abortus infection induces osteoblast apoptosis. Apoptosis induced by B. abortus infection in primary mouse osteoblasts. Osteoblastic cells were infected at different MOIs (100 to 1,000) or were treated with 4% paraformaldehyde (PFA) as a positive control, and apoptosis was evaluated by the annexin V, TUNEL, and Hoechst 33342 techniques. (A) Flow cytometry analysis of apoptotic cells by annexin V-FITC binding. (B to E) Fluorescence microscopy analysis of apoptotic cells by TUNEL (B, C) and Hoechst 33342 staining (D, E). P values for comparison with results for uninfected cells (Control) are as follows: **, P < 0.01; ***, P < 0.001. Data shown are from a representative experiment of five performed.
Fig 5.
B. abortus induces RANKL expression on osteoblasts and apoptosis of these cells in a way that is dependent on a functional T4SS, MAPKs, and caspases. (A) Analysis by fluorescence microscopy of apoptotic cells by Hoechst 33342. Primary mouse osteoblasts were infected at an MOI of 100 with either wild-type B. abortus (WT) or its isogenic virB10 mutant (VirB10). **, P < 0.01 versus the results for cells infected with the virB10 mutant. Paraformaldehyde (PFA) was used as a positive control for apoptosis. (B) Primary mouse osteoblasts were infected at an MOI of 100 with B. abortus and treated or not with ERK1/2 inhibitor PD98059 (PD), p38 inhibitor SB23850 (SB), or both inhibitors administered together (PD-SB). **, P < 0.01 versus the results for cells treated with inhibitors. (C) Primary mouse osteoblasts were treated with a general caspase inhibitor, Z-VAD-FMK, and 2 h later, cells were infected with B. abortus. **, P < 0.01 versus the results for cells treated with the general caspase inhibitor. (D) RANKL production by primary mouse osteoblasts infected with B. abortus at different MOIs (100 to 1,000) or not stimulated (Control). Staphylococcus aureus at an MOI of 100 was used as a positive control. P values for comparison with the results for uninfected cells (Control) are as follows: **, P < 0.01; ***, P < 0.001. Data shown are from a representative experiment of five performed.
B. abortus-mediated apoptosis has been reported for a variety of cells, including astrocytes and hepatocytes (13, 15). In particular, it has been demonstrated that astrocyte apoptosis induced by B. abortus infection involves caspase activation (15). To determine the role of caspases in osteoblast apoptosis induced by B. abortus, these cells were treated with a general caspase inhibitor (Z-VAD-FMK) and then infected with B. abortus at an MOI of 100. Apoptosis was assessed by staining cells with Hoechst stain and analyzing them with fluorescence microscopy. Z-VAD-FMK inhibited the osteoblast apoptosis induced by B. abortus infection (Fig. 5C). Altogether, these results indicate that B. abortus infection induces osteoblast apoptosis and that p38 MAPK and caspases participate in cell death.
B. abortus induces RANKL in osteoblasts.
RANKL is a key molecule implicated in bone remodeling. Once expressed on osteoblasts, it initiates bone resorption, since RANKL-expressing osteoblasts are the main osteoclastogenesis-supporting cells (46). Moreover, RANKL is upregulated in other bacterial osteomyelitides (10). Therefore, we investigated whether B. abortus infection leads to RANKL expression in osteoblasts. Quantitative analysis of RANKL expression on osteoblast lysates 24 h p.i. revealed that B. abortus was able to induce the upregulation of RANKL expression with respect to the level in unstimulated cells in an MOI-dependent fashion (Fig. 5D). As we expected, Staphylococcus aureus infection also induced upregulation of RANKL expression (Fig. 5D) (10). In contrast, RANKL was not found in culture supernatants from B. abortus-infected osteoblasts (not shown). This indicates that B. abortus-induced upregulation of RANKL could contribute to bone destruction through activation of osteoclasts.
B. abortus infection inhibits osteoblast differentiation.
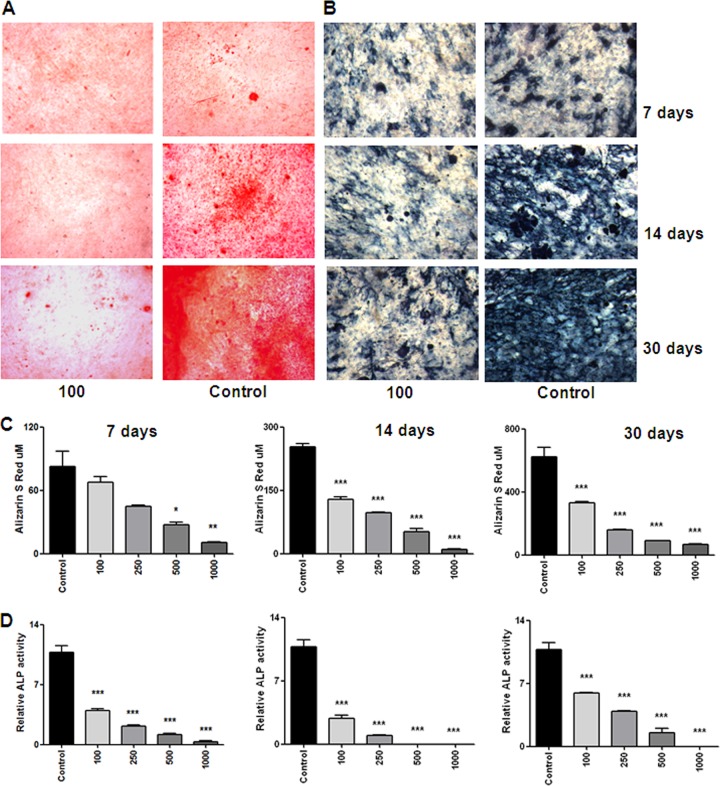
It has been reported that alkaline phosphatase (ALP) is an osteoblast phenotype marker and an essential enzyme for mineralization (4). In addition, the expression of ALP is closely associated with osteoblastic differentiation (23). We thus measured ALP activity during osteoblast differentiation. B. abortus infection reduced ALP activity in an MOI-dependent manner both in MC3T3-E1 cells and primary osteoblasts (Fig. 6B and D) on days 7, 14, and 30. These results indicate that B. abortus infection can effectively inhibit osteoblast function.
Fig 6.
B. abortus infection inhibits mineral matrix deposition by osteoblasts. Effects of B. abortus infection (MOI of 100) on mineral deposition at different days of primary mouse osteoblast cultures are shown. (A) Calcium deposition revealed by alizarin red S staining. (B) ALP activity revealed with BCIP-NBT solution. (C) Quantification of alizarin red S showed that inhibition of calcium deposition was in an MOI-dependent manner. (D) Quantification of ALP activity showed that inhibition of the activity was in an MOI-dependent manner. P values for comparison with results for uninfected cells (Control) are as follows: *, P < 0.1, **, P < 0.01; ***, P < 0.001. Data shown are from a representative experiment of five performed.
B. abortus inhibits mineralization of osteoblasts.
Osteoblast differentiation in vivo and also in vitro can be characterized by the deposition and mineralization of bone matrix, leading to the rigidity of the skeletal system (7, 30). Mineralization takes place between day 7 and day 10 and can be detected by staining the mineral deposits on the osteoblasts. We next investigated the effect of B. abortus infection on the mineralization of osteoblasts by staining calcium-rich deposits on the osteoblasts with alizarin red S staining. Both B. abortus-infected and uninfected MC3T3-E1 cells and primary osteoblasts progressively deposited more mineral with time, but the infected cells produced significantly (P < 0.001) less mineral than the control in an MOI-dependent fashion on days 7, 14, and 30 (Fig. 6A and C), demonstrating an inhibition of osteoblast mineralization by B. abortus.
B. abortus inhibits deposition of organic matrix.
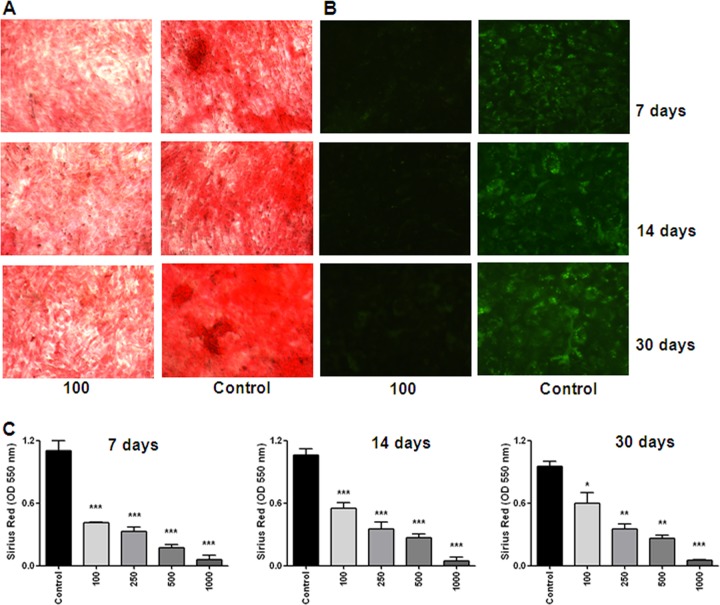
Collagen comprises 85 to 90% of the total organic bone matrix (41). Bones show a variety of structural organizations that are related to the balance between the amount of collagen and mineral (28). However, not only mineral matrix deposition is essential to ensure a healthy bone, giving strength and rigidity in the skeletal system; the adequate deposition of organic matrix also contributes to bone architecture. Therefore, experiments were conducted to determine whether B. abortus infection inhibits organic matrix deposition. Collagen was stained by adding Sirius red, a strong anionic dye that binds strongly to collagen molecules. Both B. abortus-infected and uninfected MC3T3-E1 cells progressively deposited more collagen with time, but infected cells produced significantly (P < 0.001) less collagen than the control in an MOI-dependent fashion on days 7, 14, and 30 (Fig. 7A and C), demonstrating the inhibition of osteoblast collagen deposition induced by B. abortus infection. To determine whether these results could be extended to another protein present in the organic bone matrix, we decided to study the effect of B. abortus infection on the expression of osteocalcin. Osteocalcin is a noncollagenous, highly conserved and secreted protein that is associated with the mineralized matrix of bone (30). Using a specific antiosteocalcin antibody, we demonstrated that B. abortus infection could also inhibit the deposition of osteocalcin in an MOI-dependent fashion (Fig. 7B).
Fig 7.
B. abortus infection inhibits organic matrix deposition by osteoblasts. Effects of B. abortus infection (MOI of 100) on collagen and osteocalcin deposition at different days of primary mouse osteoblast cultures. (A) Collagen deposition revealed by Sirius red staining. (B) Osteocalcin deposition revealed by immunofluorescence with a specific antibody. (C) Quantification of Sirius red showed that inhibition of collagen deposition was in an MOI-dependent manner. P values for comparison with the results for uninfected cells (Control) are as follows: *, P < 0.1**, P < 0.01; ***, P < 0.001. Data shown are from a representative experiment of five performed.
TNF-α is a key cytokine involved in RANKL expression in osteoblasts induced by supernatants from B. abortus-infected macrophages.
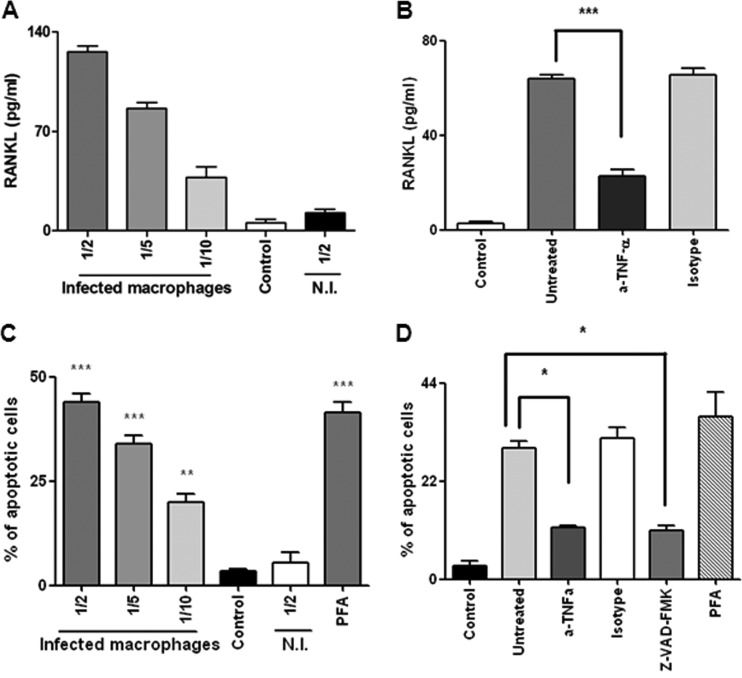
In view of the ability of Brucella-infected osteoblasts to secrete MCP-1 (Fig. 2), a key cytokine involved in monocyte/macrophage migration, we hypothesized that macrophages could be attracted to the site of infection (14). Thus, we decided to investigate the effect of cytokines present in supernatants from B. abortus-infected macrophages on the production of RANKL by osteoblasts. Therefore, supernatants from B. abortus-infected macrophages were harvested at 24 h postinfection, sterilized by filtration, and used to stimulate osteoblasts. The addition of supernatants from B. abortus-infected macrophages in different proportions (1/2 to 1/10) induced RANKL expression in osteoblasts in a dose-dependent manner compared to its expression in unstimulated cells or cells stimulated with supernatants from uninfected macrophages (Fig. 8A). It has been proposed that TNF-α may induce RANKL expression in a variety of cell types (16, 24, 40, 53, 55). To verify that the TNF-α levels present in supernatants from B. abortus-infected macrophages can stimulate RANKL expression by osteoblasts, these cells were incubated in the presence of supernatants from B. abortus-infected macrophages preincubated or not for 1 h with either an anti-TNF-α neutralizing antibody or an isotype control. As shown in Fig. 8B, neutralization of TNF-α significantly reduced (P < 0.001) the ability of supernatants to stimulate RANKL expression by osteoblasts, whereas the isotype control had no effect. Altogether, these results indicate that macrophage infection could alter bone homeostasis by inducing the expression of RANKL in osteoblasts and that TNF-α is involved.
Fig 8.
Effects of supernatants from B. abortus-infected macrophages on osteoblast differentiation and function. (A) RANKL production by primary mouse osteoblasts stimulated with supernatants from B. abortus-infected macrophages (added at a 1/2, 1/5, or 1/10 proportion) or from macrophages that were not infected (N.I.) was determined in cell lysates by ELISA. (B) Inhibition of the stimulating effect by pretreatment of supernatants with an anti-TNF-α neutralizing antibody or an isotype control for 1 h before addition to osteoblasts. (C) Fluorescence microscopy analysis of apoptosis using the Hoechst 33342 technique in primary osteoblasts stimulated with conditioned medium from macrophages infected or not with B. abortus. (D) Analysis of inhibition of apoptosis induced by supernatants from B. abortus-infected macrophages. Supernatants were pretreated with a neutralizing antibody to TNF-α or an isotype control or with a general caspase inhibitor for 1 h before addition to primary osteoblasts. Apoptotic cells were determined by fluorescence microscopy analysis using the Hoechst 33342 technique. P values for comparisons with the results for unstimulated cells (Control) are as follows: *, P < 0.1; **, P < 0.01; ***, P < 0.001. The data shown are from a representative experiment of five performed. (C, D) Paraformaldehyde (PFA) was used as a positive control for apoptosis.
TNF-α present in supernatants from B. abortus-infected macrophages induces apoptosis of osteoblasts through caspase coupling.
Macrophages have been shown to produce tissue injury in different organs, including bone, through the release of MMPs and proinflammatory cytokines (50). To analyze whether factors secreted by B. abortus-infected macrophages could induce osteoblast injury, we studied the effects of conditioned medium from infected macrophages on osteoblast cells. Supernatants from B. abortus-infected macrophages added to osteoblast cells induced osteoblast apoptosis in a dose-dependent manner. In contrast, the apoptosis levels in osteoblasts stimulated with conditioned medium from uninfected macrophages were similar to the levels in unstimulated cultures (Fig. 8C).
TNF-α is a proinflammatory cytokine that induces apoptosis in a number of cell systems, including osteoblasts (21, 50). To test the effect of TNF-α present in supernatants from B. abortus-infected macrophages on osteoblast apoptosis, we stimulated osteoblasts with supernatants from B. abortus-infected macrophages pretreated with a neutralizing anti-TNF-α antibody or an isotype control. As shown in Fig. 8D, neutralization of TNF-α partially inhibited the ability of supernatants to induce osteoblast apoptosis, whereas the isotype control had no effect.
TNF-α signaling via tumor necrosis factor receptor 1 (TNFR1) has been known to induce apoptosis through the coupling of caspase-8 with TNFR superfamily 1A (TNFRSF1A)-associated via death domain (TRADD) which, in turn, activates caspase-3 (9). To determine whether caspases are involved in the apoptosis induced by supernatants from B. abortus-infected macrophages, osteoblasts were pretreated with a general caspase inhibitor (Z-VAD-FMK) and then stimulated with supernatants from B. abortus-infected macrophages. Apoptosis was assessed by staining cells with Hoechst stain and analyzed by fluorescence microscopy. Z-VAD-FMK inhibited the osteoblast apoptosis induced by supernatants from B. abortus-infected macrophages, whereas supernatants from uninfected macrophages did not (Fig. 8D).
Supernatants from B. abortus-infected macrophages inhibit mineralization and organic matrix deposition of osteoblasts due largely to TNF-α.
Inflammation has positive effects on the immune response against pathogens; however, persistent inflammation may have negative consequences, such as inhibition of tissue regeneration. We have previously demonstrated that macrophages secrete proinflammatory cytokines in response to B. abortus infection and produce bone damage through osteoclastogenesis induction (12). In addition, these macrophages could induce bone injury inhibiting osteoblast differentiation. To test this hypothesis, we added supernatants from B. abortus-infected macrophages to osteoblast cells. The addition of supernatants from B. abortus-infected macrophages in different proportions (1/2 to 1/10) to uninfected osteoblasts significantly inhibited, in a dose-dependent manner, the capacity of the osteoblasts to secrete mineral and organic matrix deposition compared to that of osteoblasts stimulated with supernatants from uninfected macrophages or unstimulated cultures, both of which were also grown in differentiation medium (not shown).
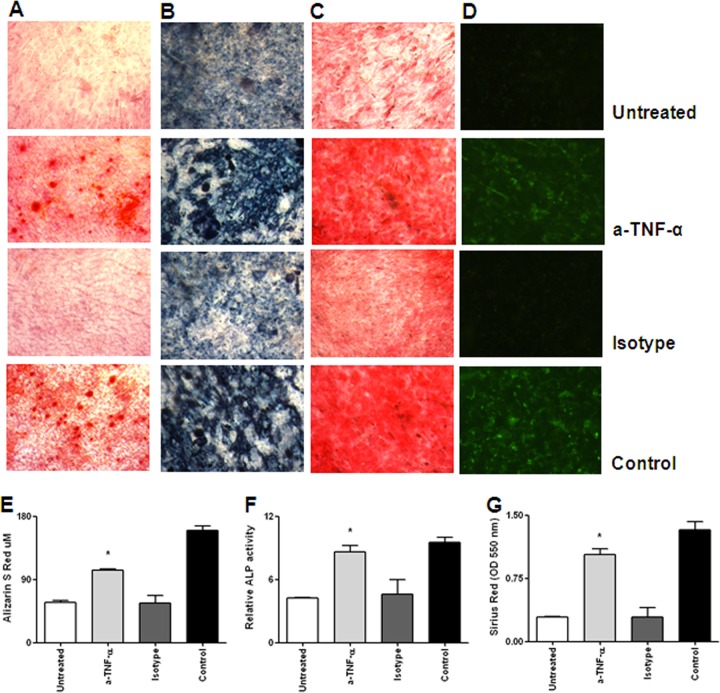
It has been reported (31) that TNF-α plays a main role in impeding osteoblast differentiation. To assess whether the levels of TNF-α present in supernatants from B. abortus-infected macrophages could inhibit osteoblast differentiation, these cells were incubated in the presence of supernatants from infected macrophages preincubated or not for 1 h with either an anti-TNF-α neutralizing antibody or an isotype control. As shown in Fig. 9, neutralization of TNF-α reduced the ability of supernatants to inhibit osteoblast differentiation, whereas the isotype control had no effect. These results indicate that TNF-α plays a key role in inhibiting osteoblast differentiation, thereby contributing in another way to bone loss.
Fig 9.
TNF-α is involved in inhibitory effects induced by supernatants from B. abortus-infected macrophages. Pretreatment of supernatants from B. abortus-infected macrophages harvested at 24 h p.i. with a neutralizing anti-TNF-α antibody inhibited the effects of the supernatants on osteoblast mineralization and organic matrix deposition. (A to D) Alizarin red S staining (A), ALP activity revealed with BCIP-NBT (B), Sirius red staining (C), and osteocalcin deposition revealed by immunofluorescence (D). (E to G) Quantitative analysis of alizarin red S (E), relative ALP activities (F), and Sirius red staining (G). *, P < 0.1 versus the results for unstimulated cells (Control). Data shown are from a representative experiment of five performed.
DISCUSSION
Bone is normally resistant to infection. However, Brucella spp. have a tropism for osteoarticular localization. Accordingly, osteoarticular brucellosis is the most common localization of active brucellar disease (1, 11, 33, 34, 57). Although the clinical aspects of this form of the disease have been described widely (33, 34), the molecular pathogenic mechanisms have only recently been partially described (14, 42).
In this article, we describe the modifications that occurred in osteoblast metabolism when these cells were infected by B. abortus and how this response inhibited osteoblast differentiation and function, leading to bone loss. First, we demonstrated that B. abortus may invade and replicate in primary mouse osteoblasts and the murine cell line MC3T3.E1, as has been previously described for human osteoblasts (14, 42). The parallelism between mice and humans underscores the usefulness of the mouse model developed in this investigation for future studies of immune mechanisms that may be implicated in the pathogenesis of the osteoarticular forms of human brucellosis.
To our knowledge, there are no reports on the ability of Brucella to infect human osteoblasts in vivo. This may be explained in part by ethical restrictions, since a biopsy of the affected bone may be justified only in very select cases. Nevertheless, in the few published studies performed in dogs in which culture of bone tissue samples was performed, Brucella organisms were isolated from such samples (22, 43). Also, during experimental infections performed on mice, intracellular bacteria have been found in the epiphysis and metaphysis of peripheral joints, as well as in the subchondral region of vertebrae (32). All these result give relevance to our in vitro finding that B. abortus is able to invade and replicate in osteoblasts.
Using primary cultures of mouse osteoblasts, we showed here that B. abortus infection readily induces p38 and ERK1/2 MAPK phosphorylation, thus enlisting these pathways among the kinase pathways that Brucella may address as it invades the osteoarticular system. Using specific inhibitors, we determined that these pathways are implicated in chemokine and MMP production and also in the induction of apoptosis of osteoblasts by B. abortus. The MAPK signaling cascade, an important group of signaling pathways, has been implicated in bacterial pathogenesis (19, 44, 47, 48), and in this model, Brucella has not been the exception.
Osteoarticular brucellosis, including spondylitis and arthritis, may be destructive and associated with osteopenia and cartilage damage (11). Such tissue damage may be due to a direct effect of Brucella infection on osteoblasts and/or a result of the ongoing inflammatory response. In line with the first proposed mechanism, we showed that B. abortus infection can induce apoptosis of primary mouse osteoblasts. Whether pathogen-induced apoptosis is harmful or beneficial to the host has been a considerable source of debate (26, 56). Osteoblast apoptosis may be responsible, at least in part, for the damage produced by Brucella infection to bone since apoptosis would reduce the number of osteoblasts in bone. It may seem contradictory that B. abortus is able to induce osteoblast apoptosis when it has been reported that Brucella species are able to inhibit macrophage apoptosis (8). However, this finding is not particularly surprising since Brucella spp. are mainly adapted to establish chronic infections in macrophages (3). Thus, our results suggest that osteoblasts do not constitute a survival niche for long-term Brucella persistence in osteoarticular localization and go along with the fact that B. abortus can induce apoptosis of cell types other than macrophages (13, 15). We hypothesize that apart from resident macrophages, other monocytes/macrophages could be attracted to the site of osteoarticular infection through MCP-1 secretion mediated by B. abortus-infected osteoblasts, increasing the likelihood of finding a replicative niche.
Mineralization is a process in which phosphate and calcium become deposited in bone, and the adequate deposition of organic matrix also contributes to bone architecture (18). This gives the bones additional strength and rigidity. During B. abortus infection, osteoblasts become less differentiated and decrease the activity of a specific marker of differentiation involved in bone mineralization, ALP. Also, mineral and organic matrix deposition are inhibited, contributing to the bone loss observed in osteoarticular pathology. Altogether, our results indicate that B. abortus infection can affect bone formation by at least two mechanisms, by rendering osteoblasts less differentiated and therefore less able to deposit mineral matrix and/or by entering the apoptotic pathway and dying.
Another mechanism to induce bone damage is through pathological induction of osteoclastogenesis; in this way, RANKL is a homotrimeric molecule displayed on the membrane of osteoblasts that stimulates differentiation of osteoclasts and is a key induction molecule involved in bone resorption, leading to bone destruction (5). As occurs with other bacterial osteoarticular infections (10, 45, 49), B. abortus induced an increase in RANKL expression in osteoblasts. The increase in RANKL is likely to trigger osteoclast-induced bone resorption and bone destruction and may help to explain why patients with brucellar osteomyelitis have significant bone loss (1, 11, 33, 34, 39, 57).
In addition, macrophages (the ones that dwell in the bone or the ones that could be attracted to the site of infection) also contributed to bone damage through apoptosis induction via TNF-α and caspases. TNF-α appeared to be the main cytokine involved in osteoblast apoptosis induced by supernatants from B. abortus-infected macrophages, because apoptosis was inhibited when supernatants from B. abortus-infected macrophages were preincubated with a neutralizing anti-TNF-α antibody. The fact that a pancaspase inhibitor also inhibited Brucella-induced apoptosis underscores the relevance of TNF-α in this phenomenon, because apoptosis induced by TNF-α has been known to involve caspase activation (9).
TNF-α from B. abortus-infected macrophages may affect osteoblast metabolism by inhibiting mineral and organic matrix deposition, since the inhibition was reversed when supernatants were pretreated with neutralizing anti-TNF-α antibody. These results are in concordance with previous reports demonstrating that TNF-α may modulate bone mineralization (37).
As mentioned above, the increase in RANKL is likely to trigger osteoclast-induced bone resorption and bone destruction; even though B. abortus infection may induce upregulation of RANKL on the osteoblast membrane, macrophages may also contribute to this mechanism. We found that soluble mediators secreted by B. abortus-infected macrophages could contribute to RANKL expression on osteoblast membrane. This induction depended on the presence of TNF-α in supernatants from B. abortus-infected macrophages, since pretreatment with a neutralizing anti-TNF-α antibody abrogated the response. These results are consistent with the observation that TNF-α triggers osteoclast differentiation and subsequent bone destruction (2).
In summary, the results presented here, together with our previous observations (12, 14, 42), are shedding light on how the interactions of B. abortus with cells involved in bone metabolism and its intermingling with cells involved in innate immunity play a role in the pathogenesis of osteoarticular brucellosis. B. abortus may infect osteoblasts, inhibiting their differentiation and function. B. abortus-infected osteoblasts also upregulate the expression of RANKL, a cytokine that has been indicated as the major cytokine regulating osteoclast differentiation by interacting with RANK on the surface of osteoclast precursors, inducing pathological bone resorption. Since osteoblasts play a key role in healthy bone balance, we submit that osteoblast dysfunction would be another mechanism contributing to the bone damage observed in osteoarticular brucellosis.
Upon infection with B. abortus, osteoblasts also release MCP-1 (14, 42), which can attract macrophages to the site of infection. In the in vivo situation, attracted and resident infected monocytes/macrophages can respond to Brucella infection with the production of proinflammatory cytokines such as TNF-α, inducing increases in the expression of RANKL by osteoblasts and osteoblast apoptosis and inhibiting osteoblast function and differentiation.
Based on the results obtained in the present study, we hypothesize that B. abortus may harm osteoblast function directly and indirectly, contributing to the bone and joint destruction observed in patients with osteoarticular complications of brucellosis.
ACKNOWLEDGMENTS
We thank Horacio Salomón and the staff of the Centro Nacional de Referencia del Sida, University of Buenos Aires, for their assistance with biosafety level 3 laboratory use.
This work was supported by grants PICT2006-0517, PICT2007-0139 and PICT2006-1335 from Agencia Nacional of Promoción Científica y Tecnológica (ANPCYT, Argentina), by grant UBACYT 20020090100083 from Universidad de Buenos Aires, and by grant PIP112-200801-02706 from Consejo Nacional de Investigación Científica y Tecnológica (CONICET). R.S. is the recipient of a fellowship from CONICET. M.V.D., P.B., C.A.F, and G.H.G. are members of the Research Career of CONICET.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Aydin M, et al. 2005. Scintigraphic findings in osteoarticular brucellosis. Nucl. Med. Commun. 26:639–647 [DOI] [PubMed] [Google Scholar]
- 2. Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. 2000. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 275:4858–4864 [DOI] [PubMed] [Google Scholar]
- 3. Baker PJ, et al. 1999. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67:2804–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellows CG, Aubin JE, Heersche JN. 1991. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 14:27–40 [DOI] [PubMed] [Google Scholar]
- 5. Boyce BF, et al. 2005. TNF-alpha and pathologic bone resorption. Keio J. Med. 54:127–131 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JA, Cooper RR. 1987. Bone structure and function. Instr. Course Lect. 36:27–48 [PubMed] [Google Scholar]
- 8. Caron E, Gross A, Liautard JP, Dornand J. 1996. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J. Immunol. 156:2885–2893 [PubMed] [Google Scholar]
- 9. Chen G, Goeddel DV. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634–1635 [DOI] [PubMed] [Google Scholar]
- 10. Claro T, et al. 2011. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS One 6:e18748 doi:10.1371/journal.pone.0018748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colmenero JD, et al. 2008. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin. Infect. Dis. 46:426–433 [DOI] [PubMed] [Google Scholar]
- 12. Delpino MV, et al. 2012. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J. Leukoc. Biol. 91:285–298 [DOI] [PubMed] [Google Scholar]
- 13. Delpino MV, Barrionuevo P, Scian R, Fossati CA, Baldi PC. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J. Hepatol. 53:145–154 [DOI] [PubMed] [Google Scholar]
- 14. Delpino MV, Fossati CA, Baldi PC. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 77:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia Samartino C, et al. 2010. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am. J. Pathol. 176:1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goto H, et al. 2011. Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Cytokine 56:662–668 [DOI] [PubMed] [Google Scholar]
- 17. Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. 1985. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 260:2493–2500 [PubMed] [Google Scholar]
- 18. Hill PA. 1998. Bone remodelling. Br. J. Orthod. 25:101–107 [DOI] [PubMed] [Google Scholar]
- 19. Hobbie S, Chen LM, Davis RJ, Galan JE. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550–5559 [PubMed] [Google Scholar]
- 20. Hughes DE, Boyce BF. 1997. Apoptosis in bone physiology and disease. Mol. Pathol. 50:132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeng GW, et al. 1997. Measurement of synovial tumor necrosis factor-alpha in diagnosing emergency patients with bacterial arthritis. Am. J. Emerg. Med. 15:626–629 [DOI] [PubMed] [Google Scholar]
- 22. Kerwin SC, et al. 1992. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980–1991). J. Am. Vet. Med. Assoc. 201:1253–1257 [PubMed] [Google Scholar]
- 23. Kim CH, et al. 2002. IL-1beta regulates cellular proliferation, prostaglandin E2 synthesis, plasminogen activator activity, osteocalcin production, and bone resorptive activity of the mouse calvarial bone cells. Immunopharmacol. Immunotoxicol. 24:395–407 [DOI] [PubMed] [Google Scholar]
- 24. Kong YY, et al. 1999. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309 [DOI] [PubMed] [Google Scholar]
- 25. Krens SF, Spaink HP, Snaar-Jagalska BE. 2006. Functions of the MAPK family in vertebrate-development. FEBS Lett. 580:4984–4990 [DOI] [PubMed] [Google Scholar]
- 26. Kunes P, et al. 2009. Neutrophil apoptosis by Fas/FasL: harmful or advantageous in cardiac surgery? Thorac. Cardiovasc. Surg. 57:1–6 [DOI] [PubMed] [Google Scholar]
- 27. Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. 2003. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J. Immunol. 170:6244–6249 [DOI] [PubMed] [Google Scholar]
- 28. Landis WJ. 1995. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16:533–544 [DOI] [PubMed] [Google Scholar]
- 29. Lawrence MC, et al. 2008. The roles of MAPKs in disease. Cell Res. 18:436–442 [DOI] [PubMed] [Google Scholar]
- 30. Lee CW, et al. 2007. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L799–L812 [DOI] [PubMed] [Google Scholar]
- 31. Li W, et al. 2010. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem. Biophys. Res. Commun. 391:1228–1233 [DOI] [PubMed] [Google Scholar]
- 32. Lyons E, Magnani D, Forde Toni S, Splitter G, Adarichev Vyacheslav A. 2011. Brucellosis-induced murine arthritis and spondylolisthesis. Arthritis Rheum. 63(Suppl 10):2096 [Google Scholar]
- 33. Madkour MM. 2001. Bone and joint imaging, p 90–132 In Madkour MM. (ed), Madkour's brucellosis, 2nd ed Springer-Verlag, Berlin, Germany [Google Scholar]
- 34. Madkour MM. 2001. Osteoarticular brucellosis, p 74–87 In Madkour MM. (ed), Madkour's brucellosis, 2nd ed Springer-Verlag, Berlin, Germany [Google Scholar]
- 35. Murphy CM, Haugh MG, O'Brien FJ. 2010. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31:461–466 [DOI] [PubMed] [Google Scholar]
- 36. Ning RD, Zhang XL, Li QT, Guo XK. 2011. The effect of Staphylococcus aureus on apoptosis of cultured human osteoblasts. Orthop. Surg. 3:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Panagakos FS, Kumar S. 1994. Modulation of proteases and their inhibitors in immortal human osteoblast-like cells by tumor necrosis factor-alpha in vitro. Inflammation 18:243–265 [DOI] [PubMed] [Google Scholar]
- 38. Plunkett NA, Partap S, O'Brien FJ. 2010. Osteoblast response to rest periods during bioreactor culture of collagen-glycosaminoglycan scaffolds. Tissue Eng. Part A 16:943–951 [DOI] [PubMed] [Google Scholar]
- 39. Rajapakse CN. 1995. Bacterial infections: osteoarticular brucellosis. Baillieres Clin. Rheumatol. 9:161–177 [DOI] [PubMed] [Google Scholar]
- 40. Redlich K, et al. 2002. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 46:785–792 [DOI] [PubMed] [Google Scholar]
- 41. Sasano Y, et al. 2000. Immunohistochemical localization of type I collagen, fibronectin and tenascin C during embryonic osteogenesis in the dentary of mandibles and tibias in rats. Histochem. J. 32:591–598 [DOI] [PubMed] [Google Scholar]
- 42. Scian R, et al. 2011. Granulocyte-macrophage colony-stimulating factor- and tumor necrosis factor alpha-mediated matrix metalloproteinase production by human osteoblasts and monocytes after infection with Brucella abortus. Infect. Immun. 79:192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smeak DD, Olmstead ML, Hohn RB. 1987. Brucella canis osteomyelitis in two dogs with total hip replacements. J. Am. Vet. Med. Assoc. 191:986–990 [PubMed] [Google Scholar]
- 44. Smith MD, et al. 1992. Immunohistochemical analysis of synovial membranes from inflammatory and non-inflammatory arthritides: scarcity of CD5 positive B cells and IL2 receptor bearing T cells. Pathology 24:19–26 [DOI] [PubMed] [Google Scholar]
- 45. Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. 2008. Staphylococcus aureus induces expression of receptor activator of NF-kappaB ligand and prostaglandin E2 in infected murine osteoblasts. Infect. Immun. 76:5120–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takayanagi H. 2010. The unexpected link between osteoclasts and the immune system. Adv. Exp. Med. Biol. 658:61–68 [DOI] [PubMed] [Google Scholar]
- 47. Tang P, Rosenshine I, Cossart P, Finlay BB. 1996. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect. Immun. 64:2359–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang P, Sutherland CL, Gold MR, Finlay BB. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang Y, et al. 2011. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J. Endod. 37:1653–1658 [DOI] [PubMed] [Google Scholar]
- 50. Thammasitboon K, Goldring SR, Boch JA. 2006. Role of macrophages in LPS-induced osteoblast and PDL cell apoptosis. Bone 38:845–852 [DOI] [PubMed] [Google Scholar]
- 51. Tierney CM, Jaasma MJ, O'Brien FJ. 2009. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J. Biomed. Mater. Res. A 91:92–101 [DOI] [PubMed] [Google Scholar]
- 52. Tullberg-Reinert H, Jundt G. 1999. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 112:271–276 [DOI] [PubMed] [Google Scholar]
- 53. Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T. 2002. The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 4:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong GL, Cohn DV. 1975. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc. Natl. Acad. Sci. U. S. A. 72:3167–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu L, Lin JH, Bao K, Li PF, Zhang WG. 2009. In vitro effects of erythromycin on RANKL and nuclear factor-kappa B by human TNF-alpha stimulated Jurkat cells. Int. Immunopharmacol. 9:1105–1109 [DOI] [PubMed] [Google Scholar]
- 56. Yamashita N, et al. 2000. Induction of apoptosis in bronchial eosinophils: beneficial or harmful? Int. Arch. Allergy Immunol. 122(Suppl 1):40–43 [DOI] [PubMed] [Google Scholar]
- 57. Young EJ. 1989. Clinical manifestations of human brucellosis. In Young EJ, Corbel MJ, (ed) Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL [Google Scholar]