Abstract
Nocardia brasiliensis is an intracellular microorganism and the most common etiologic agent of actinomycetoma in the Americas. Several intracellular pathogens induce an immunosuppressive microenvironment through increases in CD4+ Foxp3+ regulatory T cells (Treg), thus downregulating other T-cell subpopulations and assuring survival in the host. In this study, we determined whether N. brasiliensis modulates T-lymphocyte responses and their related cytokine profiles in a murine experimental model. We also examined the relationship between N. brasiliensis immunomodulation and pathogenesis and bacterial survival. In early infection, Th17/Tc17 cells were increased at day 3 (P < 0.05) in footpad tissue and spleen. Treg subpopulations peaked at days 7 and 15 (P < 0.01) in the footpad and spleen, respectively. Transforming growth factor β1 (TGF-β1) and interleuki-10 (IL-10) are cytokines known for their immunosuppressive effects. During early and chronic infections, these cytokines were elevated with increased TGF-β1 levels from days 3 to 30 (P < 0.01) and sustained IL-10 expression throughout infection compared to uninfected mice. IL-6 production was increased at day 3 (P < 0.01), whereas gamma interferon (IFN-γ), IL-17A, and IL-23 levels were highest at day 15 postinfection (P < 0.01) when a decrease in the bacterial load (>1 log) was also observed (P < 0.05). After these changes, at 30 to 60 days postinfection, IFN-γ production was decreased, whereas the expression of anti-inflammatory cytokines and the bacterial load again increased (P < 0.05). The increment in Treg cells and the related cytokine profile correlated with reduced inflammation at day 15 (P < 0.05) in the footpad. We conclude that N. brasiliensis modulates the immune system to induce an immunosuppressive microenvironment that benefits its survival during the chronic stage of infection.
INTRODUCTION
Nocardia brasiliensis is a Gram-positive, partially acid-fast bacterium belonging to the order of Actinomycetales. In Mexico, N. brasiliensis has been isolated in 86.6% of actinomycetoma cases (22). The infection occurs by accidental inoculation of bacteria in the skin or cutaneous tissue (32). Actinomycetoma caused by N. brasiliensis is a chronic disease that occurs in immunocompetent individuals; however, some studies suggest the possibility that subtle and continuous decay in immune protection may be responsible for the chronic infection (8).
Others studies have shown that the protective host immune response to N. brasiliensis involves cell-mediated immunity (24). IgM, but not IgG, monoclonal antibodies also confer protection against experimental actinomycetoma (10). In the early stages of infection, there is a local infiltration of polymorphonuclear cells, followed by phagocytic and mononuclear cells to the site of infection. N. brasiliensis is an intracellular bacterium that survives and multiplies inside macrophages (34). Little is known concerning how N. brasiliensis escapes from microbicidal mechanisms developed by the host.
Using a murine model of N. brasiliensis, Salinas-Carmona et al. (34) demonstrated that the Th1 and Th2 type immunity are involved in the host response against N. brasiliensis. The same study reported the presence of N. brasiliensis antibodies in the sera of mice after infection with N. brasiliensis (34). Studies have also reported that IgM is involved in the partial protection against experimental actinomycetoma in BALB/c mice (10, 33).
Th17 and Tc17 lymphocytes are characterized by their production of interleukin-17 (IL-17), which is a proinflammatory cytokine with an important role in chronic inflammatory diseases, such as arthritis, myocarditis, asthma, and allergies (47). IL-17 induces the secretion of antimicrobial peptides, including defensins and S100 proteins, which are important factors in the host response against bacteria (14, 18, 20). Th17 cell differentiation in murine models of chronic infection with Mycobacterium tuberculosis and Mycobacterium bovis (16, 17, 47) is dependent on transforming growth factor β (TGF-β), IL-6, and IL-23. Early protection in the lungs against influenza virus A (12) is also dependent on the presence of Tc17 and Th17 cells.
Treg cells maintain the balance between anti-inflammatory and proinflammatory responses to protect the host from exacerbated inflammation and subsequent pathology. Treg produce IL-10 and TGF-β cytokines, which are associated with anti-inflammatory processes (5). These cells can suppress Th1, Th2, and Th17 functions (9) and may also inhibit antimicrobial activity in phagocytic cells, thus promoting microorganism survival. Treg are also found during the chronic stages of M. tuberculosis infection (35), suggesting that they may contribute to the persistent stages of M. tuberculosis infection by suppressing the immune response. Little is known about the microenvironment induced by N. brasiliensis in an experimental actinomycetoma model, especially with regard to T-cell immunomodulation.
In the present study, we evaluated changes in the Treg, Th17, and Tc17 populations, along with their related cytokine profiles, during the course of an experimental intracellular N. brasiliensis infection in BALB/c mice and whether these changes play a role in the immunopathogenesis of the disease. Here, we demonstrated for the first time that N. brasiliensis induces high levels of Th17 and Tc17 lymphocytes and inflammatory cytokines (IL-6, gamma interferon [IFN-γ], IL-17A, and IL-23) in the early stages of infection in situ. However, evidence of an immunosuppressive microenvironment (IL-10 and TGF-β1) was found in both early and chronic stages of infection. Therefore, we demonstrated that N. brasiliensis modulates the immune system to favor its survival and persistence in the host.
MATERIALS AND METHODS
Mice.
Eight- to twelve-week-old female BALB/c mice were used for the present study. Animals were derived from the colony kindly donated by Carl Hansen (Small Animal Section, Veterinary Resources Branch, National Institutes of Health, Bethesda, MD) and maintained under BSL2 conditions at the Department of Immunology. Mice received sterile water and Purina rodent food ad libitum. Protocols for animal care and infections were developed according to the International Review Board regulations and Mexican regulations (NOM-062-ZOO-1999) and were approved by The Bioethics Committee of the Facultad de Medicina at the Universidad Autonoma de Nuevo Leon.
Experimental infection.
Actinomycetoma infection was induced in mice by injecting 106 CFU of N. brasiliensis into a rear footpad as previously described (5). Footpad (n = 5) and spleen tissues from each animal were surgically removed at 0, 3, 7, 15, 30, and 60 days postinfection. Footpads and spleens were processed to obtain cell suspensions for flow cytometry analysis. Footpads were homogenized for bacterial load (in 5 ml of saline solution), cytokine (in 1 ml of 10 mM phosphate-buffered saline [PBS]; pH 7.2 to 7.4) or gene expression analysis (in 1 ml of TRIzol). Inflammation observed in the footpads and spleen of each mouse was registered at each time point indicated during the course of the study. The results shown in the present study are representative of three independent experiments with similar experimental designs.
Footpad digestion.
Footpads were surgically removed and placed in complete RPMI medium (cRPMI; RPMI 1640 supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 1% nonessential amino acids, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% fetal bovine serum) containing collagenase XI (0.7 mg/ml; Sigma-Aldrich) and type IV bovine pancreatic DNase (30 μg/ml; Sigma-Aldrich) prior to incubation for 30 min at 37°C. The digested tissue was disrupted by gently pushing the tissue sample through a cell strainer (BD Biosciences, Lincoln Park, NJ). Red blood cells were lysed with Gey's solution, washed, and resuspended in cRPMI. The total cell numbers per footpad were determined by using a hemocytometer.
Flow cytometric analysis.
Cell suspensions from the footpad and spleen containing 106 cells/ml from each mouse were incubated with monoclonal antibodies (MAbs) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) purchased from eBioscience (San Diego, CA). These cells were stained for cell surface markers by incubation with MAbs recognizing CD4 (clone RM4-5) and CD8 (clone eBioH35-17.2) molecules at 4°C for 30 min in the dark. After a washing step with PBS, intracellular staining was performed with MAbs against IL-17A (clone eBio17B7) or Foxp3 (clone FJK-16S). Samples were incubated again at 4°C for 30 min in the dark and then washed with PBS and resuspended in 500 μl of FACS flow solution (BD Biosciences). The data acquisition and analysis for the present study were completed using a FACSCalibur cytometer (BD Biosciences, Mountain View, CA) and CellQuest Pro software (BD Biosciences, San Jose, CA), respectively. Analyses were performed with an acquisition of at least 10,000 total events in the cell gate. The results are reported after background subtraction.
ELISAs.
Footpad samples were homogenized and cytokine quantification was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol for TGF-β1 (R&D Systems, MN), IL-6, IL-17A, and IL-23 (eBioscience, CA). Each sample was analyzed in triplicate.
Gene expression assays.
The footpad was homogenized in TRIzol and immediately frozen at −80°C. RNA was extracted according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). DNA was digested with RQ1 RNase-free DNase (Promega, Madison, WI), and RNA was reisolated with TRIzol. Finally, the concentration of RNA in each sample was determined by spectrophotometry, and 2 μg of RNA was reverse transcribed with Moloney murine leukemia virus (M-MuLV; Invitrogen, Carlsbad, CA) and random hexamers (Invitrogen, Carlsbad, CA). Real-time PCR was performed with 1 μl of cDNA, iQ Supermix (Bio-Rad, Hercules, CA) and specific primers and TaqMan probes (Table 1) supplied by Applied Biosystems, Foster City, CA) in a CFX96 thermocycler (Bio-Rad) to evaluate the relative mRNA expression of IL-10, TGF-β1, and IFN-γ. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to normalize the expression levels. The amplification conditions were as follows: 50°C for 2 min, 95°C for 2 min, and 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 58°C for 30 s. The cytokine mRNA levels were normalized to the GAPDH levels by using the ΔΔCT method to calculate relative changes.
Table 1.
Primers and TaqMan probes purchased from Applied Biosystems
| Gene | Product (Applied Biosystems) |
|---|---|
| GAPDH | Mm03302249_g1 |
| IL-10 | Mm00439614_m1 |
| TGF-β1 | Mm00441729_g1 |
| IFN-γ | Mm01168134_m1 |
Bacterial load.
Bacterial loads in the footpads of mice were determined by plating serial dilutions of the tissues homogenized on brain heart infusion agar and counting the CFU after 1 week of incubation at 37°C.
Statistical analysis.
The results presented are representative of three experiments. The data are expressed as means ± the standards error of the mean (SEM) (n = 5) from triplicate assays. One-way analysis of variance (ANOVA) and Tukey's post hoc test were used. Calculations were performed using GraphPad Prism version 4.00 for Windows (San Diego, CA). P values of <0.05 were considered significant.
RESULTS
Treg and Th17/Tc17 lymphocytes are present during the early stages of N. brasiliensis infection.
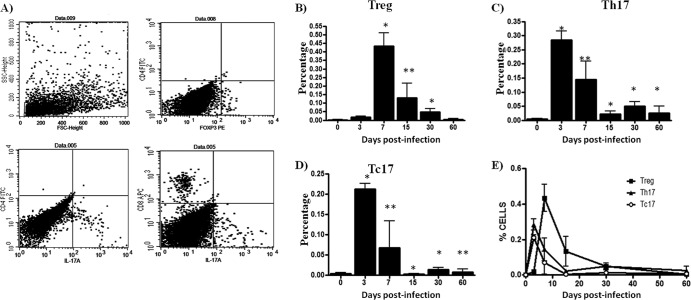
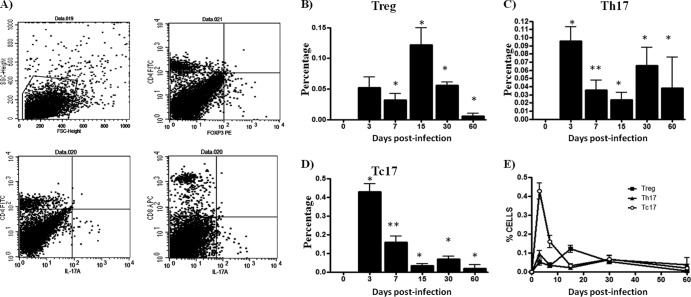
Treg and Th17/Tc17 lymphocytes in the footpads of experimentally infected BALB/c mice were studied by flow cytometry. Treg and Th17/Tc17 lymphocytes were identified by their characteristic CD4+ Foxp3+, CD4+ IL-17A+, and CD8+ IL-17A+ phenotypes, respectively. The percentages of Treg in the footpads (Fig. 1B) of N. brasiliensis-infected mice were higher in samples obtained at 7 days postinfection compared to cell suspensions obtained from the control group (0.4340% ± 0.0777 versus 0.002% ± 0.002; P < 0.001). However, the percentage of Treg lymphocytes (Fig. 2B) in spleens appeared to be highest in the samples obtained at 15 days postinfection compared to the same population from the control group mice (0.1220 ± 0.02780 versus 0 ± 0; P < 0.001). Cell suspensions obtained from the footpads at 3 days postinfection contained the highest percentages of Th17 and Tc17 cells (0.2840 ± 0.03250 versus 0.0040 ± 0.0024 [P < 0.001] and 0.2120 ± 0.0140 versus 0.0040 ± 0.0024 [P < 0.001]) compared to the control group mice (P < 0.05) (Fig. 1C and D). Meanwhile, cells analyzed from spleens demonstrated that the percentages of Th17 and Tc17 lymphocytes (Fig. 2C and D) obtained at 3 days postinfection were higher than those observed in control mice (0.0960 ± 0.0175 versus 0 ± 0 [P < 0.05] and 0.43 ± 0.04 versus 0 ± 0 [P < 0.05], respectively).
Fig 1.
T-cell dynamics during N. brasiliensis infection in mouse footpads. The percentages of T-cell subpopulations were obtained using specific monoclonal antibodies and flow cytometry analysis. (A) Representative dot plots show selected regions and specific staining used to study T-cell subpopulation. (B) CD4+ Foxp3+ (Treg) cell levels were increased at day 7 of infection. (C and D) CD4+ IL-17A+ (Th17) and CD8+ IL-17A+ (Tc17) cell levels were highest at day 3. (E) Dynamics of three subpopulations during N. brasiliensis infection. The data represent the means of five mice per group ± the SEM. Statistical analysis were performed using one-way ANOVA, and the post hoc test was Tukey's (*, P < 0.05; **, P < 0.01). The data are representative of three experiments of similar experimental design.
Fig 2.
Spleen T-cell dynamics during N. brasiliensis infection. The percentages of T-cell subpopulations were obtained using specific monoclonal antibodies and flow cytometry analysis. (A) Representative dot plots show the selected region, and specific staining was used to study the T-cell subpopulation. (B) CD4+ Foxp3+ (Treg) cell levels were increased at day 15 of infection. (C and D) CD4+ IL-17A+ (Th17) and CD8+ IL-17A+ (Tc17) cell counts were highest at day 3. (E) Dynamics of three subpopulations during N. brasiliensis infection. The data represent the means of five mice per group ± the SEM. Statistical analyses were performed using one-way ANOVA, and the post hoc test was Tukey's (*, P < 0.05; **, P < 0.01). The data presented in this figure are representative of three experiments of similar experimental design.
Immunosuppressive microenvironment during N. brasiliensis infection.
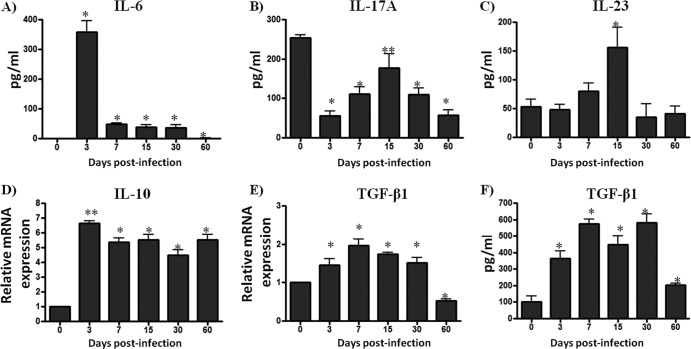
We studied whether the changes in the percentages of Treg and Th17/Tc17 cells observed in the footpad during the early stages of N. brasiliensis infection in BALB/c mice were also accompanied by changes in the cytokine profile in the same tissue. Using ELISA and quantitative PCR (qPCR), we investigated the levels of IL-6, IL-17A, IL-10, IL-23, and TGF-β1 cytokines in samples obtained from homogenized footpad tissues. The concentration of IL-6 in samples from day 3 postinfection footpads was higher than in the control group (358.1 ± 38.75 versus 1.097 ± 0.0975 [P < 0.001]). By 3 days postinfection, the concentration of IL-6 had decreased (Fig. 3A). The levels of IL-17A in control mice were higher than those observed in the footpads of mice in the early stages of infections (P < 0.05); however, once the infection was established, IL-17A increased at 15 days postinfection (P < 0.05) (Fig. 3B). The concentration of IL-23 cytokine in the same samples was found to be higher than in the control group at day 15 postinfection (155.6 ± 35.07 versus 53.15 ± 13.29 [P < 0.05]) (Fig. 3C). Interestingly, the expression of IL-10 in the N. brasiliensis-infected animals was higher than that of the controls at every time point (P < 0.05) (Fig. 3D). The concentrations of TGF-β1 protein and mRNA were elevated in all samples obtained between 3 and 30 days postinfection and decreased in samples obtained at day 60 postinfection (P < 0.05) (Fig. 3E and F).
Fig 3.
An immunosuppressive environment is predominant during N. brasiliensis infection. Footpad samples were collected and processed using ELISA or qPCR. (A) Graphs showing the concentration of IL-6 in footpad homogenates from each group of mice. (B and C) IL-17A and IL-23 levels were higher at day 15 of infection. (D) IL-10 expression was elevated during N. brasiliensis infection. (E and F) TGF-β1 levels, as determined by ELISA and qPCR, increased during the first 30 days of infection. The data represent the means of five mice per group ± the SEM. Statistical analysis were performed using one-way ANOVA, and the post hoc test was Tukey's (*, P < 0.05; **, P < 0.01). The data are representative of three experiments of similar experimental design.
Reduction in inflammation paralleled with Treg increment.
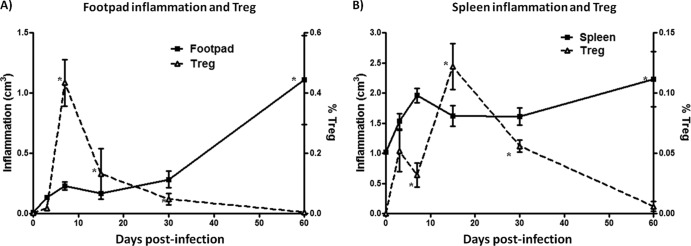
Progressive inflammation, abscess formation, and tissue destruction were observed during N. brasiliensis infection in mouse footpads as previously reported (34). The inflammation in the footpad was measured throughout the infection using a vernier, and the inflammation score was calculated using the ellipsoid equation. At 7 days postinfection, the lesions reached an average size of 0.23 ± 0.03 cm. The lesions decreased 70% in size at day 15 postinfection when Treg cells were present in high numbers compared to infected mice at day 7 (Fig. 4A). However, the lesions increased in size again at day 60 postinfection (1.10 ± 0.37 cm [P < 0.05]). Infection with N. brasiliensis also resulted in splenomegaly; thus, we measured the size of the spleen from each mouse. Interestingly, the changes in spleen size followed a trend similar to that described for the footpads. Thus, the size of the spleen was lower at 15 days postinfection than at 60 days postinfection when the size of the spleen increased by 75% (Fig. 4B).
Fig 4.
CD4+ Foxp3+ Treg cells reduce inflammation of the mouse footpad and spleen. (A) A solid line represents the foot inflammation calculated though ellipse formula, and a dashed line represents the percentage of CD4+ Foxp3+ (Treg) cells. (B) A solid line represents the spleen inflammation calculated though ellipse formula, and a dashed line represents the percentage of CD4+ Foxp3+ (Treg) cells. The data represent the means of five mice per group ± the SEM. Statistical analyses were performed using one-way ANOVA, and the post hoc test was Tukey's (*, P < 0.05). The data are representative of three experiments of similar experimental design.
IFN-γ is not capable of controlling bacterial load.
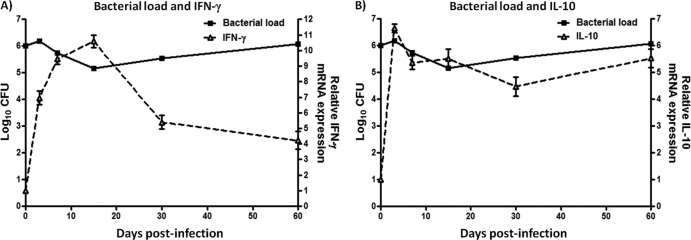
IFN-γ is essential in the control of bacteria growth due to activation of bactericidal mechanisms in macrophages (7). Thus, we questioned if the levels of expression of IFN-γ correlated with bacterial load. For this purpose, samples obtained from the footpads of each mouse were processed for qPCR or CFU analysis and used to determine the levels of IFN-γ expression and bacterial load, respectively. These analyses demonstrated that in samples obtained from the footpads at 3 to 15 days postinfection, IFN-γ expression was higher than in similar samples from the control group (P < 0.05). Increased IFN-γ expression in these samples correlated with a >1-log reduction in bacterial load (P < 0.05). Most importantly, by 15 days postinfection the bacterial load in the footpads samples had increased again, whereas IFN-γ expression decreased in the following days (P < 0.05) (Fig. 5A). Meanwhile, higher levels of IL-10 were correlated with bacterial load during chronic infection (Fig. 5B).
Fig 5.
An increase in IFN-γ did not control the bacterial load in N. brasiliensis infections. (A) A solid line represents the bacterial load obtained from mouse footpads using plating serial dilutions, and a dashed line represents the IFN-γ expression during N. brasiliensis infection. (B) A solid line represents the bacterial load obtained from mice footpad using plating serial dilutions, and a dashed line represents the IL-10 expression during N. brasiliensis infection. The data represent the means of five mice per group ± the SEM. Statistical analyses were performed using one-way ANOVA, and the post hoc test was Tukey's (*, P < 0.05). The data are representative of three experiments of similar experimental design.
DISCUSSION
Our studies have demonstrated that BALB/c mice experimentally infected with N. brasiliensis develop actinomycetoma. This chronic infection is characterized by progressive inflammation, abscess formation and tissue destruction. We found alterations in the Treg, Th17, and Tc17 cell populations and cytokine profiles in the footpads of mice at different stages of N. brasiliensis infection. Furthermore, these changes correlated with the course of the immunopathogenesis of actinomycetoma induced by N. brasiliensis.
We found that the percentages of Th17 and Tc17 cells increased at 3 days postinfection but were then downregulated when the Treg population emerged. This close relationship of Th17, Tc17, and Treg cells during the early stages of infection suggests that Treg lymphocytes may participate in the regulation of Th17 and Tc17 lymphocytes. Furthermore, the cytokine profile of immunosuppressive (TGF-β1 and IL-10) and protective cytokines (IFN-γ), along with the sustained bacterial load observed during the infection, also support a regulatory role for Treg lymphocytes during this infection. Thus, TGF-β1 and IL-10 cytokines are known to be expressed by Treg cells and can suppress IFN-γ function, with subsequent increases in bacterial load. Our data demonstrated the same trend in the upregulation of Treg numbers, TGF-β1, and IL-10 with concomitant downregulation of IFN-γ and augmentation of bacterial load in the lesions developed in the footpads of N. brasiliensis-infected mice.
It is well known that TGF-β1 and IL-6 are important cytokines involved in Th17 and Tc17 lymphocyte differentiation (11, 13). As shown here, elevated levels of TGF-β1 and IL-6 at day 3 postinfection in footpad and spleen tissues correlated with our findings of increased numbers of Th17 and Tc17 lymphocytes.
In the spleen, Tc17 lymphocytes were higher than Th17 and Treg cells, at least in the first 7 days postinfection. Differences among these three subpopulations were measured until day 30; however, N. brasiliensis has not been observed in the spleen (data not shown), which could explain the apparent T-cell activation. A possible explanation for these findings is that the soluble antigens produced by N. brasiliensis may reach the blood flow and activate T cells in this organ.
In our study, IL-6 peaked at 3 days postinfection and decreased significantly thereafter, which is in agreement with the emergence of Treg cells (mainly IL-10-producing cells) and upregulated IL-10 expression. This finding may be explained by the suppressive actions of IL-10 on IL-6 as IL-10 is known to downregulate IL-6 production in monocytes activated by lipopolysaccharide/IFN-γ in vitro (6). However, decreased IL-6 levels do not explain the presence of the Th17 and Tc17 subpopulations after 3 days postinfection. IL-23 was elevated at days 7 and 15, which may be related to Th17 lymphocyte maintenance during N. brasiliensis infection. It has been demonstrated that IL-23 produced by M. tuberculosis-infected dendritic cells stimulates the production of IL-17 by γδ T cells and other populations (21).
We found elevated levels of IL-17A expression in uninfected mice compared to N. brasiliensis-infected mice. In agreement with our results, other authors have detected high levels of IL-17A in uninfected mice with γδ T cells as the source of this cytokine (39, 48). Those findings could partially explain the high concentration of IL-17A at day 0 in our experiment. In flow cytometry analysis, we observed other cell populations positive for IL-17A. IL-17A-producing alveolar macrophages in asthmatic patients have been reported, and it has been suggested that these cells are also involved in the inflammatory process (40). In the experimental actinomycetoma model, macrophages are important cells recruited into the lesion site to phagocytose the bacteria. Our data suggest that recruited macrophages may produce IL-17A and contribute to the inflammatory process in this model. Recent publications demonstrated that IL-17-deficient mice are unable to control M. tuberculosis infection. In the present study, we report high levels of IL-17 in the early stages of N. brasiliensis infection, suggesting that this cytokine may be playing a role in early control of infection. IL-17 is a potent inducer of chemokines; some of them participate in the recruitment of neutrophil and macrophages, which in the mycobacterial animal model contribute to control infection and granuloma formation (26, 37, 44). N. brasiliensis induces granuloma formation characterized by the recruitment of neutrophils, macrophages, and lymphocytes similar to tuberculosis. In N. brasiliensis early infection, the level of IL-17 is increased, suggesting that it is responsible for recruiting neutrophils.
High levels of CD4+ Foxp3+ Treg cells were found at days 7 and 15, when the IL-10 expression and the TGF-β1 levels were also high. At day 15, footpad inflammation was reduced, which may be associated with the high levels of Treg cells and related cytokines. Therefore, the environment produced by the Treg lymphocytes could favor N. brasiliensis replication after inoculation, as has been demonstrated in several tuberculosis models. Marin et al. (23) found high levels of Treg lymphocytes in patients with active pulmonary tuberculosis compared to latent TB patients. In the same study, it was reported that Treg lymphocytes inhibited IFN-γ production, but not Th17 production. Those findings are in agreement with our results, which show the downregulation of IFN-γ expression by the immunosuppressive environment produced by Treg lymphocytes and a reduced expression of IL-17A during infection. Another study reported an association between the IL-10 produced by Treg cells with anergy status to tuberculin skin test found in patients with active tuberculosis (3). Shafiani et al. (38) reported that Treg cells depress T-cell-mediated immune responses to the protective mycobacterial antigen HBHA during active TB in humans. These data have demonstrated the role of Treg lymphocytes in infection and chronic disease. Other studies using parasites have demonstrated that Plasmodium vivax and Strongyloides ratti induce regulatory T cells, which have been associated with high parasite load and impaired parasite clearance, respectively (2, 4, 15). Furthermore, it was demonstrated that regulatory T cells favor persistent Salmonella infection (15). However, inactivation of Treg cells during M. tuberculosis infection increases cytokine production but not the bacterial load (29).
Our results suggest that there is a synergistic effect between TGF-β1 and IL-10 during N. brasiliensis infection. However, TGF-β1 is also an important cytokine involved in the differentiation of the studied lymphocyte subpopulations. In tuberculosis studies, it has been described that TGF-β1 downregulates phagocytic receptors and suppresses antimicrobial activity (43, 45, 46). Our results are in agreement with those found in tuberculosis models. Although Treg cells are important source of IL-10, many other cells produce this cytokine, including macrophages (25) and dendritic cells (42). In the early stage of infection, a high number of Treg cells and elevated IL-10 were detected; however, these cells were diminished by 15 days postinfection. These data suggest that IL-10 detected during the following days could have been produced by macrophages and dendritic cells in the lesion site. IL-10 has been associated with the susceptibility with several intracellular infections, such as M. tuberculosis (41), Listeria monocytogenes (27), and Salmonella (28). It is known that IL-10 suppresses the mycobactericide mechanisms, and IL-10-deficient mice showed an increased protection against M. tuberculosis infection (30, 36). Moreover, IL-10 has been implicated in the promotion of the tuberculosis progression (1). We demonstrated here that IL-10 levels increased during N. brasiliensis infection, and a correlation between IL-10 and bacterial load was observed in chronic infection. Redford et al. (31) proposed that IL-10 is associated with the ability of M. tuberculosis to evade the immune response and its persistence in the lung. The findings presented here are in agreement with these studies using an intracellular bacterium such as N. brasiliensis that also induces high levels of IL-10 creating an immunosuppressive environment to evade the immune response favoring its growth and persistence in the footpad.
Although Th1 lymphocytes were not assessed in our study, IFN-γ expression was analyzed (main Th1 cytokine). The IFN-γ levels were highest at 15 days postinfection, at which point the bacterial load decreased by >1 log thereafter, suggesting an effective host Th1 response. Elevated levels of IL-17A and IL-23 were found at the same time point. Interestingly, a significant increment in bacterial load in the subsequent days was associated with a reduction in the levels of IFN-γ- and Th17-related cytokines and a predominantly immunosuppressive environment in the lesion site. These findings suggest that these Th1- and Th17-related cytokines could play an important role in bacterial load control. Regulatory T cells inhibit γδ T cells to produce IFN-γ in response to M. tuberculosis antigen (19).
In summary, we report here that N. brasiliensis modulates the Th17/Tc17 and Treg cells in the early stages of infection and induces an immunosuppressive microenvironment in BALB/c mice (in the early and chronic stages of the infection) favoring bacterial growth and chronic disease.
ACKNOWLEDGMENTS
This study was supported by a grant to A.G.R.-T. from the Programa Para el Mejoramiento del Profesorado (PROMEP). P.B.-I. received a scholarship from PROMEP.
We thank Mercedes Gonzalez-Juarrero for reading the manuscript and providing insightful comments. We are grateful to Alejandra Gallegos and Reynaldo Rodriguez for technical support.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Beamer GL, et al. 2008. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J. mice. J. Immunol. 181:5545–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blankenhaus B, et al. 2011. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 186:4295–4305 [DOI] [PubMed] [Google Scholar]
- 3. Boussiotis VA, et al. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 105:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bueno LL, et al. 2010. Plasmodium vivax: induction of CD4+ CD25+ FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One 5:e9623 doi:10.1371/journal.pone.0009623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corthay A. 2009. How do regulatory T cells work? Scand. J. Immunol. 70:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding AH, Nathan CF, Stuehr DJ. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407–2412 [PubMed] [Google Scholar]
- 8. Euzeby JP. 1997. List of bacterial names with standing in nomenclature: a folder available on the Internet. Int. J. Syst. Bacteriol. 47:590–592 [DOI] [PubMed] [Google Scholar]
- 9. Fletcher JM, et al. 2009. CD39+ Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 183:7602–7610 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez-Suarez ML, Salinas-Carmona MC, Perez-Rivera I. 2009. IgM but not IgG monoclonal anti-Nocardia brasiliensis antibodies confer protection against experimental actinomycetoma in BALB/c mice. FEMS Immunol. Med. Microbiol. 57:17–24 [DOI] [PubMed] [Google Scholar]
- 11. Gottfried-Alber TK. 2007. Regulation of protective and pathogenic Th17 response. Curr. Immunol. Rev. 3:3–16 [Google Scholar]
- 12. Hamada H, et al. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182:3469–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huber M, et al. 2009. A Th17-like developmental process leads to CD8+ Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39:1716–1725 [DOI] [PubMed] [Google Scholar]
- 14. Iwakura Y, Nakae S, Saijo S, Ishigame H. 2008. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 226:57–79 [DOI] [PubMed] [Google Scholar]
- 15. Johanns TM, Ertelt JM, Rowe JH, Way SS. 2010. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 6:e1001043 doi:10.1371/journal.ppat.1001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khader SA, Cooper AM. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khader SA, et al. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175:788–795 [DOI] [PubMed] [Google Scholar]
- 18. Kolls JK, McCray PB, Jr, Chan YR. 2008. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 8:829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Wu CY. 2008. CD4+ CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M tuberculosis antigen ESAT-6. Blood 111:5629–5636 [DOI] [PubMed] [Google Scholar]
- 20. Liang SC, et al. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lockhart E, Green AM, Flynn JL. 2006. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177:4662–4669 [DOI] [PubMed] [Google Scholar]
- 22. Lopez Martinez R, et al. 1992. Epidemiology of mycetoma in Mexico: study of 2,105 cases. Gac. Med. Mex. 128:477–481 (In Spanish.) [PubMed] [Google Scholar]
- 23. Marin ND, et al. 2010. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis (Edinb.) 90:252–261 [DOI] [PubMed] [Google Scholar]
- 24. McNeil MM, Brown JM. 1994. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin. Microbiol. Rev. 7:357–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765 [DOI] [PubMed] [Google Scholar]
- 26. Okamoto Yoshida Y, et al. 2010. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184:4414–4422 [DOI] [PubMed] [Google Scholar]
- 27. Pasche B, et al. 2005. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect. Immun. 73:5952–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pie S, Matsiota-Bernard P, Truffa-Bachi P, Nauciel C. 1996. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect. Immun. 64:849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quinn KM, et al. 2006. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 84:467–474 [DOI] [PubMed] [Google Scholar]
- 30. Redford PS, et al. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40:2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Redford PS, Murray PJ, O'Garra A. 2011. The role of IL-10 in immune regulation during Mycobacterium tuberculosis infection. Mucosal Immunol. 4:261–270 [DOI] [PubMed] [Google Scholar]
- 32. Salinas-Carmona MC. 2000. Nocardia brasiliensis: from microbe to human and experimental infections. Microbes Infect. 2:1373–1381 [DOI] [PubMed] [Google Scholar]
- 33. Salinas-Carmona MC, Perez-Rivera I. 2004. Humoral immunity through immunoglobulin M protects mice from an experimental actinomycetoma infection by Nocardia brasiliensis. Infect. Immun. 72:5597–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salinas-Carmona MC, Torres-Lopez E, Ramos AI, Licon-Trillo A, Gonzalez-Spencer D. 1999. Immune response to Nocardia brasiliensis antigens in an experimental model of actinomycetoma in BALB/c mice. Infect. Immun. 67:2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott-Browne JP, et al. 2007. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204:2159–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreiber T, et al. 2009. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J. Immunol. 183:1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seiler P, et al. 2003. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 33:2676–2686 [DOI] [PubMed] [Google Scholar]
- 38. Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. 2010. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207:1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith SS, Barnum SR. 2008. Differential expression of beta 2-integrins and cytokine production between γδ and αβ T cells in experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 83:71–79 [DOI] [PubMed] [Google Scholar]
- 40. Song C, et al. 2008. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181:6117–6124 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan BM, et al. 2005. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-gamma production. J. Immunol. 175:4593–4602 [DOI] [PubMed] [Google Scholar]
- 42. Svensson M, Kaye PM. 2006. Stromal-cell regulation of dendritic-cell differentiation and function. Trends Immunol. 27:580–587 [DOI] [PubMed] [Google Scholar]
- 43. Takaki H, et al. 2006. TGF-β1 suppresses IFN-γ-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Genes Cells 11:871–882 [DOI] [PubMed] [Google Scholar]
- 44. Torrado E, Cooper AM. 2010. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 21:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tridandapani S, et al. 2003. TGF-β1 suppresses myeloid Fc gamma receptor function by regulating the expression and function of the common gamma-subunit. J. Immunol. 170:4572–4577 [DOI] [PubMed] [Google Scholar]
- 46. Tsunawaki S, Sporn M, Ding A, Nathan C. 1988. Deactivation of macrophages by transforming growth factor-beta. Nature 334:260–262 [DOI] [PubMed] [Google Scholar]
- 47. van de Veerdonk FL, et al. 2009. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 42:776–787 [DOI] [PubMed] [Google Scholar]
- 48. von Vietinghoff S, Ley K. 2009. IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. J. Immunol. 183:865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]